Abstract
Photobiomodulation (PBM) is a treatment method based on research findings showing that irradiation with certain wavelengths of red or near-infrared light has been shown to produce a range of physiological effects in cells, tissues, animals and humans. Scientific research into PBM was initially started in the late 1960s by utilizing the newly invented (1960) lasers, and the therapy rapidly became known as ‘low-level laser therapy”. It was mainly used for wound healing and reduction of pain and inflammation. Despite other light sources being available during the first 40 years of PBM research, lasers remained by far the most commonly employed device, and in fact, some authors insisted that lasers were essential to the therapeutic benefit. Collimated, coherent, highly monochromatic beams with the possibility of high power densities were considered preferable. However in recent years, non-coherent light sources such as light-emitting diodes (LEDs) and broad-band lamps have become common. Advantages of LEDs include no laser safety considerations, ease of home use, ability to irradiate a large area of tissue at once, possibility of wearable devices, and much lower cost per mW. LED photobiomodulation is here to stay.
Keywords: photobiomodulation therapy, low-level laser (light) therapy, light emitting diodes, mechanisms: medical indications
1. Introduction
Distinct wavelengths of light have been known to have various biological effects on humans. Ultraviolet-B radiation promotes vitamin D synthesis and visible light has important effects on circadian rhythm entrainment and alertness. For more than three thousand years, sunlight has been used as a medical treatment for a variety of diseases by the ancient Egyptians, Indian Ayurveda and traditional Chinese medicine, but it is only since the invention of the electric light in the latter part of the 19th century, that an alternative has emerged.
Since the beginning of the 21st century, over 2000 PubMed-indexed scientific articles have also been published focusing on the various physiological effects of red light and near-infrared radiation. These wavelengths of light have been shown to penetrate through human tissues and to locally (and possibly systemically) affect cellular metabolism, cellular signaling, inflammatory processes and growth factor production.
This treatment is nowadays called “photobiomodulation therapy” (PBM), but it has also had more than 60 other names in the scientific literature; “low-level laser therapy” (LLLT) has been the most commonly used term. The reasons to prefer the use of “PBM” over “LLLT” are twofold [1]. Firstly PBM does not imply that a laser is necessary for the therapeutic benefits to occur. Secondly PBM implies that the therapeutic effects could in some circumstances be due to inhibition effects, as well as to the more usual stimulation effects.
Table 1 illustrates various medical conditions (or their animal models), for which PBM has already been investigated, in animals and/or clinical human studies. These indications include a multitude of diseases of brain, bone, eyes, internal organs, connective tissue, skin and muscles. Most of the published results have been positive. More than 40 clinical studies are currently underway based on information currently available in the ClinicalTrials.gov database.
Table 1:
Medical indications studied in photobiomodulation research
| Acne | Crescentic glomerulonephritis | Liver regeneration | Periodontitis |
| Achilles tendinitis | Delayed hypersensitivity | Lung fibrosis | Peritonitis |
| Acute pain | Dentin regeneration | Lung hemorrhage | Pleurisy |
| Acute respiratory distress syndrome | Depression | Lung inflammation | Pressure ulcer |
| Adipose tissue inflammation | Dermal abrasions | Lung injury | Radiation injury |
| Age-related macular degeneration | Diabetic kidney | Lymphedema | Restenosis |
| Allergic asthma | Diabetic eyes | Mastitis | Retinitis pigmentosa |
| Allergic contact dermatitis | Diaphragm muscle dysfunction | Methanol toxicity of retina | Rheumatoid arthritis |
| Allergic rhinitis | Eardrum perforation | Morphine withdrawal | Sarcopenia |
| Allodynia | Endophthalmitis | Multiple sclerosis | Sciatica |
| Alzheimer’s disease | Exercise performance | Muscle injury | Spinal cord injury |
| Amyotrophic lateral sclerosis | Haemarthrosis | Myocardial infarct | Stroke |
| Aneurysm | Hair loss | Myonecrosis | Submandibular gland inflammation |
| Arthritis | Heart failure | Myopathy | Surgical wound infection |
| Atherosclerosis | Hearing loss | Nerve injury | Teeth re-implantation |
| Atrophic gastritis | Hyperalgesia | Neuropathic pain | Tendinopathy |
| Auditory neuropathy | Hypertension | Oral mucositis | Thrombocytopenia |
| Bone fracture | Kidney fibrosis | Oral ulcer by formocresol | Tinnitus |
| Bone grafts | Kidney injury | Osteoarthritis | TMJ inflammation |
| Burn injury | Laryngitis | Osteomyelitis | Tracheal incision healing |
| Cancer | Ligament injury | Osteoporosis | Traumatic brain injury |
| Colitis | Listeria infection | Parkinson’s disease | Wound healing |
| COPD | Liver cirrhosis | Paw edema |
2. Mechanisms of photobiomodulation
It has been shown that many cellular molecules are able to absorb various wavelengths of light. In photobiomodulation with visible red light and near-infrared radiation, evidence suggests that the primary cellular photoacceptors are the copper centers of cytochrome c oxidase (CCO), a complex protein functioning as unit IV in the mitochondrial electron transport chain [2, 3].
It appears that specific wavelength ranges of red light and near-infrared radiation can be utilized to promote electron transport, based on a multitude of findings showing increased mitochondrial membrane potential, oxygen consumption and ATP levels after irradiation. There is also some preliminary evidence suggesting that some other wavelengths can be used to inhibit electron transport, which could be useful in the treatment of ischemia-reperfusion damage [4].
The physiological effects of longer wavelengths than 900nm might, on the other hand, depend on transient receptor potential (TRP) calcium channels [5]. Photobiomodulation-like effects have also been observed with blue and green light, and it is hypothesized that these effects might also be mediated by calcium channels [6]. While plenty of basic research on photobiomodulation has already been published, there is a lot of room for additional experiments examining the exact molecular mechanisms of light-cell-interactions.
The initial interaction between light and cellular photoacceptors (called a “primary photoreceptor mechanism”) is followed by the activation of multiple secondary mediators. These eventually lead to broad shifts in gene expression, cell signaling, cellular metabolism and cytokine secretion. These effects been described in numerous review articles in the literature [7, 8].
While medical treatments are often able treat only location-specific diseases, the observed effects of PBM on more than a hundred different treatment indications might be related to its observed mitochondrial effects. Since majority of aging-related chronic diseases have been linked to dysfunctional mitochondria and oxidative stress, it seems plausible that improving the mitochondrial function and antioxidant defenses could also alleviate these diseases [9, 10]. Since many chronic diseases share common metabolic causes, systemic treatment methods that alter metabolism could alleviate ailments of many different body parts.
The effects of PBM could possibly be compared with the mitochondrial-boosting and health-supporting effects of supplements, such as nicotinamide riboside (NR), an NAD+ precursor. NR has been shown to protect against animal models of metabolic syndrome, liver disease, stroke, Alzheimer’s disease, heart failure and myopathy [11]. Early PBM studies also showed comparable effects between red light and a chemical called methylene blue, which has also been shown to improve mitochondrial respiration and simultaneusly protect animals from a remarkably wide range of chronic diseases [2, 12, 13].
3. Lasers and light-emitting diodes (LEDs) in photobiomodulation
3.1. Lasers
Laser is an acronym for “light amplification by stimulated emission of radiation”. Lasers are light sources that utilize the physical phenomenon of stimulated emission to create a monochromatic and coherent beam of light of low divergence. The first working laser was invented in 1960 by Theodore Maiman [14] who was the first past the finishing post in an epic race that came to be called “The race to make the laser” [15].
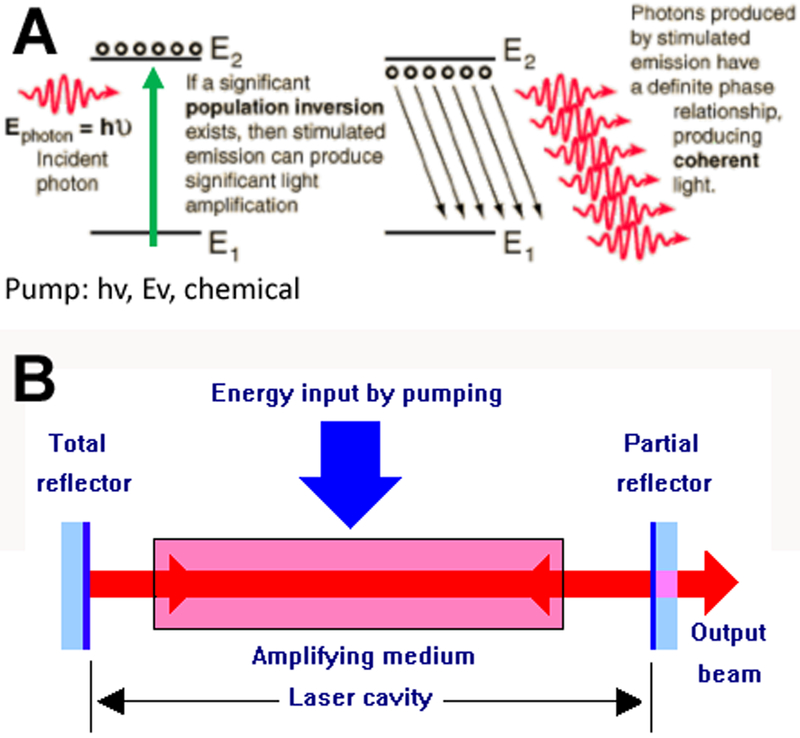
The basic mechanims of action of a laser is shown in Figure 1. It relies on pumping the electrons of a “laser gain medium” E1 to an excited state E2 using light, electricity or a chemical reaction as the energy source. Once a majority of electrons are in the excited state (population inversion) an incoming photon Ephoton will lead to stimulated emission of a torrent of new photons (coherent and polarized) and the light will be amplified (Figure 1A). Mirrors placed at either end of the laser cavity allow the light to bounce back and forth while pumping continues leading to significant aplification. One of the mirrors is only partly reflective to allow the laser beam to escape from the cavity (Figure 1B).
Figure 1. Basic mechanism of operation of a laser.
