Abstract
Cannabis use has been associated with altered sensory gating and neural oscillations. However, it is unclear which constituent in cannabis is responsible for these effects, or whether these are cannabinoid receptor 1 (CB1R) mediated. Therefore, the present study in humans and rats examined whether cannabinoid administration would disrupt sensory gating and evoked oscillations utilizing electroencephalography (EEG) and local field potentials (LFPs), respectively. Human subjects (n=15) completed four test days during which they received intravenous delta-9-tetrahydrocannabinol (Δ9-THC), cannabidiol (CBD), Δ9-THC+CBD, or placebo. Subjects engaged in a dual-click paradigm, and outcome measures included P50 gating ratio (S2/S1) and evoked power to S1 and S2. In order to examine CB1R specificity, rats (n=6) were administered the CB1R agonist CP-55940, CP-55940+AM-251 (a CB1R antagonist), or vehicle using the same paradigm. LFPs were recorded from CA3 and entorhinal cortex. Both Δ9-THC (p<0.007) and Δ9-THC+CBD (p<0.004) disrupted P50 gating ratio compared to placebo, while CBD alone had no effect. Δ9-THC (p<0.048) and Δ9-THC+CBD (p<0.035) decreased S1 evoked theta power, and in the Δ9-THC condition, S1 theta negatively correlated with gating ratios (r=-0.629, p<0.012 (p < 0.048 adjusted)). In rats, CP-55940 disrupted gating in both brain regions (p<0.0001), and this was reversed by AM-251. Further, CP-55940 decreased evoked theta (p<0.0077) and gamma (p<0.011) power to S1, which was partially blocked by AM-251.These convergent human/animal data suggest that CB1R agonists disrupt sensory gating by altering neural oscillations in the theta-band. Moreover, this suggests that the endocannabinoid system mediates theta oscillations relevant to perception and cognition.
Keywords: Cannabinoid, Neural Oscillations, Theta, EEG, P50, Sensory Gating
1. Introduction
Cannabis, via delta-9-tetrahydrocannabinol (Δ9-THC), induces disruptions in behavior, perception, and cognition (Sherif et al. 2016). Δ9-THC acts by binding to presynaptic cannabinoid receptors type 1 (CB1Rs), thus inhibiting neurotransmitter release. CB1Rs are the most highly expressed metabotropic receptors in the brain, and primarily inhibit the release of gamma-amino butyric acid (GABA) from cholecystokinin-containing interneurons (Ali and Todorova 2010; Eggan et al. 2010) and glutamate in the hippocampus and striatum (Gerdeman and Lovinger 2001; Polissidis et al. 2013; Wang 2003). Moreover, CB1Rs are implicated in the fine-tuning of GABA-mediated theta (4–7 Hz) and gamma (30–80 Hz) oscillations (Skosnik et al. 2016). As these oscillations are involved in numerous perceptual and cognitive processes (Lachaux et al. 2012; Singer and Gray 1995), it has been postulated that Δ9-THC induces its psychotropic effects by disrupting the excitatory-inhibitory balance of neural networks, which in turn, desynchronizes neural oscillations in the theta and gamma range (Cortes-Briones et al. 2015a; Skosnik et al. 2016; Skosnik et al. 2006).
Assessment of the integrity of inhibitory neural networks and their oscillatory dynamics can be achieved utilizing the auditory repetition suppression paradigm (also termed P50 sensory gating). Using either electroencephalography (EEG) in humans or local field potentials (LFPs) in animals, this highly translatable paradigm can be applied across species using the same stimuli, experimental parameters, and signal analysis methods. In the standard auditory repetition suppression or “dual-click” procedure, pairs of auditory clicks are presented, and the amplitude of the P50 event-related potential (ERP; the N40 in rodents) to the second auditory click (S2) is attenuated relative to the P50 amplitude to the first click (S1) (Patterson et al. 2008; Smucny et al. 2015). This effect is typically indexed using P50 amplitude ratio (S2/S1; higher P50 ratios indicate more disrupted sensory gating) (Edwards et al. 2009). While the P50 response to S1 is associated with basic sensory registration and processing, it is thought that the reduction of the P50 amplitude to S2 reflects the gating of irrelevant stimuli via recurrent or feed-forward inhibition (Hajos 2006; Miller and Freedman 1993; 1995).
Several studies have demonstrated P50 suppression deficits in heavy cannabis users utilizing EEG. For example, Rentzsch et al. (2007) showed that individuals studied after 28 days of abstinence demonstrated P50 gating deficits that correlated with the total years of cannabis consumption (Rentzsch et al. 2007). Studies examining individuals currently using cannabis (but not acutely intoxicated) have also shown disruptions in P50 suppression, and this effect was associated with frequency of cannabis use (Edwards et al. 2009; Patrick and Struve 2000). By contrast, Broyd et al. (2013) reported that while cannabis users did not differ from healthy controls on P50 gating, prolonged duration of chronic use was associated with greater impairment in sensory gating (Broyd et al. 2013). Germane to the current study, Edwards et al. (2009) also demonstrated that current cannabis use was associated with disruptions in evoked theta, beta, and gamma oscillations during P50 gating (Edwards et al. 2009).
Taken together, these findings suggest that cannabinoids like Δ9-THC may interfere with the inhibitory networks involved in the “gating out” of irrelevant or redundant sensory information. However, it is unclear whether these deficits are the result of a constituent in cannabis other than Δ9-THC (e.g., cannabidiol (CBD)) or premorbid differences in cannabis-seeking individuals. While several previous studies in animals utilizing LFPs have demonstrated that the administration of CB1R agonists disrupts gating (Dissanayake et al. 2008; Hajos et al. 2008; Zachariou et al. 2008), to date, no studies have examined the effect of acute cannabinoids on sensory gating in humans.
The current study therefore examined the effects of acute intravenous (IV) Δ9-THC, CBD (the second most abundant cannabinoid in cannabis), the combination of Δ9-THC+CBD, and placebo on P50 sensory gating and neural oscillations in healthy humans. In order to examine the receptor specificity of cannabinoid effects on gating and oscillations, a second study was performed utilizing a full agonist and antagonist for the CB1R. As no CB1R full agonists or antagonists are currently available for use in humans, this parallel study examined LFPs in rodents and was undertaken using the same experimental parameters and signal processing techniques (utilizing the CB1R agonist CP-55940 and antagonist AM-251).
2. Methods
2.1. Human Study
The current study utilized a randomized, placebo-controlled, double-blind, 2 (Δ9-THC or placebo) × 2 (CBD or placebo), and counterbalanced design. Subjects completed 4 test days separated by at least 72 hours to limit any carryover effects of Δ9-THC from one test day to another. The study was conducted at the Neurobiological Studies Unit (VA Connecticut Healthcare System, West Haven, CT) with the approval of the Institutional Review Boards of VA Connecticut Healthcare System and Yale University School of Medicine, the FDA (IND #51671) and in accordance with the Helsinki Declaration of 1975. Subjects were recruited by public advertisement and compensated for their participation.
2.1.1. Participants
Inclusion/exclusion criteria were the same as described previously (Cortes-Briones et al. 2014; D'Souza et al. 2004).The sample consisted of healthy individuals (n=15; 40% female; mean age in years = 29.6, S.D. = 7.0; mean years of education = 16.3, S.D. = 2.3), who underwent a structured psychiatric interview and were carefully screened for any DSM IV axis 1 lifetime psychiatric and substance use disorder and family history of major axis 1 disorder. Cannabis-naïve individuals were excluded to minimize any risk of promoting future cannabis use/abuse. A general physical and neurological examination, electrocardiogram, and laboratory tests (serum electrolytes, liver function tests, complete blood count with differential, and urine toxicology) were also conducted and subjects with ongoing medical conditions were excluded if deemed unsafe to participate. Subjects with any major current or recent stressor (< 6 weeks) were excluded to avoid decompensation under the effect of Δ9-THC. All subjects tested negative for urinary metabolites of Δ9-THC prior to the start of testing.
2.1.2. Drugs
Δ9-THC
The preparation, formulation, and storage of Δ9-THC solution have been reported elsewhere (D'Souza et al. 2004). For the placebo condition, an equivalent volume of ethanol (vehicle) was used, which has been previously shown to be undetectable in multiple post-injection blood samples (D'Souza et al. 2004). The IV route of administration was chosen to standardize the delivery of Δ9-THC as discussed previously (D'Souza et al. 2004). The dose of Δ9-THC used in this study was 0.035 mg/kg (2.5 mg in a 70 kg individual), given intravenously over 20 minutes into a rapidly flowing IV infusion of normal saline (see General Study Procedure below). Similar doses from our previous studies have been shown to produce statistically significant behavioral, cognitive, and electrophysiological effects (Cortes-Briones et al. 2015b; D'Souza et al. 2012).
CBD
CBD was manufactured and supplied by STI pharmaceuticals Ltd. The dose used in this study was 5mg, administered IV over 2 minutes. CBD powder was dissolved in 95% ethanol at a concentration of 10 mg/ml in the VA research pharmacy. This is similar to the stock solution prepared with Δ9-THC. This dose and route of administration of CBD has been demonstrated to be effective and well tolerated in previous studies (Bhattacharyya et al. 2009).
2.1.3. General Study Procedure
Subjects fasted overnight, reported to the test facility at 8 A.M., and were provided a standard breakfast. A positive urine drug screen and a positive pregnancy test resulted in exclusion. In-study safety procedures are described elsewhere (D'Souza et al. 2004). Vital signs were continuously monitored throughout the test day. A field sobriety test, mental state examination, and exit interview were conducted at the end of each test day and an exit interview was conducted on the last test day. At the −30, +20, +80, and +240 time points, blood was sampled from the IV line from the arm opposite to the one used for administering the study drug for determination of 11-nor-Δ9-THC-9-COOH (THC-COOH) and CBD levels. Immediately after collection, blood samples were placed on ice, centrifuged and the extracted plasma was aliquoted into vials for storage at −70° C until assayed.
