Abstract
The purpose of this report is to analyze long-term clinical outcomes of patients exposed to plerixafor plus granulocyte colony-stimulating factor (G-CSF) for stem cell mobilization. This was a study of patients with non-Hodgkin’s lymphoma (NHL, n = 167) and multiple myeloma (MM, n = 163) who were enrolled in the long-term follow-up of 2 pivotal phase III studies (NCT00741325 and NCT00741780) of 240 μg/kg plerixafor plus 10 μg/kg G-CSF, or placebo plus 10 μg/kg G-CSF to mobilize and collect CD34+ cells for auto-HSCT. Overall survival (OS) and progression-free survival (PFS) were evaluated over a 5-year period following the first dose of plerixafor or placebo. Probability of OS was not significantly different in patients with NHL (64% [95% CI; 56, 71] versus 56% [95% CI; 44, 67]) or patients with MM (64% [95% CI; 54, 72] versus 64% [95% CI; 53, 73]) treated with plerixafor or placebo, respectively. Additionally, there was no statistically significant difference in the probability of PFS over 5 years between treatment groups in either NHL (50% [95% CI 44, 67] versus. 43% [95% CI 31, 54]) or MM (17% [95% CI 10, 24] versus 30% [21, 40]) treated with plerixafor or placebo, respectively. In this long-term follow-up report, the addition of plerixafor to G-CSF for stem cell mobilization did not affect 5-year survival in patients with NHL or MM.
Keywords: plerixafor, multiple myeloma, non-hodgkin’s lymphoma, long-term follow-up, stem cell mobilization
INTRODUCTION
High-dose chemotherapy combined with autologous hematopoietic stem cell transplantation (auto-HSCT) is the standard of care for patients with relapsed or chemosensitive non-Hodgkin’s lymphoma (NHL) and multiple myeloma (MM) [1-3].
Auto-HSCT improves hematologic recovery in patients by reconstituting hematopoiesis following high-dose chemotherapy [3]. In patients with relapsed or chemosensitive NHL, high-dose chemotherapy with auto-HSCT has been shown to increase disease-free survival (DFS) [4], while in MM, a combination of high-dose chemotherapy with auto-HSCT improves progression-free survival (PFS) and overall survival (OS) [5,6]. The minimum number of cells generally acceptable for transplantation is ≥2 × 106 CD34+ cells/kg [7]. Transplanting less than this number of cells may result in delayed engraftment of both platelets and neutrophils [8]. The target number of cells for a single transplant was defined by Weaver et al. as ≥5 × 106 CD34+ cells/kg [9], which is important for short-term outcomes, results in earlier and more consistent neutrophil and especially platelet engraftment than transplantation with lower cell doses [10]. In some studies, transplant doses of ≥5 × 106 CD34+ cells/kg or higher have also been associated with longer DFS and OS than lower transplant doses [11-13]. One recent retrospective analysis comparing patients with NHL mobilized with chemotherapy plus granulocyte colony-stimulating factor (G-CSF) (median 12.0 × 106 CD34+ cells/kg) versus G-CSF (median 5.0 × 106 CD34+ cells/kg) did not show any difference between the groups in either event-free survival or OS [14]. It should be noted that, for patients with MM, subsequent or tandem auto-HSCT can also be considered [15].
Obtaining a sufficient quantity of cells for auto-HSCT is, however, difficult in approximately 20% to 25% of patients [3,16-18]. These include patients with NHL, elderly patients [19], heavily pretreated patients [20-22], and patients with MM who have previously received multiple cycles of lenalidomide or undergone auto-HSCT [23].
Employing an effective stem cell mobilization regimen, therefore, plays a critical role in optimizing engraftment and outcomes in patients with NHL and MM. Until recently there were two main approaches to stem cell mobilization that involved the use of growth factors, such as G-CSF alone or in conjunction with chemotherapy [24]. The administration of chemotherapy before the use of G-CSF produces a higher yield of stem cells for autologous transplantation, but this is still not effective for all patients [24].
Plerixafor is a CXCR4 receptor antagonist that is used in combination with G-CSF to mobilize hematopoietic stem cells into the peripheral blood (PB) for collection and subsequent auto-HSCT in patients with lymphoma and MM [25,26]. Plerixafor selectively and reversibly antagonizes the CXCR4 chemokine receptor and blocks binding of stromal cell-derived factor-1α (SDF-1α) [27]. The interruption of the CXCR4/SDF-1α interaction provides a mechanism for mobilization of CD34+ stem cells from the bone marrow to the PB where they can be collected for auto-HSCT. Plerixafor provides another option for transplantation, and G-CSF plus plerixafor augments mobilization of CD34+ cells, particularly in patients who are considered poor mobilizers [28-30]. The stem cells mobilized in apheresis products by the combination of G-CSF plus plerixafor have been shown to differ from those mobilized by G-CSF alone, with a higher proportion of cells in the growth phase, higher numbers of B and T lymphocytes, natural killer cells, dendritic cells and primitive CD34+ cells [24,31-35].
Each of the two pivotal, multicenter, randomized, double-blind, placebo-controlled phase III studies evaluated the efficacy and safety of plerixafor plus G-CSF, versus placebo plus G-CSF, in mobilizing and collecting hematopoietic stem cells. The trials demonstrated that plerixafor plus G-CSF can generate optimal numbers of cells for auto-HSCT in patients with NHL and MM [36,37].
Findings from the NHL study (Study 3101; NCT00103610) showed that, compared with placebo plus G-CSF, plerixafor plus G-CSF significantly increased the percentage of patients in whom the target stem cell collection (≥5 × 106 CD34+ cells/kg) was achieved (20% versus 59%) within 4 apheresis days [36].
Findings from the MM study (Study 3102; NCT00103662) also showed that plerixafor plus G-CSF significantly increased the percentage of patients in whom the target stem cell collection (≥6 × 106 CD34+ cells/kg to facilitate tandem transplantation) was achieved within 2 apheresis days, compared with placebo plus G-CSF (72% versus 34%) [37]. In both studies, auto-HSCT after mobilization with plerixafor and placebo resulted in successful engraftment of neutrophils and platelets. Durability of grafts was similar for plerixafor and placebo through 12 months follow-up, and both regimens were associated with similar survival rates at 12 months post-transplantation [36,37]. There is no evidence to date of a difference in the time to successful neutrophil or platelet engraftment, or for differences in adverse events during stem cell mobilization, or for mortality at 12 months, between G-CSF plus plerixafor compared with G-CSF alone [18,38,39].
