Abstract
A potent adjuvant without incurring any significant skin reactogenicity is urgently needed for cutaneous vaccination. Here, we report that a natural agonist of STING, 2’3’- cyclic GMP-AMP (cGAMP) robustly augmented and prolonged cellular and humoral immune responses provoked by H5N1 and 2009 H1N1 pandemic influenza vaccines after a single dose of intradermal, but not intramuscular, immunization. The potency of cGAMP for cutaneous vaccination was ascribed to a large number of antigen presenting cells resident in the skin ready for immediate activation by cGAMP injected. On the contrary, its potency was severely compromised in the muscle because antigen presenting cells could not be promptly recruited to the injection site before injected cGAMP was diffused out. The superior adjuvant effect and safety of holds great promise to be an ideal adjuvant for cutaneous vaccination.
Keywords: Cutaneous Vaccination, Adjuvant, Pandemic Influenza Vaccine, STING, cGAMP
Introduction
Current vaccines are mostly administered into the muscle despite the fact that the skin is a more potent site for vaccination. Apart from inconvenience, lack of non-inflamed, potent adjuvant remains a key issue for cutaneous vaccination (Hickling et al, 2011). As the first line of our body’s defense system, epidermis and dermis contain a large number of antigen presenting cells, making the skin not only being effective for vaccination but also prone to severe local reactogenicity. This dilemma precludes many potent adjuvants for skin vaccination due to prolonged and high levels of local inflammation. For instance, commonly used aluminum salt (Alum), squalene-based emulsions MF59 and ASO3, water-in-oil emulsions montanide ISA 51 and ISA 720, and several Toll-like receptor (TLR) agonists (e.g. R837) provoke severe local reactions including erythema, swelling, and ulceration for weeks at the injection site (Vogelbruch et al, 2000, Chen et al, 2012, Ginhoux et al, 2012). Yet a safe and effective adjuvant is indispensable for subunit- or weak vaccines in order to enhance, shape and broaden immune responses. The adjuvant is also crucial for antigen dose-sparing and rapid and strong protective immunity in very young and elder populations (Reed et al, 2013). To date, only a few adjuvants have been approved for prophylactic vaccines, including Alum, squalene-based emulsion and monophosphoryl lipid A (MPL), but all of them are approved for intramuscular (IM) administration only. There is not any adjuvant approved for skin immunization so far.
An ideal adjuvant for cutaneous vaccination should have the following properties. Firstly, it is natural and metabolizable components generated from humans so that a risk of inducing antibody against the molecule can be minimized even after repeated uses. Secondly, the adjuvant activity should be localized and transient averting unwanted adverse events in the skin while sufficiently retaining its ability to bolster vaccination. Thirdly, the adjuvant is potent and its underlying mechanism is well characterized. Understanding of the mechanisms ensures the specificity and predictability of the immune responses in different individuals, in sharp contrast to those adjuvants empirically developed like Alum. Recently, we reported a laser-based adjuvant that met these criteria and could potentially serve as a safe and effective adjuvant for intradermal (ID) vaccination (Wang et al, 2014, Chen et al, 2013). We used non-ablative factional laser (NAFL) to generate an array of micro-injuries in the skin that robustly activated sterile innate immunity. While these micro-injuries stimulate robust innate immune responses and sufficiently augment ID vaccination, the micro-injuries can be healed, concomitant with resolution of the associated micro-inflammation within 48 hours (Manstein et al, 2004, Wang et al, 2014). Our further investigation unraveled that dsDNA released from laser-damaged cells was sensed by intracellular sensors cyclic GMP-AMP synthase (cGAS) (Sun et al, 2013, Wang et al, 2015). cGAS subsequently generated 2’3’-cyclic guanosine monophosphate-adenosine monophosphate, designated as cGAMP hereafter, as a second messenger that binds the stimulator of interferon genes (STING), also known as TMEM173/MPYS/MITA/ERIS (Wu et al, 2013, Ishikawa et al, 2009, Jin et al, 2008, Zhong et al, 2008). Stimulation of STING then activate Interferon Regulatory Factor 3 (IRF3) and NFkB pathways, greatly increasing the transcription of type I interferons and other cytokines, and a strong Th1 immune response results (Paludan and Bowie, 2013). The finding raises an intriguing possibility that cGAMP may replace laser treatment as a safe, simple, and potent adjuvant for skin vaccination.
cGAMP is a natural metabolizable molecule in humans and hydrolyzed quickly by ecto-nucleotide pyrophosphatase/phosphodiesterase (ENPP1) when located outside plasma membrane (Li et al, 2014). The quick hydrolysis ensures that its adjuvant activity is transient, effectively circumventing unwanted systemic inflammation. Moreover, because cGAMP is a small, negatively charged hydrophilic molecule, induction of antibody against this small self-molecule is highly unlikely. The adjuvant effect of cGAMP has been demonstrated in mice by co-injection cGAMP and ovalbumin, a model protein vaccine, into the muscle (Li et al, 2013). Its bacterial analog, cyclic di-GMP (cdGMP) has been studied extensively as a potential vaccine adjuvant for bacteria vaccines through IM, subcutaneous, intraperitoneal or intranasal vaccinations (Karaolis et al, 2007, Ogunniyi et al, 2008, Ebensen et al, 2007a, Ebensen et al, 2007b, Blaauboer et al, 2015). Recently, modified nonhydrolyzable cGAMP analogs have also been shown to have potent anti-tumor activity when administered intratumorally (Corrales et al, 2015).
