Abstract
Background:
Domiciliary cockroaches are obnoxious pests of significant medical importance. We investigated the prevalence of human intestinal parasites in cockroaches and its attendant public health importance.
Methods:
Overall, 749 cockroaches (Periplaneta americana, 509, Blattella germanica, 240) caught by trapping from 120 households comprising 3 different housing types in Somolu, Lagos metropolis, southwest Nigeria, in 2015 were screened for human intestinal parasites using standard parasitological techniques.
Results:
The prevalence of human intestinal parasites in cockroaches was 96.4%. There was no statistically significant difference (P> 0.05) in parasite prevalences between P. americana (95.7%) and B. germanica (97.9%). Parasite species identified and their prevalence were as follows: Entamoeba histolytica/dispar (44.1%), E. coli (37.8%), Giardia lamblia (18.7%), Cryptosporidium sp. (13.8%), Ascaris lumbricoides (61.3%), Trichuris trichiura (55.8%), hookworms (11.6%), Strongyloides stercoralis (11.7%), Taenia/Echinococcus spp. (10.5%), Enterobius vermicularis (17.2%) and Hymenolepis nana (11.6%). Parasite prevalence and burdens varied with housing type; the prevalence was significantly higher statistically (P< 0.05) in cockroaches from low-cost bungalow, LCB (100%) and low-cost, 2-storey, LC2-S (100%) houses than in medium-cost flats, MCF (81.3%). Parasite burdens were also significantly higher statistically (P< 0.05) in cockroaches from LCB or LC2-S than in cockroaches from MCF. Parasite prevalences between cockroach gut and body surfaces were not statistically significant (P> 0.05) but mean parasite burdens in gut were significantly higher statistically (P< 0.05) than on body surfaces.
Conclusion:
Cockroaches types carry transmissive stages of human intestinal parasites and may act as reservoirs and potential mechanical vectors for disease transmission.
Keywords: Cockroaches, Periplaneta americana, Blattella germanica, Intestinal parasites, Lagos
Introduction
Cockroaches (Insecta: Blattaria) are insects, which have been in existence since antiquity (1), thriving in so many habitats and consuming virtually any organic matter, including fresh and processed human foods, stored products, garbage, and sewage (1, 2). About 4600 described species of cockroaches are distributed worldwide (3). However, only a few of the about 30 synanthropic species are considered as pests in homes, grocery stores, hospitals, offices, schools, warehouses and other establishments (4). The American cockroach, Periplaneta americana (Blattaria: Blattidae) and the German cockroach, Blattella germanica (Blattaria: Blattellidae) are considered two of the most common and notorious cosmopolitan pest species in Nigeria (5) and globally (6–8).
Cockroaches are pests of significant medical, veterinary and public health importance. Their presence and sight may induce psychological stress, the levels of which tend to be proportional to cockroach size and number (2). They are an important source of potent environmental aeroallergens, which provoke allergic reactions and exacerbate acute asthma, especially in predisposed atopic individuals (9, 10). Cockroaches contaminate foods to which they have access with their feces and foul-smelling secretions, thereby making them offensive and unsafe for human consumption (1, 2). They also serve as intermediate hosts to a number of helminth parasites of veterinary importance, some of which cause debilitating diseases in domestic animals (2).
Although cockroaches have yet, to be incriminated as biological vectors of human pathogens, their biology - filthy habits, indiscriminate diet, feeding mechanisms and morphology, make them vulnerable and suitable, at least, to acquire, mechanically transport and disseminate pathogens. Indeed, a variety of pathogenic and potentially pathogenic bacteria, fungi, and parasites have been isolated from body surfaces and/or gut of cockroaches in domestic, food-handling, and hospital environments (6, 11–14). The potential exists, therefore, for mechanical transmission through physical dislodgement, regurgitation, or fecal pellet deposition onto and/or into exposed human food, which may be, ready-to-eat or improperly cooked. Although the direct involvement of cockroaches in the transmission of parasites to humans remains to be fully established, their importance in parasite transport and dissemination cannot be underestimated.
