Abstract
Introduction
Clinical cohort studies suggest that mild cognitive impairment (MCI) is common in early Parkinson’s disease (PD). The objectives of this paper were to describe cognitive function in a large clinical trial of early treated PD patients at baseline and over time using two brief cognitive screening tests.
Methods
In total 1,741 participants were enrolled in the NINDS Exploratory Trials in Parkinson’s disease (NET-PD) Long-term Study-1 (LS-1). The Symbol Digit Modalities Test (SDMT) was collected annually. The SCales for Outcomes in PArkinson’s disease-COGnition (SCOPA-COG) was collected at baseline and at year 5. The trial was stopped early based on a planned interim analysis after half the cohort completed 5 years of follow-up. The median length of follow-up was 4 years (range 3 to 6 years). Predictors of cognitive change were examined using cross sectional (baseline) and longitudinal multivariable linear regression.
Results
The mean (SD) change from baseline to 5 years was −1.9 (5.1) for the SCOPA-COG and −2.1 (11.1) for the SDMT. Age and baseline UPDRS motor scores were associated with a more rapid decline in SDMT scores and 5 year SCOPA-COG scores. Male gender was associated with more rapid decline in SDMT. Self-reported income was a novel predictor of baseline cognitive function, even adjusted for educational status, although not significantly associated with change over time.
Conclusion
This large prospective cohort study demonstrated mild cognitive decline in early treated Parkinson’s disease. The study identified income level as a novel predictor of cognitive function.
Keywords: Cognitive Impairment, Parkinson’s Disease, SCOPA-COG, SDMT, MCI
Introduction
Cognitive dysfunction is an important non-motor manifestation of Parkinson’s disease (PD) [1] and contributes more to health-related Quality of Life than motor symptoms or motor complications [2, 3]. Cognitive impairment (in 3 or more cognitive domains) has been reported in as many as 18–24% of early PD patients in clinic-based cohorts using comprehensive neuropsychological testing [4–7], but is less frequently documented in clinical trial cohorts of early PD.
Several clinical trials in PD have measured cognitive function as a secondary outcome, the largest of which to date has been the Deprenyl and Tocopherol Antioxidative Therapy of Parkinsonism study (DATATOP) study. In DATATOP less than 1% of subjects had cognitive impairment at baseline (as assessed using the Mini Mental State Exam) and only 5.8% met criteria for cognitive impairment after 5 years [8]. This may have been due to the higher educational and performance status of the trial participants, as well as the insensitivity of the MMSE, a non-specific dementia screening instrument, to detect mild cognitive impairment in PD [9–11]. Despite these limitations, Uc et al. found that predominantly affected side, tremor score, dopaminergic therapy type, total daily levodopa equivalent dose, time since diagnosis, and years between symptom onset and diagnosis were all significant predictors of cognitive decline on the MMSE in the DATATOP cohort [8].
Schneider et al. studied 413 early de novo PD patients from the NET-PD FS1 and FS-TOO studies using the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS), the Frontal Assessment Battery (FAB), and Letter-Number Sequencing [12–14]. They found that none of the cognitive measures declined significantly over the 12–18 months of the trials.
The recently reported MODERATO trial of rasagiline for cognitive function in 170 participants with PD-MCI utilized the SCales for Outcomes in PArkinson’s disease-COGnition (SCOPA-COG) and the Montreal Cognitive Assessment (MoCA). Neither the treatment nor placebo groups experienced a decline in these outcome measures during the 24 month study[15]. This outcome may have been due to the sample size of the study and/or to the short duration of the trial. The recently completed NIH Exploratory Trials in Parkinson’s Disease Long-term Study-1 (NET-PD LS-1) used two screening measures: the SCOPA-COG and the Symbol Digit Modalities Test (SDMT). The SCOPA-COG was selected because it assesses multiple domains including memory, attention, executive function and visuospatial function [16–18]. The SCOPA-COG has been validated in small samples of PD dementia and PD-MCI [18, 19] but has not been shown to have high sensitivity or specificity for PD-MCI [20]. van Rooden et al examined the change in SCOPA-COG over time in the PROfiling PARKinson’s disease (PROPARK) study and found a more rapid decline in patients who were older at the time of enrollment[21]. Using the same cohort, Zhu et al. found age at enrollment, education, total daily levodopa dose and daytime sleepiness to be most predictive of incident dementia, as defined by a score of 22 or less on the SCOPA-COG [22].
The SDMT was also administered annually during the NET-PD LS1 study. It is a brief cognitive test of short-term memory and attention switching that can be administered written or orally. While this brief screening test has not been validated as a screening instrument for PD cognitive impairment, it can differentiate non-demented and demented PD from healthy controls [23–25]. As the SDMT is a timed test, it has been criticized for being confounded by PD motor impairment when completed by hand [26]. In the NET-PD LS-1 study, the SDMT was administered orally to try to reduce motor effects on performance. The Parkinson’s Progression Markers Initiative (PPMI) recently examined the performance of the SDMT over 3 years and found that participants with possible REM behavior disorder experienced a more rapid annual decline of −0.69 points/year [27].
The objective of this paper is to describe the cognitive profile of the NET-PD LS-1 study participants using the two screening measures (SCOPA-COG and SDMT), and to examine demographic and disease-related predictors of cognitive decline in this cohort. We hypothesized that the size and duration of this clinical trial cohort would permit us to detect small changes in cognitive function over time which had not been identified in previous trial cohorts.