The current study evaluates the potential of cGAMP as a safe and potent adjuvant for influenza vaccines administered intradermally. Our results showed that cGAMP could be an “ideal” adjuvant for cutaneous vaccination against both seasonal and pandemic influenza vaccines. It greatly enhanced protective humoral and cellular immune responses while evoking little local skin reactions. Strikingly, ID delivery of cGAMP showed superior adjuvant effect compared to IM vaccination, presumably owing to abundant antigen presenting cells resident in the skin and ability of the skin to better retain the small molecule than the muscle. The potency and safety of cGAMP as a cutaneous adjuvant were also confirmed in swine model.
Results
cGAMP induces superior immune responses against ID influenza vaccines
To determine adjuvanticity of cGAMP for ID influenza vaccines, Swiss Webster mice were ID immunized with a monovalent influenza vaccine, A/California/07/2009 H1N1 at a dose of 300 ng HA per mouse with or without 20 μg cGAMP. The vaccine was also IM administered either alone or along with 20 μg cGAMP or AddaVax for efficacy comparison. AddaVax is a squalene-based vaccine adjuvant with similar composition as commercial adjuvant MF59 that has been used in seasonal influenza vaccine in the elderly for a decade in Europe. MF59 or another squalene-based adjuvant AS03 has also been used in 2009 pandemic influenza vaccine in Europe and Canada. Immunization with the vaccine alone through either IM or ID did not elevate the number of CD4+ or CD8+ T cells secreting interferon gamma (IFN-γ) over unimmunized mice (Fig. 1a and b). Inclusion of cGAMP or AddaVax into IM vaccination failed to augment the cellular immune responses either (Fig. 1a and b). However, cGAMP significantly elevated the number of IFN-γ-secreting CD4+ and CD8+ T cells if it was ID delivered along with the vaccine (p<0.05, Fig. 1a and b). None of the immunizations tested augmented Th2 cellular responses, as suggested by a similar number of CD4+ T cells producing IL-4 among unimmunized and all immunized mice (data not shown).
Figure 1. cGAMP shows superior adjuvant effect on cutaneous vaccination.
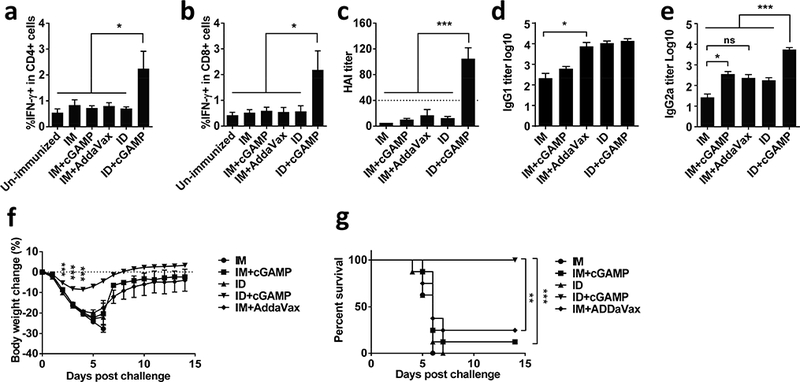
Swiss webster mice were immunized with 300 ng (HA content) 2009 H1N1 vaccine alone or with 20 μg cGAMP either intradermally (ID) or intramuscularly (IM). Un-immunized mice or mice receiving the vaccine and AddaVax adjuvant intramuscularly (IM+AddaVax) served as controls. Peripheral blood mononuclear cells were isolated one week after the immunization and stimulated by inactivated influenza virus and anti-CD28 antibody, and percentages of CD4+ (a) or CD8+ (b) secreting IFNγ were measured by flow cytometry. (c-e) HAI, IgG1, or IgG2a titers were measured in serum 4 weeks later. Mice were intranasally challenged with 10× LD50 of A/California/7/2009 H1N1 virus 5 weeks after immunization. Body weight (f) and survival (g) were monitored daily for 14 days. n=8. Data are presented as mean±s.e.m. *, p<0.05; **, p< 0.01 or ***, p<0.001, between indicated groups or between ID+cGAMP and other groups. ns, no significance.
Humoral immune responses were next measured 4 weeks later by hemagglutination inhibition (HAI) assay, a gold standard of influenza vaccination, in which a serum HAI titer >1:40 is considered protective. As shown in Fig. 1c, immunization of the vaccine alone by an ID or IM route did not give rise to protective immune responses (mean titer <1:20), neither did IM immunization of the vaccine mixed with cGAMP or AddaVax adjuvant. In marked contrast, ID immunization of the vaccine mixed with cGAMP brought about 5–10 times higher HAI titers than any other immunization strategies tested (p<0.001, Fig. 1c). Effect of cGAMP on Th1 and Th2 immune responses were subsequently assessed by measurement of influenza HA-specific IgG1 and IgG2a antibodies. Unlike AddaVax augmenting both Th1 and Th2 immune responses similarly and modestly, cGAMP preferably strengthened Th1 immune responses, resulting in a higher IgG2a titer in both IM and ID immunizations with more predominant effect on the latter (Fig. 1d and e). Consequently, the mice produced the highest level of IgG2a after receiving ID immunization with a mixture of cGAMP and the vaccine compared to all other vaccination procedures tested (Fig. 1e). While augmenting Th1 immune responses robustly, cGAMP displayed little influence on Th2 immune response irrespective of whether it was delivered via an ID or IM route. In accordance with superior immune responses elicited by ID immunization, all mice (8/8) receiving the vaccine mixed with cGAMP survived a viral challenge, concurrent with only a slight body weight loss (<10%) (Fig. 1f and g). In sharp contrast, all mice died within 7 days after the viral challenge in the absence of adjuvant irrespective of the route of immunization. IM vaccination in the presence of either cGAMP or AddaVax exhibited only 12.5% or 25% protection, respectively (Fig. 1g)
cGAMP doesn’t evoke significant skin irritation in mice
Given the superior adjuvant effect of cGAMP for skin vaccination, we next addressed its local reactogenicity by ID injection of PBS, 20 μg cGAMP, 20 μg resiquimod, 300 ng H1N1 vaccine or the vaccine plus 20 μg cGAMP. cGAMP did not evoke any overt irritations from day 1 to day 5 at the inoculation site (Fig. 2a 2nd panel). In contrast, resiquimod, a TLR7/8 agonist, provoked significant local skin reactions on day 1 and 2, forming a scar on day 5 (Fig. 2a 3rd panel). ID injection of H1N1 vaccine alone, or along with cGAMP did not cause skin irritation either (Fig. 2a last two panels). Histological examination confirmed a normal morphology of epidermis and dermis and little infiltration of inflammatory cells in the tissue 2 days after ID injection of cGAMP either alone or with H1N1 vaccine (Fig. 2b-e), whereas thickness of epidermis (Fig. 2c) and dermis (Fig. 2d) was increased apparently by resiquimod, concurrent with greatly increased cell numbers (Fig. 2e) in the skin, indicating skin infiltration of numerous inflammatory cells.