Subjects first received either CBD or placebo (administered over 2 min) followed by either Δ9-THC or placebo. Time points for behavioral assessments were −60 min, +15, +90, and +240 minutes (see details below). EEG data were collected at +25 min in order to capture electrophysiological signals during peak Δ9-THC effects.
2.1.4. Behavioral and Subjective Measures
In order to confirm Δ9-THC effects and examine possible associations of its electrophysiological effects with its behavioral and subjective effects, several outcome measures known to be sensitive to the effects of Δ9-THC (D'Souza et al. 2008a; D'Souza et al. 2004; D'Souza et al. 2008b) were also included. Perceptual alterations were measured using the Clinician Administered Dissociative Symptoms Scale (CADSS) (Bremner et al. 1998) consisting of 19 self-report items and 8 clinician-rated items rated from 0 (not at all) to 4 (extremely). The scale evaluates aspects of altered environmental (sensory) perception, time perception, body perception, feelings of unreality, and memory impairment. “High” associated with cannabis intoxication was measured using a self-reported visual analog scale (VAS) (0−100). Both of these assessments were administered at baseline (−60 min), +15, +90, and +240 minute time points, where time-point zero minutes denotes the beginning of the Δ9-THC infusion.
2.1.5. EEG Recording and Preprocessing
EEG recording and preprocessing was performed as described previously (Radhakrishnan et al. 2015). Briefly, EEG data were collected in an acoustically and electrically shielded booth, and recording was done with the commercially available Active Two acquisition system (Biosemi, the Netherlands). A sampling rate of 1024 Hz was utilized, with on-line low-pass filter of 256 Hz to prevent aliasing of high frequencies. A 64-channel electrode cap according to the extended 10–20 system was used, along with additional electrodes to record the vertical and horizontal electro-oculogram. All electrodes were referenced during recording to a commonmode signal (CMS) electrode between POz and PO3 and then subsequently re-referenced to the nose offline. EEG data were first bandpass filtered from 0.1 – 100 Hz (24 dB/oct) and notch filtered at 60 Hz. The recorded EEG was then segmented into epochs consisting of a 500 ms baseline and ending 1000 ms after stimulus onset (which captures both S1 and S2). Ocular movement correction was applied using Gratton's algorithm (Gratton et al. 1983). After baseline correction (500 ms), any trial with a voltage greater than ± 150 µV was excluded from analysis. All EEG preprocessing and analysis was performed using the software package Brain Vision Analyzer 2.0 (Brain Products GmbH, Germany).
2.1.6. Sensory Gating Paradigm
A standard P50 sensory gating paradigm was utilized which consisted of 75 trials of paired click stimuli (500 ms interstimulus interval, 80 dB SPL, binaural). The stimuli were 3 ms white noise clicks presented through Etymotic insert ER-3a earphones (Etymotic Research, Inc., Elk Grove Village, IL, USA). Each trial was separated by a variable 6–10 second intertrial interval. Participants were seated comfortably in a sound attenuated and electrically shielded booth with eyes open (focused on a fixation cross 70 cm from the subject) while passively listening to the paired click stimuli.
2.1.7. S1 and S2 Amplitudes and Sensory Gating Ratios
Following preprocessing and trial segmentation (see above), individual trials were averaged and low pass filtered (50 Hz cutoff, 24 dB/octave). In order to determine S1 and S2 amplitudes, the N40 and P50 components were detected automatically utilizing Analyzer 2.0, which were subsequently confirmed manually. The N40 window was defined as the largest negative voltage between 20 and 50 ms post click, while the P50 was defined as the largest positive voltage between 40 and 70 ms post click. S1 and S2 responses were calculated as the N40 to P50 peak-to-peak amplitude as described previously (Edwards et al. 2009). Sensory gating ratio was then determined by dividing S2/S1. Thus, lower ratios indicate more robust gating.
2.1.8. S1 and S2 Evoked Power
It has been established that evoked power is a measure of non-jittered, tightly time-locked activity commonly observed in response to simple sensory stimuli (e.g. auditory clicks, tones, etc.) (Roach and Mathalon 2008; Skosnik et al. 2016). By contrast, induced power is a measure of jittered, non-time locked activity observed during more complex higher perceptual and cognitive processing (e.g. object recognition, motion perception, semantic processing, etc.) (Roach and Mathalon 2008; Skosnik et al. 2016). Given that EEG responses during higher perception/cognition jitter from trial to trial, it is necessary to perform spectral analysis on single trials prior to averaging (otherwise, the signal of interest would be cancelled out during averaging) (Roach and Mathalon 2008; Skosnik et al. 2016). In the current study, the stimuli were simple, discrete auditory clicks, which elicited time-locked responses (i.e. ERPs). In this paradigm, any jittered non-time-locked activity likely represents noise unrelated to the stimulus. Hence, maximal signal-to-noise ratio was achieved by averaging as a first step (since non-stimulus related activity would cancel out), and then applying time-frequency analysis (see below).
Time-locked, evoked power (Skosnik et al. 2016) was determined via time-frequency analysis and was performed using a complex Morlet wavelet transform on unfiltered data in Brain Vision Analyzer 2.0. After preprocessing and individual trial averaging, a continuous wavelet transform was carried out using 1 Hz frequency steps from 1–80 Hz. A wavelet parameter of 8 was utilized, and a 500 ms baseline correction was applied. A spectral-temporal region of interest (ROI) approach was used, as the time window of interest was between 0–100 ms (corresponding to S1 and S2 amplitudes and the gating response). Traditional frequency bands between 0–100 ms (mean power) for both S1 and S2 were extracted and included delta (1–3 Hz), theta (4–7 Hz), alpha (8–12 Hz), beta (13–29 Hz), and gamma (30–80 Hz) frequencies.
2.2. Animal Study
2.2.1. Subjects
Data were collected on male Sprague-Dawley rats (n=6; 250–300 g) using an approved animal use protocol in compliance with the Animal Welfare Act Regulations and with the Guide for the Care and Use of Laboratory Animals, National Institutes of Health guidelines.
2.2.2. Drugs
CP-55940 is a selective CB1R agonist, and this compound has been tested in both auditory gating studies and network oscillation experiments (Hajos et al. 2008; Robbe and Buzsaki 2009; Robbe et al. 2006; Sales-Carbonell et al. 2013). AM-251 is a well-characterized CB1R antagonist broadly used in both in vitro and in vivo studies. Furthermore, the doses of both CP-55940 and AM-251 that affected gating and oscillations have been established in these previous studies (Hajos et al. 2008; Robbe and Buzsaki 2009; Robbe et al. 2006; Sales-Carbonell et al. 2013). CP-55940 (0.3 mg/kg; Pfizer, Groton, Connecticut) and AM-251 (3 m/kg; Tocris Bioscience, Ellisville, Missouri) were made up as suspensions in methylcellulose (2.5 mg/mL) sterile vehicle (Pharmacia & Upjohn, Kalamazoo, Michigan). Vehicle injection volumes were 1 mL/kg.
2.2.3. Sensory Gating Paradigm and LFP Recordings from the Hippocampus (CA3) and Entorhinal Cortex (ENT)
LFP recordings were carried out from the hippocampus CA3 region and the ENT since numerous previous studies have shown a relationship between auditory gating and electrophysiological activity in these brain regions (Adler et al. 1986; Boutros et al. 2008; Hajos et al. 2008; Smucny et al. 2015). Experiments were performed in chloral hydrate anesthesia (400 mg/kg, intraperitoneal) as described previously (Hajos et al. 2005). Anesthetized rats were placed in a stereotaxic frame, and the femoral vein was cannulated for administration of test compounds or additional doses of anesthetic. Craniotomy was performed above the hippocampus and the ENT. Body temperature was maintained at 37°C by an isothermal heating pad. After conclusion of experiments, animals were sacrificed; brains were removed, blocked, and frozen for histological verification of electrode placement.
Field potentials and auditory evoked potentials (AEPs) were recorded (2083 Hz sampling rate) from the CA3 region of the right hippocampus (AP = 3.5 mm, V = 4.0 mm, and L = 3.0 mm) and the ipsilateral ENT (AP = 8.0 mm, V = 6.0 mm, L = 4.4 mm). Auditory stimulation consisted of two consecutive tone bursts of 10-msec duration (sound pressure level 95 dB between the ear bars) at a frequency of 5 kHz. As in the human experiment, delay between S1 and S2 was 500 ms, and intertrial interval was 10 seconds. After baseline (vehicle), the CB1R agonist (CP-55940; 0.3 mg/kg) was injected IV. Following CP-55940, the CB1R antagonist (AM-251; 3 m/kg, IV) was administered; recordings of AEPs commenced 5 min after drug administration, and 50 paired tone trials were administered for each drug condition.
2.2.4. S1 and S2 Amplitudes, Sensory Gating Ratios, and Evoked Power
Preprocessing of the animal LFP data was performed in Analyzer 2.0 as described above, minus ocular correction. In addition, LFP data were downsampled to 1024 Hz in order to be congruent with the human EEG data.
In order to determine S1 and S2 amplitudes, the P20 and N40 components were detected automatically utilizing Analyzer 2.0, which were then subsequently confirmed manually. The P20 window was defined as the largest positive voltage between 10 and 30 ms post stimulus, while the N40 was defined as the largest negative voltage between 20 and 60 ms post stimulus. S1 and S2 responses were calculated as the P20 to N40 peak-to-peak amplitude. Sensory gating ratio was then determined by dividing S2/S1. S1 and S2 evoked power was analyzed in the same manner as the human EEG data described above.