Methods
Participants
The NINDS Exploratory Trials in Parkinson’s Disease Long-term Study-1 (NET-PD LS-1) was a large, randomized, multicenter, placebo-controlled trial of creatine as a potential disease modifying agent for PD. A total of 1741 participants were enrolled with early, treated PD. The institutional review boards of the 45 participating sites approved the study, the study protocol, and the informed consent process and documentation. All patients provided written informed consent. The primary study findings have been published [28]. Parkinson’s disease patients could be enrolled if they were within 5 years of diagnosis and 2 years of starting dopaminergic therapy. While there were no specific cognitive screening tests for enrollment, participants were excluded if they had “any unstable or clinically significant condition that would impair the subjects’ ability to comply with long-term study follow-up” and if they had “any significant features suggestive of a diagnosis of atypical parkinsonism.” Because the study terminated early, patients were followed for a minimum of 3 years and a maximum of 6 years, with annual in-person assessments. This analysis is based on the final database lock on May 5, 2014.
Assessments
The SCOPA-COG was measured twice (baseline and year 5) and the SDMT was administered annually orally to try to reduce motor effects on performance. SDMT scores of zero (29 events) were treated as missing.
Statistical Methods
The SCOPA-COG and SDMT were analyzed both at baseline and as change over time in separate multivariable linear regression models. Baseline models used baseline measures of either SCOPA-COG or SDMT as continuous dependent variables. The models included the predictor variables measured at baseline listed below:
Demographic variables: age, gender, income level (defined as the self-reported “average income for someone in your profession”), level of education, side of onset of symptoms, years since diagnosis, years since PD symptom onset.
Disease severity: UPDRS II (activities of daily living), UPDRS III (motor symptoms). Bulbar symptoms were defined as the sum of UPDRS questions 5,6,7, 18 & 19 [8], postural instability defined as the average of 5 UPDRS items 13, 14, 15, 29, 30, total tremor score defined as the average of 8 tremor items from the UPDRS questions 16, 20–21[29], orthostatic blood pressure[30], and depression as measured by the Beck Depression Inventory –II (BDI-II) score.
Type of dopaminergic therapy used at baseline (levodopa, levodopa and dopamine agonist, or dopamine agonist), total daily levodopa-equivalent dose (LED), and the ratio of levodopa to total LED (including levodopa and DA agonist).
Baseline Models
An automated selection approach was used to screen the predictor variables for the multiple linear regression models of SCOPA-COG and SDMT at baseline. A stepwise selection procedure was used allowing variables to enter and leave the model based on significance level (alpha of 0.20 to enter and alpha of 0.10 to stay in the model). The criterion for selecting among models was adjusted R-squared (PROC GLMSELECT / selection=stepwise (select=SL SLE=0.2 SLS=0.1 choose=ADJRSQ)). The following variables were forced into each model due to their a priori presumed importance: side predominantly affected, tremor score, dopaminergic therapy type, total daily levodopa equivalent dose, time since diagnosis, and years between symptom onset and diagnosis [8, 30]. Once the variables were selected, a mixed effect linear model was fit with clinical site as a random effect and the predictor variables obtained from the automated selection step as fixed effects. The final mixed effect linear models included those variables with a p-value less than 0.01 in either model. However, only variables with a Bonferroni-corrected p-value less than 0.0015 were considered statistically significant, due to the large number of hypotheses being tested. As a sensitivity analysis to the automated approach, backward and forward selection methods were also applied using the change from baseline to 5 years. Graphical and formal model diagnostic procedures indicated that no outliers were present. Variance inflation factor (VIF) was used to assess multicollinearity.
Change from Baseline Models
The following methods were used for change in SCOPA-COG at 5 years and for change in SDMT over 1–6 years. For SCOPA-COG a linear regression model of change from baseline to 5 years included only those participants who had 5 year data collected (completers only); missing data was not imputed. For the SDMT model of change from baseline, repeated measures on the same patient were included in a linear mixed model with years from baseline as a continuous predictor variable in the model. The model included all participants who had at least 1 post baseline assessment. The model assumed a first order autoregressive covariance structure between years 1–6 (via the repeated statement of SAS Proc Mixed). Firstly a model was fit for each predictor variable and for its interaction with time, adjusting for age, gender, site (random effect), treatment group (creatine or placebo). Variance inflation factor (VIF) was used to assess multicollinearity. Variables, with a p-value less than 0.2 in a simpler model of either SCOPA-COG or SDMT, were included in a multivariable model. Next, variables with a p-value less than 0.1 were retained in the final model along with treatment group, education level, gender and site, but only variables with a significance level of <0.0015 were considered statistically significant given the large number of hypotheses being tested.
Subjects missing SDMT and SCOPA-COG at 5 years (N=284) were on average older and had lower (worse) cognitive scores at baseline compared to those participants with 5 year data (N=722) and compared to participants who were not expected to have 5 year data due to early termination of the study (n=735), (see Table e1). We assumed that given age and the baseline cognitive score, the probability of having a missing cognitive assessment does not further depend on the cognitive status. Thus, missing at random (MAR) was assumed, and maximum likelihood estimation was used to handle missing SDMT assessments. Specifically in this method, the SDMT assessments collected in earlier years were used to “borrow” information for missing observations in later years while “account[ing] for the uncertainty of this projection in the calculation of the standard errors and test statistics.”[31]
Results
The SCOPA-COG was completed by 1731 participants at baseline and by 676 participants at year 5 (70% of the participants who completed their Year 5 visit). The SDMT was completed by 1736 participants at baseline and by 715 participants at year 5 (75% of the participants who completed year 5). Figure 2 shows the number of participants who completed the SDMT at each year of the study. Reasons for missing cognitive data at year 5 included 284 participants (30% of expected) who were missing (not at random) either due to withdrawal of consent, loss to follow-up, death, or some other reason. There were 737 participants (42% of the total enrolled) who were considered missing at random because of premature termination of the study. Supplementary Table 1 shows the baseline data for participants who completed the 5 year data compared to those who did not complete the study either because of early termination or other reasons. Participants who were missing not at random (missing for the reasons above) were older and had more severe UPDRS total, SDMT, and SCOPA-COG scores at baseline.