Figure 2. cGAMP evokes little local reaction in mice.
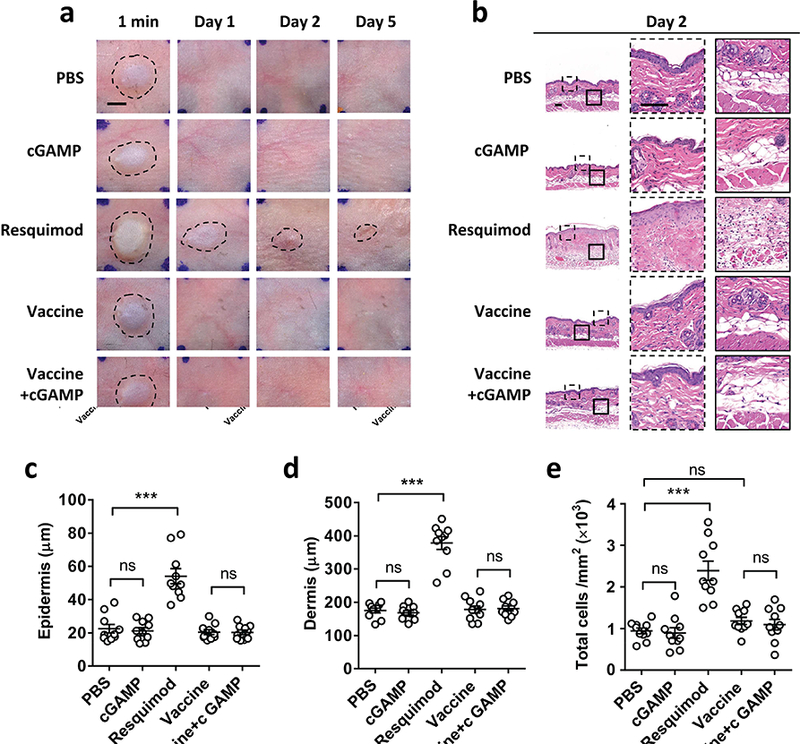
Mice were ID injected with PBS, 20 μg cGAMP, 20 μg resiquimod, 300 ng H1N1 influenza vaccine or the vaccine plus 20 μg cGAMP. Photos were taken at indicated times and representative photos are shown with 4 mice in each group (a). Scale bar, 2.5 mm. Representative H&E stained sections of the inoculation site on day 2 are shown in (b). The outlined areas in the left are enlarged showing epidermis/dermis (dashed) and dermis/hypodermis (solid), respectively. Scale bar, 100 μm. The thickness of epidermis and dermis was summarized in (c) and (d) respectively. The cell numbers in dermis and hypodermis were quantified in (e). ***, p<0.001 between indicated groups and ns, no significant difference.
cGAMP is a potent adjuvant for H5N1 avian influenza vaccine
Immunogenicity of pandemic H5N1 avian influenza vaccine is rather weak and requires two doses of vaccination to induce protective immunity or an HAI titer higher than 1:40. To test whether cGAMP could elicit protective immunity against this virus after a single dose of immunization, Swiss Webster mice were ID immunized with split H5N1 vaccine (A/Vietnam/1203/04) at 300 ng HA per mouse with or without 20 μg cGAMP. H5N1 vaccine alone exhibited a weak immune response with low HAI and IgG2a titers regardless of an immunization route, consistent with poor immunogenicity of the vaccine (Fig. 3a and b). The vaccine elevated IgG1 production strongly when given intradermally but weakly if given intramuscularly (Fig. 3c). Once again, cGAMP greatly bolstered the protective immunity of the vaccine producing much higher HAI and IgG2a titers when ID administered (Fig. 3a and b). HAI and IgG2a titers were increased to 1:58 or 1:1460 in the presence of cGAMP, from 1:13 or 1:32 in its absence, respectively (Fig. 3a and b), which should protect the mice from the viral infection, based on the current gold standard of HAI level, although further study using H5N1 viral challenge is required to conclude it. In contrast, cGAMP did not elevate HAI titers although it significantly augmented IgG2a responses when IM administered (Fig. 3a and b). Similar to H1N1 influenza vaccine, cGAMP had little effect on Th2 immune responses whether the vaccine was delivered intradermally or intramuscularly (Fig. 3c).
Figure 3. cGAMP augments ID H5N1 vaccine.