(A): Principle of population inversion and stimulated emission; when a majority of the atoms in a laser material are pumped to a higher electronic state, an incoming photon can cause release of the excess energy as coherent photons of the same wavelength. (B) Principle of a laser cavity confined by two mirrors, one of which is partially transmissive.
Modern photobiomodulation literature is founded on the basis of original findings by Endre Mester, a physician from Hungary, who published a report describing hair-growing effects in mice treated with ruby laser (694 nm) in the late 1960s [16]. After that, he supplemented these findings by clinical reports suggesting that red laser light could improve the healing of various ulcers in humans [17].
These initial observations soon led to additional scientific studies investigating the effects of red and near-infrared wavelengths of laser light in the treatment of a huge variety of chronic diseases. The Soviet Union was a pioneering country by conducting early research with helium-neon (He-Ne laser) in the 1960s and 1970s. Tiina Karu working in Troitsk in Russia published over 100 papers in this field (https://www.isan.troitsk.ru/dls/karu.htm). By the end of 1980s, many research groups around the world (eg. USA, Japan, Sweden, Israel, Italy) had already started their photobiomodulation research projects. Nowadays PBM has been studied in approximately 40 different countries [18, 19].
To this day, more than 3500 scientific articles on photobiomodulation have been published. Approximately 85–90% of the original research has utilized lasers as light sources. Practically all of the photobiomodulation research before the 21th century was based on lasers.
This laser-centered history of photobiomodulation has been the reason fot the assumption that the beneficial physiological effects of red and near-infrared light are somehow dependent of the “laser properties” of light, such as monochromaticity, coherence, collimation or polarization. According to current knowledge, this is certainly debatable and probably not true, and will be critically discussed in this review.
3.2. LEDs
Light-emitting diodes (LEDs) are light sources based on the phenomenon of electroluminescence of semiconductor materials, most often InGaN (60%) and AlInGaP (38%) [20]. The earliest historical accounts of LEDs were written by Henry Round and Oleg Losev in the 1907 and 1927, respectively. These scientists showed that crystals of a semiconductor material, silicon carbide (SiC), glowed when an electrical current passed through them.
Nick Holonyak, Jr. (born November 3, 1928) invented the first visible LED in 1962 while working as a consulting scientist at a General Electric Company laboratory in Syracuse, New York, and he has been called “the father of the light-emitting diode” [21]. A few decades later, electroluminescence properties of other semiconductors were studied especially in the United States, which eventually led to the invention of orange, yellow and green LEDs in the 1960s and 1970s. Since then, LED technology has undergone many improvements, and LEDs represent a growing portion of the sales of indoor, and even outdoor lighting. Nowadays one of the most important challenges in the industry is to improve the luminous efficacy of LEDs [22].
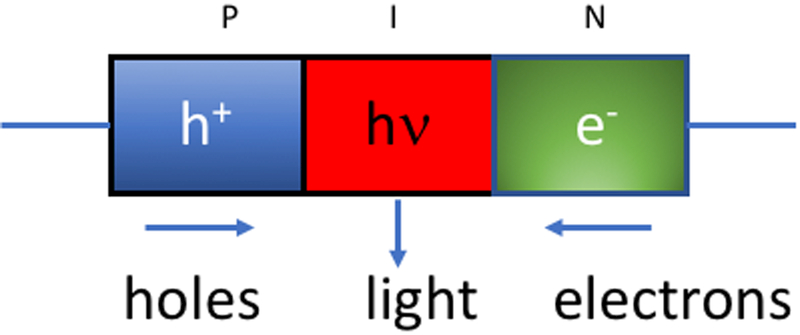
It should be noted that the basic principle of operation is the same in LEDs and diode lasers, and is termed the PIN semiconductor diode (Figure 2). An electric potential applied to the semiconductor causes separation of electrons in the N (negative)-section and holes in the P (positive)-section. When the electrons and holes recombine in the I (intrinsic)-section, light is produced whose wavelength depends on the energy of the electrons. In order to produce a laser diode a waveguide is applied to the outside of the PIN diode which acts in the same way as the mirrors in the traditional laser cavity.
Figure 2. Basic structure of an LED.
In a PIN-type semiconductor, positive holes occur in the P-region, negative electrons in the N-region, and these recombine in the I (intrinsic)-region to give non-coherent light whose wavelength is determined by the semiconductor composition
Unlike incandescent and halogen lamps, which are based on electrical resistive heating and subsequent thermal radiation including both visible wavelengths and infrared radiation, LED light emission is based on non-thermal emission of light.
An important difference between laser light and LED light (in addition to coherence discussed below) is the band width. Lasers can have a very narrow bandwidth; for instance in gas lasers it can be a fraction of a nanometer, while in diode lasers the bandwidth is typically 1–2 nm.
Photobiomodulation by light-emitting diodes is a relatively new phenomenon. With the exception of a few papers published towards the end of the 20th century, LED-LLLT (or LED-PBM) has started appearing regularly in the literature only since 2001. In these early years, some of the basic research with LEDs was conducted by Harry Whelan’s group located in Wisconsin-Milwaukee [23]. Because the development of these LEDs was funded by the US National Aeronautic and Space Administration (NASA) as a light source for plant growth experiments in space, they were often referred to as NASA LEDs [24].
Nowadays the use of LEDs in photobiomodulation and other healthcare applications has been quite well established and their efficacy has been demonstrated in many reports [25].
LEDs are much cheaper than laser devices on average. As a rule of thumb, the cost per mW of optical power is approximately one hundred times lower for LEDs compared to lasers. In the past, laser light sources were predominantly marketed to clinicians, who could cover the high costs of their devices by treating a large number of patients. However, during the recent years, patients themselves or even healthy individuals, have been able to buy their own LED devices for personal use at home. Various LED-based quasimonochromatic in-home devices are nowadays widely available, and the prices appear to be steadily falling due to increasing demand and competition between companies.
One of the most important factors hindering the acceptance of PBM/LLLT in the healthcare is cost-effectiveness. In addition to the high device prices, additional costs come when the therapeutic session is carried out by healthcare practitioners such as physical therapists, chiropractors, nurses or physicians. Only in few cases is this cost-effective when the lasers are used [26]. In this sense, the adoption of LED lights into PBM/LLLT treatment practices could support the wider acceptance of PBM/LLLT by the medical community.
Also, multiple LEDs can be arranged into planar arrays. This increases the beam area significantly, making it easier to treat large body areas, which has been a limitation of lasers that typically have tiny to small spot sizes. The only limit on the power output of a LED array is caused by the need to remove heat from the actual diodes. Since the typical LED is only 20–30% efficient in converting electrical energy to light energy, this means that heat is generated and excessive heat can lead to degradation of the semiconductior material and reduction in its lifetime. Moreover if the LED array is designed to be used in contact with the tissue, it cannot be allowed to get too hot. Heat is removed by heat-sinks (heat conducting metal substrates) or in some cases by incorporation of a small fan to cool the diodes.
Flexible wearable LED arrays are becoming available for use as bandages (to be wrapped around joints for instance). There is a flexible LED belt designed to be wrapped around the abdominal area for fat reduction. Light emitting clothing might become a future way to apply light [27]. Therapeutic lasers have been integrated into caps, helmets, and hair combs for stimulation of hair regrowth [28]. With LEDs, the same applications would cost less to the customers. However the laser hair growth industry has so far remained committed to lasers at the expense of LEDs, and several manufacturers maintain that red lasers (~650 nm) are the best light source to stimulate the hair follicle and its progenitor cells.
4. What the research literature says about laser vs non-coherent light?
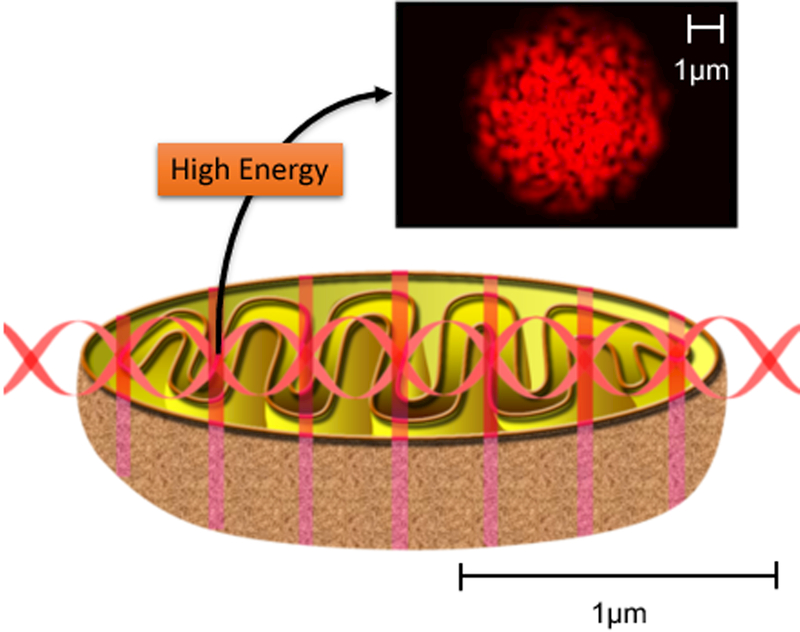
There are several properties of lasers that proponents claim may be reasons why laser light is superior to LED light for PBM. The most often discussed property is that of coherence. Laser devices generate coherent light with various coherence lengths depending on the band-width of the specific laser. Coherence lengths of lasers can range from many meters for the He-Ne laser to only a few mm for diode lasers. When coherent laser light interacts with tissue, small imperfections in the tissue structure lead to different phases occurring in the individual wavefronts leading to mutual interference patterns. These interference patterns are called “laser speckles” and the size of the speckles is related to the wavelength of the light. In the visible range speckles are less than 1 micron in diameter. Subcellular organelles (such as mitochondria) have dimensions of this order and one theory proposes that the laser speckles are better able to stimulate mitochondria than non-coherent LED light [29–31] as illustrated in Figure 3.
Figure 3. Proposed mechanism of mitochondrial stimulation by laser speckles.
The size of the laser speckles approximately matches the size of mitochondria inside the cell.
