Abstract
Microglia are the first responders of the central nervous system, acting as the key modulators of neuroinflammation observed during neurotoxic insults as well as in the pathophysiology of several neurodegenerative disorders including Alzheimer’s (AD), Parkinson’s (PD), and Huntington’s diseases (HD). The number of publications on microglia has increased steadily throughout the past decade because of immense interests in the neuroinflammation that precedes the neurodegenerative process. To study microglial biology and its role in modulating neuroinflammation, immortalized microglial cell lines derived from mice, rats, and humans have been developed. Among these, the BV2 mouse microglial cell line is the most well characterized and widely used cell culture model. However, even unstimulated BV2 cells exhibit an amoeboid, hypertrophied morphology, indicating a highly activated and inflammatory state compared to primary microglia, thus making them less than ideal for studying the low-dose effects of toxicants on microglial activation. Therefore, we performed an in-depth characterization of a recently developed mouse microglial cell (MMC) line, which we compared with primary mouse microglia (PMG) and BV2s to identify which cell line was best suited for studying the microglial response to neurotoxicants. Comparative analyses reveal that MMCs are strikingly more similar to PMGs in basal activity, morphology, and sensitivity, than are BV2s. Furthermore, basal nitrite and inflammatory cytokine levels are significantly higher in BV2s compared to MMCs. BV2 cells are also less reactive to the inflammagen LPS compared to MMCs, due to the higher basal activation state of BV2s. Collectively, our in-depth analyses of morphology, basal activity, and responsivity to two different stimuli (LPS, aggregated α-synuclein) demonstrate that MMCs closely mimic neonatal PMGs, and are discernibly more suitable than BV2s for studying the neuroinflammatory mechanisms of neurotoxicants.
Keywords: Neuroinflammation, Microglia, Metals, Aggregated Protein, Cell Signaling
Introduction
Microglia are the first immune responders in the brain. Persistently active and unregulated microglia are key modulators of chronic neuroinflammation observed in several neurodegenerative disorders. Activated microglia are distinct from resting microglia both morphologically as well as functionally with respect to the production and secretion of pro-inflammatory factors (Glass et al., 2010; Panicker et al., 2015). Lipopolysaccharide (LPS)-activated microglia undergo morphological changes, in general, from a ramified (surveillant) type to a hypertrophic, amoeboid type that is typically observed releasing ROS, nitrite, and various other pro-inflammatory mediators and cytokines that can be toxic over time. Like macrophages, microglia can also undergo an alternative activation, which leads to the production of anti-inflammatory factors. For example, IL-4 can induce this alternative anti-inflammatory phenotype in microglial cells (Nakagawa and Chiba, 2014). Walker and Lue (2015) pondered the probable existence of a third microglial phenotype, known as M3. Such a polarization may be induced via CSF-1 or IL-34 signaling, either of which can activate CSF-1R, which could tightly regulate microglial cell division; however, the evidence is still scant. Thus, microglial activation is complex, and the molecular footprints underlying the various phenotypes are the focus of much research.
Several lines of evidence, from cell culture to animal models to postmortem tissue analyses to genetic linkage analyses, conclusively demonstrate that sustained neuroinflammation contributes to neuronal dysfunction and death (Block et al., 2007; Gordon et al., 2016; Kirkley et al., 2017; Panicker et al., 2015; Sarkar et al., 2017b). Microglial activation leading to neurodegeneration has been well documented in AD, PD, and other neurodegenerative disease (Block et al., 2007; Glass et al., 2010; Solito and Sastre, 2012). Misfolded proteins, including aggregated α-synuclein (αSynAgg) and amyloid-β, the respective hallmarks of PD and AD pathologies, can hyper-activate microglia, leading to chronic inflammation and loss of neurons (Halle et al., 2008; Heneka et al., 2013).
The number of publications on microglial cells in the last decade has increased steadily with every passing year (Fig. 1A), especially the importance of microglia in AD, PD, multiple sclerosis (MS), and other neurodegenerative disorders. To study microglial biology and its role in modulating neuroinflammation, several immortalized microglial cell lines have been developed (Fig. 1B), including BV2, N9, and EOC (mouse microglial cell lines); HAPI (rat microglial cell line); and the HMC3 and HMO6 (human microglial cell lines). Among the six prevalent cell lines, BV2 cell lines have been used in ~75% of publications (Fig. 1B). Since 2010, the number of publications reporting on BV2s has substantially increased (Fig. 1C). Though BV2 microglial cell lines are not identical to primary microglia in morphology or activation state, the prevalent use of BV2s may be attributed to the following: i) lack of good, easily obtainable cell lines; ii) cost of primary culture; iii) low yield of primary microglial cells; and iv) difficulty in isolating primary cells. Blasi et al. (1990) created the BV2 cells via the J2 retrovirus carrying the v-myc/v-raf genes from C57BL/6 primary mouse microglia. In this study, we performed a more in-depth characterization of a newly developed microglial cell line that was also immortalized with the J2 retrovirus from C57BL/6 primary mouse microglia (Halle et al., 2008). We compared this new mouse microglial cell line (MMC) with the predominantly used BV2 microglia. Our results demonstrate that the basal inflammatory level of BV2 microglia is higher than that of MMCs, and that the responsiveness of MMCs to LPS and αSynAgg stimulation far exceeds that of BV2s, suggesting that MMCs more closely mimic PMGs than do BV2s.
Figure 1: Morphological comparison between the mouse microglial cell (MMC) line, the BV2 cell line, and primary microglia (PMG).
A) Number of yearly publications on microglia in PubMed. B) Distribution of the number of PubMed-indexed publications on various microglial cell lines. C) Number of yearly publications on BV2 microglia in PubMed. D) Immunostaining for IBA1 on BV2, MMC, and PMG cells after a 6-h LPS (1 μg/mL) exposure. Scale bar is 10 μM.