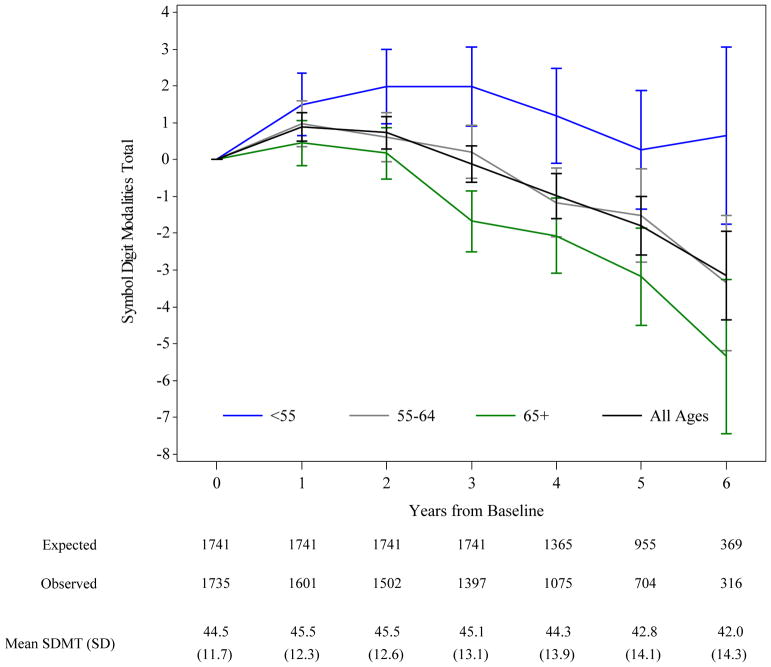
Figure 2. Mean Change (95% CI) from Baseline in SDMT by Age Group.
Plot of change from baseline in SDMT scores over 5 years by baseline age. At baseline n=378 (22%) were less than 55 years old, n=663 (38%) were 55–64 years, and n=694 (40%) were 65 or older. The number of participants at each time point is shown below the graph in tabular form. Expected= the number of subjects expected to have the annual visit based on the subject’s enrollment and date of study termination. Observed= the actual number of participants with SDMT data at each time point. SD= Standard Deviation.
Among completers, the mean (SD) SCOPA-COG score decreased from 30.3 (5.4) at baseline to 28.6 (7.1) at Year 5 (for a mean change of −1.9 (5.1) points over 5 years). The mean (SD) SDMT score decreased from 44.4 (11.7) at baseline to 42.4 (14.8) at Year 5 (mean change of −2.1 (11.1) points over 5 years). As shown in Figure 1, the distribution of both the SCOPA-COG and SDMT became more highly skewed to the left at year 5, suggesting either that there was a subset of participants who experienced a more rapid decline, or that the subjects with missing data were more likely to have a lower score at baseline. As shown in Figure 2, there was an initial increase in SDMT scores at Year 1, consistent with a practice effect, which then declined in a curvilinear manner over time.
Figure 1.
Distribution of SCOPA-COG (N=1731) and Symbol Digit Modalities (N=1736) total scores in participants at baseline and Year 5 in the NET-PD LS-1 study. For both scales higher scores are “better”. At baseline the SCOPA-COG distribution (1A) shows a slight left skew, while the SDMT distribution (1B) lacks significant skew or kurtosis. At Year 5, the SCOPA-COG (1C, N=676) and SDMT (1D, N=715) distributions show a stronger left skew compared to baseline possibly due to missing data for those patients with worse baseline scores, or due to more rapid decline in participants with lower baseline scores.
First we examined predictors of cognitive function measured at baseline. Table 1 shows the parameter estimates from the multivariable linear regression models of SCOPA-COG and SDMT at baseline. The results of the two separate models were similar and confirmed previous reports of predictors of cognitive function in early PD [8, 30]. Age, gender, education, UPDRS part III and the BDI-II depression score were all significantly associated with both the baseline SCOPA-COG total score and the baseline SDMT score, consistent with prior reports. On average, women scored 3.49 points on the SDMT (S.E. 0.52, p<0.0001) and 1.76 points on the SCOPA-COG (S.E. 0.24 p<0.0001) higher than men at baseline. Unexpectedly, disease duration, time since diagnosis, and side of onset were not significant in our multivariable model. Finally lower self-reported income was associated with lower (worse) cognitive scores in both models, even after adjusting for years of education.
Table 1.