Another very common assertion is that lasers penetrate deeper than LEDs. The so-called “superpulsed” gallium-arsenide laser at 905 nm emits light with a pulse duration of around 100–200 nsec. The pulse frequency can be varied from 25 Hz to 5000 Hz. The typical average power is 60 mW and the peak power is therefore about 20W. The depth into tissue at which a threshold power density is obtained, is directly related to the power density at the surface, and this means that manufacturers claim deeper penetration. However it is usually not mentioned that the actual amonut of energy that penetrates to a depth is only a fraction since the pulses are only “on” a small fraction of the time. Another way to generate pulsed laser light is simply to “chop” the beam, i.e. turn the laser on and off. A review paper [32] examined the effect of pulsing in PBM and concluded “There is some evidence that pulsed light does have effects that are different from those of continuous wave light. However further work is needed to define these effects for different disease conditions and pulse structures.”
There is also an argument that a collimated laser beam is more likely to be forward scattered in tissue than a divergent LED beam.
In some published editorials and review articles, it has been emphasized that photobiomodulation is a “photobiological phenomenon” and coherence is not necessarily needed [31, 33–35]. Some old laboratory studies with cell cultures also have concluded that coherence is not needed for photobiological effects of red light [36, 37]
Following the understanding that the photobiological effects of red and near-infrared light do not apply solely to laser light, some authors have started renaming the “LLLT” from low-level laser therapy to low-level light therapy [38].
However, other authors have still continued to insist that coherence is still important, making even bold statements that “[w]henever compared, coherent light has so far demonstrated better results than non-coherent light”, however softening these statements by admitting that “[t]he superiority of coherent light is shown to be relevant only for bulk tissue” and that “[t]he pain-relieving and healing effect in superficial wounds may be good also for both coherent and non-coherent polarized light” [30].
In the most extreme end, supporters of laser sources have used statements such as “would you take a knife to a gunfight - would you use LEDs instead of a laser”, claiming that the effectiveness of LED-based photobiomodulation is negligible compared to lasers. Our counter-word to that kind of argument would be that PBM is not about fights or cutting; instead it is ultimately a photobiological phenomenon dependent on light absorption to the photoacceptor molecule.
This debate about the importance of coherence or other laser-specific properties for photobiomodulation has been ongoing for more than 30 years already, as can be seen from Table 2 which presents some relevant quotes from the literature of the field.
Table 2:
Quotes showing variable opinions regarding the importance of coherence in PBM
| Ref | Quote |
|---|---|
| Greguss (1984) [64] | “[We] concluded that low-level laser irradiation, when having a biostimulating effect, is not laser specific” |
| Karu (1988) [65] | “Renewed interest in the effects of visible light action on biological objects occurred in the sixties after appearance of the first lasers, particularly the He-Ne laser. The He-Ne laser was the first widely accessible source of coherent light. No wonder that the stimulating effect of light, red in particular, was rediscovered with the use of the coherent light source. Recent literature suggest experiments on the action of incoherent light on biological objects performed in the twenties and the thirties [6–8] were either forgotten or were not known. So, the observed effects were attributed to a unique property of the He-Ne laser light, coherence of of its radiation. Actually there were no physical grounds for such a conclusion [2].” |
| Devor (1990) [66] | “If a red flashlight with mystical labels is as effective as a $10,000 laser instrument, who wins when the laser is purchased?” |
| Ohshiro (1990) [67] | “‘Laser is important/Laser is not important/polarization is important/wavelength is important’ are also very common arguments heard at congresses and in papers: however, they are basics, and are fundamental to the correct understanding of what LLLT is or is not. Perhaps ‘Low reactive-Level Laser Therapy’ will become ‘Low reactive-Level Light Therapy’, fortunately they will share the same acronym!” |
| Laakso (1993) [35] | “Whether the effects gained by laser could be obtained as easily by cheaper (near-monochromatic) light emitting sources is yet to be fully established. The weight of research evidence to date indicates that there are few convincing arguments for the use of true laser. Photostimulation occurs using both true laser light and near-monochromatic but non-coherent, non-collimated light. On this basis, it seems unnecessary for the clinician to spend large sums of money on laser apparatus when simpler and inexpensive light sources may suffice.” |
| Karu (1999) [2] | “Conventional light sources generating the appropriate wavelength can also be used (...) Laser sources are just handy tools providing many practical advantages (e.g., efficient fiber-optic coupling to irradiate interior body parts, high monochromaticity and easy wavelength tunability, simplicity of use and electrical safety in the case of semiconductor lasers.” |
| Hode&Tunér (2000) [41] | “Till today, there is no study showing that LEDT is better than LLLT; and there is no study showing that LEDT is as good as LLLT.” |
| Smith (2005) [31] | “More and more papers are appearing in the therapy literature using non-coherent light sources such as LEDs. In general, they are less expensive than lasers, and as discussed above, in phototherapy it is the wavelength of the light that is important, not the coherence or lack of same.” |
| Enwemeka (2005) [34] | “Coherence may influence light distribution in tissue, and it can be demonstrated that lasers produce light speckles or pockets of intense light within tissues; noncoherent monochromatic and polychromatic lights do not.7 However, this physical difference has not been shown to produce any additional benefits in favor of treatment with lasers.” |
| Hashmi (2010) [32] | “In recent years, the development of light-emitting diodes (LEDs) as alternative light sources for LLLT has added to the confusion. These devices produce light with wavelengths similar to those of lasers, but they have broader output peaks (ie, they are less monochromatic) and lack the coherence that is a particular feature of laser light. LEDs have the advantage of being significantly less expensive than laser diodes (by a factor of approximately 100 on a milliwatt basis), and the LLLT community is engaged in a vigorous ongoing debate about their respective benefits.” |
| Smith (2010) [68] | “Phototherapy, whether using low intensity radiation of the proper wavelength from a laser, an LED, or a filtered incandescent lamp, can be beneficial in a munber of clinical situations, from pain remission to wound healing.” |
| Moskvin (2017) [40] | “[All] studies, in which the comparison is carried out correctly and close parameters of the impact and the model are used, have a firm conclusion that laser light is much more effective. (…) [It] is uniquely identified that the most important parameter that determines the efficiency of lasers is monochromaticity, i.e., a much narrower spectral width than for all other light sources. Only laser light sources can be used for LLLT!” |
| Brochetti et al. (2017) [69] | “Laser is characterized by coherence while LED is non-coherent. However, the coherence of laser light is not responsible for the effects of therapy, because this property is lost in the first layers of biological tissues [5, 6, 7]. Thus, despite of the light emission of laser to be different from LED, we admit based on the literature that the effects of both are similar.” |
| Henderson and Morries (2017) [70] | “These milliwatt LED things — that yahoos here in town and elsewhere say they’re using to treat traumatic brain injury — don’t even get through the skin,” he declares. “LEDs are great if you’re trying to treat the skin. LEDs for acne? Wonderful. But if you’re trying to treat the brain, you’d better get serious.” |
| Salehpour et al. (2018) [71] | “Overall, LED therapy appears to be similarly effective as laser therapy for superficial tissue, but for transcranial brain PBM, comparisons between these sources seem to be in favor of lasers for deeper penetration. On the other hand, LED arrays are less expensive and can irradiate much larger surface areas of tissue (particularly use- ful for the head, e.g., frontal region).” |
In their book on low-level laser therapy, Tunér and Hode presented an argument about the superiority of laser compared to LEDs, supported by approximately 15 study references published in the years 1973–2000, which they also described concisely. These same studies have been also been referenced in other recent articles with similar claims about photobiomodulation light sources [39–41].
We summarize some of these papers in the Table 3. Despite our efforts, we could not find the full texts of the papers by Bihari (1989), Kubota (1989), Nicola (1989), Onac (1998) and Paolini (2000) mentioned in these papers. We also excluded Lederer (1982) and Nicola (1994) due low quality of data reporting.
Table 3:
Laser versus non-coherent comparison trials 1982–1996
| Study | Study type | Parameters (non-laser) | Parameters (laser) | Results |
|---|---|---|---|---|
| Haina (1982) [72] | Rat | 630nm; 4 J/cm2 | 633 nm; 90 mW; 50 mW/cm2; 0.5, 1.5, 4, 10 or 20 J | Both He-Ne laser and non-coherent red light with similar parameters increased granulation tissue in wounds significantly, but He-Ne laser had a notably more pronounced effect. |
| Muldiyarov (1983) [73] | Rat | “Ordinary incandescent lamp with a simple red filter” | 633 nm; 1 – 1.5 mW/cm2; 120 sec | He-Ne laser decreased synovitis, while ordinary red light had no effect. |
| Berki (1988) [74] | In vitro, lymphoid cels and macrophages | 633 nm; 5.6 mW; 0.14 – 14.0 J/cm2 | 633 nm; 5.6 mW; 1 J; 0.14 – 28.0 J/cm2; 180 sec | Laser light appeared to kill cells on higher doses, while non-coherent filtered light from xenon arc lamp with similar parameters didn’t have this effect in this study. |
| Rosner (1993) [75] | Rat | 904 nm; 10 or 15 mW; 2 min | 633 nm; 3.5–10.5 mW; 1–10 min; many experimental groups with different dose parameters | He-Ne laser showed beneficial effects on the action potential amplitude with several of the studied parameters, although there were also multiple ineffective parameters. The non-coherent light did not show beneficial effects with the studied parameters. There were also some groups treated with He-Ne laser or non-coherent light that showed lower action potential amplitude than the nonirradiated controls |
| Laakso (1994) [76] | Human, RCT, double-blind | 660 nm; 9.5 mW; 1 or 5 J/cm2 | 670 nm; 10 mW; 1 or 5 J/cm2 | It was reported that (only) coherent light was able to potentiate the plasma levels of ACTH and β-endorphin. However, while there were some statistically significant changes in individual timepoints, the changes didn’t follow any clear patterns and the magnitude of changes was relatively low to make clear clinical interpretations. |
| Antipa (1996) [77] | Human | 750 nm; 9 mW; 1.08 J/cm2; 0.50 cm2; 60 s | 720 nm; 3 mW; 1.08 J/cm2; 0.50 cm2; 180 s | Laser light had better efficacy (66.7%) than noncoherent light (52%) and placebo light (36.4%). However, the difference between laser and noncoherent light was not statistically significant |
While most of these studies have not been indexed in PubMed and they represent fairly old and low-quality photobiomodulation literature, they indeed lend some support to the idea that laser light could be more effective than narrow-band non-coherent light with comparable main parameters (wavelength, power output, energy density). However, some papers with more neutral results regarding the coherence appear to have been left out from this list [42–44].