2.3. Statistical analysis
Initially, data were examined descriptively using means, standard deviations, and graphs. Each outcome was assessed for normality visually with histograms, normal probability plots, and Kolmogorov test statistics. CADSS and VAS data were examined at time point +15 min in order to examine subjective effects as close as possible to EEG data collection and peak Δ9-THC effects. For the human EEG data (S1 and S2 amplitude, P50 gating ratio, and S1 and S2 spectral power; all at electrode FCz, where P50 was largest), linear mixed models were utilized with drug condition (4; Δ9-THC, CBD, Δ9-THC+CBD, placebo) as a within-subjects factor. For the animal LFP data (S1 and S2 amplitude, P50 gating ratio, and S1 and S2 spectral power) linear mixed models were utilized with drug condition (3; CP-55940, CP-55940+AM-251, placebo) and brain region (2; CA3 and ENT) as a within-subject factors. Every model included random effects and unstructured variance-covariance matrices. The best fitting variance-covariance structure was determined using information criteria. Tukey’s post-hoc procedure was used to determine significant pair-wise group differences The mixed effects approach is advantageous as it is unaffected by randomly missing data and allows greater flexibility in modeling the correlation structure of repeated measures data (Gueorguieva and Krystal 2004). Pearson correlations were utilized to examine relationships between variables. All analyses were conducted using SAS version 9.1 (SAS Institute Inc., Cary, NC).
3. Results
3.1. Human Subjective/Behavioral Results
Subjective/behavioral data exhibited a non-normal distribution. Hence, a nonparametric approach utilizing ANOVA-Type Statistics (ATS) was performed for the VAS and CADSS data (Brunner et al. 2002).
For VAS “high” a main effect of drug condition was observed (ATS = 20.42, df = 1.9, p < 0.0001) (data not shown). Post hoc tests revealed that Δ9-THC significantly increased VAS “high” compared to placebo (ATS = 13.42, df = 1, p < 0.0002) and CBD alone (ATS = 22.35, df = 1, p < 0.0001). Moreover, the combination of Δ9-THC+CBD induced increases in VAS “high” compared to placebo (ATS = 40.86, df = 1.9, p < 0.0001) and CBD alone (ATS = 47.35, df = 1.9, p < 0.0001). No differences were observed for Δ9-THC compared to Δ9-THC+CBD (ATS = 0.2, df = 1, p = 0.659) or CBD compared to placebo (ATS = 1.58, df = 1.9, p = 0.209).
For CADSS patient-rated (CADSS-PR), a main effect of drug condition was observed (ATS = 8.15, df = 2.31, p < 0.0001) (data not shown). Post hoc tests revealed that Δ9-THC significantly increased CADSS-PR compared to placebo (ATS = 12.57, df = 1, p < 0.0004) and CBD alone (ATS = 10.75, df = 1, p < 0.001). Moreover, the combination of Δ9-THC+CBD induced increases in CADSS-PR compared to placebo (ATS = 12.6, df = 1, p < 0.0004) and CBD alone (ATS = 8.13, df = 1, p < 0.0044). No differences were observed for Δ9-THC compared to Δ9-THC+CBD (ATS = 0, df = 1, p = 0.956) or CBD compared to placebo (ATS = 0.02, df = 1, p = 0.8935).
For CADSS clinician-rated (CADSS-CR), a main effect of drug condition was observed (ATS = 16.8, df = 2.61, p < 0.0001) (data not shown). Post hoc tests revealed that Δ9-THC significantly increased CADSS-CR compared to placebo (ATS = 48.33, df = 1, p < 0.0001) and CBD alone (ATS = 27.6, df = 1, p < 0.0001). Moreover, the combination of Δ9-THC+CBD increased CADSS-CR scores compared to placebo (ATS = 28.28, df = 1, p < 0.0001) and CBD alone (ATS = 11.9, df = 1, p < 0.0006). No differences were observed for Δ9-THC compared to Δ9-THC+CBD (ATS = 1.52, df = 1, p = 0.217) or CBD compared to placebo (ATS = 0.11, df = 1, p = 0.7383).
3.2. Human S1 and S2 Amplitudes, and P50 Gating Ratios
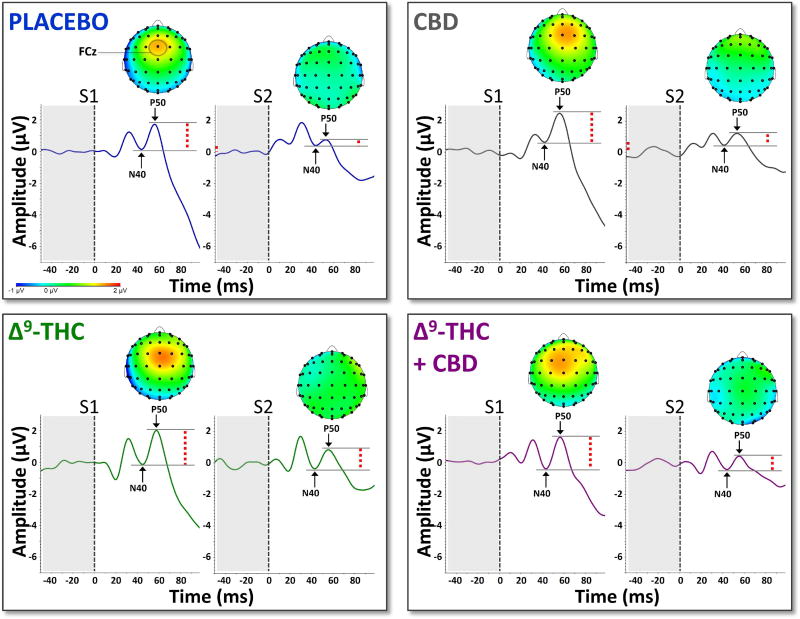
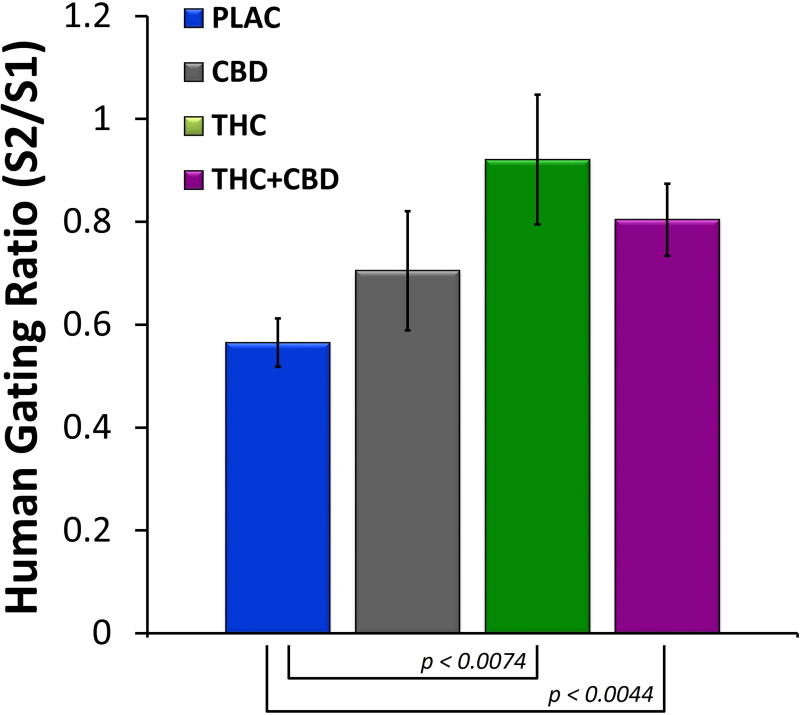
Grand averaged ERPs (electrode FCz) from the human sensory gating paradigm can be seen in Figure 1 For S1 amplitude, no main effect of drug condition was observed (F(3,42) = 0.61, p = 0.61). Likewise, no main effect of S2 amplitude was observed (F(3,42) = 0.64, p = 0.59). However, a significant main effect of drug condition was observed for P50 gating ratio (F(3,42) = 4.65, p < 0.007). Post hoc tests revealed that gating ratios were significantly increased (disrupted) by Δ9-THC (t(42) = 2.82, p < 0.007) and Δ9-THC+CBD (t(42) = 3.01, p < 0.004) compared to placebo. Mean gating ratios for the four drug conditions can be seen in Figure 2.
Figure 1.
Grand-averaged AEPs from electrode FCz across the four drug conditions. Dashed red lines indicate peak-to-peak amplitudes of the N40-P50 components. Grand-averaged topographic maps indicate peak P50 amplitudes for both S1 and S2.
Figure 2.
Mean gating ratios (± S.E.M.) across the four drug conditions derived from AEPs from electrode FCz. Both Δ9-THC and Δ9-THC+CBD disrupted P50 gating ratios compared to placebo, while CBD alone had no effect.