Material and Methods
Chemicals, Reagents and Instruments used
Dulbecco’s modified Eagle’s medium (DMEM), DMEM-F12, fetal bovine serum (FBS), L-glutamine (Q), penicillin/streptomycin (P/S), Sodium Pyruvate (SP), and Non-Essential Amino Acids (NEAA) were obtained from Invitrogen (Carlsbad, CA). CellTiter Glo Luminescent Cell Viability Assay kit was obtained from Promega (Madison, WI). The CD11b magnetic separation kit was purchased from Stem Cell Technologies (Vancouver, Canada). All standards used for the Luminex multiplex cytokine assay were purchased from PeproTech, Inc (Rocky Hill, NJ). Streptavidin-Biotin and biotinylated antibodies used for Luminex were purchased from eBioSciences (San Diego, CA). Phagocytosis assay kit was purchased from Cayman Chemicals (Ann Arbor, MI).
For qRT-PCR, the QuantStudio3 Real Time PCR system from Thermo Fisher (Waltham, MA) was used. For MTS and Griess assays, a Molecular Devices (Sunnyvale, CA) plate reader was used. For scanning Western blot, a Li-COR Odyssey gel imager (Lincoln, NE) was used. Cell culture supplies were purchased from Corning (Corning, NY). For immunocytochemistry studies, the images were obtained using a Nikon (Model: TE-2000U) inverted fluorescent microscope and images were obtained using a Spot Digital Camera (Sterling Heights, MI).
Literature Review
To assess the use of microglial cell lines in peer-reviewed studies, the following terms were searched in PubMed: microglia, BV2 microglia, HAPI microglia, N9 microglia, HMO6 microglia, HMC3 microglia, EOC microglia.
Cell culture and Treatments
Primary Microglia:
Primary microglia were obtained from 1- to 3-day postnatal mouse pups, as described in our published protocol (Sarkar et al., 2017c). The brains were excised, immersed in 0.25% trypsin-EDTA (TE), and incubated for 15 min in a 37° C water bath with occasional mixing. The TE was neutralized with growth medium (10% FBS with 1% P/S, Q, SP, NEAA in DMEM-F12), and the brains were washed 4 times by adding and removing fresh growth medium. The brains were then homogenized via trituration, filtered through a 70-μm filter, and then the cell suspension was plated in T75 flasks (2 T75 flasks per brain). After 6 days, the growth medium was decanted and replaced with fresh growth medium. On the 16th day, the cells were collected, subjected to an antibody CD11b positive selection, and isolated using a magnetic column that binds magnetic spheres complexed onto the CD11b surface marker. Once the cells are seeded appropriately, they are ready for treatments after 48 h. All experiments were performed in treatment medium (2% FBS with 1% P/S, Q, SP, NEAA in DMEM-F12).
BV2:
BV2 microglia were maintained in 10% FBS with 1% P/S and Q in RPMI 1640. All experiments were performed in treatment medium (2% FBS with 1% P/S and Q in RPMI 1640). These cells were a kind gift from Dr. Debomoy K. Lahiri, professor of neurobiology at the Indiana University School of Medicine.
Wild-type mouse microglial cell (MMC) line:
MMCs were derived by viral transduction of primary microglia, which were a kind gift from Dr. D. Golenbock (University of Massachusetts). These cells have similar morphology and surface markers as primary microglial cells (Halle et al., 2008). MMCs were maintained in 10% FBS with 1% P/S, Q, and SP in DMEM-F12. All experiments were performed in treatment medium (2% FBS with 1% P/S, Q, and SP in DMEM-F12) (Sarkar et al., 2017b).
Immunocytochemistry (ICC)
For ICC, coverslips were placed in 24-well plates, immersed in poly-D-Lysine, and then washed with phosphate-buffered saline (PBS). Next, cells were seeded with their respective growth medium. After the experiment, the treatment medium was decanted and the cells on the coverslips were fixed by incubating at RT with 4% paraformaldehyde for 25 min. The cells were then washed with PBS 4 times. Next, the cells were blocked with blocking buffer (2% BSA with 0.05% Triton-X100 and 0.005% Tween-20 in PBS) for 45 min to 1 h. Primary antibodies were made with 1% BSA in PBS, incubated overnight at 4° C, and then washed for 30 min with PBS. All secondary antibodies were made with 1% BSA in PBS, incubated at RT for 90 min, and then washed for 30 min with PBS. After washing, the coverslips were mounted on pre-coated slides using Fluoromount (Sigma-Aldrich). The following antibodies were used: IBA1 (Wako AB_2314667, 1:1000), iNOS (Abcam AB_2152867, 1:500). Alexa dye-conjugated secondary antibodies were used for ICC experiments.
Recombinant human α-synuclein purification and aggregation
Recombinant human α-synuclein was purified following our previous publication (Sarkar et al., 2017a). Briefly, E. coli cells were transformed with human α-synuclein plasmid and grown on an agar plate with ampicillin (Amp). Pre-culture was prepared by inoculation of a single bacterial colony from the agar plate into a tube containing 10 mL of LB broth with Amp and incubated overnight at 37 °C. Next, the pre-culture was inoculated into 1 L of LB medium containing Amp, and every hour the OD600 was taken until the culture reached an OD of 0.5. The α-synuclein expression was induced by adding 1 mM isopropyl β-D-1-thiogalactopyranoside (Invitrogen), and the cells were further incubated at 37 °C for 8 h before harvesting. Cells were lysed and recombinant α-synuclein was purified and aggregated as previously described (Sarkar et al., 2017a).