Our current review is based on our self-made PBM research database including approximately 3500 research articles. The spreadsheet has been assembled within the past two years by repeatedly scanning the research literature with a large variety of keywords such as “photobiomodulation”, “LLLT”, “low-level laser irradiation”, “cold laser”, “He-Ne laser”, “soft laser”, “transcranial laser”, “670nm light”, “low-level light”, “near-infrared”, “light-emitting diode”, “LED phototherapy”, “narrow-band light”, “photobioactivation”, “photobiostimulation”, “photo-enchancement”, “photoradiation”, “photostimulation” and “far-red light” among others.
From this above-mentioned spreadsheet, we were able to extract another spreadsheet of approximately 350 scientific articles examining specifically LED photobiomodulation in humans, animals or cell cultures. Most of these studies showed positive results, confirming that LED-based photobiomodulation can also bring observable therapeutic effects (Supplementary file 1).
In this sample, we also found approximately 40 newer papers comparing the effectiveness of laser photobiomodulation to LED photobiomodulation in either animals, cell cultures or humans. The results of these studies are shortly presented in tables 4, 5 and 6, respectively. The tables are not comprehensive, since we excluded a few papers due to difficulties in interpreting either the methods or the results [45–48].
Table 4:
Laser versus non-coherent light comparisons (animal research)
| Study | Animal | Indication | LED/non-coherent | LASER parameters | Results |
|---|---|---|---|---|---|
| Campos (2016) [78] | Hamster | Oral mucositis | 635 nm; 120 mW; 1.2 J/cm2; 1.2 J; 10 s; 0.04 cm2 | 660 nm; 40 mW; 6 J/cm2; 1.2J; 36 s; 1 cm2 | LED and laser both were effective in decreasing oral mucositis severity and TNF-α concentration. |
| Freire Mdo (2014) [79] | Hamster | Oral mucositis | 670 nm; 150 mW; 4 J/cm2; 4.8 J; 16 s; 0.5 cm2 | 660 nm; 40 mW; 4.8 J/cm2; 16 J, 30 s, 4 mm2 | LED and laser both were effective in decreasing oral mucositis severity. |
| Nadur-Andrade (2014) [80] | Mouse | Paw edema from snake venom | 635 or 945 nm; 4 or 3.8 J/cm2; 41 or 38 s | 685 nm, 2.2 J/cm2; 15 s | LED and laser both were effective in reducing edema formation after snake venom injection. |
| Nadur-Andrade (2012) [81] | Mouse | Edema and hemorrhage from snake venom | 635 or 945 nm; 110 or 120 mW; 4 J/cm2; 4.5 J/point; 41 or 38 s; 1.2 cm2 | 685 nm; 30 mW; 2.2 J/cm2; 0.45 J; 15 s; 0.2 cm2 | LED and laser both were effective in decreasing venom-induced edema and hemorrhage. |
| Demidova-Rice (2007) [82] | Mouse | Excisional wound | 635, 670, 720 and 820 nm; 2 J/cm2 | 633 nm; 2 J/cm2 | LED and laser both had a similar beneficial effect on wound closure. |
| Comunian (2017) [83] | Rabbit | Mandibular socket healing after tooth extraction | 830 nm; 26 mW; 30 J/cm2; 150 s | 780 nm; 30 J/cm2; 50 s | LED and laser were both associated with improved clinical and histological signs, but only LED was associated with improved alveolar bone density. |
| Takhtfooladi & Sharifi (2015) [84] | Rabbit | Transected sciatic nerve | 650 nm; 2.4 J/cm2; 1.5 cm2 at 1 point | 680 nm; 10 mW; 10 J/cm2; 600 s; 4 mm2 at 3 points | Laser group showed signs of improved nerve regeneration, while LED group showed no improvement compared to control group. |
| Rosa (2017) [85] | Rat | Rapid maxillary expansion -related bone repair in midpalatal suture | 850 nm; 150 mW; 36 J/cm2; 18 J; 120 s; 0.5 cm2 | 780 nm; 70 mW; 450 J/cm2; 18 J; 257 s; 0.04 cm2 | LED and laser both were effective in increasing hydroxyapatite. |
| Silveira (2016) [86] | Rat | Burn wound | 632 and 850 nm; 8.4 and 19.8 J/cm2; 160 cm2; 10 min | 660 and 904 nm; 10 and 3 J/cm2; 0.10 cm2; 20 and 9 s | Only 660 nm laser and 850 nm LED were effective in reducing inflammatory response and improving wound repair. |
| de Carvalho (2015) [87] | Rat | Oral ulcer induced by formocresol | 630 nm; 150 mW; 4.8 J/cm2; 0.8 cm2 | 660 nm; 40 mW; 4.8 J/cm2; 4 mm2; | LED and laser were bot heffective in accelerating the healing of oral ulcers. |
| El-Bialy (2015) [88] | Rat | Mandibular growth | 655 nm; 10 mW/cm2; 6 J/cm2 | 655 nm; 10 mW/cm2; 6 J/cm2 | LED and laser both were effective. LED groups showed most pronounced results. |
| de Castro (2015) [89] | Rat | TMJ inflammation | 850 nm; 100 mW; 0.5 cm2 | 780 nm; 70 mW; 0.04 cm2 | LED and laser both appeared to be effective in attenuating the inflammatory infiltrate in the temporomandibular joint of rat. |
| Wu (2015) [90] | Rat (+ in vitro) |
Wound healing: wounds | 623 nm; 7–10 mW/cm2; 0.2, 1 or 5 J/cm2 | 635 nm; 10 mW/cm2; 5 J/cm2 | Organic LED and laser both were effective in accelerating the wound closure and improving total histological scores. |
| De Castro (2014) [91] | Rat | Surgical wound on dorsum | 630 nm; 10 J/cm2; 115 mW; 87 s | 660 nm; 10 J/cm2; 40 mW; 252 s | LED and laser groups showed decreased mast cells in the healing process, but in LED group this was evident earlier. Irradiation did not have any significant effect on amount of myofibroblasts. |
| Rosa (2014) [92] | Rat | Rapid maxillary expansion -related bone formation | 850 nm; 150 mW; 36 J/cm2; 18 J; 120 s; 0.5 cm2 | 780 nm; 70 mW; 450 J/cm2; 18 J; 257 s; 0.04 cm2 | LED and laser both were effective in increasing hydroxyapatite in the midpalatal suture. |
| de Sousa (2013) [93] | Rat | Cutaneous wound (angiogenesis) | 700 nm; 15 mW; 10 J/cm2; 2 cm2 | 660 or 790 nm; 60 or 50 mW; 10 J/cm2; 0.03 cm2 | LED and laser both were effective in stimulatin angiogenesis in cutaneous wounds. |
| de Oliveira (2013) [94] | Rat | Thoracic incision | 640 nm; 70 mW; 6 or 10 J/cm2; 10.1 or 18 J; 152 or 253 s; 1.77 cm2 | 660 nm; 40 mW; 6 or 10 J/cm2; 2.4 or 4 J; 60 or 100 s; 0.4 cm2 | LED and laser both were effective in increasing wound break strength and decreasing tissue deformation strength. |
| Oliveira Sampaio (2013) [95] | Rat | Cutaneous wound | 700 nm; 15 mW; 10 J/cm2; 1 cm2 | 660 nm; 40 mW; 10 J/cm2; | LED increased wound fibroblast number in anemic animals. Laser increased it in non-anemic animals. |
| De Castro (2012) [96] | Rat | Cutaneous wound (hypothyroid rats) |
630 nm; 150 mW; 24 J/cm2; 0.5 cm2 | 660 nm; 40mW; 24 J/cm2; 0.04 cm2 | LED and laser both were effective in decreasing wound contraction on the 7th day. |
| Nishioka (2012) [97] | Rat | Skin flap | 670 nm; 2.49 J; 5 J/cm2; 17 s | 660 nm; 0.14 or 2.49 J; 5 or 89 J/cm2; 2 or 42 s | LED and laser both were effective in decreasing the necrotic area, when the total energy (2.49 J) was same in both groups. |
| de Morais (2010) [98] | Rat | Arthritis | 628 nm; 20 mW; 2.5 J/cm2; 2 J; 100 s; 0.8 cm2 | 685 or 830 nm; 2.5 J/cm2; 2 J; 100 s; 0.8 cm2 | Laser was more effective than LED in reducing edema, vascular permeability and hyperalgesia. |
| Dall Agnol (2009) [99] | Rat | Dorsal wound | 640 nm; 30 mW; 6 J/cm2; 0.5 cm2; 100 s | 660 nm; 30 mW; 6 J/cm2; 0.5 cm2; 100 s | LED and laser both were effective in reducing the amount of inflammatory cells. LED was more effective than laser in reducing the wound diameter of diabetic animals. |
| Bastos (2009) [100] | Rat | Tendon healing | 630 or 880 nm; 25 mW; 6 J/cm2; 0.2826 cm2 | 685 or 830 nm; 15 mW; 6 J/cm2; 0.0028 cm2 | LED and laser both were effective in improving the organization of collagen fiber on day 5. On day 10, the results remained, though without statistical significance. |
| Corazza (2007) [101] | Rat | Wound healing | 635 nm; 90 mW; 1058 mW/cm2; 0.085 cm2 | 660 nm; 40 mW; 1000 mW/cm2; 0.04 cm2; | LED and laser both were effective in increasing in increasing angiogenesis in wound healing. |
| Klebanov (2006) [102] | Rat | Wound exudate lipid peroxiidation | 630 nm; 1.5 J/cm2; 5 min | 633 nm; 1.5 J/cm2; 5 min | LED and laser both decreased lipid peroxidation products in wound fluid. |
| Kana (1981) [42] | Rat | Wound healing | 630 nm; 45 mW/cm2; 4 J/cm2 | 633 nm; 25 mW; 45 mW/cm2; 4 J/cm2 | Laser and non-coherent both were effective in enchancing collagen synthesis, when compared to contralateral non-irradiated wounds. However, when compared to one of the control animal groups, there appears to be no difference. It can also be interpreted that both groups showed improved wound closure, though the data regarding the noncoherent red light is not shown in the study report. |
Table 5:
Laser versus non-coherent comparisons (in vitro research)
| Study | Cell type | LED/non-coherent | LASER parameters | Results |
|---|---|---|---|---|
| Khan & Arany (2016) [103] | Human dermal keratinocyte; Human normal oral keratinocyte (NOKSI) | 660 and 850 nm; 1 and 3 J/cm2 for both wavelengths; | 810 nm; 1 or 3 J/cm2 | Laser appeared to have some effects on the number and size of mucosal colonies, while LED appeared to be ineffective. However, statistical significances were not calculated in these comparisons. |
| Pagin (2014) [104] | Pre-osteoblast MC3T3 cell | 630 nm; 60 mW/cm2; 3 and 5 J/cm2; 0.31 cm2; 3 and 5 s | 660 and 780 nm; 1 W/cm2; 3 and 5 J/cm2; 0.042 cm2; 3 and 5 s | LED and laser both had only limited effects on pre-osteoblast growth (at 24h), but laser was more effective. Neither showed effects on pre-osteoblast differentiation. |