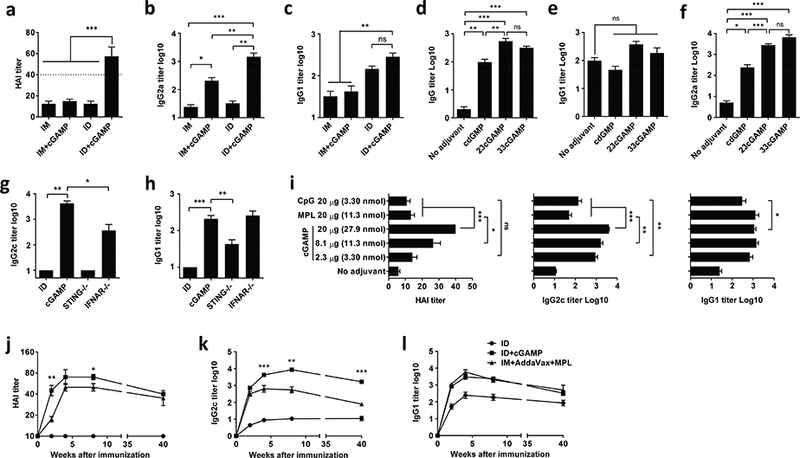
Swiss webster mice were ID or IM immunized with 300 ng (HA content) H5N1 pandemic influenza vaccine alone (ID) or with 20 μg cGAMP (a-c). Separate groups of the mice were also ID immunized similarly with H5N1 vaccine alone or the vaccine mixed with 20 μg cdGMP, 2’3’-cGAMP or 3’3’-cGAMP (d-f). (g, h), STING−/−, IFNAR−/− and C57BL/6 control mice were ID immunized as (a). Serum HAI (a), IgG2b or IgG2c (b, f, g), and IgG1 (c, e, h) and total IgG (d) were measured 2 weeks after immunization. (i), C57BL/6 mice were ID immunized with H5N1 vaccine plus varying doses of cGAMP, 20 μg MPL or 20 μg CpG. Humoral immune responses were measured 2 weeks after immunization as (a-c). (j-l) C57BL/6 mice were ID immunized or IM immunized as (a) with H5N1 vaccine adjuvantated by AddaVax+MPL adjuvant. Humoral immune responses were measured 2, 4, 8 and 40 weeks later. n=8. Data are presented as mean±s.e.m. *, p<0.05; **, p< 0.01 or ***, p<0.001, respectively.
cGAMP displays more potent adjuvant effect than its bacterial analogs
The adjuvant effect of cGAMP (2’3’-cGAMP) was further compared with its bacterial analogs, cdGMP and 3’3’-cGAMP. The same amount (20 μg) of cyclic di-nucleotides (CDNs) was ID injected along with H5N1 vaccine. As shown in Fig. 3d, 2’3’-cGAMP showed similar adjuvanticity as 3’3’-cGAMP but more robust than cdGMP, although all three CDNs profoundly augmented Th1 immune responses, not Th2 immune responses (Fig. 3e and f). Early studies showed that mouse STING responded similarly to bacterial 3’3’-cGAMP and vertebrate 2’3’-cGAMP, but 2’3’-cGAMP bound human STING at a higher affinity. We thus used 2’3’-cGAMP in our studies.
cGAMP is a more potent cutaneous adjuvant than CpG and MPL
The adjuvant effect of ID cGAMP was independent of mouse strains. When inbred C57BL/6 mice were ID immunized with a mixture of 300 ng HA H5N1 vaccine and 20μg cGAMP, the mice generated higher IgG2c titers (Fig. 3g) as well as IgG1 (Fig. 3h), slightly different from Swiss Webster mice (Fig. 3c). STING deficiency (STING−/−) completely abrogated the adjuvant effect of cGAMP on Th1 immune responses, but only partially impaired Th2 immune responses (Fig. 3g and h). The adjuvant effect of cGAMP on Th2 immune responses appeared to be independent of type I Interferons (IFNs) and interferon stimulating genes (ISGs), since the interferon-α/β receptor (IFNAR) knockout mice generated similar levels of IgG1 as wild-type controls (Fig. 3h). On the contrary, IFNs and ISGs contributed only partially to the adjuvant effect of cGAMP on Th1 immune responses (Fig. 3g).
We next compared the adjuvant effect of cGAMP with other prominent experimental adjuvants in ID vaccination. Our previous study showed that most of adjuvants including alum, oil-in-water emulsion, etc. induced severe local reactions and were excluded from skin immunization, except for CpG and MPL (Chen et al, 2012). Therefore CpG and MPL were included for comparison study. C57BL/6 mice were ID immunized with H5N1 vaccine and varying doses of cGAMP, 20 μg CpG or 20 μg MPL. The adjuvant doses selected are commonly used in mouse study (MacLeod et al, 2011, Wegmann et al, 2012). As shown in Fig. 3i, at the same dose of 20 μg, cGAMP induced robustly higher HAI titer (p<0.001) and IgG2c titer (p<0.001) than either CpG or MPL. At the same molar dose of 11.3 nmol, cGAMP still induced significantly higher HAI (p<0.05) and IgG2c (p<0.01) titer than MPL. Although there was no significant difference in HAI titers, the IgG2c titer induced by 3.3 nmol of cGAMP was significantly (p<0.01) higher than that induced by the same molar of CpG. All adjuvants showed similar effects on IgG1 production. Apparently, cGAMP is a more potent adjuvant than CpG or MPL in cutaneous vaccination.