To examine clinical outcomes beyond 1 year, a long-term, observational study of patients with NHL and MM enrolled in each of the phase III pivotal studies was undertaken. The study assessed OS and PFS over a 5-year period following the first dose of study drug (ie, plerixafor or placebo) administered in studies 3101 and 3102. Further, since the use of autologous PB stem cells for transplantation could be associated with the risk of contamination of the graft with tumor cells [40], the assessment of OS and PFS may serve as a surrogate for determining the risk that plerixafor may have in mobilizing malignant cells from the bone marrow during hematopoietic stem cell mobilization. Very limited data from small numbers of myeloma and lymphoma patients indicate that tumor cell trafficking to the peripheral blood is not significantly increased after mobilization with G-CSF plus plerixafor compared with G-CSF alone, in MM and NHL patients [41,42]. It should be also noted that the clinical significance of tumor cell mobilization or of myeloma tumor cell contamination of mobilized apheresis products on long-term outcomes is unclear.
METHODS
Study Design
This study was a long-term, observational follow-up (hereafter referred to as the ‘LTF study’) to the 2 phase III, multicenter, randomized, double-blind, placebo-controlled, comparative trials of plerixafor plus G-CSF (hereafter referred to as ‘plerixafor’) versus placebo plus G-CSF (hereafter referred to as ‘placebo’) to mobilize and collect CD34+ cells for auto-HSCT in patients with NHL (Study 3101) [36], and patients with MM (Study 3102) [37]. Patients were eligible if they previously received at least 1 dose of study treatment (placebo or plerixafor) in either study and had a signed informed consent for follow-up data collection. Patients who failed to mobilize or who did not achieve at least 2 × 106CD34+ cells/kg in 4 or less days of apheresis after treatment with either placebo or plerixafor were allowed to enter a “rescue” procedure. These patients received open-label plerixafor with the aim of collecting a transplantable dose of CD34+ cells, and were assigned to the plerixafor treatment group for analysis. Data were collected for OS evaluation in studies 3101 and 3102 at 100 days, 6 months, and 12 months following auto-HSCT. The initial objective of the LTF study was to assess OS over a 5-year period following administration of the first dose of plerixafor or placebo in studies 3101 and 3102, and was later expanded to include PFS.
These studies were registered at www.clinicaltrials.gov as #NCT00741325 and #NCT00741780 (3101-LTF and 3102-LTF, respectively) and conducted in accordance with ICH GCP, the principles specified in the Declaration of Helsinki and its amendments, and all applicable national and international laws. All patients who had consented and received at least 1 dose of plerixafor or placebo in studies 3101 and 3102 were eligible for inclusion in the LTF study. After consenting to long-term data collection, patients were free to withdraw consent or discontinue participation at any time at the discretion of the investigator or sponsor. There were no formal exclusion criteria.
Endpoints
The primary endpoint analysis was OS and PFS. This analysis included all patients enrolled in the 3101 and 3102 studies, and patients enrolled in the LTF studies. Data for survival and disease state were collected up to 5 years after the first study drug treatment administered in studies 3101 and 3102. Data from patients who did not enroll for the LTF study was included up to either their last reported follow-up date during the original 3101 and 3012 study periods, or until the date of their LTF study registration form.
Overall survival was defined as the time from the date of first study drug exposure (placebo or plerixafor) until the date of death due to any cause. PFS was defined as the time from the date of first study drug exposure until the date of reported disease progression, disease relapse, or death due to any cause, whichever occurred first. If a disease progression/relapse or death event occurred, but the date of the event was missing, the date of follow-up contact at which the event was reported was used as the best approximation.
PFS was determined utilizing Revised Response Criteria for Malignant Lymphoma [43] and the International Uniform Response Criteria for Multiple Myeloma [44]. Time points and testing requirements were not specified for assessment of PFS, and data were not collected in the case report form to document the determination of PFS.
Long-Term Follow-Up Schedule
The LTF schedule was determined by whether or not the patient underwent auto-HSCT during studies 3101 and 3102. For patients who underwent auto-HSCT (including those who withdrew or were lost to follow-up), follow-up began at 18 months post auto-HSCT or at LTF study entry (ie, signed informed consent) and occurred every 6 months (± 3 months) for 5 years after the first dose of study treatment. For patients who did not undergo auto-HSCT in studies 3101 or 3102, follow-up occurred every 6 months (± 3 months) for a total follow-up period of 5 years following the first dose of study treatment (placebo or plerixafor).
Data Collection
For this observational study, data for disease progression/relapse and death were collected from the original study data for 3101 and 3102, LTF study registration forms, and the LTF study itself. Data from patients who did not enroll in the LTF study were limited to that collected during studies 3101, 3102, and/or the LTF study registration form.
Assessments
The principal population assessed for efficacy was the primary intent-to-treat (ITT) population, which consisted of all randomized patients. Data analyzed for the ITT population were based on the actual randomization assignment. The Per Protocol population consisted of all ITT patients who received any fraction of study treatment (plerixafor or placebo), completed the apheresis period, and did not have any major protocol deviations that significantly impacted the assessment of efficacy and included all patients who had received at least 1 dose of study drug or placebo, as well as those patients who failed stem cell collection on study and had elected to enter the ‘rescue protocol’.
Death and disease progression/relapse status was captured at study entry and at each follow-up contact, which occurred every 6 months until 5 years from the date of first dose. In addition, the investigators or treating physicians were encouraged to record any additional treatments (eg, transplant, chemotherapy, or radiotherapy) received since the last study contact.
Not all sites provided information on the nature of additional therapy and its reason for administration. Patients could have received maintenance/adjuvant therapy either in another study or as a result of study site clinical practice, and it was necessary to evaluate the effect of presumed maintenance/adjuvant treatment on disease progression rates. Consequently, a post hoc analysis was performed using a “composite (triple) endpoint” event, defined by death (yes or no), progression/relapse (yes or no), or introduction of any additional treatments for the management of MM, whichever occurred first.
Statistical Analysis
Overall survival, PFS and the “composite (triple) endpoint” were estimated using the Kaplan-Meier (KM) method. The 25th, 50th (median), and 75th OS and PFS percentiles (along with 95% confidence intervals [CI] for these quartiles, if estimable) were determined. The OS and PFS probabilities along with 95% CI at 12, 24, 36, 48, and 60 months were estimated from the KM method. The log-rank test and the Wilcoxon test (Breslow test) were used to compare treatment groups (plerixafor versus placebo). The statistical analysis system was used to perform all analyses. When inferential statistics were performed, a p-value of <0.05 was considered to be statistically significant. The p-values of all tests are reported without any correction for the multiplicity of tests performed.
Safety assessments of adverse events other than death or progression were not collected for this observational study.
RESULTS
Patient Disposition and Baseline Disease Characteristics
The disposition of patients with NHL and MM included in the LTF study is described in Figure 1 and Figure 2, respectively. Patients from Study 3101 and 3102 were included in the LTF study if they enrolled for the study, or for some analyses (OS and PFS), data were included from patients who had died before the initiation of the study.
Figure 1.