| Spitler & Berns (2014) [105] | A549 adenocarcinoma human alveolar epithelial cell; PtK2 rat kangaroo renal epithelial cell; U2OS human osteosarcoma cell | 637 and 901 nm; 5.57 and 1.30 mW/cm2; 10.02 and 2.334 J/cm2; 1800 s | 652 and 806 nm; 5.57 and 1.30 mW/cm2; 10.02 and 2.334 J/cm2; 1800 s | LED and laser both had a comparable effects on cell migration and wound closure. |
| Vinck (2003) [106] | Fibroblast | 660 and 950 nm; 0.53 J/cm2 | 830 nm; 0.196 cm2; 1 J/cm2 | LED and laser both showed significant effects on fibroblast proliferation in most of the individual experiments in this study. |
Table 6:
Laser versus non-coherent comparisons (clinical trials)
| Study | Methodology | Indication | LED/non-coherent | LASER parameters | Results |
|---|---|---|---|---|---|
| Panhoca (2015) [107] | Comparison trial, uncontrolled | Temporomandibular disorder | 630 and 850; 150 mW; 300 mW/cm2; 18 J/cm2; 9 J/point | 780 nm; 70 mW; 1700 mW/cm2; 105 J/cm2; 4.2 J/point | There were no significant differences in pain scores and maximum oral aperture between groups at baseline or any periods after treatment. |
| Freitas (2014) [108] | Comparison trial, uncontrolled | Oral mucositis | 630 nm; 80mW; 0.24 J/point; 1 cm2 | 660 nm; 40 mW; 6.6 J/cm2; 0.24 J/point; 0.036 cm2 | LED and laser both were effective in alleviating oral mucositis scores, but LED had more pronounced effects. |
| Ammar (2014) [109] | Comparison trial, uncontrolled | Knee osteoarthritis | 890 nm; 62.4 J/cm2; 180 cm2 | 850 nm; 100 mW; 0.76 mm2 | LED and laser both appeared to be similarly effective in reducing pain and increasing physical function. |
| Esper (2011) [110] | RCT | Orthodontic pain | 640 nm; 100 mW; 4 J/cm2; 70 s | 660 nm; 30 mW; 4 J/cm2; 25 s | LED was effective in reducing orthodontic pain while laser was not. Laser dose (radiant energy) might have been too small. |
| Lizarelli (2010) [111] | RCT, double-blind | Dentin hypersensitivity | 630 nm; 25 mW; 5.4 J/cm2; 4 mm2 | 660 nm; 25 mW; 5.4 J/cm2; 4 mm2 | LED and laser were equally effective in the treatment of dentin hypersensitivity. |
| Lima (2016) [112] | RCT, double-blind | Pain after surgery | 640 nm; 70 mW; 10.1 J; 6 J/cm2; 1.77 cm2; 1216 s [Note: Wavelength reported in abstract contradicts with the wavelength provided in the full text.] |
660 nm; 40 mW; 2.4 J; 6 J/cm2; 0.4 cm2; 480 s [Note: Wavelength reported in abstract contradicts with the wavelength provided in the full text.] |
LED and laser both were effective in decreasing pain on the 6th and 8th postoperative day. |
| Leal Junior (2009) [113] | RCT, double-blind, crossover | Exercise physiology | 660 + 850 nm; 34 red diodes and 35 near-infrared diodes; 1390 mW; 83.4 J; 6.0 J/cm2; 60 s | 810 nm; 1 laser diode; 200 mW; 12 J; 164.84 J/cm2; 60 s | LED decreased post-exercise creatine kinase, but neither LED or laser had effects on exercise performance or blood lactate levels. |
| Lima (2017) [114] | RCT, double-blind | Sternotomy healing | 640 nm; 70 mW; 10.1 J; 6 J/cm2; 1.77 cm2; 1216 s [Note: Wavelength reported in abstract contradicts with the wavelength provided in the full text.] |
660 nm; 40 mW; 2.4 J; 6 J/cm2; 0.4 cm2; 480 s [Note: Wavelength reported in abstract contradicts with the wavelength provided in the full text.] |
LED and laser both were effective in decreasing hyperemia and incision bleeding or dehiscencce. |
As can be noted from these tables, most of the comparisons have a very high risk of bias due to differing key parameters between the LED and laser groups. In almost every study the wavelengths, power outputs and spot sizes are different between the groups, which makes is it impossible to make reliable comparisons between lasers and LEDs in photobiomodulation. Despite these notable shortcomings, most of these comparisons provisionally suggest that lasers could indeed be replaced by LEDs without significant worsening of the results.
5. Can broadband light also be used for photobiomodulation?
If the experimental evidence suggests that non-coherent and non-monochromatic light from LEDs can be used for photobiomodulation, then it is reasonable to asssume that even the natural broadband light (blackbody radiation) originating from a heated object (such as a tungsten filament or the sun) could have similar biological effects. So far, there exists limited yet tentatively positive evidence related to beneficial effcts of broadband light in PBM.
The earliest writings on PBM were published in the very early 20th century, when several authors described that visible light, red and infrared wavelengths produced by incandescent lamps appeared to have beneficial effects in the treatment of many different diseases such as syphilis, smallpox, tuberculosis, chronic fatigue, diabetes and obesity (Figure 4) [49, 50].
Figure 4. Incandescent light bath by physician John Harvey Kellogg.
In the early 20th century, incandescent bulbs were used as a source of therapeutic light in an “electric light bath”. Kellogg was one of the notable inventors and authors of this era. [Fuzheado / Wikimedia Commons / CC-SA-3.0]. No permission needed.
While the photobiomodulation research has mainly focused on monochromatic laser and quasimonochromatic LED lights, some research groups have also been using broadband (polychromatic) light sources in photobiomodulation research. Some commonly used wavelength ranges have been visible light (400 – 800 nm) or water-filtered infrared A (760 – 1400 nm). In a some cases the broadband light has been polarized [51]. Some of these studies are summarized in Table 7.
Table 7:
Broadband light research
| Indication | Animal | Reference |
|---|---|---|
| Arthritic joints | Rat | [115] |
| Atherosclerosis | Rabbit | [116] |
| Back pain | Human | [117] |
| Burns | Human, rat | [118–120] |
| Colitis | Mice | [121] |
| Foot ulcer | Human | [53] |
| Oral mucositis | Human | [52] |
| Skin rejuvenation | Human | [122] |
| Surgical wounds | Human, rat | [123, 124] |
| Tennis elbow | Human | [125, 126] |
| Wounds and ulcers | Human, rat | [54, 127–130] |
Visible light irradiation has been investigated in numerous in vitro and in vivo trials as well as in small clinical trials. While the data is generally of low methodological quality, the results have been mostly positive and the treatment effectiveness appears to be comparable to laser or LED photobiomodulation [52, 53].
Water-filtered infrared A has been investigated for the treatment of wound healing in humans [54] with positive results. However, some of the beneficial effects might be related to the thermal effects of infrared wavelengths, independently of athermic photobiomodulation mechanisms. It should be pointed out that in the case of longer wavelength IR light (>980 nm) where the primary photoacceptor is thought to be water, then the difference between thermal and non-thermal mechanisms tends to disappear. Santana-Blank has written extensively on this subject [55] and suggested that “exclusion zone (EZ) water may act as an electrolytic bio-battery, which can efficiently and selectively transfer light energy to sites expressing redox injury potentials, as found in cancer and other complex diseases”. Some researchers have also been arguing that some of the cellular effects of water-filtered infrared A might be harmful instead of being beneficial. Therefore it has been suggested that in addition to protecting skin from ultraviolet radiation, it would be helpful to also protect it from near-infrared radiation. However, those detrimental effects have been mostly noted in cell cultures receiving remarkably high irradiation doses of infrared A [56]
Because of the apparent success of these broadband light sources, it could be hypothesized that natural daylight or sunlight could also have health effects related to photobiomodulation. In cohort studies, sunlight exposure and low latitude have been associated with better health, eg. decreased mortality, lower cholesterol levels and lower incidence of cancer, fractures and type 2 diabetes [57–62].
While these findings have usually been attributed to increased vitamin D synthesis due to sunlight exposure, recent randomized clinical trials of vitamin D supplementation have been unable to substantiate those assumptions [63]. We suggest an alternative hypothesis, where vitamin D could act as a surrogate marker for photobiomodulation from sunlight exposure.
6. Conclusions
The current total evidence appears to support the idea that photobiomodulation is not dependent on lasers or coherence, but quasimonochromatic LED devices and even broad-wavelength light sources such as water-filtered infrared-A can also yield physiological effects. The comparisons between lasers and LEDs lend support to this idea. However, the quality of these comparisons is low for the most part, because of the difficulty of arranging the parameters so that the beam from a LED is identical to the beam from a laser, with regard to spot-size, band-width and power density. Nevertheless, even today the debate about the equivalence of laser and LED reamins the single most controversial topic in the PBM field.