H5N1 vaccine induces long-lasting protective immunity in the presence of cGAMP
We went on to evaluate how long the protective immunity could last. C57BL/6 mice were ID immunized with H5N1 vaccine with or without cGAMP as above, followed by collection of sera in 2, 4, 8 and 40 weeks post-immunization. A combination of squalene-based adjuvant and TLR4 agonist has been demonstrated to be the most promising adjuvant for H5N1 vaccine so far (Clegg et al, 2012) and thus was also set up for comparison, in which AddaVax and an FDA-approved TLR4 agonist MPL were combined. ID immunization of the vaccine alone was totally ineffective, but ID immunization of H5N1 vaccine mixed with cGAMP elicited a protective HAI titer >1:40 within two weeks, whereas it took 4 weeks to achieve similar HAI titers by the vaccine adjuvanted with AddaVax+MPL (Fig. 4j). The HAI titer was sustained over 1:40 for at least 40 weeks after ID immunization of the vaccine mixed with cGAMP, despite a slight drop from the peak at the end of study (Fig. 4j). The mice also produced 1000-fold or 10fold higher IgG2c titers than mice receiving the vaccine alone or the vaccine plus AddaVax+ MPL, respectively, throughout the experimental period of 40 weeks (Fig. 4k), although cGAMP and AddaVax+MPL boosted IgG1 production similarly (Fig. 4l). The data suggests that cGAMP if injected intradermally, is at least similar, if not more potent than AddaVax+MPL in adjuvantation of H5N1 vaccine.
Figure 4. Efficacy and safety of ID cGAMP in swine.
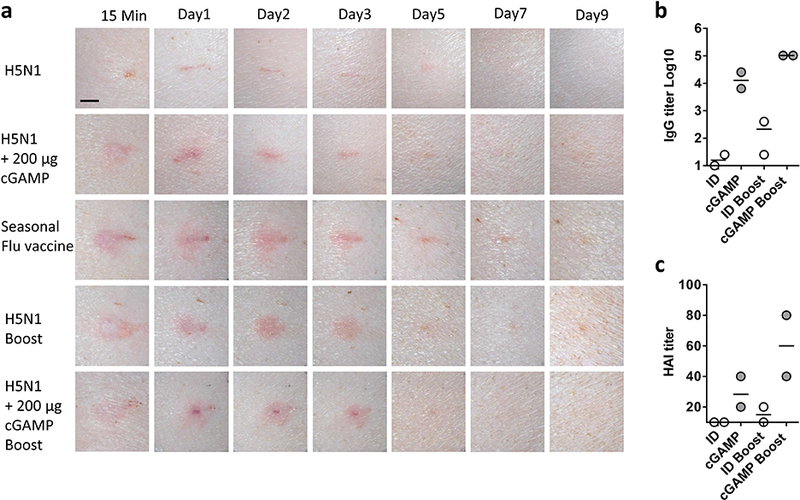
The exterior hind legs of Yorkshire pigs were ID vaccinated with 6 μg (HA content) H5N1 influenza vaccine alone or with 200 μg cGAMP. A booster immunization was performed similarly 2 weeks later. Pigs receiving the same amount of seasonal influenza vaccine once served as controls. Local reactions were photographed in 15 minutes, 1, 2, 3, 5, 7 and 9 days after priming and boosting (a). Scale bar, 5 mm. Serum IgG (b) and HAI (c) titers were measured 14 days after priming or 7 days after boosting. Each symbol represents data from individual animals, and horizontal bars indicate the mean.
cGAMP evokes little skin inflammation in swine model
The skin response to influenza vaccines is similar between swine and humans and swine is commonly used to assess skin reaction to ID influenza vaccines. Hence, four-month-old Yorkshire pigs were ID administered H5N1 vaccine at a dose of 6 μg HA per pig either alone or along with 200 μg cGAMP. H5N1 vaccine mixed with cGAMP evoked slightly higher levels of local reactions than the vaccine alone in the first two days, as shown by a slightly larger area of erythema (Fig. 4a, 1st and 2nd panel), but it was completely resolved by day 5. Yet, the skin reactogenicity was less severe than that provoked by ID seasonal influenza vaccine (Fig. 4a, 3rd panel), which has been FDA approved for humans for years, suggesting acceptability of the skin reaction in humans. After boosting immunizations, H5N1 vaccine provoked severer local reactions than priming, and once again the skin reaction was completely resolved by day 5 (Fig. 4a, 4th panel). The skin reaction induced by the vaccine was not worsened by cGAMP (Fig. 4a, fifth panel). Immunologically, cGAMP greatly augmented the immune responses induced by H5N1 vaccine in both priming and boosting vaccinations with more prominent effect on the latter in terms of HAI titers (Fig. 4b and c). Importantly, sera from the two animals had HAI titers >1:40. Although the number of swine was too low to conclude protective immunity in the animals, the trend appeared to point this possibility positively.
cGAMP activates antigen presenting cells in the skin
In accordance with strong adjuvanticity in the skin, cGAMP stimulated vigorous cytokine and chemokine productions at the inoculation site and in draining lymph nodes 6 hours after ID vs. IM administration (Fig. 5a and b). These included cytokines important for Th1 immune responses such as IFN-β, CXCL10, CXCL9, CCL2, IL-6, and IL-β, except for IL-12 (Fig. 5a and b). ID cGAMP did not raise the level of TGF-β over IM delivery. By using mice expressing green fluorescent protein (GFP) infused with MHC-II (Boes et al, 2002), we found that cGAMP not only robustly stimulated cytokine and chemokine productions at the inoculation site, but also enhanced the velocity of antigen presenting cells. The velocity of these antigen presenting cells peaked during 4–6 hours after cGAMP injection (Fig. 5c) but was completely blunted in STING-deficient mice (Fig. 5c), confirming the movement of these antigen presenting cells dependent on the adjuvanticity of cGAMP. Moreover, cGAMP at a concentration as low as 0.04 μg/ml could significantly stimulate CXCL10 expression in dendritic cells derived from bone marrow cells (p<0.001, Fig. 5d) and the response was detectable as early as 1 hour of the stimulation (Fig. 5e), during which a majority of injected cGAMP remained in the skin in sharp contrast to the muscle. As shown in Fig. 5f, fluorescently labeled cGAMP was sustained locally at a much higher concentration and a longer time after ID delivery than IM delivery. cGAMP concentration in the skin was above 10 or 1 μg/g of wet tissue after 3 or 6 hours of injection, respectively, whereas cGAMP declined precipitously in the muscle within 3 hours of IM injection (Fig. 5f). The injected cGAMP drained into lymph nodes also significantly higher after ID compared to IM delivery, in particular, at 3 hours of the injection (Fig. 5g). The high sensitivity, quick responses, and the ability of the skin to strongly retain cGAMP explain why cGAMP is so effective in ID but not in IM immunization.