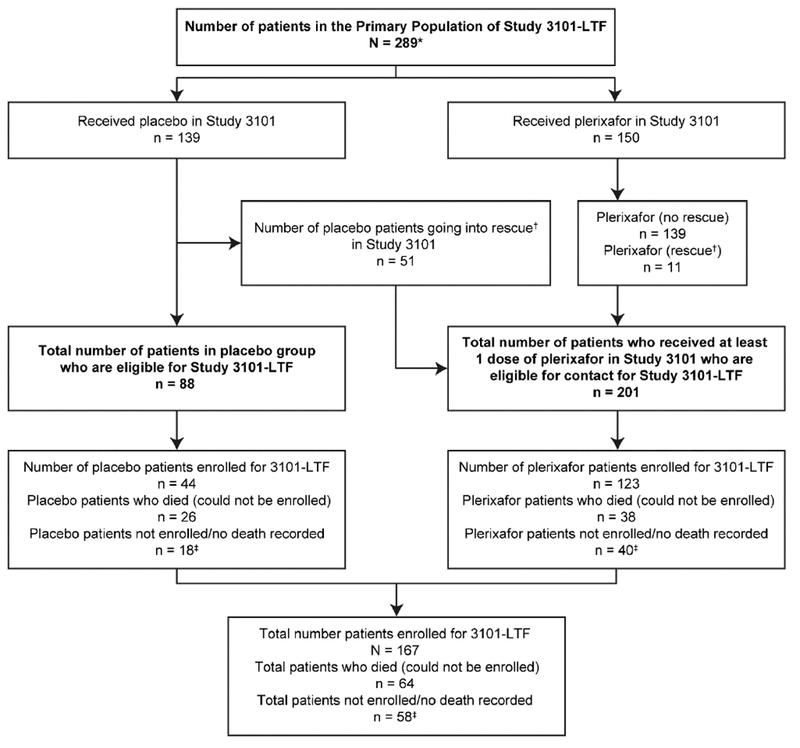
Disposition of patients with NHL included in the 3101 LTF study
*‘Does not include 13 patients who received rituximab (patients were part of a Study 3101 substudy) or 9 patients who withdrew prior to receiving plerixafor or placebo.
†All rescue patients received plerixafor.
‡Patients censored in the OS analysis at last date where data were available.
Figure 2.
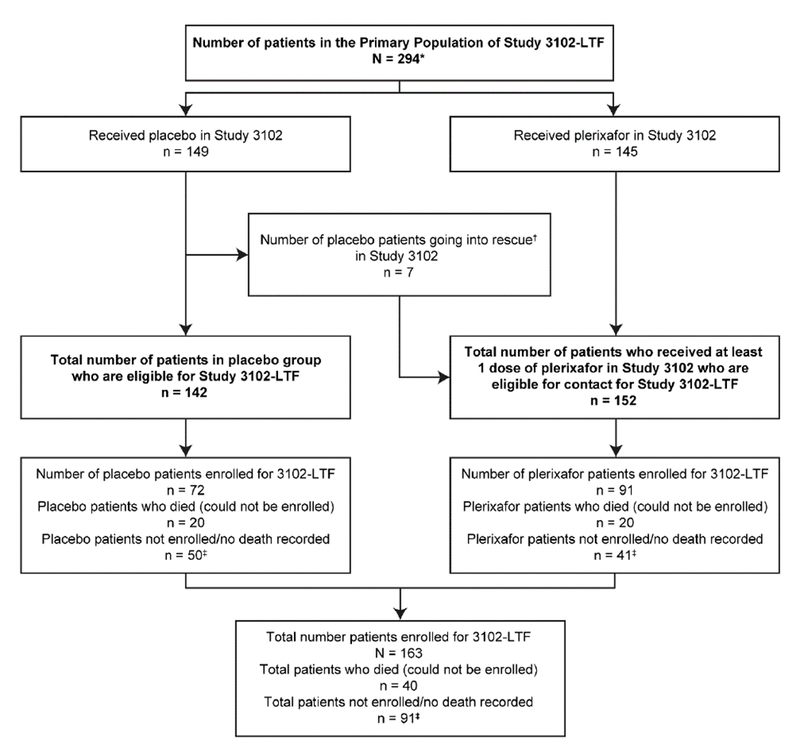
Disposition of patients with MM included in the 3102 LTF study
* ‘Does not include the 1 patient who received post-transplant cyto-reductive chemotherapy treatment (patient 25-401) or 8 patients who withdrew prior to receiving plerixafor or placebo.
†All rescue patients received plerixafor.
‡Patients censored in the OS analysis at last date where data were available.
From the original 289 patients with NHL from the Study 3101, a total of 167 (58%) were enrolled in the LTF study. Of these 167 patients, 44 (26%) had received placebo and 123 (74%) had received plerixafor either initially or following rescue. Of the 122 patients (42%) who did not enter the LTF study, 64 (26 placebo, 38 plerixafor), had died before initiation of the LTF study, 31 (22 plerixafor) had been lost to follow-up, 21 (14 plerixafor) refused to participate and 6 (4 plerixafor) patients from sites that declined to participate in the study.
Of the 294 patients with MM in Study 3102, 163 (55%) enrolled in the LTF study. Of these 163 patients 72 received placebo and 91 received plerixafor either as allocated treatment (85) or as a rescue (6). The remaining 131 (45%) patients who did not enroll in the LTF study comprised 40 who had died during and after Study 3102, 45 patients were lost to follow-up during or after Study 3102, 27 patients were at sites that declined to participate in the study, and 19 patients refused to participate.
Demographics and baseline disease characteristics for patients with NHL and MM who enrolled in the LTF study, stratified by treatment group, are summarized in Table 1 and Table 2, respectively. Demographics and baseline data for patients with NHL and MM who enrolled in the original 3101 and 3102 studies are summarized in Supplementary Table 1 and Supplementary Table 2, respectively.
Table 1.