Nevertheless, more high-quality head-to-head comparison studies should be conducted in order to figure out whether there are significant differences between the dose response or physiological effects of LED photobiomodulation and laser photobiomodulation, and whether LED-based treatments could be carried out based on the treatment parameters adopted from laser-based studies.
Supplementary Material
Acknowledgements
MRH was supported by US-NIH grants R01AI050875 and R21AI121700
Footnotes
Conflict of Interest
The authors declare no conflict of Interest
Supplementary material (.PDF and .XLSX)
The excel database of 350 LED photobiomodulation studies. Below are the links to the files:
https://drive.google.com/file/d/0B1ming8_i64HM3RrTGp3SUltQnB0TlMxZXRKS3NTeTBTMzY0/view?usp=sharing .PDF file
https://drive.google.com/file/d/1MMj6BNQD5_XEdhV1zXYZ_kD0FCqCB_vt/view?usp=sharing .XLSX file
References
- 1.Anders JJ, Lanzafame RJ, and Arany PR, Low-level light/laser therapy versus photobiomodulation therapy. Photomed Laser Surg, 2015. 33(4): p. 183–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karu T, Primary and secondary mechanisms of action of visible to near-IR radiation on cells. J Photochem Photobiol B, 1999. 49(1): p. 1–17. [DOI] [PubMed] [Google Scholar]
- 3.Karu TI, et al. , Absorption measurements of a cell monolayer relevant to phototherapy: reduction of cytochrome c oxidase under near IR radiation. Journal of Photochemistry and Photobiology B: Biology, 2005. 81(2): p. 98–106. [DOI] [PubMed] [Google Scholar]
- 4.Sanderson TH, et al. , Inhibitory modulation of cytochrome c oxidase activity with specific near-infrared light wavelengths attenuates brain ischemia/reperfusion injury. Sci Rep, 2018. 8(1): p. 3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Y, et al. , Photobiomodulation of human adipose-derived stem cells using 810nm and 980nm lasers operates via different mechanisms of action. Biochim Biophys Acta, 2017. 1861(2): p. 441–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Y, et al. , Photobiomodulation (blue and green light) encourages osteoblastic-differentiation of human adipose-derived stem cells: role of intracellular calcium and light-gated ion channels. Sci Rep, 2016. 6: p. 33719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Freitas LF and Hamblin MR, Proposed Mechanisms of Photobiomodulation or Low-Level Light Therapy. IEEE J Sel Top Quantum Electron, 2016. 22(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prindeze NJ, Moffatt LT, and Shupp JW, Mechanisms of action for light therapy: a review of molecular interactions. Exp Biol Med (Maywood), 2012. 237(11): p. 1241–8. [DOI] [PubMed] [Google Scholar]
- 9.Pieczenik SR and Neustadt J, Mitochondrial dysfunction and molecular pathways of disease. Exp Mol Pathol, 2007. 83(1): p. 84–92. [DOI] [PubMed] [Google Scholar]
- 10.Camps J and Garcia-Heredia A, Introduction: oxidation and inflammation, a molecular link between non-communicable diseases. Adv Exp Med Biol, 2014. 824: p. 1–4. [DOI] [PubMed] [Google Scholar]
- 11.Katsyuba E and Auwerx J, Modulating NAD(+) metabolism, from bench to bedside. Embo j, 2017. 36(18): p. 2670–2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rojas JC and Gonzalez-Lima F, Neurological and psychological applications of transcranial lasers and LEDs. Biochem Pharmacol, 2013. 86(4): p. 447–57. [DOI] [PubMed] [Google Scholar]
- 13.Tucker D, Lu Y, and Zhang Q, From Mitochondrial Function to Neuroprotection-an Emerging Role for Methylene Blue. Mol Neurobiol, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maiman TH, Stimulated optical radiation in ruby. Nature, 1960. 187: p. 493–494. [Google Scholar]
- 15.Hecht J, Beam: The Race to Make the Laser. 2005, Oxford, UK: Oxford University Press. [Google Scholar]
- 16.Mester E, Szende B, and Gartner P, The effect of laser beams on the growth of hair in mice. Radiobiol Radiother (Berl), 1968. 9(5): p. 621–6. [PubMed] [Google Scholar]
- 17.Mester E, Mester AF, and Mester A, The biomedical effects of laser application. Lasers Surg Med, 1985. 5(1): p. 31–9. [DOI] [PubMed] [Google Scholar]
- 18.Gamaleya NF, Laser Biomedical Research in the USSR, in Laser Applications in Medicine and Biology: Volume 3, Wolbarsht ML, Editor. 1977, Springer US: Boston, MA: p. 1–173. [Google Scholar]
- 19.INTERNATIONAL UPDATE IN LLLT. LASER THERAPY, 1990. 2(1): p. 10–13. [Google Scholar]
- 20.Renk KF, Basics of laser physics. 2012: Springer. [Google Scholar]
- 21.Courtland R, No Nobel for the Father of the LED. IEEE Spectrum, 2014. 8 October. [Google Scholar]
- 22.Pust P, Schmidt PJ, and Schnick W, A revolution in lighting. Nature Materials, 2015. 14: p. 454. [DOI] [PubMed] [Google Scholar]
- 23.Desmet KD, et al. , Clinical and experimental applications of NIR-LED photobiomodulation. Photomed Laser Surg, 2006. 24(2): p. 121–8. [DOI] [PubMed] [Google Scholar]
- 24.Whelan HT, et al. , Effect of NASA light-emitting diode irradiation on wound healing. J Clin Laser Med Surg, 2001. 19(6): p. 305–14. [DOI] [PubMed] [Google Scholar]
- 25.Kim WS and Calderhead RG, Is light-emitting diode phototherapy (LED-LLLT) really effective? Laser Ther, 2011. 20(3): p. 205–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Antunes HS, et al. , Cost-effectiveness of low-level laser therapy (LLLT) in head and neck cancer patients receiving concurrent chemoradiation. Oral Oncol, 2016. 52: p. 85–90. [DOI] [PubMed] [Google Scholar]
- 27.Hamblin MR, et al. Low level laser (light) therapy and photobiomodulation: the path forward in SPIE BiOS. 2015. SPIE. [Google Scholar]
- 28.Avci P, et al. , Low-level laser (light) therapy (LLLT) for treatment of hair loss. Lasers Surg Med, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zalevsky Z and Belkin M, Coherence and speckle in photomedicine and photobiology. Photomed Laser Surg, 2011. 29(10): p. 655–6. [DOI] [PubMed] [Google Scholar]
- 30.Hode L, The importance of the coherency. Photomed Laser Surg, 2005. 23(4): p. 431–4. [DOI] [PubMed] [Google Scholar]
- 31.Smith KC, Laser (and LED) therapy is phototherapy. Photomed Laser Surg, 2005. 23(1): p. 78–80. [DOI] [PubMed] [Google Scholar]
- 32.Hashmi JT, et al. , Role of low-level laser therapy in neurorehabilitation. Pm r, 2010. 2(12 Suppl 2): p. S292–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karu T, Photobiology of low-power laser effects. Health phys, 1989. 56(5): p. 691–704. [DOI] [PubMed] [Google Scholar]
- 34.Enwemeka CS, The place of coherence in light induced tissue repair and pain modulation. Photomed Laser Surg, 2006. 24(4): p. 457. [DOI] [PubMed] [Google Scholar]
- 35.Laakso L, Richardson C, and Cramond T, Quality of light - is laser necessary for effective photobiostimulation? Aust J Physiother, 1993. 39(2): p. 87–92. [DOI] [PubMed] [Google Scholar]
- 36.Lubart R, et al. , LIGHT EFFECT ON FIBROBLAST PROLIFERATON. LASER THERAPY, 1993. 5(2): p. 55–57. [Google Scholar]
- 37.Vacca RA, et al. , Increase in cytosolic and mitochondrial protein synthesis in rat hepatocytes irradiated in vitro by He-Ne laser. J Photochem Photobiol B, 1996. 34(2–3): p. 197–202. [DOI] [PubMed] [Google Scholar]
- 38.Ohshiro T, NEW CLASSIFICATION FOR SINGLE-SYSTEM LIGHT TREATMENT. LASER THERAPY, 2011. 20(1): p. 11–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hode L and Tunér J, Laser phototherapy-clinical practice and scientific background. Grängesberg: Prima Books AB, 2014. [Google Scholar]
- 40.Moskvin SV, Only lasers can be used for low level laser therapy. Biomedicine (Taipei), 2017. 7(4): p. 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hode L and Tuner J. Low-level laser therapy (LLLT) versus light-emitting diode therapy (LEDT): What is the difference? in Laser Florence ‘99. 2000. SPIE. [Google Scholar]
- 42.Kana JS, et al. , Effect of low-power density laser radiation on healing of open skin wounds in rats. Arch Surg, 1981. 116(3): p. 293–6. [DOI] [PubMed] [Google Scholar]
- 43.Young S, et al. , Macrophage responsiveness to light therapy. Lasers Surg Med, 1989. 9(5): p. 497–505. [DOI] [PubMed] [Google Scholar]
- 44.Karu TĬ, et al. , Biological action of low-intensity visible light on HeLa cells as a function of the coherence, dose, wavelength, and irradiation regime. Soviet Journal of Quantum Electronics, 1982. 12(9): p. 1134. [Google Scholar]
- 45.Pinheiro AL, et al. , Biochemical changes on the repair of surgical bone defects grafted with biphasic synthetic micro-granular HA + beta-tricalcium phosphate induced by laser and LED phototherapies and assessed by Raman spectroscopy. Lasers Med Sci, 2017. 32(3): p. 663–672. [DOI] [PubMed] [Google Scholar]
- 46.Pinheiro AL, et al. , Raman ratios on the repair of grafted surgical bone defects irradiated or not with laser (lambda780 nm) or LED (lambda850 nm). J Photochem Photobiol B, 2014. 138: p. 146–54. [DOI] [PubMed] [Google Scholar]