Parameter estimates of the regression models of SCOPA-COG and SDMT at Baseline
Parameter estimates of the final linear regression models for total SCOPA-COG and SDMT at baseline adjusted for age and education level. PD=Parkinson’s disease; UPDRS ADL= Unified Parkinson’s Disease Rating Scale Part II; UPDRS Motor= Unified Parkinson’s Disease Rating Scale Part III; BDI-II= Beck Depression Inventory-II; LED= Levodopa equivalent daily dose; DA=dopamine agonist (including pramipexole, ropinirole, rotigotine, and bromocriptine (one subject))
| Baseline SDMT (N=1714) | Baseline SCOPA-COG (n=1707) | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Baseline Characteristics | Estimate | S.E. | Pr > |t| | Estimate | S.E. | Pr > |t| | |
| Gender (Male reference) | 3.49 | 0.52 | <.0001 | 1.76 | 0.24 | <.0001 | |
|
| |||||||
| Age | −0.44 | 0.03 | <.0001 | −0.12 | 0.01 | <.0001 | |
|
| |||||||
| Education (High school is reference) | Graduate | 4.60 | 0.74 | <.0001 | 3.40 | 0.34 | <.0001 |
| Bachelors | 3.35 | 0.74 | <.0001 | 2.60 | 0.34 | <.0001 | |
| Associate | 3.75 | 1.01 | 0.0002 | 1.30 | 0.47 | 0.005 | |
| Some college | 1.38 | 0.81 | 0.08 | 1.23 | 0.37 | 0.001 | |
|
| |||||||
| Income level (>85K reference) | Refused to disclose | −1.98 | 0.78 | 0.011 | −1.39 | 0.36 | 0.0001 |
| ≤45k | −1.17 | 0.72 | 0.11 | −1.08 | 0.34 | 0.0013 | |
| 45k–85K | −1.07 | 0.62 | 0.085 | −0.73 | 0.29 | 0.011 | |
|
| |||||||
| Duration since diagnosis | −0.44 | 0.22 | 0.049 | −0.06 | 0.10 | 0.54 | |
|
| |||||||
| Dominant Side (Right is reference) | Symmetric | −3.17 | 2.24 | 0.16 | −0.40 | 1.03 | 0.70 |
| Left | −0.24 | 0.47 | 0.60 | 0.52 | 0.22 | 0.02 | |
|
| |||||||
| BDI-II total | −0.15 | 0.05 | 0.0014 | −0.08 | 0.02 | 0.0001 | |
|
| |||||||
| UPDRS ADL part II | 0.05 | 0.08 | 0.51 | 0.17 | 0.04 | <.0001 | |
|
| |||||||
| UPDRS motor part III | −0.28 | 0.04 | <.0001 | −0.12 | 0.02 | <.0001 | |
|
| |||||||
| Average Tremor Score | 1.18 | 0.81 | 0.14 | 1.04 | 0.37 | 0.006 | |
|
| |||||||
| Type of Dopaminergic Therapy (compared to DA only) | levodopa only | −1.22 | 0.57 | 0.03 | −0.83 | 0.27 | 0.002 |
| levodopa + DA | −0.51 | 0.66 | 0.44 | −0.30 | 0.31 | 0.33 | |
We then examined predictors of change over time using longitudinal regression models for the SDMT and linear regression models for the change from baseline to year 5 in the SCOPA-COG. Table 2 shows parameter estimates of the final, longitudinal regression models of the change from baseline over 1–6 years for the SDMT and the change from baseline to year 5 for the SCOPA-COG. The results of the two multivariable models were fairly similar. The predicted annual rate of decline was −0.63 points per year for SDMT and the adjusted mean change for SCOPA-COG over 5 years was −1.82 when adjusting for the mean value of each covariate in the final model. On the SDMT, on average, the difference in the change from baseline between males and females was −1.27 at any given year (males scored lower at every time point). The adjusted mean change in SDMT from baseline to 5 years was −1.0 for females and −2.3 for males. Gender was not a significant predictor of change in SCOPA-COG although the trend was in the same direction as in the SDMT model. Older age at baseline was associated with worse change (more rapid decline) on the SCOPA-COG. On average, the change from baseline to 5 years in SCOPA-COG was 1.08 points greater for each 10 year increase in baseline age. Similarly, older age was associated with faster decline on the SDMT; the adjusted mean change in SDMT from baseline to 5 years was −1.38 for patients enrolled at age 60 vs. −4.36 for patients enrolled at age 70 (See figure 2). As expected, higher (worse) UPDRS part III scores were associated with a greater (worse) change in SDMT and SCOPA-COG. Symmetric symptom onset and depression as measured using the BDI-II were marginally associated with lower SDMT over time however neither was statistically significant (p=0.003 for both) and neither variable entered into the SCOPA-COG model. Participants who started the study on levodopa containing regimens appeared to have a more rapid rate of decline over time on SDMT than participants on dopaminergic agonists alone, however this did not reach our Bonferroni-adjusted level of significance (p=0.006). Finally, neither education nor income level were statistically significant in these models, although there was a trend towards lower SCOPA-COG scores at 5 years in participants earning <45K (−1.007, p=0.09).
Table 2.
Parameter estimates of the regression models for Change in SDMT and SCOPA-COG
Parameter estimates of the regression models for change in SCOPA-COG over 5-years and change in SDMT scores over 1–6 years. Values <0.0015 are bold. BDI-II= Beck Depression Inventory-II; UPDRS Motor= Unified Parkinson’s Disease Rating Scale Part III; DA=dopamine agonist (including pramipexole, ropinirole, rotigotine, and bromocriptine). Parameter estimates provided are adjusted for all variables listed in the table and for treatment (not significant) and clinical site (random effect). The SDMT model assumes a first-order autoregressive covariance structure for repeated measurements within subject.