3.3. Human S1 and S2 Evoked Power
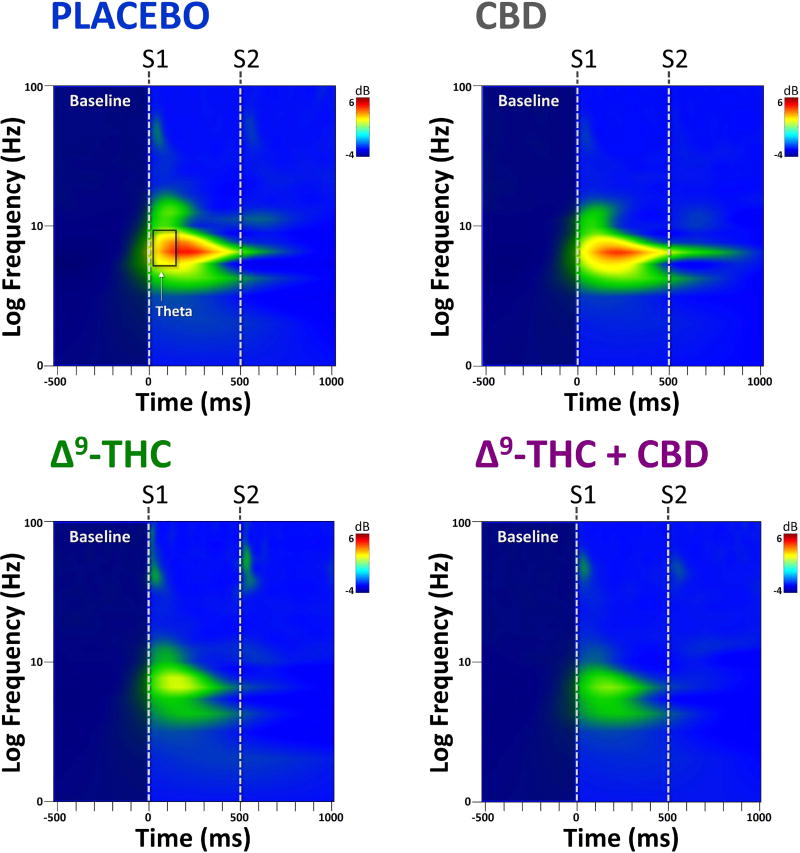
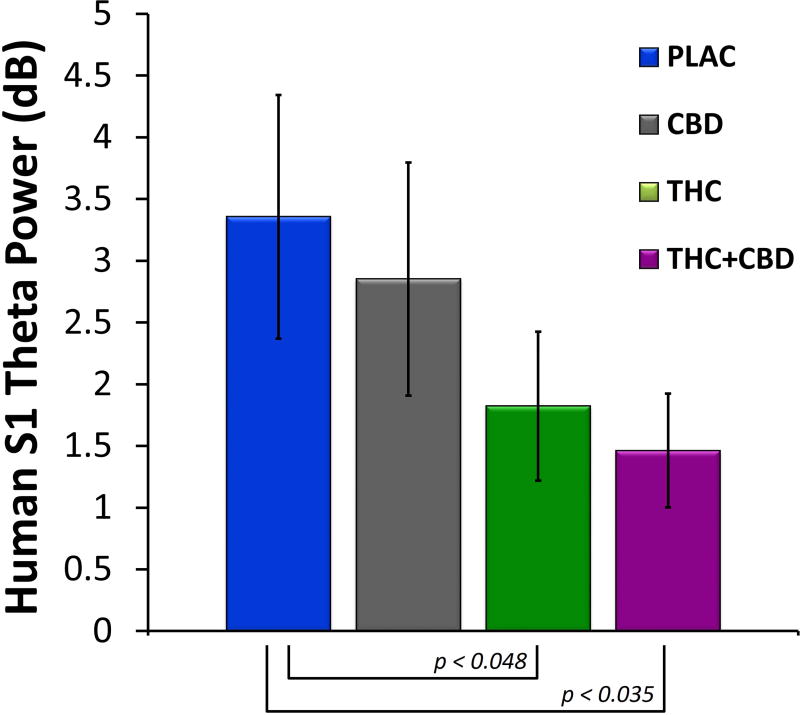
Time-frequency plots of evoked power at FCz from the human sensory gating paradigm can be seen Figure 3. For S1, no significant differences were observed for evoked power, although there was a trend towards significance for drug condition at the theta frequency (F(3,42) = 2.09, p = 0.14). Post hoc tests revealed that theta power following S1 was significantly decreased for both Δ9-THC (t(42) = 2.17, p < 0.048) and Δ9-THC+CBD (t(42) = 2.31, p < 0.035) compared to placebo. Mean S1 evoked theta power for the four drug conditions can be seen in Figure 4. No effect of drug on evoked power was observed for S2.
Figure 3.
Grand-averaged time×frequency plots from electrode FCz across the four drug conditions. Both Δ9-THC and Δ9-THC+CBD decreased S1 evoked theta power compared to placebo, while CBD alone had no effect.
Figure 4.
Mean evoked theta power (± S.E.M.) at S1 across the four drug conditions from electrode FCz. Both Δ9-THC and Δ9-THC+CBD decreased S1 evoked theta power compared to placebo, while CBD alone had no effect.
3.4. Human Correlational Results
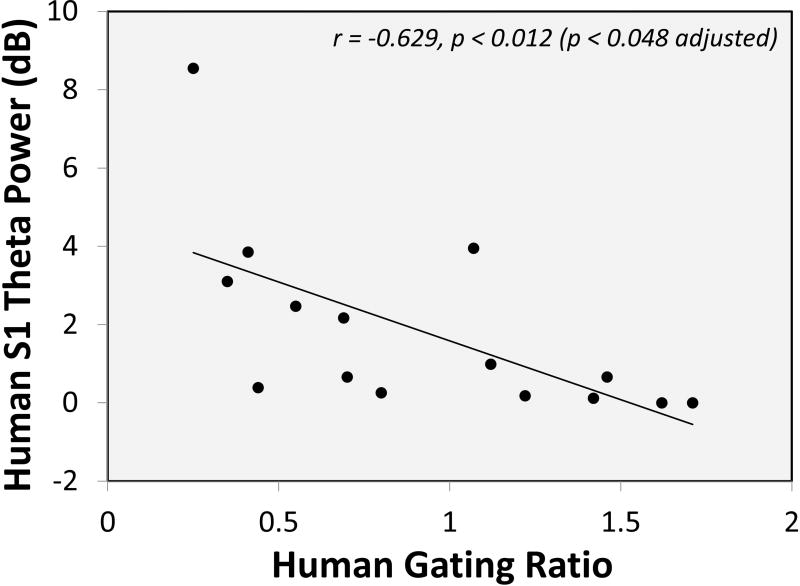
Given the apparent selective role of Δ9-THC in gating ratios and S1 theta power described above, potential associations between gating ratios, S1 theta power, and sensory effects (CADSS-PR and CADSS-CR) were examined within the Δ9-THC condition. While no correlations were observed for subjective measures, a significant negative correlation between gating ratio and S1 theta was found (r = −0.629, p < 0.012 [p < 0.048 adjusted for four comparisons]) (Figure 5). Interestingly, gating ratio did not correlate with S1 theta in the placebo condition (r = −0.271, p = 0.329) (data not shown). In other words, those individuals with the lowest S1 theta power during Δ9-THC administration exhibited the largest (most disrupted) gating ratios.
Figure 5.
Negative correlation between S1 theta and gating ratios in the THC condition (electrode FCz). Individuals with lower S1 theta exhibited poorer gating ratios.
3.5. Animal S1 and S2 Amplitudes, and P50 Gating Ratios
Grand averaged AEPs (from CA3 and ENT) from the animal sensory gating paradigm can be seen in Figure 6. For S1 amplitude, a main effect of drug condition (F(2,25) = 8.34, p < 0.0017) and region was observed (lower S1 amplitudes at CA3) (F(1,25) = 6.15, p < 0.02). However there was no drug×region interaction (F(2,25) = 1.08, p = 0.35). Post hoc tests averaged over region indicated that while CP-55940 significantly decreased S1 amplitude compared to vehicle (F(1,25) = 8.44, p < 0.008), the combination of CP-55940+AM-251 was not different from vehicle, indicating that AM-251 reversed the effects of CP-55940 on S1 amplitude (F(1,25) = 1.07, p = 0.31). There was also a significant difference between CP-55940 alone and CP-55940+AM-251 (F(1,25) = 15.51, p < 0.0006).
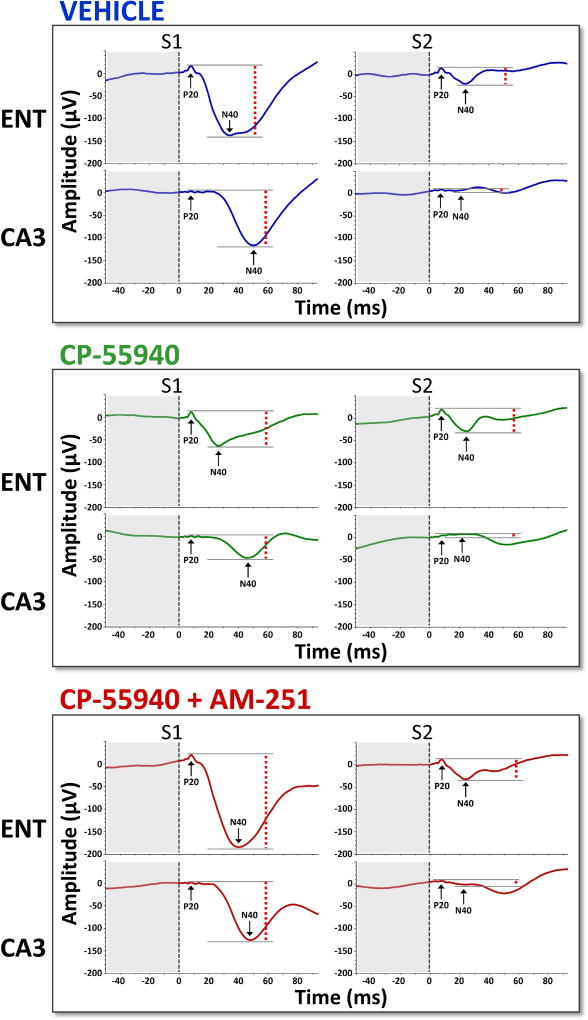
Figure 6.