Figure 5. cGAMP activates antigen presenting cells in the skin.
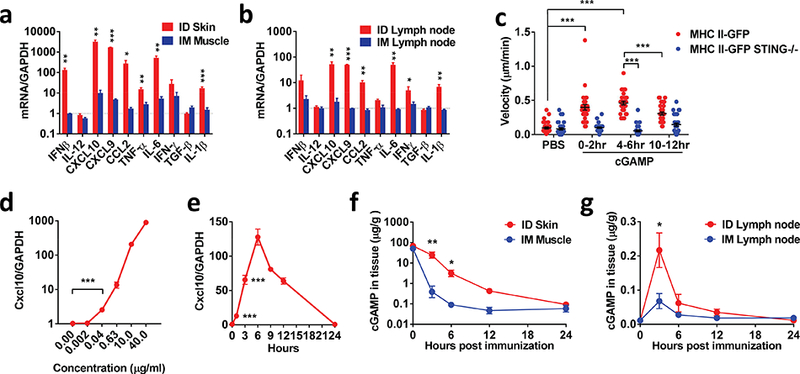
cGAMP was ID or IM injected into mice followed by measurement of cytokine and chemokine expressions by real-time PCR 6 hours later at the injection site (skin or muscle) (a) and in draining lymph nodes (b). n=4. The ears of MHC II-GFP mice and MHC II-GFP STING−/− mice received ID injection of 10 μg cGAMP or PBS, followed by tracking the velocity of antigen presenting cells by intravital two-photon microscopy for 12 hours. The velocities were summarized in (c) where each dot represents one cell with a total of 31 analyzed in each sample. Bone marrow derived dendritic cells were incubated for 6 hrs with difference concentrations of cGAMP, after which CXCL10 mRNA was measured. n=4. The cells were also stimulated with 20 μg/ml cGAMP for indicated times before measuring CXCL10 mRNA as (a). n=4. Skin, muscle or inguinal lymph nodes involved were collected immediately or at indicated times after FITC-labeled cGAMP (20 μg) were ID or IM injected into the lower dorsal skin or legs of mice. The local concentration of cGAMP was measured by fluorescence plate reader. n=4. Data are presented as mean±s.e.m. *, p<0.05; **, p< 0.01 or ***, p<0.001, respectively.
Discussion
A new generation adjuvant should have a well-defined component and action mode in addition to its efficacy and safety. The present study demonstrates that natural STING agonist cGAMP can be such an adjuvant for skin vaccination. In contrast to many vaccine adjuvants like Alum whose action modes are just emerging after more than 90 years of clinical applications, the mechanism whereby cGAMP stimulates type I interferon-associated pathways is well defined to date, which is a major anti-viral pathway in humans (Cai et al, 2014). cGAMP binds to STING, activates the downstream IRF3 and NFkB pathways, and triggers the transcription and expression of a great deal of cytokines and chemokines pivotal for Th1 immune responses. The ability of selectively stimulating immunological pathways to obtain the desired immune response warrants its safety, tolerability and efficacy in clinical applications and helps understanding of any variations among different populations. Secondly, cGAMP is a negatively charged, hydrophilic, small molecule in contrast to many other adjuvants that are not homogeneous and their compositions are not well characterized. Because of its small and hydrophilicity, cGAMP diffuses from the injection site quickly, averting prolonged local inflammation that remains a major issue for hydrogel, emulsion, nano/microparticles or positively charged molecule adjuvants in skin vaccination (Ginhoux et al, 2012, Chen et al, 2012). These adjuvants tend to remain at the injection site for a long time causing severe skin inflammation for an unnecessarily long period of time. Several studies, including ours, have shown that brief inflammation at the inoculation site is sufficient to “educate” antigen presenting cells for effective skin vaccination (Hutchison et al, 2012, Wang et al, 2014). In fact, surgical removal of the inoculation site containing alum 2 hours after vaccination did not affect the adjuvanticity of Alum (Hutchison et al, 2012), an argument strongly supported by our current and previous investigations (Wang et al, 2014, Wang et al, 2015). Thirdly, cGAMP is a metabolizable molecule, whose systemic exposure could be well confined by intrinsic enzymes (e.g. ENPP1) that are located abundantly in serum, liver and spleen, further limiting its systemic distribution and any unwanted effects (Li et al, 2014).
However, a quick diffusion from the inoculation site confers a very narrow window of opportunity for this molecule to stimulate antigen presenting cells, which presents a significant challenge of IM immunization as few antigen presenting cells reside in the tissue. The first wave of immune cells, neutrophils and monocytes, recruited into the muscle occurs 3–6 hrs after vaccination and dendritic cells appear atthe vaccination site as late as 16 hours post-vaccination (Calabro et al, 2011), at which time point more than 99% cGAMP already leaves the injection site in the muscle (Fig. 5f), explaining a relatively weak adjuvanticity of cGAMP when IM administered. The quick diffusion is likely a common property for all CDNs and the analogs in light of their similar chemical structures and solubility. Several strategies have been developed to overcome this limitation of CDNs and their analogs, one of which is to encapsulate cdGMP into nanoparticles to limit its diffusion and prolong its local exposure (Hanson et al, 2015). Another approach is to design nonhydrolyzable analogs to prevent enzymatic digestion (Li et al, 2014, Corrales et al, 2015). In contrast, our investigation suggests a simple but effective strategy to overcome the limitation while preserving the safety of cGAMP by delivering it into the skin. Skin contains a large number of antigen presenting cells that are ready to be activated by cGAMP immediately upon injection. Skin appears to be able to sustain cGAMP much longer than the muscle, maintaining a cGAMP concentration above 1 μg/ml for at least 6 hours as opposed to only 1 hour in the muscle after injection (Fig. 5f). ID injection also results in a high level of cGAMP in the draining lymph nodes in comparison with IM delivery. Taken together, ID immunization offers abundant antigen presenting cells and sufficient retention time for cGAMP to sufficiently activate innate immune system and fully realize its adjuvant potential in the skin.