| Longitudinal Model of Change in SDMT over 1–6 years N=1592 |
Model of 5-year Change in Scopa-Cog N=668 |
||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Effect | Estimate | SE | two sided p-value | Estimate | SE | two sided p-value | |
|
| |||||||
| Intercept | 25.913 | 2.288 | <.0001 | 13.145 | 2.244 | <.0001 | |
|
| |||||||
| Baseline Cognition Score (SDMT or SCOPA-COG) | −0.286 | 0.017 | <.0001 | −0.206 | 0.042 | <.0001 | |
|
| |||||||
| Age (years) | −0.153 | 0.031 | <.0001 | −0.108 | 0.024 | <.0001 | |
|
| |||||||
| Year | 1.151 | 0.546 | 0.03 | ||||
|
| |||||||
| Age*Year | −0.029 | 0.009 | 0.002 | ||||
|
| |||||||
| Gender (male is reference) | 1.274 | 0.338 | 0.0002 | 0.625 | 0.432 | 0.15 | |
|
| |||||||
| Education (high school is reference) | Graduate | 0.772 | 0.493 | 0.12 | 0.559 | 0.611 | 0.36 |
| Bachelors | 0.259 | 0.503 | 0.61 | 0.054 | 0.611 | 0.93 | |
| Associate | −0.503 | 0.692 | 0.47 | 0.604 | 0.888 | 0.50 | |
| Some college | −0.250 | 0.554 | 0.65 | −0.814 | 0.667 | 0.22 | |
|
| |||||||
| Income level (>85K reference) | Refused to disclose | −0.454 | 0.644 | 0.48 | |||
|
|
|||||||
| ≤ 45k | −1.007 | 0.598 | 0.09 | ||||
|
|
|||||||
| 45k–85K | −0.628 | 0.499 | 0.21 | ||||
|
| |||||||
| Dominant Side (right is reference) | Symmetric | −4.917 | 1.630 | 0.003 | −3.012 | 1.772 | 0.09 |
| Left | −0.536 | 0.314 | 0.09 | 0.137 | 0.382 | 0.72 | |
|
| |||||||
| Baseline BDI-II total | −0.092 | 0.031 | 0.003 | ||||
|
| |||||||
| Baseline UPDRS motor | −0.161 | 0.023 | <.0001 | −0.089 | 0.027 | 0.001 | |
|
| |||||||
| Baseline Average Tremor Score | 3.084 | 0.540 | <.0001 | ||||
|
| |||||||
| Baseline Type of Dopaminergic Therapy (DA alone is reference) | levodopa only | 0.550 | 0.639 | 0.39 | −0.821 | 0.476 | 0.08 |
| levodopa+DA | 0.185 | 0.741 | 0.80 | 0.080 | 0.536 | 0.88 | |
|
| |||||||
| Baseline Type of Dopaminergic Therapy *Year (DA alone is reference) | levodopa only | −0.521 | 0.189 | 0.006 | |||
|
| |||||||
| levodopa+DA | −0.513 | 0.218 | 0.019 | ||||
Discussion
This study presents longitudinal cognitive data from a large well-characterized early cohort of treated PD study participants. The main strength of our study was the large number of participants enrolled, allowing us to detect weak determinants of cognitive function. Overall participants exhibited very little change on the SCOPA-COG and SDMT over time, however this is likely to be an under-estimate of the true cognitive decline in PD patients related to: 1) recruitment bias into this randomized controlled trial excluding PD patients with early cognitive symptoms; 2) early dropout of subjects due to cognitive impairment (as evidenced by the lower cognitive function at baseline in those with missing follow-up visits); 3) the limitations of these brief cognitive screening instruments [32].
Limitations of our study include the fact that the SCOPA-COG was administered only twice during the study, unlike the SDMT, and that there was a high degree of missing SDMT and SCOPA-COG data at year 5. Nearly half of the total enrolled cohort was followed for less than 5 years due to the fact that the study was terminated prematurely. However, 30% of the expected 5 year data was missing due to drop out for various reasons including death. The longitudinal SDMT model considered the relationship of missingness to baseline cognitive function and adjusted for missingness accordingly. However, it is possible that the baseline characteristics included in the model do not fully explain the reasons for missing SDMT cognition scores; in other words, those with missing follow-up assessments may have not only been worse at baseline, but had faster rates of decline. The SCOPA-COG model of change from baseline included completers only. Since there was only one post-baseline SCOPA-COG assessment performed, it was not possible to use the same approach to implicitly impute missing data. Hence this analysis may be more likely to be biased towards patients who are declining more slowly and the predictors of cognitive decline identified in our analysis may not be the same predictors for more severe patients.
Another limitation of the study is that although the SDMT was administered orally, it is still possible that bradyphrenia, slow visual saccades and bradyphemia could have confounded the SDMT results [33–35]. Indeed, improved performance on the manual version of the SDMT after treatment with levodopa has previously been published [26]. We note that in the NET-PD LS-1 study, participants on levodopa (only) at baseline had lower cognitive screening scores and lower scores over time, however this may have been due to reverse causation (i.e. participants with lower baseline cognition were more likely to be prescribed levodopa only).
In our multivariable analysis, we were able to confirm previously reported predictors of cognitive decline including male gender, age and motor severity [8] [21]. One novel predictor of baseline cognitive performance in our study was income level, even adjusted for educational status. Baseline self-reported income was significantly associated with cognitive performance and marginally associated with cognition over time. This may have been due to reverse causation (lower pre-morbid cognitive status leading to lower income) although interestingly this was adjusted for highest education level achieved. Income has been reported to predict cognitive decline in other cohorts [36–38], however it has not been previously examined in studies of cognition in Parkinson’s disease.
In summary, this study provides a description of cognitive function over time in a large cohort of early treated PD patients, and explores demographic and clinical predictors of cognitive function in PD. These data support prior studies showing that PD patients experience mild cognitive decline early in their disease course. The study identified income level (even adjusted for educational status) as a novel predictor of cognitive function, suggesting that this variable should be collected in future studies.
Supplementary Material
Acknowledgments
LS1 participants: The authors thank the patients and families who participate in the NET-PD LS-1 study.