Grand-averaged AEPs from entorhinal cortex (ENT) and hippocampus (CA3) across the three drug conditions. Dashed red lines indicate peak-to-peak amplitudes of the P20-N40 components.
For S2 amplitude, a main effect of drug condition (F(2,25) = 5.68, p < 0.0092) and region was observed (lower S2 amplitudes at CA3) (F(1,25) = 12.91, p < 0.0014). However there was no drug×region interaction (F(2,25) = 0.95, p = 0.4). Post hoc tests averaged over region indicated that while CP-55940 significantly increased S2 amplitude compared to vehicle (F(1,25) = 11.35, p < 0.0025), the combination of CP-55940+AM-251 was not different from vehicle, indicating that AM-251 reversed the effect of CP-55940 on S2 amplitude (F(1,25) = 0.69, p = 0.41). There was no significant difference between CP-55940 alone and CP-55940+AM-251 (F(1,25) = 1.01, p = 0.33).
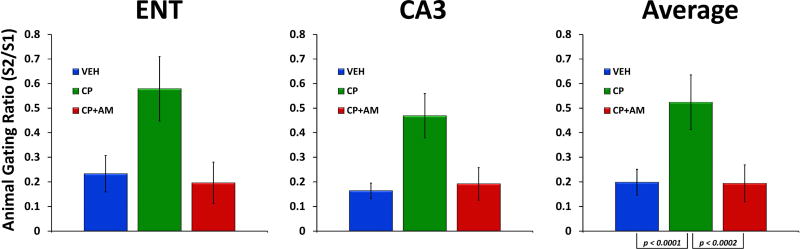
Mean gating ratios for the three drug conditions can be seen in Figure 7. For P50 gating ratio, a main effect of drug condition (F(2,25) = 18.27, p < 0.0001) and region (F(1,25) = 7.52, p < 0.011) was observed. However, no drug × region interaction was observed (F(2,25) = 0.47, p = 0.63). Post hoc tests averaged over region indicated that while CP-55940 significantly increased (disrupted) P50 gating ratio compared to vehicle (F(1,25) = 32.37, p < 0.0001), the combination of CP-55940+AM-251 was not different from vehicle, indicating that AM-251 reversed the effects of CP-55940 on gating ratio (F(1,25) = 0.02, p = 0.89). Moreover, there was a significant difference between CP-55940 alone and CP-55940+AM-251 (F(1,25) = 18.99, p < 0.0002).
Figure 7.
Mean gating ratios (± S.E.M.) from entorhinal cortex (ENT), hippocampus (CA3), and averaged across regions for the three drug conditions (Vehicle (VEH); CP-55940 (CP); AM-251 (AM)). CP-55940 disrupted gating in both brain regions, and this was reversed by AM-251.
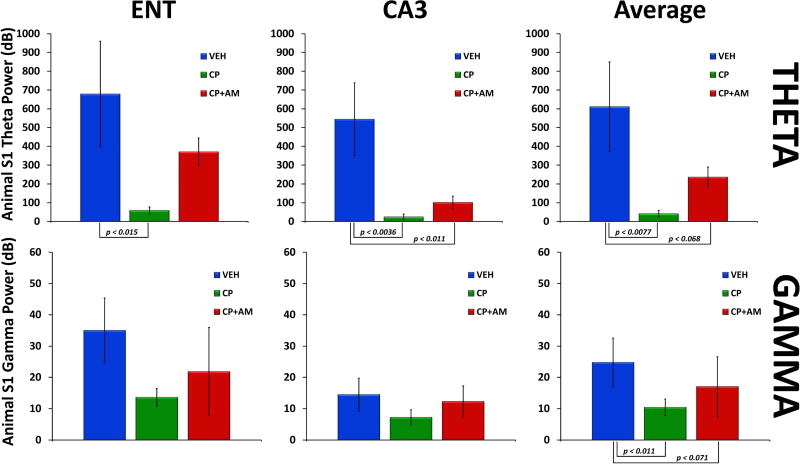
3.6. Animal S1 and S2 Evoked Power
Time-frequency plots of evoked power from ENT and CA3 from the animal sensory gating paradigm can be seen in Figure 8. Main effects for condition were only observed in the theta and gamma bands, and mean S1 evoked theta and gamma power for the three drug conditions can be seen in Figure 9. Specifically, for S1 evoked theta power, a main effect of drug condition (F(2,25) = 4.34, p < 0.024) and region (F(1,25) = 11.32, p < 0.0025) was observed. There was also a trend towards a condition×region interaction (F(2,25) = 2.42, p = 0.11). Post hoc tests averaged over region revealed that CP-55940 significantly decreased evoked theta power compared to vehicle (F(1,25) = 8.40, p < 0.0077). There was also a trend-level difference between vehicle and CP-55940+AM-251 (F(1,25) = 3.65, p = 0.068). No differences were observed in evoked theta power between CP-55940 alone and CP-55940+AM-251 (F(1,25) = 0.98, p = 0.33). Examining each region separately, post hoc tests revealed that CP-55940 decreased evoked theta power compared to vehicle in both ENT (F(1,25) = 6.76, p < 0.015) and CA3 (F(1,25) = 10.33, p < 0.0036). There was also a significant difference in theta power between CP-55940+AM-251 and vehicle in CA3 (F(1,25) = 7.47, p < 0.011).
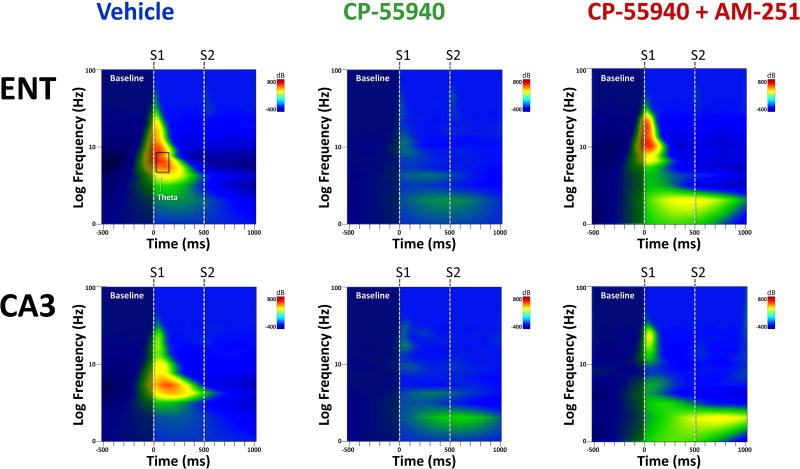
Figure 8.
Grand-averaged time×frequency plots from entorhinal cortex (ENT) and hippocampus (CA3) across the three drug conditions. CP-55940 decreased evoked theta and gamma power to S1, which was partially blocked by AM-251.
Figure 9.
Mean evoked theta and gamma power (± S.E.M.) from entorhinal cortex (ENT), hippocampus (CA3), and averaged across regions for the three drug conditions (Vehicle (VEH); CP-55940 (CP); AM-251 (AM)). CP-55940 decreased evoked theta and gamma power to S1, which was partially blocked by AM-251.
For S1 evoked gamma power, a main effect of drug condition was observed (F(2,25) = 6.63, p < 0.0049). Post hoc tests averaged over region revealed a significant difference between CP-55940 and vehicle (F(1,25) = 3.65, p < 0.011) and a trend level difference between vehicle and CP-55940+AM-251 (F(1,25) = 3.56, p = 0.071).
For S2 evoked power, a main effect of region was observed for theta (F(1,25) = 4.35, p < 0.047), alpha (F(1,25) = 4.29, p < 0.049), and gamma (F(1,25) = 22.83, p < 0.0001). No main effects of drug condition or condition×region interactions were observed for S2.
4. Discussion
The current study found that both Δ9-THC and Δ9-THC+CBD disrupted P50 gating ratios compared to placebo, while CBD alone had no effect. Δ9-THC and Δ9-THC+CBD also decreased S1 evoked theta power, and in the Δ9-THC condition, S1 theta negatively correlated with gating ratios (larger ratios indicate more disruption). In rats, CP-55940 disrupted gating in both brain regions, and this was reversed by AM-251. Further, CP-55940 decreased evoked theta and gamma power to S1, which was partially blocked by AM-251.These convergent human and animal data suggest that cannabinoid agonists disrupt sensory gating by altering neural oscillations in the theta-band, and that these effects are CB1R-mediated.
To our knowledge, this is the first study demonstrating that acute Δ9-THC disrupts P50 sensory gating in humans. The results of the current study are congruent with previous work demonstrating that chronic cannabis use is associated with altered P50 gating ratios (Broyd et al. 2013; Edwards et al. 2009; Patrick and Struve 2000; Rentzsch et al. 2007). However, given the limitations of previous cross-sectional studies, the specific role of Δ9-THC and CB1Rs in P50 gating had remained equivocal. The current study now provides direct evidence that perturbation of the endogenous cannabinoid system by exogenous Δ9-THC disrupts inhibitory networks involved in auditory repetition suppression/sensory gating. Of note, deficits in P50 gating were not observed by the administration of cannabidiol, a phytocannabinoid with minimal activity at the CB1R (Morales et al. 2017). Interestingly, CBD has been shown to attentuate many of the effects of Δ9-THC, particularly it’s psychotomimetic effects (Bhattacharyya et al. 2010; Englund et al. 2013; Zuardi et al. 1982). However, in the current study, CBD failed to reverse the Δ9-THC-induced disruption of sensory gating and theta oscillations. This finding, and the fact that CP-55940-mediated disruptions in sensory gating were reversed by the CB1R antagonist AM-251 in the animal portion of this study provides converging evidence that cannabinoid-related deficits in auditory repetition suppression/sensory gating are directly linked to alterations in CB1R function.