qRT-PCR
RNA extraction and qRT-PCR were performed as described previously (Sarkar et al., 2017b; Seo et al., 2014). Briefly, cells were plated in 6-well plates and treated. Following treatment, TRIzol reagent was used to isolate total RNA from the cells and the concentration was measured using NanoDrop. Affinity Script qPCR cDNA synthesis system (Agilent Technologies) was used to convert RNA to cDNA. Real-time PCR was performed with the RT2 SYBR Green master mix (Thermo Fisher #K0172). The following genes from QuantiTect Primer Assay (Qiagen) were used for qRT-PCR: pro-il-1β (QT01048355), pro-il-18 (QT00171129), il-12b (QT00153643), Tnf-α (QT00104006), il-6 (QT00098875), and Nos2 (QT00100275). The housekeeping gene 18s rRNA (Qiagen #PPM57735E) was used as the reference for all qRT-PCR experiments. The results are reported as fold change in gene expression, which was determined via the ΔΔCt method using the threshold cycle (Ct) value for the housekeeping gene and for the respective gene of interest in each sample (Lawana et al., 2017).
Western Blotting
Western blot analysis was performed according to previous published protocols (Langley et al., 2017). Briefly, cell samples were lysed using modified RIPA buffer and sonicated using a refrigerated cup-sonicator. Bradford assay was performed for protein estimation. Next, 25 ¼g of protein was loaded in each well of 10% SDS-acrylamide gel and ran for 2-2.5 h at 110 V (Panicker et al., 2015). Proteins were transferred to a nitrocellulose membrane at 27 V for 18 h at 4° C. The nitrocellulose membranes were blocked using LI-COR blocking buffer for 1 h. The blocked nitrocellulose membranes were incubated in the primary antibody for gp91-phox (BD Biosciences) for 3 to 18 h, washed with PBS-Tween (0.01%), incubated in infrared LI-COR secondary antibodies for 1 h, washed again with PBS-Tween, and imaged using a LI-COR scanner. Secondary antibodies were used according to manufacturer’s instructions. We used β-actin as the loading control.
Luminex Multiplex Cytokine Assay
Luminex cytokine assay was performed according to our previous publications (Panicker et al., 2015; Sarkar et al., 2017a). Briefly, PMGs and MMCs (100,000 cells/well) were treated in 96-well plates. After treatment, 40 μL of the medium was collected and added to 40 μL of primary antibody conjugated to magnetic microspheres and incubated overnight at 4°C in a clear-bottomed, black 96-well plate. Following incubation in primary antibodies, each well was triple-washed using a magnetic washer, incubated for 1 h with biotin-conjugated secondary antibodies, triple-washed again, incubated with streptavidin/phycoerythrin for 30 min and washed two more times. A Bio-Plex reader (Bio-Rad) was used to read the 96-well plates.
MTS Assay
MTS assay was performed according to our previous publication (Sarkar et al., 2017a). Briefly, 100,000 cells/well were plated in a 96-well tissue culture plate and treated with 1–3000 ng/mL of LPS for 24 h. After treatment, 10 μL of MTS dye was added to each well and incubated for 60 min at 37° C. Absorbance readings were measured using a plate reader at 490 nm, and a 640-nm readout was used for background subtraction.
Griess Assay
Griess assay was performed according to our previous publication (Langley et al., 2017). Briefly, 100,000 cells/well were plated in 96-well plates. Cells were treated with 1–3000 ng/mL of LPS. Following treatment, 50 ¼L of media was mixed with an equal volume of Griess reagent and incubated for 10 min. The plate was read using a plate reader at 540 nM. Sodium nitrite was used to make the standard curve.
Data Analysis
Prism 7.0 (GraphPad) was used for statistical analysis with p≤0.05 considered statistically significant. One-way ANOVA was used for comparison among multiple groups. In most cases, Tukey post-hoc analysis was applied. For comparing 2 groups, Student’s t-test was performed.
Results
LPS effects on the morphology of BV2s, MMCs, and PMGs
Classical activation of microglia leads to an amoeboid morphology with enlarged soma and fewer processes. MMCs are lightly heterogeneous cells, typically smaller than BV2s, with two or more ramifications. In this experiment, we determined the morphological changes associated with LPS treatment in BV2s, MMCs, and PMGs. Cell lines were treated with 1 ¼g/mL of LPS for 6 h, fixed and subjected to ICC (Fig. 1D). Microscopic analysis demonstrates a change in morphology of MMCs and PMGs upon LPS exposure from a ramified to a more amoeboid shape exhibiting drastic hypertrophy. On the contrary, the widely used microglial cell line, BV2, largely exhibits amoeboid shapes prior to treatment, suggesting that they are already activated and LPS treatment does not discernibly alter their morphology (Fig. 1D). These results suggest that LPS-induced morphological changes in MMCs are more similar to the morphological changes seen in PMGs. Therefore, although lacking in BV2s, the combined hypertrophic and amoeboid-like morphological changes observed in MMCs and PMGs could be used as a measure of microglial activation.
Comparison of LPS-induced iNOS expression and nitrite production in microglial cell lines
Hallmarks of microglial inflammation include iNOS induction and subsequent nitrite production (Gordon et al., 2016; Panicker et al., 2015). Nitrite released from activated glial cells is neurotoxic and contributes to the neurodegenerative process (Mander and Brown, 2005). Probing for basal iNOS expression revealed that BV2 microglial cells exhibited higher iNOS levels when compared to both MMCs and PMGs (Fig. 2A). Upon LPS treatment (1 μg/mL) for 6 h, iNOS expression was rapidly induced in MMCs and PMGs relative to controls, whereas iNOS induction in BV2 cells was not strikingly different from controls.