Demographics and Baseline Characteristics of Patients With NHL Enrolled in the LTF Study
| Variable/Statistics | Placebo (n = 44) | Plerixafor† (n = 123) | Total (n = 167) |
|---|---|---|---|
| Sex, n (%) | |||
| Male | 29 (66) | 85 (69) | 114 (68) |
| Female | 15 (34) | 38 (31) | 53 (32) |
| Ethnic origin, n (%) | |||
| Caucasian | 43 (98) | 118 (96) | 161 (96) |
| African-American | 0 | 1 (1) | 1 (1) |
| Asian | 1 (2) | 1 (1) | 2 (1) |
| Hispanic/Latino | 0 | 3 (2) | 3 (2) |
| Other | 0 | 0 | 0 |
| Age (y) | |||
| Mean (SD) | 58.4 (10.26) | 57.1 (9.83) | 57.4 (9.93) |
| Median | 58.5 | 59.0 | 59.0 |
| Min, Max | 27, 74 | 29, 75 | 27, 75 |
| Weight (kg) | |||
| Mean (SD) | 87.5 (21.95) | 87.3 (18.82) | 87.4 (19.63) |
| Median | 87.8 | 85.6 | 86.3 |
| Min, Max | 56.5, 178.0 | 48.6, 159.6 | 48.6, 178.0 |
| Underwent auto-HSCT following treatment and apheresis, n (%) | 42 (95) | 121 (98) | 163 (98) |
| Disease stage at baseline*, n (%) | |||
| I | 1 (2) | 6 (5) | 7 (4) |
| II | 8 (18) | 12 (10) | 20 (12) |
| III | 14 (32) | 28 (23) | 42 (25) |
| IV | 13 (30) | 54 (44) | 67 (40) |
| Missing | 8 (18) | 23 (19) | 31 (19) |
| Remission status at baseline*, n (%) | |||
| First/second CR | 25 (57) | 70 (57) | 95 (57) |
| Relapse/second PR | 17 (38) | 53 (43) | 70 (42) |
| Missing | 2 (5) | 0 | 2 (1) |
Baseline represents the point immediately before study drug was administered.
The plerixafor group included the patient initially randomized to placebo and subsequently underwent rescue with plerixafor.
Abbreviations: CR, complete remission; HSCT, hematopoietic stem cell transplantation; PR, partial remission; SD, standard deviation.
Table 2.
Demographics and Baseline Characteristics of Patients With MM Enrolled in the LTF Study
| Variable/Statistics | Placebo (n = 72) | Plerixafor (n = 91) | Total (n = 163) |
|---|---|---|---|
| Sex, n (%) | |||
| Male | 53 (74) | 64 (70) | 117 (72) |
| Female | 19 (26) | 27 (30) | 46 (28) |
| Ethnic origin, n (%) | |||
| Caucasian | 60 (83) | 73 (80) | 133 (82) |
| African-American | 6 (8) | 11 (12) | 17 (10) |
| Asian | 1 (1) | 1 (1) | 2 (1) |
| Hispanic/Latino | 2 (3) | 5 (5) | 7 (4) |
| Other | 3 (4) | 1 (1) | 4 (2) |
| Age (y) | |||
| Mean (SD) | 59.3 (9.06) | 59.6 (8.18) | 59.4 (8.56) |
| Median | 62.0 | 60.0 | 61.0 |
| Min, Max | 28, 74 | 37, 76 | 28, 76 |
| Weight (kg) | |||
| Mean (SD) | 87.3 (18.51) | 84.4 (18.48) | 85.7 (18.49) |
| Median | 85.1 | 84.5 | 84.8 |
| Min, Max | 53.1, 130.6 | 48.1, 135.5 | 48.1, 135.5 |
| Underwent auto-HSCT following treatment and apheresis, n (%) | 72 (100) | 91 (100) | 163 (100) |
| Disease stage at baseline*, n (%) | |||
| I | 8 (11) | 15 (16) | 23 (14) |
| II | 18 (25) | 16 (18) | 34 (21) |
| III | 40 (56) | 50 (55) | 90 (55) |
| IV | 0 | 0 | 0 |
| Missing | 6 (8) | 10 (11) | 16 (10) |
| Remission status at baseline*, n (%) | |||
| First/second CR | 11 (15) | 8 (9) | 19 (12) |
| Relapse/second PR | 61 (85) | 83 (91) | 144 (88) |
| Missing | 0 | 0 | 0 |
Baseline represents the point immediately before study drug was administered.
The plerixafor group included the patient initially randomized to placebo and subsequently underwent rescue with plerixafor.
Abbreviations: CR, complete remission; HSCT, hematopoietic stem cell transplantation; PR, partial remission; SD, standard deviation.
The majority of patients who enrolled in the LTF study were male (NHL, 68%; MM, 72%); mean age was 57.4 years (standard deviation [SD], 9.9 years) in the NHL patient population and 59.4 years (SD, 8.6 years) in the MM group. Demographics were similar when stratified by treatment group and rescue for both NHL and MM groups. The most common baseline disease characteristics in the NHL population were Stage III disease (32%) and Stage IV disease (30%) in the placebo group, and Stage IV disease (44%) in the plerixafor group. The most common remission status at baseline was the first complete remission (CR) for placebo- (43%) and plerixafor-treated (31%) patients. The majority of patients with MM who enrolled in the LTF study had Stage III disease in both plerixafor (55%) and placebo (56%) groups, and most had a remission status of first partial remission (PR) at baseline (82%).
Overall Survival
All 289 patients with NHL and 294 patients with MM from the Studies 3101 and 3102, respectively, contributed to the OS and PFS analyses. Patients who did not enroll in the LTF study were censored on either the date of their LTF study registration form or by their last reported follow-up date within the 3101 or 3102 study periods.
Based on KM analysis, median OS was not reached within 5 years of follow-up for either the placebo or plerixafor group (Figure 3a). In the NHL population, 93 of 289 (32%) of patients died, 32 of 88 (36%) patients in the placebo group and 61 of 201 (30%) patients in the plerixafor group. There was no statistically significant difference in OS over 5 years between the placebo- and plerixafor-treated patients (log-rank, P = .273; Wilcoxon, P = .308). Findings were similar for rescue and non-rescue patients (data not presented). The estimated 12, 24, 36, 48, and 60 month OS probabilities for the placebo and plerixafor group are shown in Table 3.
Figure 3.
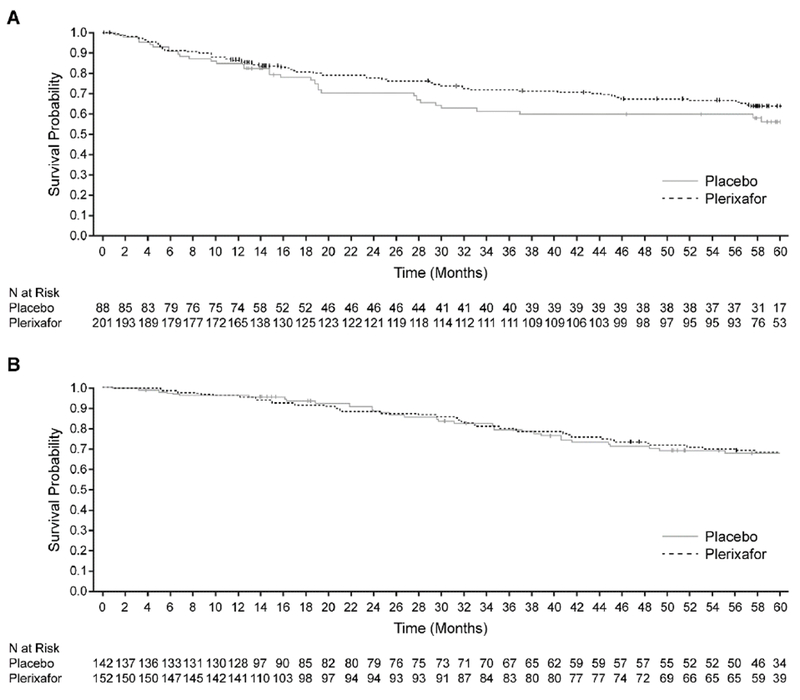
Overall survival in patients with NHL (A) and MM (B) stratified by treatment group
Table 3.