The following additional NINDS grants supported the Net-PD LS-1 study: U10 NS044547, U10 NS044425 U10 NS044462, U10 NS053379, U10 NS044483, U10 NS044479, U10 NS 044474, U10 NS 044545, U10 NS044460, U10 NS053381, U10 NS044453, U10 NS053370, U10 NS053380, U10 NS044475, U10 NS044431, U10 NS044466, U10 NS044451, U10 NS044465, U10 NS044482, U10 NS044484, U10 NS044450, U10 NS044504, U10 NS053369, U10 NS044437, U10 NS053372, U10 NS044448, U10 NS044426, U10 NS044455, U10 NS044446, U10 NS044501, U10 NS053377, U10 NS044469, U10 NS053368, U10 NS044471, U10 NS044454, U10 NS044481, U10 NS044441, U10 NS044464, U10 NS044505, U10 NS053387, U10 NS044427, U10 NS044555, U10 NS044458, U10 NS044415, U10 NS044472
Funding/Support: The NET-PD LS-1 Trial Investigators were supported, in part, by grants U01NS043127, U01NS043128, and U10NS44415-44555 from the National Institute of Neurologic Disorders and Stroke.
Footnotes
Authors’ Roles:
- Conception: Wills, Elm, Chou, Parashos, Schneider;
- Organization and Execution: Wills, Elm;
- Execution: Elm, Ye
- Review and Critique: Wills, Elm, Chou, Parashos, Schneider;
- Writing of the first draft: Wills, Elm
- Review and Critique: Chou, Parashos, Schneider; Hauser, Christine, Hinson, Bodis-Wollner
Financial Disclosure/COI: The authors report no other financial disclosures related to the content of this manuscript.
Financial Disclosures of all authors (for the preceding 12 months):
Anne-Marie A. Wills M.D. M.P.H. has received research support from the NIH, ALSA, has consulting agreements with Accordant, a CVS/Caremark disease management company and with Asubio pharmaceuticals and has participated in clinical trials funded by Pfizer, Acorda/Civitas. Jordan J. Elm, Ph.D., has received research support from NIH/NINDS, research support from Remedy pharmaceuticals, and received compensation from TEVA for her role as a member of an Advisory board. Rong Ye M.S. could not provide any disclosure information. Kelvin L. Chou, M.D. has received research support from the NIH (NS44504-08, NS091856-01) and the Michael J. Fox Foundation, has participated as a site-PI in clinical trials sponsored by the Huntington Study Group (2CARE) and Osmotica, receives royalties from UpToDate and Demos Health and serves as a consultant for Medtronic, Inc. and Accordant. Sotirios A. Parashos, M.D., Ph.D. owns stock in St. Jude Medical, is on the Advisory Boards of Struthers Parkinson’s Center; National Parkinson Foundation Minnesota; and the Minneapolis Clinic of Neurology Foundation, is a Partner in the Minneapolis Clinic of Neurology Ltd.; Minneapolis Clinic of Neurology Building Company LLC, has received honoraria from Northshore University HealthSystem, royalties from Oxford University Press, and grants from Adamas Pharmaceuticals; Astellas Pharma US; Civitas Therapeutics (now Acorda Therapeutics); NINDS, and NPF. Robert A. Hauser, M.D.6, reports consulting for AbbVie, Allergan Neuroscience, Neurocrine, Pfizer, UCB Biosciences, Teva, Chelsea, Auspex Pharma, serves on the Steering Committee for Impax Pharmaceuticals, Advisory Board for Lundbeck, AstraZeneca, Acadia, UCB Biosciences, and has received speaking fees from UCB. Ivan Bodis-Wollner M.D., D.Sc. receives royalties from Springer Verlag and consulting fees from Boehringer Ingelheim. Vanessa K. Hinson, MD, PhD is on the Advisory Board of Teva Pharmaceuticals and receives grant support from the NIH, Michael J Fox Foundation. Chadwick W. Christine MD reports salary support for Research studies from NIH for NET-PD and SteadyPD3; Salary support for research studies from Michael J Fox Foundation. Jay S. Schneider, Ph.D.
References
- 1.NINDS. Parkinsons Disease Research Summit. Non-Motor and Other Complications of Parkinson’s Disease. 2006 Available from: http://www.ninds.nih.gov/research/parkinsonsweb/PD_Plan_2006.htm.