Figure 2: BV2s have higher basal nitrite levels compared to MMCs and PMGs.
A)ICC analysis showing BV2 cells have a higher iNOS level compared to MMCs and PMGs. Scale bar is 10 μM. B) Griess assay showing BV2 cells produce higher nitrite compared to MMCs and PMGs. C) Griess assay post LPS (1 μg/mL for 6 h) treatment showing that BV2s produce lower nitrite relatively compared to MMCs and PMGs. This fold change was calculated after comparing the controls versus LPS for each cell type. Data analyzed via ANOVA with Tukey post-hoc analysis and BV2 was compared with MMC and PMG, **p<0.01, ***p<0.001. Data represented as mean±SEM with 3-4 biological replicates.
Since increased iNOS expression will result in increased nitrite production, we quantified basal, extracellularly released nitrite levels by Griess assay. BV2 cells released significantly higher nitrite than did MMCs or PMGs (Fig. 2B). Extracellularly released nitrite levels did not differ significantly between PMGs and MMCs (Fig. 2B). Relative to their respective controls, MMCs and PMGs produced more LPS (1 μg/mL)-induced nitrites than the BV2s (Fig. 2C), indicating the basally activated state of BV2. Together, these findings show that BV2 cells are already in an activated state under basal conditions compared to MMCs and PMGs.
BV2 cells have higher basal level of pro-inflammatory factors
Similar to nitrite production, the production and secretion of pro-inflammatory cytokines are also major hallmarks of microglial inflammation (Gordon et al., 2016; Panicker et al., 2015). Pro-inflammatory cytokines released from activated glial cells are neurotoxic and contribute to the neurodegenerative process. We performed qRT-PCR to compare the basal expression levels of pro-inflammatory cytokines and Nos2 (Fig. 3). The qRT-PCR analysis revealed that BV2s have significantly higher basal gene expression of the pro-inflammatory factors pro-il-1β (Fig. 3A), il-6 (Fig. 3B), il-12 (Fig. 3C), Tnf-α (Fig. 3D) and Nos2 (Fig. 3E), with one exception being pro-il-18 (Fig. 3F), when compared to MMCs and PMGs as evident from the lower ΔCt value. These data suggest significantly higher mRNA expression levels of pro-inflammatory factors in BV2s compared to MMCs and PMGs. The basal expression of these pro-inflammatory factors was remarkably similar in MMCs and PMGs. Furthermore, Luminex analysis reveals that basal levels of the secreted pro-inflammatory cytokines IL-1β (Fig. 4A), IL-6 (Fig. 4B), and TNFα (Fig. 4C) were significantly higher in BV2 cells compared to MMCs and PMG. These data together suggest that BV2 microglial cells are basally more active than MMCs and PMGs.
Figure 3: BV2s have higher basal pro-inflammatory factors compared to MMCs and PMGs.
A-F) qRT-PCR analyses of basal levels of A) pro-il-1β, B) il-6, C) il-12, D) Tnf-α, E) Nos2, and F) pro-il-18 in BV2, MMC and PMG cells. Data analyzed via ANOVA with Tukey post-hoc analysis and BV2 was compared with MMC and PMG, *p<0.05, **p<0.01, ***p<0.001. Data represented as mean±SEM with 3-8 biological replicates.
Figure 4: BV2s have higher basal secreted pro-inflammatory factors compared to MMCs and PMGs.
A-C) Luminex analysis of basal levels of secreted A) IL-1β, B) IL-6 and C) TNF-α in BV2, MMCs and PMG cells. Data analyzed via ANOVA with Tukey post-hoc analysis and BV2 was compared with MMC and PMG, ***p<0.001. Data represented as mean±SEM with 4 biological replicates.
LPS-stimulated pro-inflammatory response is weaker in BV2s compared to MMCs and PMGs
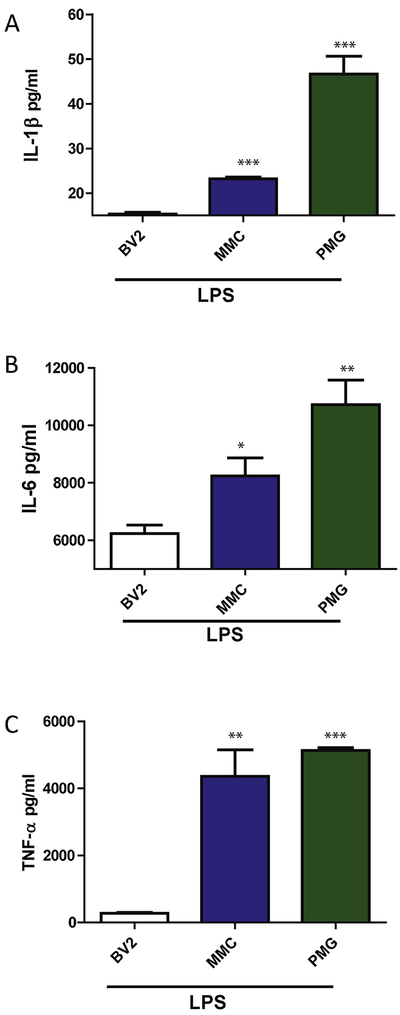
MMCs, BV2s and PMGs were exposed to LPS for 6 h, following which qPCR was performed for pro-inflammatory factors. Each cell type was compared to their respective untreated controls. When compared to LPS-stimulated BV2 cells, pro-il-1β (Fig. 5A), il-6 (Fig. 5B), il-12b (Fig. 5C), pro-il-18 (Fig. 5D), Nos2 (Fig. 5E) and Tnf-α (Fig. 5F) were rapidly upregulated in MMCs as well as in PMGs. Furthermore, the multiplex assay of the treatment media revealed that LPS induced significantly higher levels of the pro-inflammatory factors IL-1β (Fig. 6A), IL-6 (Fig. 6B) and TNFα (Fig. 6C) in MMCs and PMGs compared to BV2 cells. These data collectively suggest that MMCs are a good model system for understanding the mechanism of LPS-induced microglial inflammation.