Kaplan-Meier (KM) Overall Survival, Progression-Free Survival, and Triple-Endpoint Survival Probabilities at 12, 24, 36, 48, and 60 Months
| NHL | MM | |||
|---|---|---|---|---|
| Placebo | Plerixafor | Placebo | Plerixafor | |
| Overall Survival (OS) | ||||
| KM 12 month OS Probability, % (95% CI) | 85 (76, 91) | 87 (81, 91) | 96 (91, 98) | 95 (90, 98) |
| KM 24 month OS Probability, % (95% CI) | 71 (59, 80) | 78 (71, 83) | 87 (79, 92) | 87 (79, 92) |
| KM 36 month OS Probability, % (95% CI) | 61 (49, 72) | 72 (65, 78) | 77 (67, 84) | 77 (69, 84) |
| KM 48 month OS Probability, % (95% CI) | 60 (48, 70) | 68 (60, 74) | 67 (57, 76) | 70 (60, 77) |
| KM 60 month OS Probability, % (95% CI) | 56 (44, 67) | 64 (56, 71) | 64 (53, 73) | 64 (54, 72) |
| Progression-Free Survival (PFS) | ||||
| KM 12 month PFS Probability, % (95% CI) | 72 (62, 81) | 75 (68, 80) | 88 (81, 92) | 83 (76, 88) |
| KM 24 month PFS Probability, % (95% CI) | 66 (55, 76) | 66 (58, 72) | 63 (53, 72) | 57 (48, 65) |
| KM 36 month PFS Probability, % (95% CI) | 54 (42, 65) | 58 (50, 65) | 46 (35, 55) | 38 (30, 47) |
| KM 48 month PFS Probability, % (95% CI) | 48 (36, 59) | 54 (46, 61) | 34 (25, 44) | 24 (17, 32) |
| KM 60 month PFS Probability, % (95% CI) | 43 (31, 54) | 50 (42, 58) | 30 (21,40) | 17 (10, 24) |
| Triple-Endpoint Survival (TES) | ||||
| KM 12 month TES Probability, % (95% CI) | 60 (49, 69) | 60 (52, 66) | 64 (56, 72) | 64 (56, 71) |
| KM 24 month TES Probability, % (95% CI) | 55 (44, 65) | 49 (42, 56) | 43 (34, 53) | 42 (33, 50) |
| KM 36 month TES Probability, % (95% CI) | 44 (33, 55) | 41 (34, 49) | 28 (19, 38) | 27 (20, 35) |
| KM 48 month TES Probability, % (95% CI) | 38 (27, 49) | 40 (32, 47) | 19 (11, 28) | 16 (10, 24) |
| KM 60 month TES Probability, % (95% CI) | 31 (20, 42) | 37 (29, 44) | 14 (7, 23) | 11 (6, 17) |
In the MM population, 76 of 294 (26%) patients died, 35 of 142 (25%) patients in the placebo group and 41 of 152 (27%) patients in the plerixafor group. Median OS was not reached for either the placebo or plerixafor groups within 5 years of follow-up (Figure 3b) and there was no statistically significant difference in OS over 5 years between the placebo- and plerixafor-treated patients (log-rank, P = .936; Wilcoxon P = .970). The estimated 12, 24, 36, 48, and 60 month OS probabilities for each treatment group are listed in Table 3.
Progression-Free Survival
Overall, 129 of 289 (45%) patients with NHL reported a PFS event, 43 of 88 (49%) patients in the placebo group and 86 of 201 (43%) patients in the plerixafor group. Median PFS was reached for the placebo group at 39 months but was not reached for the plerixafor group within 5 years of follow-up. However, the assessment of PFS over 5 years (Figure 4a) showed no significant difference between treatment groups (log-rank, P = .343; Wilcoxon, P = .396). The estimated 12, 24, 36, 48, and 60 month PFS probabilities for the placebo and plerixafor groups are summarized in Table 3. One case of acute myeloid leukemia (AML) was observed in an NHL patient who had received plerixafor plus G-CSF but was considered by the treating physician to be unrelated to plerixafor.
Figure 4.
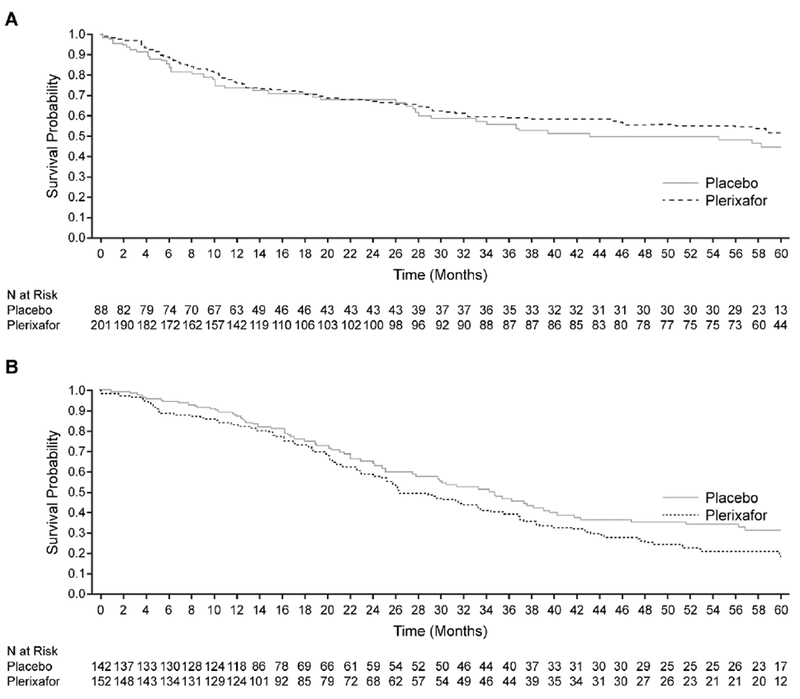
Progression-free survival in patients with NHL (A) and MM (B) stratified by treatment group
A progression-free survival (PFS) event was defined as a report of disease progression, disease relapse, or death due to any cause. Assessment of PFS over 5 years showed no statistically significant difference in patients with non-Hodgkin’s lymphoma (A) (log-rank, P = .343; Wilcoxon, P = .396) or multiple myeloma (MM) (B) between the 2 treatment groups. In patients with MM, a non-significant (log-rank, P = .061; Wilcoxon, P = .138) trend toward shorter PFS in patients treated with plerixafor than in those receiving placebo.