- 2.Marras C, McDermott MP, Rochon PA, Tanner CM, Naglie G, Lang AE. Predictors of deterioration in health-related quality of life in Parkinson’s disease: results from the DATATOP trial. Mov Disord. 2008;23(5):653–9. doi: 10.1002/mds.21853. quiz 776. [DOI] [PubMed] [Google Scholar]
- 3.Visser M, Verbaan D, van Rooden S, Marinus J, van Hilten J, Stiggelbout A. A longitudinal evaluation of health-related quality of life of patients with Parkinson’s disease. Value Health. 2009;12(2):392–6. doi: 10.1111/j.1524-4733.2008.00430.x. [DOI] [PubMed] [Google Scholar]
- 4.Aarsland D, Bronnick K, Larsen JP, Tysnes OB, Alves G. Cognitive impairment in incident, untreated Parkinson disease: the Norwegian ParkWest study. Neurology. 2009;72(13):1121–6. doi: 10.1212/01.wnl.0000338632.00552.cb. [DOI] [PubMed] [Google Scholar]
- 5.Aarsland D, Andersen K, Larsen JP, Perry R, Wentzel-Larsen T, Lolk A, Kragh-Sorensen P. The rate of cognitive decline in Parkinson disease. Arch Neurol. 2004;61(12):1906–11. doi: 10.1001/archneur.61.12.1906. [DOI] [PubMed] [Google Scholar]
- 6.Cooper JA, Sagar HJ, Jordan N, Harvey NS, Sullivan EV. Cognitive impairment in early, untreated Parkinson’s disease and its relationship to motor disability. Brain. 1991;114(Pt 5):2095–122. doi: 10.1093/brain/114.5.2095. [DOI] [PubMed] [Google Scholar]
- 7.Muslimovic D, Post B, Speelman JD, Schmand B. Cognitive profile of patients with newly diagnosed Parkinson disease. Neurology. 2005;65(8):1239–45. doi: 10.1212/01.wnl.0000180516.69442.95. [DOI] [PubMed] [Google Scholar]
- 8.Uc EY, McDermott MP, Marder KS, Anderson SW, Litvan I, Como PG, Auinger P, Chou KL, Growdon JC. Incidence of and risk factors for cognitive impairment in an early Parkinson disease clinical trial cohort. Neurology. 2009;73(18):1469–77. doi: 10.1212/WNL.0b013e3181bf992f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mamikonyan E, Moberg PJ, Siderowf A, Duda JE, Have TT, Hurtig HI, Stern MB, Weintraub D. Mild cognitive impairment is common in Parkinson’s disease patients with normal Mini-Mental State Examination (MMSE) scores. Parkinsonism Relat Disord. 2009;15(3):226–31. doi: 10.1016/j.parkreldis.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kulisevsky J, Pagonabarraga J. Cognitive impairment in Parkinson’s disease: tools for diagnosis and assessment. Mov Disord. 2009;24(8):1103–10. doi: 10.1002/mds.22506. [DOI] [PubMed] [Google Scholar]
- 11.Chou KL, Amick MM, Brandt J, Camicioli R, Frei K, Gitelman D, Goldman J, Growdon J, Hurtig HI, Levin B, Litvan I, Marsh L, Simuni T, Troster AI, Uc EY. A recommended scale for cognitive screening in clinical trials of Parkinson’s disease. Mov Disord. 25(15):2501–2507. doi: 10.1002/mds.23362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schneider JS, Elm JJ, Parashos SA, Ravina BM, Galpern WR. Predictors of cognitive outcomes in early Parkinson disease patients: The National Institutes of Health Exploratory Trials in Parkinson Disease (NET-PD) experience. Parkinsonism Relat Disord. 2010;16(8):507–12. doi: 10.1016/j.parkreldis.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Investigators, N.N.P. A randomized, double-blind, futility clinical trial of creatine and minocycline in early Parkinson disease. Neurology. 2006;66(5):664–71. doi: 10.1212/01.wnl.0000201252.57661.e1. [DOI] [PubMed] [Google Scholar]
- 14.NINDS NET-PD Investigators. A randomized clinical trial of coenzyme Q10 and GPI-1485 in early Parkinson disease. Neurology. 2007;68(1):20–8. doi: 10.1212/01.wnl.0000250355.28474.8e. [DOI] [PubMed] [Google Scholar]
- 15.Weintraub D, Hauser RA, Elm JJ, Pagan F, Davis MD, Choudhry A. Rasagiline for mild cognitive impairment in Parkinson’s disease: A placebo-controlled trial. Movement disorders : official journal of the Movement Disorder Society. 2016;31(5):709–14. doi: 10.1002/mds.26617. [DOI] [PubMed] [Google Scholar]
- 16.Marinus J, Visser M, Verwey NA, Verhey FR, Middelkoop HA, Stiggelbout AM, van Hilten JJ. Assessment of cognition in Parkinson’s disease. Neurology. 2003;61(9):1222–8. doi: 10.1212/01.wnl.0000091864.39702.1c. [DOI] [PubMed] [Google Scholar]
- 17.Verbaan D, Marinus J, Visser M, van Rooden SM, Stiggelbout AM, Middelkoop HA, van Hilten JJ. Cognitive impairment in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2007;78(11):1182–7. doi: 10.1136/jnnp.2006.112367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Verbaan D, Jeukens-Visser M, Van Laar T, van Rooden SM, Van Zwet EW, Marinus J, van Hilten JJ. SCOPA-cognition cutoff value for detection of Parkinson’s disease dementia. Mov Disord. 2011 doi: 10.1002/mds.23750. [DOI] [PubMed] [Google Scholar]
- 19.Isella V, Mapelli C, Morielli N, Siri C, De Gaspari D, Pezzoli G, Antonini A, Poletti M, Bonuccelli U, Picchi L, Napolitano A, Vista M, Appollonio IM. Diagnosis of possible mild cognitive impairment in Parkinson’s disease: validity of the SCOPA-Cog. Parkinsonism & related disorders. 2013;19(12):1160–3. doi: 10.1016/j.parkreldis.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 20.Marras C, Armstrong MJ, Meaney CA, Fox S, Rothberg B, Reginold W, Tang-Wai DF, Gill D, Eslinger PJ, Zadikoff C, Kennedy N, Marshall FJ, Mapstone M, Chou KL, Persad C, Litvan I, Mast BT, Gerstenecker AT, Weintraub S, Duff-Canning S. Measuring mild cognitive impairment in patients with Parkinson’s disease. Movement disorders : official journal of the Movement Disorder Society. 2013;28(5):626–33. doi: 10.1002/mds.25426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Rooden SM, Verbaan D, Stijnen T, Marinus J, van Hilten JJ. The influence of age and approaching death on the course of nondopaminergic symptoms in Parkinson’s disease. Parkinsonism & related disorders. 2016;24:113–8. doi: 10.1016/j.parkreldis.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 22.Zhu K, van Hilten JJ, Marinus J. Predictors of dementia in Parkinson’s disease; findings from a 5-year prospective study using the SCOPA-COG. Parkinsonism & related disorders. 2014;20(9):980–5. doi: 10.1016/j.parkreldis.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 23.Goldman WP, Baty JD, Buckles VD, Sahrmann S, Morris JC. Cognitive and motor functioning in Parkinson disease: subjects with and without questionable dementia. Arch Neurol. 1998;55(5):674–80. doi: 10.1001/archneur.55.5.674. [DOI] [PubMed] [Google Scholar]