Intestinal parasitoses are among the most common and widespread diseases of humans globally responsible for considerable morbidity and mortality, especially in children, the most vulnerable population (15). They remain a serious threat to public health worldwide, particularly in communities in resource-poor developing countries in the tropics and subtropics where high prevalences are attributable to poverty, poor living conditions, lack of potable water supply, inadequate waste disposal, poor sanitation and environmental hygiene (16). While a few of these parasites require intermediate hosts, many are transmitted by direct ingestion of infective cysts, and oocysts (protozoa) or eggs and/or larvae (helminths) in foods (especially fruits and vegetables), water, soil, pica, or on hands so contaminated.
The incidence of human intestinal parasitoses has continued to increase in recent years, in spite of concerted efforts at reduction. Moreover, the transmissive/human-infective stages of some of these parasites can survive in the environment for considerable lengths of time. Because cockroaches carry the same pathogens found in substrates with which they have contact (17), it is plausible that cockroaches, in environments contaminated with parasite cysts, oocysts, eggs and or larvae, may pick up these stages for transport and dissemination.
Poor household hygiene and inadequate environmental sanitation provide congenial atmosphere for cockroach infestation. Somolu, Lagos metropolis, southwest Nigeria, typifies a cosmopolitan setting in a developing economy such as Nigeria, where poor sanitary conditions, together with ecology and demography, provide congenial atmosphere for cockroach infestation and contact with pathogens. In spite of the above, and the heterogeneity of Somolu in terms of human population and physical infrastructures, and considering the medical and public health importance of cockroaches, there is yet, no information on prevalence of human intestinal parasites in domiciliary cockroaches from this locality.
The objective of this study was to determine and compare prevalences and species composition of human intestinal parasites in cockroaches from different residential buildings in Somolu, Lagos State, southwest Nigeria.
Materials and Methods
Study Area
The study was carried out between Aug and Nov 2015 in Somolu (geographical coordinates, 6°32’ N and longitude 3°22’ E), a densely populated cosmopolitan area of Lagos metropolis, and headquarters of Somolu Local Government Area (LGA) of Lagos State, southwest Nigeria. The LGA which has a land size of 11615km2 and a population of 403569 (18) is inhabited predominantly by people of the Yoruba extraction, although all tribes and sub-tribes of the Nigerian nationality and expatriates are also resident.
Climate in the area is typical of that in the State and is characterized generally by daily temperatures of 24–34 °C and monthly relative humidity of 64–93% during the rainy season, usually from Apr to Oct, and 34–49% during the dry season from Nov to Mar.
Housing Types
Houses in Somolu are typical, a mix of old and modern architecture. One hundred and twenty residential buildings were selected for the study using a stratified random sampling procedure. They comprised 40 each, of buildings classified as low-cost bungalow (LCB), low-cost, 2-storey (LC2-S), and medium-cost flat (MCF), based on building architecture and socio-economic status of residents. Each LCB or LC2-S had between 5 and 7 rooms on either side of a story and each room was occupied by low-income class individual(s)/family who shared the only kitchen and toilet/bathroom facilities on either side of a story. Each MCF consists of a 3-bedroom apartment with a kitchen and at least one toilet facility, in a semi-detached building inhabited by a medium-income class family. Inclusion criterion was that no insecticide and/or trapping device was used to treat cockroach infestation in the one week prior to the commencement of the study. Advocacy visits were made to residents of selected houses to explain the objective(s) of the study and to seek for their participation, cooperation, and understanding in the execution of the study.
Cockroach collection and identification
Cockroaches were trapped live, using sterile jars baited with pieces of bread soaked in a small amount of beer. The jars, whose inside upper portions were coated with a thin film of petroleum jelly (Vaseline®) to prevent cockroach escape, were placed indoors at 19:00h and retrieved at 07:00h the next morning, for 1–3 consecutive days. Cockroaches were transported in the jars to the laboratory where they were anaesthetized and killed by exposure to chloroform fume. They were examined under a dissecting microscope and identified to the lowest taxon possible, using standard taxonomic keys (19). They cockroaches were counted and sorted by capture site (housing type), and the appropriate taxon.