In the MM patient population, 172 of 294 (59%) patients reported a PFS event, 70 of 142 (49%) patients in the placebo group and 102 of 152 (67%) patients in the plerixafor group. Median PFS was reached at 34 months for the placebo group and at 26 months for the plerixafor group. A trend towards a shorter PFS for patients treated with plerixafor than for those receiving placebo (Figure 4b) was observed (log-rank, P = .061; Wilcoxon, P = .138). Table 3 shows the estimated 12, 24, 36, 48, and 60 month PFS probabilities for each treatment group. There were no cases of secondary MDS or AML among patients with MM who had received plerixafor plus G-CSF.
Composite Endpoint Analysis
During the period covering the end of recruitment to the phase III studies and the observational phase data analysis, several large studies were conducted to evaluate the possible benefit of adding some form of maintenance or adjuvant therapy post transplantation to attempt to improve the overall outcome for patients with MM. To evaluate this effect on studies 3101, 3102 and the LTF study, a “composite (triple) endpoint” analysis was undertaken (Supplementary Figures 1 and 2).
Overall, 166 of 289 (57%) patients with NHL had a triple endpoint event; 53 of 88 (60%) patients in the placebo group and 113 of 201 (56%) patients in the plerixafor group. Median triple endpoint was reached at 28 months and 24 months for the placebo and plerixafor groups, respectively. No significant difference between placebo and plerixafor was noted (log-rank, P = .702; Wilcoxon P = .836). Table 3 summarizes the estimated 12, 24, 36, 48, and 60 month triple endpoint survival probabilities for plerixafor and placebo treatment groups.
In the MM patient population, 207 of 294 (70%) patients had a triple endpoint event; 91 of 142 (64%) patients in the placebo group and 116 of 152 (76%) patients in the plerixafor group. Based on KM analysis, median triple endpoint was reached at 20 months for both treatment groups. There was no significant difference between placebo and plerixafor for the triple endpoint (log-rank, P = .752, Wilcoxon, P = .944). The estimated 12, 24, 36, 48, and 60 month triple endpoint survival probabilities for both treatment groups are described in Table 3.
DISCUSSION
This is the first long-term observational study to assess OS and PFS in patients with NHL and MM for a period of 5 years following the first dose of study treatment (plerixafor or placebo) in studies 3101 and 3102. The results from this LTF study suggest that the addition of plerixafor to G-CSF did not have a detrimental effect on long-term survival.
The main limitation of this study was that it was observational in nature. Additionally, only 58% of patients with NHL and 55% of patients with MM enrolled for the LTF study. However, as data from patients who had previously died was included within the analysis, 80% of patients with NHL and 69% of patients with MM contributed to data for OS and PFS. The finding that PFS data trended in opposite directions for patients with NHL and MM was difficult to interpret. The progression analyses contained limitations associated with data collection and reporting during the study period, making determination of true progression and progression timeline difficult to ascertain. There was also an imbalance in patients who achieved complete remission following ablative therapy in studies 3101 and 3102. Progression data may also have been confounded by inconsistent reporting of additional treatments used for maintenance or disease recurrence to improve progression-free intervals [45,46]. However, findings from the “composite (triple) endpoint analysis” which took into account any reported potential maintenance medication, showed no statistically significant difference in the incidence of “triple endpoint” events between plerixafor and placebo groups. A further limitation of the study is that the sample size provided limited power, and could only detect a relatively large difference between the two groups.
Evaluating differences in OS and PFS between treatment groups in the present investigation also served as a surrogate for the risk of potential detrimental outcome of the mobilization of malignant cells from the bone marrow during hematopoietic stem cell mobilization for auto-HCST. Our finding that there was no statistically significant difference in OS or PFS following treatment with plerixafor plus G-CSF versus placebo G-CSF, would seem to suggest that plerixafor does not exert long-term deleterious effects as a result of tumor cell mobilization. This observation is consistent with findings from previous investigations that have demonstrated that plerixafor does not contribute to tumor cell mobilization any more than G-CSF alone. A registry study is also being undertaken in collaboration with the European Group for Blood and Marrow Transplant to compare the outcomes of patients transplanted with plerixafor mobilized and non-plerixafor-mobilized hematopoietic stem cells.
Other studies examining the effect of stem cell mobilization agents for auto-HSCT on long-term disease outcomes in patients with NHL and patients with MM are limited, and the paucity of long-term follow-up studies addressing OS and PFS in patients with NHL and MM following stem cell mobilization and auto-HSCT highlights the importance of the present study. Although the scarcity of published data restricts direct comparison of this study to others, a retrospective analysis that evaluated long-term outcomes of plerixafor plus G-CSF, and G-CSF alone in patients with MM and lymphoma (NHL and Hodgkin’s lymphoma), showed that the median PFS in the plerixafor plus G-CSF group was 22.5 months in patients with MM, comparable to the present study, and median OS was 40 months. However, the authors did not compare OS and PFS between plerixafor plus G-CSF, and G-CSF alone because of the retrospective nature of the study and the small sample size [15]. Within the wider context of other studies examining long-term outcomes following auto-HSCT, our findings, which show that more than one-half of patients remained alive 5 years following transplantation, echo those of several previous studies, ie, that auto-HSCT is associated with extended OS [5,6,46].
In conclusion, though limited by its observational design, the results of this follow-up study suggest that the use of plerixafor plus G-CSF does not have a negative outcome on the overall survival and progression free survival at 5 years in these patients with NHL or MM.
Supplementary Material
Highlights.
Addition of plerixafor to G-CSF did not affect clinical or LT safety outcomes
No differences were noted in 5 year OS/PFS for plerixafor treated patients
No difference in composite endpoint of death, progression, or additional treatment
ACKNOWLEDGMENTS
The study was sponsored by Sanofi Oncology, Cambridge, Massachusetts, USA. Peter D. Cheverton was the clinical lead, and along with Martin Struijs, was responsible for analyzing the data. Rita Vargo was responsible for clinical trial management. This study was conducted by the following investigators: Ivana N. Micallef, Patrick J. Stiff, Auayporn P. Nademanee, Richard T. Maziarz, Mitchell E. Horwitz, Edward A. Stadtmauer, Jonathan L. Kaufman, John M. McCarty, Brian Bolwell, and John F. DiPersio. All authors had full access to the data and contributed to the development of the manuscript. The authors thank the nurse coordinators and patients who participated in this study. Editorial support, funded by Sanofi, was provided by MedLogix Communications, LLC, Schaumburg, Illinois, USA, and Envision Scientific Solutions, Horsham, West Sussex, UK.