- 47.Soares LG, et al. , Do laser/LED phototherapies influence the outcome of the repair of surgical bone defects grafted with biphasic synthetic microgranular HA + beta-tricalcium phosphate? A Raman spectroscopy study. Lasers Med Sci, 2014. 29(5): p. 1575–84. [DOI] [PubMed] [Google Scholar]
- 48.de Carvalho ME, et al. , Low intensity laser and LED therapies associated with lateral decubitus position and flexion exercises of the lower limbs in patients with lumbar disk herniation: clinical randomized trial. Lasers Med Sci, 2016. 31(7): p. 1455–63. [DOI] [PubMed] [Google Scholar]
- 49.Cleaves MA, Light energy: its physics, physiological action and therapeutic applications. 1904: Rebman. [Google Scholar]
- 50.Kellogg JH, Light therapeutics: a practical manual of phototherapy for the student and the practitioner. 1910: Sanitarium and Hospital Equipment Company. [Google Scholar]
- 51.Dimitrios S and Stasinopoulos L, Treatment of Carpal Tunnel Syndrome in pregnancy with Polarized Polychromatic Non-coherent Light (Bioptron Light): A Preliminary, Prospective, Open Clinical Trial. Laser Ther, 2017. 26(4): p. 289–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Elad S, et al. , A randomized controlled trial of visible-light therapy for the prevention of oral mucositis. Oral Oncol, 2011. 47(2): p. 125–30. [DOI] [PubMed] [Google Scholar]
- 53.Landau Z, et al. , Visible light-induced healing of diabetic or venous foot ulcers: a placebo-controlled double-blind study. Photomed Laser Surg, 2011. 29(6): p. 399–404. [DOI] [PubMed] [Google Scholar]
- 54.Hoffmann G, Hartel M, and Mercer JB, Heat for wounds - water-filtered infrared-A (wIRA) for wound healing - a review. Ger Med Sci, 2016. 14: p. Doc08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Santana-Blank L, et al. , “Quantum Leap” in Photobiomodulation Therapy Ushers in a New Generation of Light-Based Treatments for Cancer and Other Complex Diseases: Perspective and Mini-Review. Photomed Laser Surg, 2016. 34(3): p. 93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Barolet D, Christiaens F, and Hamblin MR, Infrared and skin: Friend or foe. J Photochem Photobiol B, 2016. 155: p. 78–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lindqvist PG, et al. , Avoidance of sun exposure is a risk factor for all-cause mortality: results from the Melanoma in Southern Sweden cohort. J Intern Med, 2014. 276(1): p. 77–86. [DOI] [PubMed] [Google Scholar]
- 58.Lindqvist PG, Olsson H, and Landin-Olsson M, Are active sun exposure habits related to lowering risk of type 2 diabetes mellitus in women, a prospective cohort study? Diabetes Res Clin Pract, 2010. 90(1): p. 109–14. [DOI] [PubMed] [Google Scholar]
- 59.Grant WB and Mohr SB, Ecological studies of ultraviolet B, vitamin D and cancer since 2000. Ann Epidemiol, 2009. 19(7): p. 446–54. [DOI] [PubMed] [Google Scholar]
- 60.Grimes DS, Hindle E, and Dyer T, Sunlight, cholesterol and coronary heart disease. Qjm, 1996. 89(8): p. 579–89. [DOI] [PubMed] [Google Scholar]
- 61.Wong A, Incident solar radiation and coronary heart disease mortality rates in Europe. Eur J Epidemiol, 2008. 23(9): p. 609–14. [DOI] [PubMed] [Google Scholar]
- 62.Iwamoto J, Takeda T, and Matsumoto H, Sunlight exposure is important for preventing hip fractures in patients with Alzheimer’s disease, Parkinson’s disease, or stroke. Acta Neurol Scand, 2012. 125(4): p. 279–84. [DOI] [PubMed] [Google Scholar]
- 63.Allan GM, et al. , Vitamin D: A Narrative Review Examining the Evidence for Ten Beliefs. J Gen Intern Med, 2016. 31(7): p. 780–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Greguss P, Low-level laser therapy — reality or myth? Optics & Laser Technology, 1984. 16(2): p. 81–85. [Google Scholar]
- 65.Karu T, Molecular mechanism of the therapeutic effect of low-intensity laser radiation. Lasers Life Sci, 1988. 2(1): p. 53–74. [Google Scholar]
- 66.Devor M, What’s in a laser beam for pain therapy? Pain, 1990. 43(2): p. 139. [DOI] [PubMed] [Google Scholar]
- 67.Ohshiro T, TERMINOLOGY... AGAIN! LASER THERAPY, 1990. 2(3): p. 99–100. [Google Scholar]
- 68.Smith KC, LASER AND LED PHOTOBIOLOGY. LASER THERAPY, 2010. 19(2): p. 72–78. [Google Scholar]
- 69.Brochetti RA, et al. , Photobiomodulation therapy improves both inflammatory and fibrotic parameters in experimental model of lung fibrosis in mice. Lasers Med Sci, 2017. 32(8): p. 1825–1834. [DOI] [PubMed] [Google Scholar]
- 70.Henderson T and Morries L. http://www.westword.com/news/colorado-doctors-using-lasers-as-a-weapon-against-tbi-other-brain-injuries-9223729. 2017. [cited 2018 Mar 26].
- 71.Salehpour F, et al. , Brain Photobiomodulation Therapy: a Narrative Review. Mol Neurobiol, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Haina D, et al. , Animal experiments on light-induced woundhealing, in Optoelectronics in Medicine. 1982, Springer; p. 164–169. [Google Scholar]
- 73.Mul’diyarov PY and Tsurko V, Effect of monochromatic red light of a helium-neon laser on the morphology of zymosan arthritis in rats. Bulletin of Experimental Biology and Medicine, 1983. 95(1): p. 140–143. [PubMed] [Google Scholar]
- 74.Berki T, Nemeth P, and Hegedüs J, Biological effect of low-power helium-neon (HeNe) laser irradiation. Lasers in Medical Science, 1988. 3(1–4): p. 35–39. [Google Scholar]
- 75.Rosner M, et al. , Dose and temporal parameters in delaying injured optic nerve degeneration by low-energy laser irradiation. Lasers Surg Med, 1993. 13(6): p. 611–7. [DOI] [PubMed] [Google Scholar]
- 76. Laakso EL, et al. , PLASMA ACTH AND β-ENDORPHIN LEVELS IN RESPONSE TO LOW LEVEL LASER THERAPY (LLLT) FOR MYOFASCIAL TRIGGER POINTS. LASER THERAPY, 1994. 6(3): p. 133–141. [Google Scholar]
- 77.Antipa C, et al. Low-power coherent and noncoherent light in clinical practice in Effects of Low-Power Light on Biological Systems II. 1996. International Society for Optics and Photonics. [Google Scholar]
- 78.Campos L, et al. , Comparative study among three different phototherapy protocols to treat chemotherapy-induced oral mucositis in hamsters. J Biophotonics, 2016. 9(11–12): p. 1236–1245. [DOI] [PubMed] [Google Scholar]
- 79.Freire Mdo R, et al. , LED and laser photobiomodulation in the prevention and treatment of oral mucositis: experimental study in hamsters. Clin Oral Investig, 2014. 18(3): p. 1005–13. [DOI] [PubMed] [Google Scholar]
- 80.Nadur-Andrade N, et al. , Photobiostimulation reduces edema formation induced in mice by Lys-49 phospholipases A2 isolated from Bothrops moojeni venom. Photochem Photobiol Sci, 2014. 13(11): p. 1561–7. [DOI] [PubMed] [Google Scholar]
- 81.Nadur-Andrade N, et al. , Effects of photobiostimulation on edema and hemorrhage induced by Bothrops moojeni venom. Lasers Med Sci, 2012. 27(1): p. 65–70. [DOI] [PubMed] [Google Scholar]
- 82.Demidova-Rice TN, et al. , Low-level light stimulates excisional wound healing in mice. Lasers Surg Med, 2007. 39(9): p. 706–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Comunian CR, et al. , Photobiomodulation with LED and laser in repair of mandibular socket rabbit: clinical evaluation, histological, and histomorphometric. Oral Maxillofac Surg, 2017. 21(2): p. 201–206. [DOI] [PubMed] [Google Scholar]
- 84.Takhtfooladi MA and Sharifi D, A comparative study of red and blue light-emitting diodes and low-level laser in regeneration of the transected sciatic nerve after an end to end neurorrhaphy in rabbits. Lasers Med Sci, 2015. 30(9): p. 2319–24. [DOI] [PubMed] [Google Scholar]
- 85.Rosa CB, et al. , Laser and LED phototherapy on midpalatal suture after rapid maxilla expansion: Raman and histological analysis. Lasers Med Sci, 2017. 32(2): p. 263–274. [DOI] [PubMed] [Google Scholar]
- 86.Silveira PC, et al. , Effect of Low-Power Laser (LPL) and Light-Emitting Diode (LED) on Inflammatory Response in Burn Wound Healing. Inflammation, 2016. 39(4): p. 1395–404. [DOI] [PubMed] [Google Scholar]
- 87.de Carvalho FB, et al. , Effect of laser (lambda 660 nm) and LED (lambda 630 nm) photobiomodulation on formocresol-induced oral ulcers: a clinical and histological study on rodents. Lasers Med Sci, 2015. 30(1): p. 389–96. [DOI] [PubMed] [Google Scholar]
- 88.El-Bialy T, et al. , The effect of light-emitting diode and laser on mandibular growth in rats. Angle Orthod, 2015. 85(2): p. 233–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.de Castro IC, et al. , Assessment of different energy delivery settings in laser and LED phototherapies in the inflammatory process of rat’s TMJ induced by carrageenan. Lasers Med Sci, 2015. 30(8): p. 2105–13. [DOI] [PubMed] [Google Scholar]
- 90.Wu X, et al. , Organic light emitting diode improves diabetic cutaneous wound healing in rats. Wound Repair Regen, 2015. 23(1): p. 104–14. [DOI] [PubMed] [Google Scholar]
- 91.De Castro IC, et al. , Do laser and led phototherapies influence mast cells and myofibroblasts to produce collagen? Lasers Med Sci, 2014. 29(4): p. 1405–10. [DOI] [PubMed] [Google Scholar]