In addition to Δ9-THC’s effects on gating, the current study showed that evoked theta power to S1 was disrupted in both the Δ9-THC and Δ9-THC+CBD conditions. This finding was corroborated by the animal study, as CP-55940 significantly reduced S1 theta power. This may have functional implications regarding the mechanism whereby cannabinoids disrupt sensory gating. It is thought that gating occurs via recurrent for feed-forward inhibition whereby neuronal ensembles activated by S1 stimulate inhibitory circuits which serve to diminish excitatory responses to S2 (Fritz et al. 2005; Mears et al. 2006; Miller and Freedman 1993; 1995; Tan et al. 2004). Germane to the current theta findings, it has been shown that septal input to the hippocampus plays a major role in the inhibition of S2 (Miller and Freedman 1993), and GABAergic input from the septum is known to be the primary generator of hippocampal theta (Bender et al. 2015; Dragoi et al. 1999; Hangya et al. 2009; Mysin et al. 2015; Pilly and Grossberg 2013). Moreover, CB1R agonists have been shown to disrupt septal theta oscillations (Hajos et al. 2008). Hence, S1 may generate a travelling wave of inhibition in the theta band, which may serve to alter the firing thresholds in circuits responding to S2. Through the inhibition of GABA release (and perhaps acetylcholine), cannabinoids like Δ9-THC and CP-55940 would desynchronize these theta oscillations, thus disrupting the “gating out” of S2. Indeed, the correlation between S1 theta and P50 gating ratios in the Δ9-THC condition in the current study provides support for this notion, as does the finding that septal input to the hippocampus may be fine-tuned by endocannabinoid signaling (Nyiri et al. 2005).
More generally, the observed finding regarding cannabinoids and theta are consistent with previous reports, and suggests a common mechanism whereby cannabinoids disrupt sensory, perceptual, and cognitive processes. As pointed out by Kucewicz et al. (2011), disruption of theta “provides an intuitive neural mechanism for cannabinoid-induced cognitive impairment that Melges et al. (1970) called ‘temporal disintegration’ and described as ‘difficulty in retaining, coordinating and serially indexing those memories, perceptions and expectations that are relevant to the goal one is pursuing’” (Kucewicz et al. 2011; Melges et al. 1970). It has already been established that both chronic cannabis use and acute Δ9-THC disrupt sensory-related gamma oscillations (Cortes-Briones et al. 2015a; Skosnik et al. 2015; Skosnik et al. 2012; Skosnik et al. 2006; Skosnik et al. 2014). However, due to longer conduction delays between distant brain regions, it is thought that gamma-range oscillations act to synchronize the activity of local circuits, while lower frequency oscillations serve to coordinate the activity of more widely distributed brain networks (as would be necessary for higher perceptual and cognitive functions) (Siegel et al. 2012). Theta oscillations may serve this purpose, and if disrupted by cannabinoids, could disrupt perceptual and memory processes. Indeed, previous work has demonstrated that acute cannabinoids can disrupt non stimulus-related theta power in both humans (Bocker et al. 2010; Morrison et al. 2011) and animals (Hajos et al. 2008). Interestingly, alterations in resting theta power have been associated with polymorphisms of the CB1R gene (Heitland et al. 2014). More importantly, several studies in animals have shown that cannabinoid agonists can disrupt theta oscillations during memory tasks (Kucewicz et al. 2011; Robbe and Buzsaki 2009; Robbe et al. 2006). Here we show that stimulus-evoked theta power during auditory repetition suppression/P50 sensory gating is also perturbed by CB1R agonists in both humans and animals. Hence, a confluence of data suggests that perturbation of normal CB1R function by exocannabinoids disrupts functionally relevant neural oscillations in the theta-range. By extension, this indicates that the endocannabinoid system may modulate theta oscillations relevant to behavior, perception, and perhaps, cognition.
Several limitations and possible future directions are worth discussing. The first and most obvious limitation is the fact that dissimilar cannabinoid compounds were used in the human versus the animal study. While Δ9-THC is a partial agonist at the CB1R, synthetic cannabinoids like CP-55940 work as full agonists (Tai and Fantegrossi 2017). Moreover, the human study utilized ethanol for drug delivery, while in the animal study, drugs were made up as suspensions in methylcellulose. These factors may limit the generalizability and translational nature of the animal study, and could also explain why S1 gamma disruption was observed in rodents but not in humans. Future studies could replicate and extend these results by examining sensory gating and neural oscillations in non-human animals utilizing Δ9-THC. Further limitations from the animal portion of this study include the use of anesthetized rats and the small number of implanted electrodes. Hence, prospective studies should consider the use of free-moving animals and with a greater number of electrodes (e.g., in prefrontal cortex, auditory cortex, etc.). Limitations to the human portion of the study include the low spatial resolution of EEG, making assertions regarding the anatomical substrates of the observed effects difficult. Future studies examining Δ9-THC utilizing intracranial EEG or simultaneous EEG/fMRI could shed light on the neuroanatomical correlates of cannabinoid-induced alterations on sensory gating and neural oscillations. A second limitation to the human portion of the study relates to the correlation observed between gating ratios and S1 theta power. Given that both gating ratios and S1 theta are outcomes derived after averaging (i.e. ERPs and evoked power), it is possible that this association reflects the fact that the measures may be partially dependent. However, it should be noted that gating ratio takes into account P50 amplitude at S2 as well as S1, and the P50 response itself is likely a higher frequency transient oscillation (e.g., gamma) (Basar et al. 1987; Clementz et al. 1997). This suggests that these measures may represent different (albeit related) underlying neural processes. Nonetheless, given this potential limitation, the correlation between gating ratio and S1 theta in the Δ9-THC condition should be interpreted with caution. Lastly, while the IV route of administration yields the most reliable delivery of Δ9-THC (D'Souza et al. 2004; Sherif et al. 2016), studies using more ecologically valid methods of drug administration (i.e. pyrolyzed cannabis) would yield results more generalizable to cannabis use disorders.
These limitations notwithstanding, the current study in both humans and animals suggests that exogenous cannabinoids disrupt sensory gating and the ability to generate neural oscillations in the theta range, which may have implications for understanding the short- and long-term neurobiological effects of cannabis. Moreover, this study illustrates the translational potential of the signals derived from the auditory repetition/sensory gating paradigm. Hence, gating ratios and evoked power could be utilized as translational biomarkers in the design of “proof-of-pharmacology” trials (Soares 2010), which are urgently needed in the fields of neuroscience and neuropharmacology.
Both Δ9-THC and Δ9-THC + CBD disrupted P50 gating ratio compared to placebo, while CBD alone had no effect.
Δ9-THC and Δ9-THC + CBD decreased S1 evoked theta power, and in the Δ9-THC condition, S1 theta negatively correlated with gating ratios.
In rats, CP-55940 disrupted gating in both brain regions, and this was reversed by AM-251.
Further, CP-55940 decreased evoked theta and gamma power to S1, which was partially blocked by AM-251.
These convergent human/animal data suggest that CB1R agonists disrupt sensory gating by altering neural oscillations in the theta-band. Moreover, this suggests that the endocannabinoid system mediates theta oscillations relevant to perception and cognition.
Acknowledgments
Patrick D. Skosnik and Mihaly Hajos have received grant support from Forum Pharmaceuticals in the past 3 years. Deepak C. D'Souza has in the past 3 years received or currently receives research grant support administered through the Yale University School of Medicine from AstraZeneca, Abbott Laboratories, Eli Lilly Inc., Organon, Pfizer Inc., and Sanofi; he is also consultant for Bristol Meyers-Squibb. Mohini Ranganathan has received in the past 3 years or currently receives research grant support administered through the Yale University School of Medicine from Insys Therapeutics and Pfizer Inc.
All authors have reviewed the contents of the manuscript being submitted, approve of its contents, and validate the accuracy of the data. No part of this material has been published previously nor is being considered for publication elsewhere.
This work was supported in part from a grant from NIH (R21 DA030696-01; PI: Ranganthan).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure
All other authors declare that they do not have any potential conflicts of interest.