- 24.Starkstein SE, Preziosi TJ, Berthier ML, Bolduc PL, Mayberg HS, Robinson RG. Depression and cognitive impairment in Parkinson’s disease. Brain. 1989;112(Pt 5):1141–53. doi: 10.1093/brain/112.5.1141. [DOI] [PubMed] [Google Scholar]
- 25.Starkstein SE, Bolduc PL, Preziosi TJ, Robinson RG. Cognitive impairments in different stages of Parkinson’s disease. J Neuropsychiatry Clin Neurosci. 1989;1(3):243–8. doi: 10.1176/jnp.1.3.243. [DOI] [PubMed] [Google Scholar]
- 26.Growdon JH, Kieburtz K, McDermott MP, Panisset M, Friedman JH. Levodopa improves motor function without impairing cognition in mild non-demented Parkinson’s disease patients. Parkinson Study Group. Neurology. 1998;50(5):1327–31. doi: 10.1212/wnl.50.5.1327. [DOI] [PubMed] [Google Scholar]
- 27.Chahine LM, Weintraub D, Hawkins KA, Siderowf A, Eberly S, Oakes D, Seibyl J, Stern MB, Marek K, Jennings D. Cognition in individuals at risk for Parkinson’s: Parkinson associated risk syndrome (PARS) study findings. Movement disorders : official journal of the Movement Disorder Society. 2016;31(1):86–94. doi: 10.1002/mds.26373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kieburtz K, Tilley BC, Elm JJ, Babcock D, Hauser R, Ross GW, Augustine AH, Augustine EU, Aminoff MJ, Bodis-Wollner IG, Boyd J, Cambi F, Chou K, Christine CW, Cines M, Dahodwala N, Derwent L, Dewey RB, Jr, Hawthorne K, Houghton DJ, Kamp C, Leehey M, Lew MF, Liang GS, Luo ST, Mari Z, Morgan JC, Parashos S, Perez A, Petrovitch H, Rajan S, Reichwein S, Roth JT, Schneider JS, Shannon KM, Simon DK, Simuni T, Singer C, Sudarsky L, Tanner CM, Umeh CC, Williams K, Wills AM. Effect of creatine monohydrate on clinical progression in patients with Parkinson disease: a randomized clinical trial. Jama. 2015;313(6):584–93. doi: 10.1001/jama.2015.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jankovic J, McDermott M, Carter J, Gauthier S, Goetz C, Golbe L, Huber S, Koller W, Olanow C, Shoulson I, et al. Variable expression of Parkinson’s disease: a base-line analysis of the DATATOP cohort. The Parkinson Study Group. Neurology. 1990;40(10):1529–34. doi: 10.1212/wnl.40.10.1529. [DOI] [PubMed] [Google Scholar]
- 30.Anang JB, Gagnon JF, Bertrand JA, Romenets SR, Latreille V, Panisset M, Montplaisir J, Postuma RB. Predictors of dementia in Parkinson disease: a prospective cohort study. Neurology. 2014;83(14):1253–60. doi: 10.1212/WNL.0000000000000842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Allison PD. SAS Global Forum 2012. Haverford; PA, USA: 2012. Handling Missing Data by Maximum Likelihood. [Google Scholar]
- 32.Kiely KM, Butterworth P, Watson N, Wooden M. The Symbol Digit Modalities Test: Normative data from a large nationally representative sample of Australians. Archives of clinical neuropsychology : the official journal of the National Academy of Neuropsychologists. 2014;29(8):767–75. doi: 10.1093/arclin/acu055. [DOI] [PubMed] [Google Scholar]
- 33.Kulisevsky J, Avila A, Barbanoj M, Antonijoan R, Berthier ML, Gironell A. Acute effects of levodopa on neuropsychological performance in stable and fluctuating Parkinson’s disease patients at different levodopa plasma levels. Brain. 1996;119(Pt 6):2121–32. doi: 10.1093/brain/119.6.2121. [DOI] [PubMed] [Google Scholar]
- 34.Fera F, Nicoletti G, Cerasa A, Romeo N, Gallo O, Gioia MC, Arabia G, Pugliese P, Zappia M, Quattrone A. Dopaminergic modulation of cognitive interference after pharmacological washout in Parkinson’s disease. Brain Res Bull. 2007;74(1–3):75–83. doi: 10.1016/j.brainresbull.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 35.Hanna-Pladdy B, Heilman KM. Dopaminergic modulation of the planning phase of skill acquisition in Parkinson’s disease. Neurocase. 16(2):182–90. doi: 10.1080/13554790903379609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cagney KA, Lauderdale DS. Education, wealth, and cognitive function in later life. The journals of gerontology. Series B, Psychological sciences and social sciences. 2002;57(2):P163–72. doi: 10.1093/geronb/57.2.p163. [DOI] [PubMed] [Google Scholar]
- 37.Koster A, Penninx BW, Bosma H, Kempen GI, Newman AB, Rubin SM, Satterfield S, Atkinson HH, Ayonayon HN, Rosano C, Yaffe K, Harris TB, Rooks RN, Van Eijk JT, Kritchevsky SB. Socioeconomic differences in cognitive decline and the role of biomedical factors. Annals of epidemiology. 2005;15(8):564–71. doi: 10.1016/j.annepidem.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 38.Lee S, Buring JE, Cook NR, Grodstein F. The relation of education and income to cognitive function among professional women. Neuroepidemiology. 2006;26(2):93–101. doi: 10.1159/000090254. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.