Figure 5: LPS-stimulated pro-inflammatory response is weaker in BV2s compared to MMCs and PMGs.
A-F) qRT-PCR analysis of levels of A) pro-il-1β, B) il-6, C) il-12b, D) pro-il-18, E) Nos2, and F) Tnf-α in BV2s, MMCs and PMGs 6 h after LPS (1 μg/mL) treatment. Data analyzed via ANOVA with Tukey post-hoc analysis and BV2 was compared with MMC and PMG, *p<0.05, **p<0.01, ***p<0.001. Data represented as mean±SEM with 2-3 biological replicates.
Figure 6: LPS-stimulated pro-inflammatory factor release was weaker in BV2s compared to MMCs and PMGs.
Luminex analysis of levels of secreted A) IL-1β, B) IL-6 and C) TNF-α in BV2, MMCs and PMGs 6 h after LPS (1 μg/mL) treatment. Data analyzed via ANOVA with Tukey post-hoc analysis and BV2 was compared with MMC and PMG, *p<0.05, **p<0.01, ***p<0.001. Data represented as mean±SEM with 3-4 biological replicates.
LPS induces gp-91phox expression in MMCs
Gp91-phox is a key component of the NADPH oxidase (Nox-2) complex and has been identified as one of the major downstream effectors of LPS-induced inflammation. LPS-induced free-radical formation is diminished in Nox2/gp91-phox deficient mice (Clement et al., 2010), and gp91-phox plays a role in neuroinflammation in PD (Langley et al., 2017). NOX2 facilitates ROS generation (Belarbi et al., 2017) and recent studies from our group and others have shown that ROS formation can lead to inflammation in microglial cells (Park et al., 2015; Sarkar et al., 2017a; Sarkar et al., 2017b). NOX2 inhibition has further been shown to reduce the release of pro-inflammatory factors from microglial cells (Langley et al., 2017). We have also previously shown that LPS stimulation leads to transport of p47 to the membrane and activation of NOX2 (Langley et al., 2017). To further verify the downstream effector of LPS-induced inflammation in MMCs, cells were treated with LPS for 1 h, as inflammatory signaling cascades are known to be activated at early time points (Panicker et al., 2015). Western blot analysis revealed that LPS induced gp91-phox in the MMCs (Fig. 7A), which was corroborated by ICC analysis (Fig. 7B). These data support previous findings in glial cells, further showing that this cell line can be used for studying Nox-2 signaling mechanisms underlying microglial activation.
Figure 7: LPS induces gp-91phox in MMCs.
A) Western blot and B) ICC analysis reveal increased gp91-phox in MMCs following LPS exposure (1 μg/mL) for 1 h. Data were quantified using ImageJ and analyzed via Student’s t-test, *p<0.05. Data represented as mean±SEM with 3-4 biological replicates.
LPS induces a dose-dependent pro-inflammatory response in MMCs
To further characterize the response of MMCs upon exposure to an inflammatory stimulus, we performed a dose-response analysis of various LPS-induced inflammatory parameters. Given that LPS treatment can induce mitochondrial dysfunction (Kuwabara and Imajoh-Ohmi, 2004), we treated MMCs with different doses of LPS (1-3000 ng/mL) for 24 h. An MTS assay revealed that LPS dose-dependently reduced metabolic activity (Fig. 8A). The 24 h EC50 concentration was calculated to be 8.6 ng/mL LPS. Moreover, a Griess assay revealed that LPS induced a dose-dependent increase in nitrite release (Fig. 8B). Our Luminex assay demonstrated that low-dose LPS exposure (1 ng/mL) produced pro-inflammatory factors including IL-6 (Fig. 8C), IL-12 (Fig. 8D), TNF-α (Fig. 8E), and IL-1β (Fig. 8F). Together, these data suggest that LPS stimulation can induce a broad dose-dependent inflammatory response in MMCs.
Figure 8: MMCs dose-dependentlv respond to LPS.
A) MTS assay performed on MMCs after treatment with 1, 3, 10, 30, 100, 300, 1000 and 3000 ng/mL LPS for 24 h reveals dose-dependent decrease in metabolic activity. B) Griess assay performed on MMCs after treatment with 1, 3, 10, 30, 100, 300, 1000 and 3000 ng/mL LPS for 24 h demonstrating dose-dependent increase in nitrite release. C-F) Luminex assay performed on MMCs after treatment with 1, 3, 10, 30, 100, 300, 1000 and 3000 ng/mL LPS for 24 h demonstrating increase in secreted C) IL-6 (control = 184.44 pg/mL), D) IL-12 (control = 20.15 pg/mL), E) TNF-α (control = 0.44 pg/mL), and F) IL-1 β (control = 0.025 pg/mL). Data analyzed via ANOVA with Tukey post-hoc analysis. Data represented as mean±SEM with 8-32 biological replicates.