Pandemic influenza poses a huge threat to global public health due to its unpredictability in its onset, duration, and severity. In the 2014–2015 flu season, the worst animal influenza virus outbreak in U.S. history killed 48 million birds and the virus may come back any time. Although no humans have been infected yet, there is a serious concern that the bird influenza virus may mutate and make a jump from animals to humans, which is rare but within the realm of the possible. These potential threats urge development of effective pandemic influenza vaccine. However, most people do not have preexisting immunity to newly emerged viral strains and immunogenicity of some pandemic antigens are extremely poor. For instance, H5N1 vaccine in the absence of adjuvant requires two doses in an interval of two weeks each at 90 μg that is 6 times higher than seasonal influenza vaccine, to induce protective immunity in only 58% recipients (Treanor et al, 2006). However, the current vaccine manufacturing capacity is ~ 1 billion doses per year, which only meets 1/10th of global need to vaccinate 70% population for two doses. Therefore, effective and safe adjuvants are urgently needed for pandemic influenza vaccine to shorten the time of immunization and to spare the antigen dose. cGAMP, if ID delivered only once, along with H5N1 pandemic influenza vaccine, elicited higher HAI titers than that induced by AddaVax plus MPL delivered intramuscularly. The high level of HAI was induced faster and maintained longer as well (Fig. 3). The study concludes that cGAMP is at least comparable, if not superior to the combination of AddaVax plus MPL, which has been considered a most effective adjuvant for H5N1 vaccine so far.
Besides safety and potency, cGAMP doesn’t increase any viscosity of a vaccine and thus it is readily incorporated into any vaccine formulation designed for skin vaccination, which is always required to be concentrated into a very small volume. cGAMP is also highly compatible with new transcutaneous delivery methods such as biodegradable microneedles or fractional laser delivery owing to its small molecule mass, hydrophilic property, and excellent stability against extreme temperature or lyophilization. Taken together, cGAMP shows great promise to be an “ideal” adjuvant for skin vaccination and merits further clinical studies.
Materials and Methods
IFNAR−/− mice (Muller et al, 1994) were purchased from Jackson Laboratories. MHC II-GFP mice expressing MHC class II molecule infused into enhanced green fluorescent protein (GFP) and STING−/− mice were kindly gifts of Dr. Hidde Ploegh, Massachusetts Institute of Technology and Dr. Glen Barber, University of Miami, respectively (Boes et al, 2002, Ishikawa et al, 2009). MHC II-GFP STING−/− mice were generated by breeding MHC II-GFP mice with STING−/− mice. Male Yorkshire pigs at 4 months of age were obtained from the Teaching and Research Resources at Tufts University. All studies were reviewed and approved by the MGH Institutional Animal Care and Use Committee. Data are presented as mean ± s.e.m. p value was calculated by PRISM software (GraphPad, CA) and a difference was regarded significant if p value was less than 0.05. Full details of methods are described in Supplemental Information.
Supplementary Material
Acknowledgments
The authors would like to thank Photopathology Core for their help in the histology analysis during this project. This work is supported in part by the National Institutes of Health grants AI089779, AI070785, and AI097696 to M.X.W.
Footnotes
Author contributions
M.X.W. has designed and supervised the research. J.W. and P.L. designed and performed the experiments. M.X.W. and J.W. wrote the manuscript.