FINANCIAL DISCLOSURES
Ivana N. Micallef received research support from Sanofi and was an uncompensated advisory board member; Patrick J. Stiff received research support from Sanofi. Mitchell Horwitz, Richard Maziarz and John McCarty also received research support and honoraria for advisory board participation and speaking engagements from Sanofi. Peter D. Cheverton, Martin Struijs and Rita Vargo were employees of Sanofi at the time of the study. The remaining authors have nothing to disclose.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Bensinger W, DiPersio JF, McCarty JM. Improving stem cell mobilization strategies: future directions. Bone Marrow Transplant. 2009;43:181–195. [DOI] [PubMed] [Google Scholar]
- 2.Flomenberg N, DiPersio J, Calandra G. Role of CXCR4 chemokine receptor blockade using AMD3100 for mobilization of autologous hematopoietic progenitor cells. Acta Haematol. 2005;114:198–205. [DOI] [PubMed] [Google Scholar]
- 3.Josefsen D, Rechnitzer C, Parto K, Kvalheim G. The use of Plerixafor for peripheral blood stem cell mobilisation reduces the frequency of mobilisation failure in patients planned to undergo autologous transplantation. European Haematology. 2010;4:24–29. [Google Scholar]
- 4.Sehn LH, Fenske TS, Laport GG. Follicular lymphoma: prognostic factors, conventional therapies, and hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2012;18:S82–91. [DOI] [PubMed] [Google Scholar]
- 5.Child JA, Morgan GJ, Davies FE, et al. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med 2003;348:1875–1883. [DOI] [PubMed] [Google Scholar]
- 6.Wach M, Cioch M, Hus M, et al. Treatment of multiple myeloma patients with autologous stem cell transplantation - a fresh analysis. Folia Histochem Cytobiol 2011;49:248–254. [DOI] [PubMed] [Google Scholar]
- 7.Jillella AP, Ustun C. What is the optimum number of CD34+ peripheral blood stem cells for an autologous transplant? Stem Cells Dev 2004;13:598–606. [DOI] [PubMed] [Google Scholar]
- 8.Stockerl-Goldstein KE, Reddy SA, Horning SF, et al. Favorable treatment outcome in non-Hodgkin’s lymphoma patients with “poor” mobilization of peripheral blood progenitor cells. Biol Blood Marrow Transplant. 2000;6:506–512. [DOI] [PubMed] [Google Scholar]
- 9.Weaver CH, Hazelton B, Birch R, et al. An analysis of engraftment kinetics as a function of the CD34 content of peripheral blood progenitor cell collections in 692 patients after the administration of myeloablative chemotherapy. Blood. 1995;86:3961–3969. [PubMed] [Google Scholar]
- 10.Shpall EJ, Champlin R, Glaspy JA. Effect of CD34+ peripheral blood progenitor cell dose on hematopoietic recovery. Biol Blood Marrow Transplant. 1998;4:84–92. [DOI] [PubMed] [Google Scholar]
- 11.Blystad AK, Delabie J, Kvaloy S, et al. Infused CD34 cell dose, but not tumour cell content of peripheral blood progenitor cell grafts, predicts clinical outcome in patients with diffuse large B-cell lymphoma and follicular lymphoma grade 3 treated with high-dose therapy. Br J Haematol 2004;125:605–612. [DOI] [PubMed] [Google Scholar]
- 12.Bolwell BJ, Pohlman B, Rybicki L, et al. Patients mobilizing large numbers of CD34+ cells (‘super mobilizers’) have improved survival in autologous stem cell transplantation for lymphoid malignancies. Bone Marrow Transplant. 2007;40:437–441. [DOI] [PubMed] [Google Scholar]
- 13.Toor AA, Ayers J, Strupeck J, et al. Favourable results with a single autologous stem cell transplant following conditioning with busulphan and cyclophosphamide in patients with multiple myeloma. Br J Haematol 2004;124:769–776. [DOI] [PubMed] [Google Scholar]
- 14.Tuchman SA, Bacon WA, Huang LW, et al. Cyclophosphamide-based hematopoietic stem cell mobilization before autologous stem cell transplantation in newly diagnosed multiple myeloma. J Clin Apher 2015;30:176–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moreb JS, Salmasinia D, Hsu J, Hou W, Cline C, Rosenau E. Long-term outcome after autologous stem cell transplantation with adequate peripheral blood stem cell mobilization using plerixafor and G-CSF in poor mobilizer lymphoma and myeloma patients. Adv Hematol 2011;2011:517561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boeve S, Strupeck J, Creech S, Stiff PJ. Analysis of remobilization success in patients undergoing autologous stem cell transplants who fail an initial mobilization: risk factors, cytokine use and cost. Bone Marrow Transplant. 2004;33:997–1003. [DOI] [PubMed] [Google Scholar]
- 17.Nademanee AP, DiPersio JF, Maziarz RT, et al. Plerixafor plus granulocyte colony-stimulating factor versus placebo plus granulocyte colony-stimulating factor for mobilization of CD34(+) hematopoietic stem cells in patients with multiple myeloma and low peripheral blood CD34(+) cell count: results of a subset analysis of a randomized trial. Biol Blood Marrow Transplant. 2012;18:1564–1572. [DOI] [PubMed] [Google Scholar]
- 18.Maziarz RT, Nademanee AP, Micallef IN, et al. Plerixafor plus granulocyte colony-stimulating factor improves the mobilization of hematopoietic stem cells in patients with non-Hodgkin lymphoma and low circulating peripheral blood CD34+ cells. Biol Blood Marrow Transplant. 2013;19:670–675. [DOI] [PubMed] [Google Scholar]
- 19.Lysak D, Koristek Z, Gasova Z, Skoumalova I, Jindra P. Efficacy and safety of peripheral blood stem cell collection in elderly donors; does age interfere? J Clin Apher 2011;26:9–16. [DOI] [PubMed] [Google Scholar]
- 20.Auner HW, Mazzarella L, Cook L, et al. High rate of stem cell mobilization failure after thalidomide and oral cyclophosphamide induction therapy for multiple myeloma. Bone Marrow Transplant 2011;46:364–367. [DOI] [PubMed] [Google Scholar]
- 21.Kumar S, Dispenzieri A, Lacy MQ, et al. Impact of lenalidomide therapy on stem cell mobilization and engraftment post-peripheral blood stem cell transplantation in patients with newly diagnosed myeloma. Leukemia. 2007;21:2035–2042. [DOI] [PubMed] [Google Scholar]
- 22.Mazumder A, Kaufman J, Niesvizky R, Lonial S, Vesole D, Jagannath S. Effect of lenalidomide therapy on mobilization of peripheral blood stem cells in previously untreated multiple myeloma patients [letter]. Leukemia. 2008;22:280–1281. [DOI] [PubMed] [Google Scholar]