The αSynAgg-induced pro-inflammatory factors are dampened in BV2 cells compared to MMCs and PMGs
Misfolded αSyn and its aggregates are major pathological features of PD. We have previously shown that αSynAgg classically activates microglial cells, producing pro-inflammatory cytokines and nitrite in BV2 cells and primary microglia (Gordon et al., 2016). To determine if MMCs can be activated by αSynAgg, we treated MMC cells with 1 μM αSynAgg for 6 h and compared them with PMGs as well as BV2s. The qPCR analysis revealed that αSynAgg-induced upregulation of the pro-inflammatory factors pro-il-1β (Fig. 9A), il-6 (Fig. 9B), il-12 (Fig. 9C), pro-il-18 (Fig. 9D), Nos2 (Fig. 9E) and Tnf-α (Fig. 9F) was significantly higher in MMCs and PMGs than in BV2s. Also in contrast to BV2s, Luminex analysis of IL-1 β (Fig. 10A), IL-6 (Fig. 10B) and TNF-α (Fig. 10C) release from the collected treatment media revealed a significant inflammatory response in MMCs comparable to that of PMGs. Although the gene and protein expression differs between MMCs and PMGs, their treatment responses are phenotypically more similar than either is to BV2s. These data further demonstrate that the MMCs may be used as a microglial model for neuroinflammation in diseases associated with protein misfolding.
Figure 9: αSynAgg induces comparable pro-inflammatory response in MMCs and PMGs.
A-F) qRT-PCR analysis of levels of A) pro-il-1β, B) il-6, C) il-12, D) pro-il-18, E) Nos2, and F) Tnf-α in BV2s, MMCs and PMGs 6 h after αSynAgg (1 μM) treatment. Data analyzed via ANOVA with Tukey post-hoc analysis and BV2 was compared with MMC and PMG, *p<0.05, **p<0.01, ***p<0.001. Data represented as mean±SEM with 3-5 biological replicates.
Figure 10: αSynAgg-stimulated pro-inflammatory factor release was weaker in BV2s compared to MMCs and PMGs.
Luminex analysis of levels of secreted A) IL-1β, B) IL-6 and C) TNF-α in BV2, MMC and PMG cells 6 h after αSynAgg (1 μM) treatment. Data analyzed via ANOVA with Tukey post-hoc analysis and BV2 was compared with MMC and PMG, **p<0.01, ***p<0.001. Data represented as mean±SEM with 3-4 biological replicates.
Discussion
Notably, in the past two decades, chronic neuroinflammation has been the subject of intense research (Fig. 1A). Elucidating the role of microglia in CNS pathophysiology is now regarded as fundamental in therapeutically targeting neurodegenerative disorders such as PD, AD, and HD (Schapansky et al., 2015). Optimal in vitro models are thus indispensable for delineating cellular pathways and identifying molecular targets that could be exploited for such therapeutics. Herein, we provide an in-depth comparative analysis of the inflammatory states of the most widely used cell line, BV2 (Fig. 1C), and a novel, immortalized wild-type mouse microglial cell line, MMC. We argue that the MMCs are more advantageous for studies of neuroinflammation because of their inherent tendency to remain basally surveillant, yet more sensitive and comparable to neonatal mouse primary microglia than the widely used BV2 cell line.
As evidenced in knockout animals, iNOS is a critical intracellular mediator of inflammation, conferring resistance against MPTP-induced loss of substantia nigral neurons (Liberatore et al., 1999). Furthermore, iNOS converts arginine to citrulline while also forming the byproduct nitric oxide (NO), a powerful signaling molecule (Hallemeesch et al., 2003; Moehle and West, 2015). However, in highly oxygenated conditions, NO can react with superoxide to form peroxynitrite, which can exert cytotoxic effects. Levels of its breakdown product, nitrite, correlate with the degree of inflammation (Hunter et al., 2013; Pacher et al., 2007). In this study, basal or unstimulated BV2s exhibited higher levels of iNOS compared to MMCs and PMGs, which was corroborated by measuring nitrite levels in Griess assays (Fig. 2A-B). Additionally, after stimulation with LPS, BV2s do not exhibit as much nitrite release as do MMCs and PMGs, revealing that BV2s are more desensitized (Fig. 2C). Significantly lower levels of basal iNOS and nitrite, but higher levels post-stimulation, make MMCs far more suitable for signaling studies involving iNOS, NO, and potentially other unrelated oxidative stress mechanisms. Since oxidative stressors are prime factors that contribute to the neuroinflammatory and degenerative processes (Sarkar et al., 2017b), MMCs appear to be the more appropriate immortalized cell culture model for studying microglial activation.
Both the BV2s and the MMCs were immortalized from C57BL/6 mice utilizing the J2 retrovirus carrying the v-myc/v-raf genes (Blasi et al., 1990; Halle et al., 2008). However, basal gene expression for various cytokines, as well as Nos2, were all significantly higher in BV2s except for pro-il-18 (Fig. 3). Analysis of IL-1 β, TNF-α, and IL-6 at the protein level corroborated the gene expression results (Fig. 4). Upon stimulation with LPS, MMCs demonstrated significantly greater sensitivity by exerting a more robust transcriptional burst of inflammatory genes and protein translation (Figs. 5-6). This sensitivity and robustness may be crucial to more effectively defining signaling mechanisms of inflammation spurred by microglia, especially at earlier time points and in low-dose neurotoxicant exposure studies. Although the two cell lines were developed using the same retrovirus, the culturing conditions differed. BV2s were cultured in RPMI 1640 while the MMCs were cultured in DMEM (Blasi et al., 1990; Halle et al., 2008). Our own choice to utilize DMEM-F12 might also explain the disparities observed basally and in treated cells. Importantly, BV2 cells cultured in DMEM-F12 were nonviable. Thus, the cell culturing conditions demand further scrutiny, especially when searching for appropriate in vitro models.