The authors have no conflict of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Blaauboer SM, Mansouri S, Tucker HR, Wang HL, Gabrielle VD, Jin L. The mucosal adjuvant cyclic di-GMP enhances antigen uptake and selectively activates pinocytosis-efficient cells in vivo. Elife 2015; 4: 10.7554/eLife.06670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boes M, Cerny J, Massol R, Op dB, Kirchhausen T, Chen J, et al. T-cell engagement of dendritic cells rapidly rearranges MHC class II transport. Nature 2002; 418: 983–8. [DOI] [PubMed] [Google Scholar]
- Cai X, Chiu YH, Chen ZJ. The cGAS-cGAMP-STING pathway of cytosolic DNA sensing and signaling. Mol Cell 2014; 54: 289–96. [DOI] [PubMed] [Google Scholar]
- Calabro S, Tortoli M, Baudner BC, Pacitto A, Cortese M, O’Hagan DT, et al. Vaccine adjuvants alum and MF59 induce rapid recruitment of neutrophils and monocytes that participate in antigen transport to draining lymph nodes. Vaccine 2011; 29: 1812–23. [DOI] [PubMed] [Google Scholar]
- Chen X, Pravetoni M, Bhayana B, Pentel PR, Wu MX. High immunogenicity of nicotine vaccines obtained by intradermal delivery with safe adjuvants. Vaccine 2012; 31: 159–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Wang J, Shah D, Wu MX. An update on the use of laser technology in skin vaccination. Expert Rev Vaccines 2013; 12: 1313–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg CH, Roque R, Van Hoeven N, Perrone L, Baldwin SL, Rininger JA, et al. Adjuvant solution for pandemic influenza vaccine production. Proc Natl Acad Sci U S A 2012; 109: 17585–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrales L, Glickman LH, McWhirter SM, Kanne DB, Sivick KE, Katibah GE, et al. Direct activation of STING in the tumor microenvironment leads to potent and systemic tumor regression and immunity. Cell Rep 2015; 11: 1018–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebensen T, Schulze K, Riese P, Link C, Morr M, Guzman CA. The bacterial second messenger cyclic diGMP exhibits potent adjuvant properties. Vaccine 2007a; 25: 1464–9. [DOI] [PubMed] [Google Scholar]
- Ebensen T, Schulze K, Riese P, Morr M, Guzman CA. The bacterial second messenger cdiGMP exhibits promising activity as a mucosal adjuvant. Clin Vaccine Immunol 2007b; 14: 952–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginhoux F, Ng LG, Merad M. Understanding the murine cutaneous dendritic cell network to improve intradermal vaccination strategies. Curr Top Microbiol Immunol 2012; 351: 1–24. [DOI] [PubMed] [Google Scholar]
- Hanson MC, Crespo MP, Abraham W, Moynihan KD, Szeto GL, Chen SH, et al. Nanoparticulate STING agonists are potent lymph node-targeted vaccine adjuvants. J Clin Invest 2015; 125: 2532–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickling JK, Jones KR, Friede M, Zehrung D, Chen D, Kristensen D. Intradermal delivery of vaccines: Potential benefits and current challenges. Bull World Health Organ 2011; 89: 221–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison S, Benson RA, Gibson VB, Pollock AH, Garside P, Brewer JM. Antigen depot is not required for alum adjuvanticity. FASEB J 2012; 26: 1272–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa H, Ma Z, Barber GN. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature 2009; 461: 788–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin L, Waterman PM, Jonscher KR, Short CM, Reisdorph NA, Cambier JC. MPYS, a novel membrane tetraspanner, is associated with major histocompatibility complex class II and mediates transduction of apoptotic signals. Mol Cell Biol 2008; 28: 5014–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaolis DK, Means TK, Yang D, Takahashi M, Yoshimura T, Muraille E, et al. Bacterial c-di-GMP is an immunostimulatory molecule. J Immunol 2007; 178: 2171–81. [DOI] [PubMed] [Google Scholar]
- Li L, Yin Q, Kuss P, Maliga Z, Millan JL, Wu H, et al. Hydrolysis of 2’3’-cGAMP by ENPP1 and design of nonhydrolyzable analogs. Nat Chem Biol 2014; 10: 1043–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XD, Wu J, Gao D, Wang H, Sun L, Chen ZJ. Pivotal roles of cGAS-cGAMP signaling in antiviral defense and immune adjuvant effects. Science 2013; 341: 1390–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod MK, McKee AS, David A, Wang J, Mason R, Kappler JW, et al. Vaccine adjuvants aluminum and monophosphoryl lipid A provide distinct signals to generate protective cytotoxic memory CD8 T cells. Proc Natl Acad Sci U S A 2011; 108: 7914–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manstein D, Herron GS, Sink RK, Tanner H, Anderson RR. Fractional photothermolysis: A new concept for cutaneous remodeling using microscopic patterns of thermal injury. Lasers Surg Med 2004; 34: 426–38. [DOI] [PubMed] [Google Scholar]
- Muller U, Steinhoff U, Reis LF, Hemmi S, Pavlovic J, Zinkernagel RM, et al. Functional role of type I and type II interferons in antiviral defense. Science 1994; 264: 1918–21. [DOI] [PubMed] [Google Scholar]
- Ogunniyi AD, Paton JC, Kirby AC, McCullers JA, Cook J, Hyodo M, et al. C-di-GMP is an effective immunomodulator and vaccine adjuvant against pneumococcal infection. Vaccine 2008; 26: 4676–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paludan SR, Bowie AG. Immune sensing of DNA. Immunity 2013; 38: 870–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed SG, Orr MT, Fox CB. Key roles of adjuvants in modern vaccines. Nat Med 2013; 19: 1597–608. [DOI] [PubMed] [Google Scholar]
- Sun L, Wu J, Du F, Chen X, Chen ZJ. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science 2013; 339: 786–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treanor JJ, Campbell JD, Zangwill KM, Rowe T, Wolff M. Safety and immunogenicity of an inactivated subvirion influenza A (H5N1) vaccine. N Engl J Med 2006; 354: 1343–51. [DOI] [PubMed] [Google Scholar]
- Vogelbruch M, Nuss B, Korner M, Kapp A, Kiehl P, Bohm W. Aluminium-induced granulomas after inaccurate intradermal hyposensitization injections of aluminium-adsorbed depot preparations. Allergy 2000; 55: 883–7. [PubMed] [Google Scholar]
- Wang J, Li B, Wu MX. Effective and lesion-free cutaneous influenza vaccination. Proc Natl Acad Sci U S A 2015; 112:5005–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Shah D, Chen X, Anderson RR, Wu MX. A micro-sterile inflammation array as an adjuvant for influenza vaccines. Nat Commun 2014; 5: 4447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegmann F, Gartlan KH, Harandi AM, Brinckmann SA, Coccia M, Hillson WR, et al. Polyethyleneimine is a potent mucosal adjuvant for viral glycoprotein antigens. Nat Biotechnol 2012; 30: 883–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Sun L, Chen X, Du F, Shi H, Chen C, et al. Cyclic GMP-AMP is an endogenous second messenger in innate immune signaling by cytosolic DNA. Science 2013; 339: 826–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong B, Yang Y, Li S, Wang YY, Li Y, Diao F, et al. The adaptor protein MITA links virus-sensing receptors to IRF3 transcription factor activation. Immunity 2008; 29: 538–50. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