References
- Adler LE, Rose G, Freedman R. Neurophysiological studies of sensory gating in rats: effects of amphetamine, phencyclidine, and haloperidol. Biological psychiatry. 1986;21:787–98. doi: 10.1016/0006-3223(86)90244-1. [DOI] [PubMed] [Google Scholar]
- Ali AB, Todorova M. Asynchronous release of GABA via tonic cannabinoid receptor activation at identified interneuron synapses in rat CA1. The European journal of neuroscience. 2010;31:1196–207. doi: 10.1111/j.1460-9568.2010.07165.x. [DOI] [PubMed] [Google Scholar]
- Basar E, Rosen B, Basar-Eroglu C, Greitschus F. The associations between 40 Hz-EEG and the middle latency response of the auditory evoked potential. Int J Neurosci. 1987;33:103–17. doi: 10.3109/00207458708985933. [DOI] [PubMed] [Google Scholar]
- Bender F, Gorbati M, Cadavieco MC, Denisova N, Gao X, Holman C, Korotkova T, Ponomarenko A. Theta oscillations regulate the speed of locomotion via a hippocampus to lateral septum pathway. Nat Commun. 2015;6:8521. doi: 10.1038/ncomms9521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya S, Morrison PD, Fusar-Poli P, Martin-Santos R, Borgwardt S, Winton-Brown T, Nosarti C, CM OC, Seal M, Allen P, Mehta MA, Stone JM, Tunstall N, Giampietro V, Kapur S, Murray RM, Zuardi AW, Crippa JA, Atakan Z, McGuire PK. Opposite Effects of Delta-9-Tetrahydrocannabinol and Cannabidiol on Human Brain Function and Psychopathology. Neuropsychopharmacology. 2009 doi: 10.1038/npp.2009.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya S, Morrison PD, Fusar-Poli P, Martin-Santos R, Borgwardt S, Winton-Brown T, Nosarti C, CM OC, Seal M, Allen P, Mehta MA, Stone JM, Tunstall N, Giampietro V, Kapur S, Murray RM, Zuardi AW, Crippa JA, Atakan Z, McGuire PK. Opposite effects of delta-9-tetrahydrocannabinol and cannabidiol on human brain function and psychopathology. Neuropsychopharmacology. 2010;35:764–74. doi: 10.1038/npp.2009.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocker KB, Hunault CC, Gerritsen J, Kruidenier M, Mensinga TT, Kenemans JL. Cannabinoid modulations of resting state EEG theta power and working memory are correlated in humans. Journal of cognitive neuroscience. 2010;22:1906–16. doi: 10.1162/jocn.2009.21355. [DOI] [PubMed] [Google Scholar]
- Boutros NN, Mears R, Pflieger ME, Moxon KA, Ludowig E, Rosburg T. Sensory gating in the human hippocampal and rhinal regions: regional differences. Hippocampus. 2008;18:310–6. doi: 10.1002/hipo.20388. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Krystal JH, Putnam FW, Southwick SM, Marmar C, Charney DS, Mazure CM. Measurement of dissociative states with the Clinician-Administered Dissociative States Scale (CADSS) Journal of Traumatic Stress. 1998;11:125–36. doi: 10.1023/A:1024465317902. [DOI] [PubMed] [Google Scholar]
- Broyd SJ, Greenwood LM, Croft RJ, Dalecki A, Todd J, Michie PT, Johnstone SJ, Solowij N. Chronic effects of cannabis on sensory gating. Int J Psychophysiol. 2013;89:381–9. doi: 10.1016/j.ijpsycho.2013.04.015. [DOI] [PubMed] [Google Scholar]
- Brunner E, Domhof S, Langer F. J. Wiley. New York, NY: 2002. Nonparametric analysis of longitudinal data in factorial experiments. [Google Scholar]
- Clementz BA, Blumenfeld LD, Cobb S. The gamma band response may account for poor P50 suppression in schizophrenia. Neuroreport. 1997;8:3889–93. doi: 10.1097/00001756-199712220-00010. [DOI] [PubMed] [Google Scholar]
- Cortes-Briones J, Skosnik PD, Mathalon D, Cahill J, Pittman B, Williams A, Sewell RA, Ranganathan M, Roach B, Ford J, D'Souza DC. Delta9-THC Disrupts Gamma (gamma)-Band Neural Oscillations in Humans. Neuropsychopharmacology. 2015a;40:2124–34. doi: 10.1038/npp.2015.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortes-Briones JA, Cahill JD, Ranganathan M, Sewell RA, D'Souza DC, Skosnik PD. Testing differences in the activity of event-related potential sources: Important implications for clinical researchers. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 2014 doi: 10.1016/j.clinph.2014.04.008. [DOI] [PubMed] [Google Scholar]
- Cortes-Briones JA, Cahill JD, Skosnik PD, Mathalon DH, Williams A, Sewell RA, Roach BJ, Ford JM, Ranganathan M, D'Souza DC. The Psychosis-like Effects of Delta-Tetrahydrocannabinol Are Associated with Increased Cortical Noise in Healthy Humans. Biological psychiatry. 2015b doi: 10.1016/j.biopsych.2015.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Souza DC, Braley G, Blaise R, Vendetti M, Oliver S, Pittman B, Ranganathan M, Bhakta S, Zimolo Z, Cooper T, Perry E. Effects of haloperidol on the behavioral, subjective, cognitive, motor, and neuroendocrine effects of Delta-9-tetrahydrocannabinol in humans. Psychopharmacology. 2008a;198:587–603. doi: 10.1007/s00213-007-1042-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Souza DC, Fridberg DJ, Skosnik PD, Williams A, Roach B, Singh N, Carbuto M, Elander J, Schnakenberg A, Pittman B, Sewell RA, Ranganathan M, Mathalon D. Dose-Related Modulation of Event-Related Potentials to Novel and Target Stimuli by Intravenous Delta(9)-THC in Humans. Neuropsychopharmacology. 2012 doi: 10.1038/npp.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Souza DC, Perry E, MacDougall L, Ammerman Y, Cooper T, Wu YT, Braley G, Gueorguieva R, Krystal JH. The psychotomimetic effects of intravenous delta-9-tetrahydrocannabinol in healthy individuals: implications for psychosis. Neuropsychopharmacology. 2004;29:1558–72. doi: 10.1038/sj.npp.1300496. [DOI] [PubMed] [Google Scholar]
- D'Souza DC, Ranganathan M, Braley G, Gueorguieva R, Zimolo Z, Cooper T, Perry E, Krystal J. Blunted psychotomimetic and amnestic effects of delta-9-tetrahydrocannabinol in frequent users of cannabis. Neuropsychopharmacology. 2008b;33:2505–16. doi: 10.1038/sj.npp.1301643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dissanayake DW, Zachariou M, Marsden CA, Mason R. Auditory gating in rat hippocampus and medial prefrontal cortex: effect of the cannabinoid agonist WIN55,212-2. Neuropharmacology. 2008;55:1397–404. doi: 10.1016/j.neuropharm.2008.08.039. [DOI] [PubMed] [Google Scholar]
- Dragoi G, Carpi D, Recce M, Csicsvari J, Buzsaki G. Interactions between hippocampus and medial septum during sharp waves and theta oscillation in the behaving rat. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1999;19:6191–9. doi: 10.1523/JNEUROSCI.19-14-06191.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards CR, Skosnik PD, Steinmetz AB, O'Donnell BF, Hetrick WP. Sensory gating impairments in heavy cannabis users are associated with altered neural oscillations. Behavioral neuroscience. 2009;123:894–904. doi: 10.1037/a0016328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggan SM, Melchitzky DS, Sesack SR, Fish KN, Lewis DA. Relationship of cannabinoid CB1 receptor and cholecystokinin immunoreactivity in monkey dorsolateral prefrontal cortex. Neuroscience. 2010;169:1651–61. doi: 10.1016/j.neuroscience.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englund A, Morrison PD, Nottage J, Hague D, Kane F, Bonaccorso S, Stone JM, Reichenberg A, Brenneisen R, Holt D, Feilding A, Walker L, Murray RM, Kapur S. Cannabidiol inhibits THC-elicited paranoid symptoms and hippocampal-dependent memory impairment. Journal of Psychopharmacology. 2013;27:19–27. doi: 10.1177/0269881112460109. [DOI] [PubMed] [Google Scholar]
- Fritz J, Shamma S, Elhilali M. One click, two clicks: the past shapes the future in auditory cortex. Neuron. 2005;47:325–7. doi: 10.1016/j.neuron.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Gerdeman G, Lovinger DM. CB1 cannabinoid receptor inhibits synaptic release of glutamate in rat dorsolateral striatum. J Neurophysiol. 2001;85:468–71. doi: 10.1152/jn.2001.85.1.468. [DOI] [PubMed] [Google Scholar]
- Gratton G, Coles MG, Donchin E. A new method for off-line removal of ocular artifact. Electroencephalogr Clin Neurophysiol. 1983;55:468–84. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- Gueorguieva R, Krystal JH. Move over ANOVA: progress in analyzing repeated-measures data and its reflection in papers published in the Archives of General Psychiatry. Archives of general psychiatry. 2004;61:310–7. doi: 10.1001/archpsyc.61.3.310. [DOI] [PubMed] [Google Scholar]
- Hajos M. Targeting information-processing deficit in schizophrenia: a novel approach to psychotherapeutic drug discovery. Trends Pharmacol Sci. 2006;27:391–8. doi: 10.1016/j.tips.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Hajos M, Hoffmann WE, Kocsis B. Activation of cannabinoid-1 receptors disrupts sensory gating and neuronal oscillation: relevance to schizophrenia. Biological psychiatry. 2008;63:1075–83. doi: 10.1016/j.biopsych.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Hajos M, Hurst RS, Hoffmann WE, Krause M, Wall TM, Higdon NR, Groppi VE. The selective alpha7 nicotinic acetylcholine receptor agonist PNU-282987 [N-[(3R)-1-Azabicyclo[2.2.2]oct-3-yl]-4-chlorobenzamide hydrochloride] enhances GABAergic synaptic activity in brain slices and restores auditory gating deficits in anesthetized rats. J Pharmacol Exp Ther. 2005;312:1213–22. doi: 10.1124/jpet.104.076968. [DOI] [PubMed] [Google Scholar]
- Hangya B, Borhegyi Z, Szilagyi N, Freund TF, Varga V. GABAergic neurons of the medial septum lead the hippocampal network during theta activity. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:8094–102. doi: 10.1523/JNEUROSCI.5665-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitland I, Kenemans JL, Bocker KB, Baas JM. Genetic variability in the human cannabinoid receptor 1 is associated with resting state EEG theta power in humans. Behavioural brain research. 2014;274:344–8. doi: 10.1016/j.bbr.2014.08.003. [DOI] [PubMed] [Google Scholar]