Although MMCs were initially generated by Golenbock and colleagues, the cell model was not well characterized for neurotoxicity studies (Halle et al., 2008). Here, we provide a more comprehensive analysis of their fundamental responsivity to stimulants such as LPS and αSynAgg. LPS is known to dysregulate the mitochondrial membrane potential (Kuwabara and Imajoh-Ohmi, 2004) and to modulate mitochondrial bioenergetics at early time points (Sarkar et al., 2017b). As such, we employed the MTS assay to assess LPS’s effects on the mitochondrially related metabolic activity of MMCs. At the traditional 24-h time point, an effective dose of 8.6 ng/mL of LPS inhibited metabolic activity (Fig. 8A). This was further supported by the dose-dependent release of nitrite, in which 10 ng/mL LPS was sufficient to produce ~15 μΜ of nitrite (Fig. 8B). Similarly, inflammatory cytokine analysis revealed that LPS treatment dose-dependently increased IL-1β levels. Although a dose response for IL-6, IL-12, or TNFα was lacking, 10 ng/mL was sufficient to elicit a large cytokine release. Interestingly, IL-6 appears to function as the primary cytokine effector since a major release was observed even at 1 ng/mL. Based on this analysis, low-dose LPS for time points of 24 h or longer should be appropriate without significant cytotoxicity. On the other hand, higher doses should be effective at earlier time points. This is apparent from the significant increase in gp91-phox protein, a key generator of superoxide that reacts with NO to produce peroxynitrite (Fig. 7) (Pacher et al., 2007).
Others have shown that αSynAgg induces microgliosis (Sanchez-Guajardo et al., 2013; Wang et al., 2015), which promotes inflammatory signaling through critical kinases such as protein kinase Cδ (Gordon et al., 2016). Comparatively MMCs are not drastically different from PMGs in releasing the major cytokines IL-1β, IL-6, and TNFα after being exposed to αSynAgg (Fig. 9-10). Similar results were obtained for IL-1β by stimulating the MMCs and PMGs with fibrillar amyloid-β (Halle et al., 2008). These results further highlight the flexibility of MMCs, making them ideally suited for various in vitro paradigms. Collectively, these results support adopting MMCs to supplant BV2s as the model system of choice.
In conclusion, we demonstrate that the newly available MMC line is an excellent in vitro cell culture model that can appropriately supplant the widely used BV2 cell line for testing neurotoxic chemical-induced neuroinflammatory responses. MMCs mirror primary microglia exceptionally well when compared to BV2s. While unstimulated, they resemble a surveillant state of primary microglia, but under stimulation, their responsivity is remarkably similar to activated primary microglia. Their inherent sensitivity enables them to generate similar inflammatory mediators under various inflammatory stimuli that encompass classical, chronic, and environmental paradigms. Thus, MMCs are a better alternative to BV2s for studying the cellular and molecular mechanisms underlying microglial activation during neurotoxic insults.
Highlights.
BV2s have a high basal inflammatory state and are desensitized cells.
MMCs are more suitable than BV2s for studying neuro-inflammation and - toxicology.
MMCs are more similar to neonatal primary mouse microglia than BV2s.
Acknowledgements
This study was supported by NIH grants NS088206, ES026892, and NS100090. Special thanks to Dr. Golenbock of U. Mass Med School for providing the MMC cell line. The W. Eugene and Linda Lloyd Endowed Chair and Eminent Scholar to AGK and the Salisbury Endowed Chair to AK are also acknowledged. We also thank Mr. Gary Zenitsky for assistance in preparing this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
A.G.K. and V.A. are shareholders of PK Biosciences Corporation (Ames, IA), which is interested in identifying novel biomarkers and potential therapeutic targets for PD. A.G.K and V.A. do not have any commercial interests in the work presented in here.
References
- Belarbi K, Cuvelier E, Destee A, Gressier B, Chartier-Harlin MC, 2017. NADPH oxidases in Parkinson’s disease: a systematic review. Mol Neurodegener 12(1), 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasi E, Barluzzi R, Bocchini V, Mazzolla R, Bistoni F, 1990. Immortalization of murine microglial cells by a v-raf/v-myc carrying retrovirus. J Neuroimmunol 27(2-3), 229–237. [DOI] [PubMed] [Google Scholar]
- Block ML, Zecca L, Hong JS, 2007. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci 8(1), 57–69. [DOI] [PubMed] [Google Scholar]
- Clement HW, Vazquez JF, Sommer O, Heiser P, Morawietz H, Hopt U, Schulz E, von Dobschutz E, 2010. Lipopolysaccharide-induced radical formation in the striatum is abolished in Nox2 gp91phox-deficient mice. J Neural Transm (Vienna) 117(1), 13–22. [DOI] [PubMed] [Google Scholar]
- Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH, 2010. Mechanisms underlying inflammation in neurodegeneration. Cell. 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon R, Singh N, Lawana V, Ghosh A, Harischandra DS, Jin H, Hogan C, Sarkar S, Rokad D, Panicker N, Anantharam V, Kanthasamy AG, Kanthasamy A, 2016. Protein kinase Cdelta upregulation in microglia drives neuroinflammatory responses and dopaminergic neurodegeneration in experimental models of Parkinson's disease. Neurobiol Dis 93, 96–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halle A, Hornung V, Petzold GC, Stewart CR, Monks BG, Reinheckel T, Fitzgerald KA, Latz E, Moore KJ, Golenbock DT, 2008. The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat Immunol 9(8), 857–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallemeesch MM, Janssen BJ, de Jonge WJ, Soeters PB, Lamers WH, Deutz NE, 2003. NO production by cNOS and iNOS reflects blood pressure changes in LPS-challenged mice. Am J Physiol Endocrinol Metab 285(4), E871–875. [DOI] [PubMed] [Google Scholar]