Isolation of parasites from cockroach body surface
In order to dislodge parasite stages (cysts, oocysts, eggs and/or larvae) from body surfaces, cockroaches were washed individually by submersion in 5–10ml sterile physiological saline and vortexing at low speed for 2 min. Cockroaches were removed from wash solutions using sterile forceps, fixed in 70% alcohol for 5min and air-dried at room temperature. Wash solutions were centrifuged at 2000g for 5min, supernatants were decanted and the bottom 0.5–1ml processed further using the formol-ether concentration technique (20). The resulting sediment mixed with the bottom 0.5ml was placed on slide, stained with Lugol’s iodine and examined microscopically for human intestinal parasite stages.
For the demonstration of Cryptosporidium oocysts, a modified Ziehl-Neelsen staining method (21) was used. Briefly, air-dried smears prepared from processed body surface washings were fixed with methanol and stained with carbol-fuchsin for 30min. Smears were washed with tap water, decolorized with 1% acid alcohol for 1min, washed again with tap water and counter-stained with 1% methylene blue for 1min. Smears were rinsed finally in tap water and air-dried.
Cockroach specimens which could not be processed immediately were kept in the freezer at −4 °C.
Isolation of parasites from cockroach gut
Following washing in physiological saline, fixing in 70% alcohol and subsequent air-drying, each cockroach was placed in a sterile Petri dish and dissected under a dissecting microscope using sterile entomological needles. Whole gut was removed and homogenized in 2–5ml physiological saline. The homogenate was filtered through gauze and centrifuged at 2000g for 5min, following which the supernatant was decanted. The bottom 0.5–1ml was processed further using the formol-ether concentration technique. The resulting sediment mixed with the bottom 0.5ml was placed on slide, stained with Lugol’s iodine and examined microscopically as described above.
Cryptosporidium oocysts were identified following the modified Ziehl-Neelsen staining method described above.
Parasite Identification
Cysts, oocysts, eggs and/or larvae of human intestinal parasites were identified microscopically using bench aids (22) and their numbers recorded. A cockroach was considered a carrier if any parasite stage was detected in preparations from body surface and/or gut contents.
Data analysis
Data were input into Microsoft Excel and analyzed using the “R: A Language and Environment for Statistical Computing” software package (23). Analysis of variance (ANOVA) was used to test for differences in overall prevalence of parasites between cockroach species, in overall burdens of parasites on cockroach body surfaces and in gut, and in overall burdens of protozoan and helminth groups of parasites. The Tukey’s HSD test was used for multiple comparisons of overall prevalences or burdens of protozoan and/or helminth parasites between pairs of residential building types. All tests were carried out at 95% significance level; in all cases, a P< 0.05 was considered statistically significant.
Results
Prevalence of human intestinal parasites by cockroach species
Overall, 749 cockroaches comprising two species, P. americana (509) and B. germanica (240) were caught and identified. Human intestinal parasite stages were identified in 96.4% of the cockroaches (Table 1). There was no statistically significant difference (F-statistic= 2.354, P= 0.125) in overall prevalence of parasites between P. americana (95.7%) and B. germanica (97.9%). Parasites were more frequently isolated from cockroaches trapped from LCB and LC2-S households than in cockroaches from MCF (81.3%) (Table 1). There was no statistically significant difference (P= 1.00) in prevalence of parasites between cockroaches from LCB (100%) and LC2-S (100%) households while there were statistically significant differences in prevalences of parasites between cockroaches from LCB and MCF (P=0.001) and LC2-S and MCF (P=0.001).
Table 1.
Prevalence of human intestinal parasite stages in cockroaches
| Housing Type | Number of cockroaches examined (% parasite +ve) | Total | |
|---|---|---|---|
| Periplaneta americana | Blattella germanica | ||
| LCB | 207 (100) | 91 (100) | 298 (100)a |
| LC2-S | 202 (100) | 105 (100) | 307 (100)a |
| MCF | 100 (78.0) | 44 (88.6) | 144 (81.3)b |
| Total | 509 (95.7)a | 240 (97.9)a | 749 (96.4) |
Values with same superscript along the same column or row are not significantly different statistically at α= 0.05
Species diversity of human intestinal parasites in cockroaches
Eleven human intestinal parasites, comprising four protozoan and seven helminth species were identified on body surfaces and/or in gut of cockroaches. The species and their respective prevalences in both P. americana and B. germanica are as follows: E. histolytica/dispar (44.1%), E. coli (37.8%), G. lamblia (18.7%), Cryptosporidium sp. (13.8%), A. lumbricoides (61.3%), T. trichiura (55.8%), hookworms (11.6%), S. stercoralis (11.7%), Taenia/Echinococcus spp. (10.5%), E. vermicularis (17.2%) and H. nana (11.6%). Prevalences of these parasite species in P. americana and B. germanica, trapped from different housing types are shown in Figs. 1 and 2, respectively. The helminths, A. lumbricoides and T. trichiura, and the protozoans, E. histolytica/dispar and E. coli were the four most prevalent species in both species of cockroaches, across all housing types, save B. germanica from MCF wherein G. lamblia was the fourth most prevalent species (25.0%). Their respective overall prevalences in P. americana were 59.5%, 56.8%, 43.2% and 36.7% (Fig. 1) while the corresponding overall prevalences in B. germanica were 65.0%, 53.8%, 45.8% and 40.0% (Fig. 2).