- 92.Rosa CB, et al. , Effect of the laser and light-emitting diode (LED) phototherapy on midpalatal suture bone formation after rapid maxilla expansion: a Raman spectroscopy analysis. Lasers Med Sci, 2014. 29(3): p. 859–67. [DOI] [PubMed] [Google Scholar]
- 93.de Sousa AP, et al. , Laser and LED phototherapies on angiogenesis. Lasers Med Sci, 2013. 28(3): p. 981–7. [DOI] [PubMed] [Google Scholar]
- 94.Oliveira R.d.A.d., et al. , Low-intensity laser therapy and led (light emitting diode) therapy in mechanical resistance of Rattus norvegicus chest inscision with implant of steel wire for sternal suture. Revista Brasileira de Engenharia Biomédica, 2013. 29: p. 166–174. [Google Scholar]
- 95.Oliveira Sampaio SC, et al. , Effect of laser and LED phototherapies on the healing of cutaneous wound on healthy and iron-deficient Wistar rats and their impact on fibroblastic activity during wound healing. Lasers Med Sci, 2013. 28(3): p. 799–806. [DOI] [PubMed] [Google Scholar]
- 96.Castro ICVD, et al. , Assessment of the effects of laser or LED photobiomodulation on hypothyroid rats of cutaneous wound healing: A morphometric study. AIP Conference Proceedings, 2012. 1486(1): p. 95–99. [Google Scholar]
- 97.Nishioka MA, et al. , LED (660 nm) and laser (670 nm) use on skin flap viability: angiogenesis and mast cells on transition line. Lasers Med Sci, 2012. 27(5): p. 1045–50. [DOI] [PubMed] [Google Scholar]
- 98.de Morais NC, et al. , Anti-inflammatory effect of low-level laser and light-emitting diode in zymosan-induced arthritis. Photomed Laser Surg, 2010. 28(2): p. 227–32. [DOI] [PubMed] [Google Scholar]
- 99.Dall Agnol MA, et al. , Comparative analysis of coherent light action (laser) versus non-coherent light (light-emitting diode) for tissue repair in diabetic rats. Lasers Med Sci, 2009. 24(6): p. 909–16. [DOI] [PubMed] [Google Scholar]
- 100.Bastos JLN, Lizarelli RFZ, and Parizotto NA, Comparative study of laser and LED systems of low intensity applied to tendon healing. Laser Physics, 2009. 19(9): p. 1925–1931. [Google Scholar]
- 101.Corazza AV, et al. , Photobiomodulation on the angiogenesis of skin wounds in rats using different light sources. Photomed Laser Surg, 2007. 25(2): p. 102–6. [DOI] [PubMed] [Google Scholar]
- 102.Klebanov GI, et al. , A comparative study of the effects of laser and LED radiation on lipid peroxidation in rat wound fluid. Biophysics, 2006. 51(2): p. 285–291. [Google Scholar]
- 103.Khan I and Arany PR, Photobiomodulation Therapy Promotes Expansion of Epithelial Colony Forming Units. Photomed Laser Surg, 2016. 34(11): p. 550–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pagin MT, et al. , Laser and light-emitting diode effects on pre-osteoblast growth and differentiation. Lasers Med Sci, 2014. 29(1): p. 55–9. [DOI] [PubMed] [Google Scholar]
- 105.Spitler R and Berns MW, Comparison of laser and diode sources for acceleration of in vitro wound healing by low-level light therapy. J Biomed Opt, 2014. 19(3): p. 38001. [DOI] [PubMed] [Google Scholar]
- 106.Vinck EM, et al. , Increased fibroblast proliferation induced by light emitting diode and low power laser irradiation. Lasers Med Sci, 2003. 18(2): p. 95–9. [DOI] [PubMed] [Google Scholar]
- 107.Panhoca VH, et al. , Comparative clinical study of light analgesic effect on temporomandibular disorder (TMD) using red and infrared led therapy. Lasers Med Sci, 2015. 30(2): p. 815–22. [DOI] [PubMed] [Google Scholar]
- 108.Freitas AC, et al. , Chemotherapy-induced oral mucositis: effect of LED and laser phototherapy treatment protocols. Photomed Laser Surg, 2014. 32(2): p. 81–7. [DOI] [PubMed] [Google Scholar]
- 109.Ammar TA, Monochromatic Infrared Photo Energy versus Low Level Laser Therapy in Patients with Knee Osteoarthritis. J Lasers Med Sci, 2014. 5(4): p. 176–82. [PMC free article] [PubMed] [Google Scholar]
- 110.Esper MA, Nicolau RA, and Arisawa EA, The effect of two phototherapy protocols on pain control in orthodontic procedure--a preliminary clinical study. Lasers Med Sci, 2011. 26(5): p. 657–63. [DOI] [PubMed] [Google Scholar]
- 111.Lizarelli RFZ, et al. , Dentin hypersensitivity clinical study comparing LILT and LEDT keeping the same irradiation parameters. Laser Physics Letters, 2010. 7(11): p. 805–811. [Google Scholar]
- 112.Lima AC, et al. , Low-Level Laser and Light-Emitting Diode Therapy for Pain Control in Hyperglycemic and Normoglycemic Patients Who Underwent Coronary Bypass Surgery with Internal Mammary Artery Grafts: A Randomized, Double-Blind Study with Follow-Up. Photomed Laser Surg, 2016. 34(6): p. 244–51. [DOI] [PubMed] [Google Scholar]
- 113.Leal EC Junior, et al. , Comparison between single-diode low-level laser therapy (LLLT) and LED multi-diode (cluster) therapy (LEDT) applications before high-intensity exercise. Photomed Laser Surg, 2009. 27(4): p. 617–23. [DOI] [PubMed] [Google Scholar]
- 114.Lima AC, et al. , Photobiomodulation (Laser and LED) on Sternotomy Healing in Hyperglycemic and Normoglycemic Patients Who Underwent Coronary Bypass Surgery with Internal Mammary Artery Grafts: A Randomized, Double-Blind Study with Follow-Up. Photomed Laser Surg, 2017. 35(1): p. 24–31. [DOI] [PubMed] [Google Scholar]
- 115.Araki H, et al. , Reduction of interleukin-6 expression in human synoviocytes and rheumatoid arthritis rat joints by linear polarized near infrared light (Superlizer) irradiation. Laser Ther, 2011. 20(4): p. 293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Park D, et al. , Anti-hypercholesterolemic and anti-atherosclerotic effects of polarized-light therapy in rabbits fed a high-cholesterol diet. Lab Anim Res, 2012. 28(1): p. 39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gale GD, Rothbart PJ, and Li Y, Infrared therapy for chronic low back pain: a randomized, controlled trial. Pain Res Manag, 2006. 11(3): p. 193–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Monstrey S, et al. , A conservative approach for deep dermal burn wounds using polarised-light therapy. Br J Plast Surg, 2002. 55(5): p. 420–6. [DOI] [PubMed] [Google Scholar]
- 119.Oliveira PC, et al. , The use of light photobiomodulation on the treatment of second-degree burns: a histological study of a rodent model. Photomed Laser Surg, 2008. 26(4): p. 289–99. [DOI] [PubMed] [Google Scholar]
- 120.Karadag CA, et al. , The efficacy of linear polarized polychromatic light on burn wound healing: an experimental study on rats. J Burn Care Res, 2007. 28(2): p. 291–8. [DOI] [PubMed] [Google Scholar]
- 121.Hiratsuka T, et al. , Phototherapy with artificial light suppresses dextran sulfate sodium-induced colitis in a mouse model. J Gastroenterol Hepatol, 2014. 29(4): p. 749–56. [DOI] [PubMed] [Google Scholar]
- 122.Wunsch A and Matuschka K, A controlled trial to determine the efficacy of red and near-infrared light treatment in patient satisfaction, reduction of fine lines, wrinkles, skin roughness, and intradermal collagen density increase. Photomed Laser Surg, 2014. 32(2): p. 93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hartel M, et al. , Randomized clinical trial of the influence of local water-filtered infrared A irradiation on wound healing after abdominal surgery. Br J Surg, 2006. 93(8): p. 952–60. [DOI] [PubMed] [Google Scholar]
- 124.Medeiros JL, et al. , Healing of surgical wounds made with lambda970-nm diode laser associated or not with laser phototherapy (lambda655 nm) or polarized light (lambda400–2000 nm). Photomed Laser Surg, 2010. 28(4): p. 489–96. [DOI] [PubMed] [Google Scholar]
- 125.Stasinopoulos D, et al. , Comparing the effects of exercise program and low-level laser therapy with exercise program and polarized polychromatic non-coherent light (bioptron light) on the treatment of lateral elbow tendinopathy. Photomed Laser Surg, 2009. 27(3): p. 513–20. [DOI] [PubMed] [Google Scholar]
- 126.Stasinopoulos D, The use of polarized polychromatic non-coherent light as therapy for acute tennis elbow/lateral epicondylalgia: a pilot study. Photomed Laser Surg, 2005. 23(1): p. 66–9. [DOI] [PubMed] [Google Scholar]
- 127.Lubart R, et al. , A NEW APPROACH TO ULCER TREATMENT USING BROADBAND VISIBLE LIGHT. LASER THERAPY, 2007. 16(1): p. 7–10. [Google Scholar]
- 128.Monstrey S, et al. , The effect of polarized light on wound healing. European Journal of Plastic Surgery, 2002. 24(8): p. 377–382. [Google Scholar]
- 129.Pinheiro AL, et al. , Biomodulative effects of polarized light on the healing of cutaneous wounds on nourished and undernourished Wistar rats. Photomed Laser Surg, 2006. 24(5): p. 616–24. [DOI] [PubMed] [Google Scholar]
- 130.Al-Watban FA and Andres BL, Polychromatic LED in oval full-thickness wound healing in non-diabetic and diabetic rats. Photomed Laser Surg, 2006. 24(1): p. 10–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.