- Heneka MT, Kummer MP, Stutz A, Delekate A, Schwartz S, Vieira-Saecker A, Griep A, Axt D, Remus A, Tzeng TC, Gelpi E, Halle A, Korte M, Latz E, Golenbock DT, 2013. NLRP3 is activated in Alzheimer's disease and contributes to pathology in APP/PS1 mice. Nature 493(7434), 674–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter RA, Storm WL, Coneski PN, Schoenfisch MH, 2013. Inaccuracies of nitric oxide measurement methods in biological media. Anal Chem 85(3), 1957–1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkley KS, Popichak KA, Afzali MF, Legare ME, Tjalkens RB, 2017. Microglia amplify inflammatory activation of astrocytes in manganese neurotoxicity. Journal of Neuroinflammation 14(1), 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwabara T, Imajoh-Ohmi S, 2004. LPS-induced apoptosis is dependent upon mitochondrial dysfunction. Apoptosis 9(4), 467–474. [DOI] [PubMed] [Google Scholar]
- Langley M, Ghosh A, Charli A, Sarkar S, Ay M, Luo J, Zielonka J, Brenza T, Bennett B, Jin H, Ghaisas S, Schlichtmann B, Kim D, Anantharam V, Kanthasamy A, Narasimhan B, Kalyanaraman B, Kanthasamy AG, 2017. Mito-Apocynin Prevents Mitochondrial Dysfunction, Microglial Activation, Oxidative Damage, and Progressive Neurodegeneration in MitoPark Transgenic Mice. Antioxid Redox Signal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawana V, Singh N, Sarkar S, Charli A, Jin H, Anantharam V, Kanthasamy AG, Kanthasamy A, 2017. Involvement of c-Abl Kinase in Microglial Activation of NLRP3 Inflammasome and Impairment in Autolysosomal System. J Neuroimmune Pharmacol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberatore GT, Jackson-Lewis V, Vukosavic S, Mandir AS, Vila M, McAuliffe WG, Dawson VL, Dawson TM, Przedborski S, 1999. Inducible nitric oxide synthase stimulates dopaminergic neurodegeneration in the MPTP model of Parkinson disease. Nat Med 5(12), 1403–1409. [DOI] [PubMed] [Google Scholar]
- Mander P, Brown GC, 2005. Activation of microglial NADPH oxidase is synergistic with glial iNOS expression in inducing neuronal death: a dual-key mechanism of inflammatory neurodegeneration. J Neuroinflammation 2, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moehle MS, West AB, 2015. M1 and M2 immune activation in Parkinson's Disease: Foe and ally? Neuroscience 302, 59–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa Y, Chiba K, 2014. Role of microglial m1/m2 polarization in relapse and remission of psychiatric disorders and diseases. Pharmaceuticals (Basel) 7(12), 1028–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacher P, Beckman JS, Liaudet L, 2007. Nitric oxide and peroxynitrite in health and disease. Physiol Rev 87(1), 315–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panicker N, Saminathan H, Jin H, Neal M, Harischandra DS, Gordon R,Kanthasamy K, Lawana V, Sarkar S, Luo J, Anantharam V, Kanthasamy AG, Kanthasamy A, 2015. Fyn Kinase Regulates Microglial Neuroinflammatory Responses in Cell Culture and Animal Models of Parkinson's Disease. J Neurosci 35(27), 10058–10077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Min JS, Kim B, Chae UB, Yun JW, Choi MS, Kong IK, Chang KT, Lee DS, 2015. Mitochondrial ROS govern the LPS-induced pro-inflammatory response in microglia cells by regulating MAPK and NF-kappaB pathways. Neurosci Lett 584, 191–196. [DOI] [PubMed] [Google Scholar]
- Sanchez-Guajardo V, Barnum CJ, Tansey MG, Romero-Ramos M, 2013. Neuroimmunological processes in Parkinson's disease and their relation to alpha-synuclein: microglia as the referee between neuronal processes and peripheral immunity. ASN Neuro 5(2), 113–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar S, Malovic E, Harischandra DS, Ngwa HA, Ghosh A, Hogan C, Rokad D, Zenitsky G, Jin H, Anantharam V, Kanthasamy AG, Kanthasamy A, 2017a. Manganese exposure induces neuroinflammation by impairing mitochondrial dynamics in astrocytes. Neurotoxicology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar S, Malovic E, Harishchandra DS, Ghaisas S, Panicker N, Charli A, Palanisamy BN, Rokad D, Jin H, Anantharam V, Kanthasamy A, Kanthasamy AG, 2017b. Mitochondrial impairment in microglia amplifies NLRP3 inflammasome proinflammatory signaling in cell culture and animal models of Parkinson's disease. NPJ Parkinsons Dis 3, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar S, Malovic E, Plante B, Zenitsky G, Jin H, Anantharam V, Kanthasamy A, Kanthasamy AG, 2017c. Rapid and Refined CD11b Magnetic Isolation of Primary Microglia with Enhanced Purity and Versatility. J Vis Exp(122). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schapansky J, Nardozzi JD, LaVoie MJ, 2015. The complex relationships between microglia, alpha-synuclein, and LRRK2 in Parkinson's disease. Neuroscience 302, 74–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo J, Ottesen EW, Singh RN, 2014. Antisense methods to modulate pre-mRNA splicing. Methods Mol Biol 1126, 271–283. [DOI] [PubMed] [Google Scholar]
- Solito E, Sastre M, 2012. Microglia function in Alzheimer's disease. Front Pharmacol 3, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DG, Lue LF, 2015. Immune phenotypes of microglia in human neurodegenerative disease: challenges to detecting microglial polarization in human brains. Alzheimers Res Ther 7(1), 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Chu CH, Stewart T, Ginghina C, Wang Y, Nie H, Guo M, Wilson B, Hong JS, Zhang J, 2015. alpha-Synuclein, a chemoattractant, directs microglial migration via H2O2-dependent Lyn phosphorylation. Proc Natl Acad Sci U S A 112(15), E1926–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]