- 23.Basak GW, Jaksic O, Koristek Z, et al. Haematopoietic stem cell mobilization with plerixafor and G-CSF in patients with multiple myeloma transplanted with autologous stem cells. Eur J Haematol 2011;86:488–495. [DOI] [PubMed] [Google Scholar]
- 24.Giralt S, Costa L, Schriber J, et al. Optimizing autologous stem cell mobilization strategies to improve patient outcomes: consensus guidelines and recommendations. Biol Blood Marrow Transplant. 2014;20:295–308. [DOI] [PubMed] [Google Scholar]
- 25.Fowler G, Maziarz RT. Clinical use of plerixafor in combination with granulocyte-colony stimulating factor in hematopoietic stem cell transplantation. Transplant Research and Risk Management. 2010;2:47–58. [Google Scholar]
- 26.Gerlach LO, Skerlj RT, Bridger GJ, Schwartz TW. Molecular interactions of cyclam and bicyclam non-peptide antagonists with the CXCR4 chemokine receptor. J Biol Chem 2001;276:14153–14160. [DOI] [PubMed] [Google Scholar]
- 27.Hubel K, Liles WC, Broxmeyer HE, et al. Leukocytosis and mobilization of CD34+ hematopoietic progenitor cells by AMD3100, a CXCR4 antagonist. Support Cancer Ther 2004;1:165–172. [DOI] [PubMed] [Google Scholar]
- 28.D’Addio A, Curti A, Worel N, et al. The addition of plerixafor is safe and allows adequate PBSC collection in multiple myeloma and lymphoma patients poor mobilizers after chemotherapy and G-CSF. Bone Marrow Transplant. 2011;46:356–363. [DOI] [PubMed] [Google Scholar]
- 29.Danylesko I, Sareli R, Varda-Bloom N, et al. Plerixafor (Mozobil): A Stem Cell-Mobilizing Agent for Transplantation in Lymphoma Patients Predicted to Be Poor Mobilizers - A Pilot Study. Acta Haematol 2016;135:29–36. [DOI] [PubMed] [Google Scholar]
- 30.Lanza F, Lemoli RM, Olivieri A, et al. Factors affecting successful mobilization with plerixafor: an Italian prospective survey in 215 patients with multiple myeloma and lymphoma. Transfusion (Paris). 2014;54:331–339. [DOI] [PubMed] [Google Scholar]
- 31.Gazitt Y, Freytes CO, Akay C, Badel K, Calandra G. Improved mobilization of peripheral blood CD34+ cells and dendritic cells by AMD3100 plus granulocyte-colony-stimulating factor in non-Hodgkin’s lymphoma patients. Stem Cells Dev. 2007;16:657–666. [DOI] [PubMed] [Google Scholar]
- 32.Varmavuo V, Mantymaa P, Kuittinen T, Nousiainen T, Jantunen E. Blood graft lymphocyte subsets after plerixafor injection in non-Hodgkin’s lymphoma patients mobilizing poorly with chemotherapy plus granulocyte-colony-stimulating factor. Transfusion (Paris). 2012;52:1785–1791. [DOI] [PubMed] [Google Scholar]
- 33.Devine SM, Vij R, Rettig M, et al. Rapid mobilization of functional donor hematopoietic cells without G-CSF using AMD3100, an antagonist of the CXCR4/SDF-1 interaction. Blood. 2008;112:990–998. [DOI] [PubMed] [Google Scholar]
- 34.Fruehauf S, Veldwijk MR, Seeger T, et al. A combination of granulocyte-colony-stimulating factor (G-CSF) and plerixafor mobilizes more primitive peripheral blood progenitor cells than G-CSF alone: results of a European phase II study. Cytotherapy. 2009;11:992–1001. [DOI] [PubMed] [Google Scholar]
- 35.Larochelle A, Krouse A, Metzger M, et al. AMD3100 mobilizes hematopoietic stem cells with long-term repopulating capacity in nonhuman primates. Blood. 2006;107:3772–3778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.DiPersio JF, Micallef IN, Stiff PJ, et al. Phase III prospective randomized double-blind placebo-controlled trial of plerixafor plus granulocyte colony-stimulating factor compared with placebo plus granulocyte colony-stimulating factor for autologous stem-cell mobilization and transplantation for patients with non-Hodgkin’s lymphoma. J Clin Oncol 2009;27:4767–4773. [DOI] [PubMed] [Google Scholar]
- 37.DiPersio JF, Stadtmauer EA, Nademanee A, et al. Plerixafor and G-CSF versus placebo and G-CSF to mobilize hematopoietic stem cells for autologous stem cell transplantation in patients with multiple myeloma. Blood. 2009;113:5720–5726. [DOI] [PubMed] [Google Scholar]
- 38.Hartmann T, Hubel K, Monsef I, Engert A, Skoetz N. Additional plerixafor to granulocyte colony-stimulating factors for haematopoietic stem cell mobilisation for autologous transplantation in people with malignant lymphoma or multiple myeloma. Cochrane Database Syst Rev 2015:CD010615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Varmavuo V, Rimpilainen J, Kuitunen H, et al. Engraftment and outcome after autologous stem cell transplantation in plerixafor-mobilized non-Hodgkin’s lymphoma patients. Transfusion (Paris). 2014;54:1243–1250. [DOI] [PubMed] [Google Scholar]
- 40.DiPersio JF, Ho AD, Hanrahan J, Hsu FJ, Fruehauf S. Relevance and clinical implications of tumor cell mobilization in the autologous transplant setting. Biol Blood Marrow Transplant. 2011;17:943–955. [DOI] [PubMed] [Google Scholar]
- 41.Fruehauf S, Ehninger G, Hubel K, et al. Mobilization of peripheral blood stem cells for autologous transplant in non-Hodgkin’s lymphoma and multiple myeloma patients by plerixafor and G-CSF and detection of tumor cell mobilization by PCR in multiple myeloma patients. Bone Marrow Transplant. 2010;45:269–275. [DOI] [PubMed] [Google Scholar]
- 42.Tricot G, Cottler-Fox MH, Calandra G. Safety and efficacy assessment of plerixafor in patients with multiple myeloma proven or predicted to be poor mobilizers, including assessment of tumor cell mobilization. Bone Marrow Transplant. 2010;45:63–68. [DOI] [PubMed] [Google Scholar]
- 43.Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol 2007;25:579–586. [DOI] [PubMed] [Google Scholar]
- 44.Durie BG, Harousseau JL, Miguel JS, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20:1467–1473. [DOI] [PubMed] [Google Scholar]
- 45.Reece DE. Posttransplantation maintenance therapy and optimal frontline therapy in myeloma. Hematology Am Soc Hematol Educ Program. 2011;2011:197–204. [DOI] [PubMed] [Google Scholar]
- 46.Laudi N, Arora M, Burns LJ, et al. Long-term follow-up after autologous hematopoietic stem cell transplantation for low-grade non-Hodgkin lymphoma. Biol Blood Marrow Transplant. 2005;11:129–135. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