- Kucewicz MT, Tricklebank MD, Bogacz R, Jones MW. Dysfunctional prefrontal cortical network activity and interactions following cannabinoid receptor activation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:15560–8. doi: 10.1523/JNEUROSCI.2970-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachaux J-P, Axmacher N, Mormann F, Halgren E, Crone NE. High-frequency neural activity and human cognition: past, present and possible future of intracranial EEG research. Progress in neurobiology. 2012;98:279–301. doi: 10.1016/j.pneurobio.2012.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mears RP, Klein AC, Cromwell HC. Auditory inhibitory gating in medial prefrontal cortex: Single unit and local field potential analysis. Neuroscience. 2006;141:47–65. doi: 10.1016/j.neuroscience.2006.03.040. [DOI] [PubMed] [Google Scholar]
- Melges FT, Tinklenberg JR, Hollister LE, Gillespie HK. Marihuana and temporal disintegration. Science. 1970;168:1118–20. doi: 10.1126/science.168.3935.1118. [DOI] [PubMed] [Google Scholar]
- Miller CL, Freedman R. Medial septal neuron activity in relation to an auditory sensory gating paradigm. Neuroscience. 1993;55:373–80. doi: 10.1016/0306-4522(93)90506-b. [DOI] [PubMed] [Google Scholar]
- Miller CL, Freedman R. The activity of hippocampal interneurons and pyramidal cells during the response of the hippocampus to repeated auditory stimuli. Neuroscience. 1995;69:371–81. doi: 10.1016/0306-4522(95)00249-i. [DOI] [PubMed] [Google Scholar]
- Morales P, Reggio PH, Jagerovic N. An Overview on Medicinal Chemistry of Synthetic and Natural Derivatives of Cannabidiol. Frontiers in pharmacology. 2017;8:422. doi: 10.3389/fphar.2017.00422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison PD, Nottage J, Stone JM, Bhattacharyya S, Tunstall N, Brenneisen R, Holt D, Wilson D, Sumich A, McGuire P, Murray RM, Kapur S, Ffytche DH. Disruption of frontal theta coherence by Delta(9)-tetrahydrocannabinol is associated with positive psychotic symptoms. Neuropsychopharmacology. 2011;36:827–36. doi: 10.1038/npp.2010.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mysin IE, Kitchigina VF, Kazanovich Y. Modeling synchronous theta activity in the medial septum: key role of local communications between different cell populations. Journal of computational neuroscience. 2015;39:1–16. doi: 10.1007/s10827-015-0564-6. [DOI] [PubMed] [Google Scholar]
- Nyiri G, Szabadits E, Cserep C, Mackie K, Shigemoto R, Freund TF. GABAB and CB1 cannabinoid receptor expression identifies two types of septal cholinergic neurons. The European journal of neuroscience. 2005;21:3034–42. doi: 10.1111/j.1460-9568.2005.04146.x. [DOI] [PubMed] [Google Scholar]
- Patrick G, Struve FA. Reduction of auditory P50 gating response in marihuana users: further supporting data. Clinical Electroencephalography. 2000;31:88–93. doi: 10.1177/155005940003100207. [DOI] [PubMed] [Google Scholar]
- Patterson JV, Hetrick WP, Boutros NN, Jin Y, Sandman C, Stern H, Potkin S, Bunney WE., Jr P50 sensory gating ratios in schizophrenics and controls: a review and data analysis. Psychiatry Res. 2008;158:226–47. doi: 10.1016/j.psychres.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Pilly PK, Grossberg S. How reduction of theta rhythm by medial septum inactivation may covary with disruption of entorhinal grid cell responses due to reduced cholinergic transmission. Front Neural Circuits. 2013;7:173. doi: 10.3389/fncir.2013.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polissidis A, Galanopoulos A, Naxakis G, Papahatjis D, Papadopoulou-Daifoti Z, Antoniou K. The cannabinoid CB1 receptor biphasically modulates motor activity and regulates dopamine and glutamate release region dependently. The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum. 2013;16:393–403. doi: 10.1017/S1461145712000156. [DOI] [PubMed] [Google Scholar]
- Radhakrishnan R, Skosnik PD, Cortes-Briones J, Sewell RA, Carbuto M, Schnakenberg A, Cahill J, Bois F, Gunduz-Bruce H, Pittman B, Ranganathan M, D'Souza DC. GABA Deficits Enhance the Psychotomimetic Effects of Delta(9)-THC. Neuropsychopharmacology. 2015;40:2047–56. doi: 10.1038/npp.2015.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rentzsch J, Penzhorn A, Kernbichler K, Plockl D, Gomez-Carrillo de Castro A, Gallinat J, Jockers-Scherubl MC. Differential impact of heavy cannabis use on sensory gating in schizophrenic patients and otherwise healthy controls. Exp Neurol. 2007;205:241–9. doi: 10.1016/j.expneurol.2007.02.004. [DOI] [PubMed] [Google Scholar]
- Roach BJ, Mathalon DH. Event-related EEG time-frequency analysis: an overview of measures and an analysis of early gamma band phase locking in schizophrenia. Schizophrenia bulletin. 2008;34:907–26. doi: 10.1093/schbul/sbn093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbe D, Buzsaki G. Alteration of theta timescale dynamics of hippocampal place cells by a cannabinoid is associated with memory impairment. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:12597–605. doi: 10.1523/JNEUROSCI.2407-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbe D, Montgomery SM, Thome A, Rueda-Orozco PE, McNaughton BL, Buzsaki G. Cannabinoids reveal importance of spike timing coordination in hippocampal function. Nature neuroscience. 2006;9:1526–33. doi: 10.1038/nn1801. [DOI] [PubMed] [Google Scholar]
- Sales-Carbonell C, Rueda-Orozco PE, Soria-Gomez E, Buzsaki G, Marsicano G, Robbe D. Striatal GABAergic and cortical glutamatergic neurons mediate contrasting effects of cannabinoids on cortical network synchrony. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:719–24. doi: 10.1073/pnas.1217144110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherif M, Radhakrishnan R, D'Souza DC, Ranganathan M. Human Laboratory Studies on Cannabinoids and Psychosis. Biological psychiatry. 2016;79:526–38. doi: 10.1016/j.biopsych.2016.01.011. [DOI] [PubMed] [Google Scholar]
- Siegel M, Donner TH, Engel AK. Spectral fingerprints of large-scale neuronal interactions. Nat Rev Neurosci. 2012;13:121–34. doi: 10.1038/nrn3137. [DOI] [PubMed] [Google Scholar]
- Singer W, Gray CM. Visual feature integration and the temporal correlation hypothesis. Annual Review of Neuroscience. 1995;18:555–86. doi: 10.1146/annurev.ne.18.030195.003011. [DOI] [PubMed] [Google Scholar]
- Skosnik PD, Cortes-Briones JA, Hajos M. It's All in the Rhythm: The Role of Cannabinoids in Neural Oscillations and Psychosis. Biological psychiatry. 2015 doi: 10.1016/j.biopsych.2015.12.011. [DOI] [PubMed] [Google Scholar]
- Skosnik PD, Cortes-Briones JA, Hajos M. It's All in the Rhythm: The Role of Cannabinoids in Neural Oscillations and Psychosis. Biological psychiatry. 2016;79:568–77. doi: 10.1016/j.biopsych.2015.12.011. [DOI] [PubMed] [Google Scholar]
- Skosnik PD, D'Souza DC, Steinmetz AB, Edwards CR, Vollmer JM, Hetrick WP, O'Donnell BF. The effect of chronic cannabinoids on broadband EEG neural oscillations in humans. Neuropsychopharmacology. 2012;37:2184–93. doi: 10.1038/npp.2012.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skosnik PD, Krishnan GP, Aydt EE, Kuhlenshmidt HA, O'Donnell BF. Psychophysiological evidence of altered neural synchronization in cannabis use: relationship to schizotypy. Am J Psychiatry. 2006;163:1798–805. doi: 10.1176/ajp.2006.163.10.1798. [DOI] [PubMed] [Google Scholar]
- Skosnik PD, Krishnan GP, D'Souza DC, Hetrick WP, O'Donnell BF. Disrupted Gamma-Band Neural Oscillations During Coherent Motion Perception in Heavy Cannabis Users. Neuropsychopharmacology. 2014 doi: 10.1038/npp.2014.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smucny J, Stevens KE, Olincy A, Tregellas JR. Translational utility of rodent hippocampal auditory gating in schizophrenia research: a review and evaluation. Translational psychiatry. 2015;5:e587. doi: 10.1038/tp.2015.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares HD. The use of mechanistic biomarkers for evaluating investigational CNS compounds in early drug development. Current opinion in investigational drugs. 2010;11:795–801. [PubMed] [Google Scholar]
- Tai S, Fantegrossi WE. Pharmacological and Toxicological Effects of Synthetic Cannabinoids and Their Metabolites. Current topics in behavioral neurosciences. 2017;32:249–262. doi: 10.1007/7854_2016_60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan AY, Zhang LI, Merzenich MM, Schreiner CE. Tone-evoked excitatory and inhibitory synaptic conductances of primary auditory cortex neurons. J Neurophysiol. 2004;92:630–43. doi: 10.1152/jn.01020.2003. [DOI] [PubMed] [Google Scholar]
- Wang SJ. Cannabinoid CB1 receptor-mediated inhibition of glutamate release from rat hippocampal synaptosomes. Eur J Pharmacol. 2003;469:47–55. doi: 10.1016/s0014-2999(03)01734-5. [DOI] [PubMed] [Google Scholar]
- Zachariou M, Dissanayake DW, Coombes S, Owen MR, Mason R. Sensory gating and its modulation by cannabinoids: electrophysiological, computational and mathematical analysis. Cognitive neurodynamics. 2008;2:159–70. doi: 10.1007/s11571-008-9050-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuardi AW, Shirakawa I, Finkelfarb E, Karniol IG. Action of cannabidiol on the anxiety and other effects produced by delta 9-THC in normal subjects. Psychopharmacology. 1982;76:245–50. doi: 10.1007/BF00432554. [DOI] [PubMed] [Google Scholar]