Fig. 1.
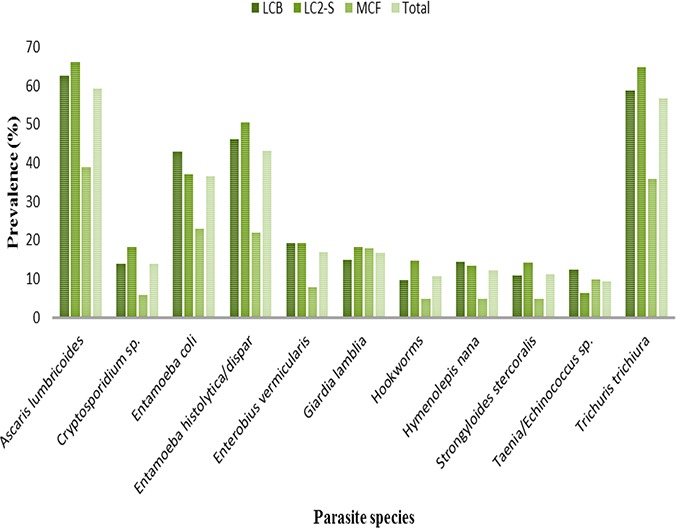
Prevalence of human intestinal parasites in Periplaneta americana from different housing types
Fig. 2.
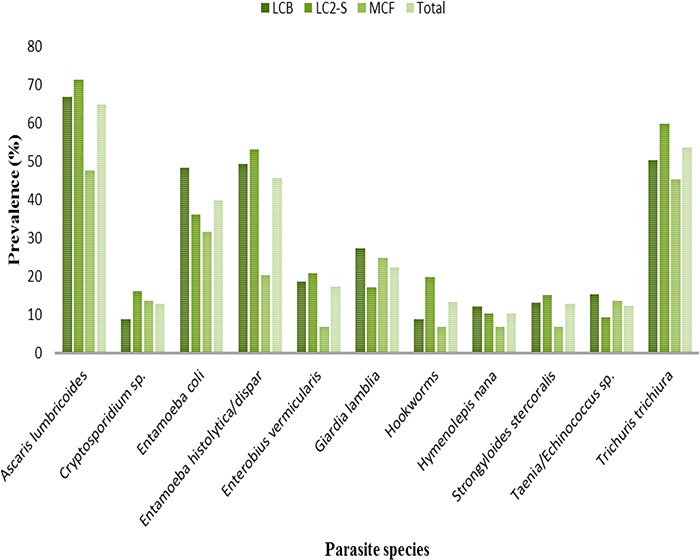
Prevalence of human intestinal parasites in Blattella germanica from different housing type
Prevalence and burden of human intestinal parasites on cockroach body surfaces and in the gut
Parasite prevalence in cockroach gut was either a little higher than or identical numerically, to the corresponding prevalence on body surface. The ANOVA test showed no statistically significant differences between prevalences in gut and on body surfaces in P. americana (F= 1.363, P= 0.243), B. germanica (F= 0.344, P= 0.558) or both (F= 1.671, P= 0.196).
Overall parasite prevalences were significantly higher statistically with helminths than protozoans in P. americana (93.9% vs. 83.7%, F= 28.25, P= 1.31e-07), B. germanica (97.5% vs. 83.3%, F= 29.38, P= 9.46e-08), or both (95.1% vs. 83.6%, F= 54.52, P= 2.54e-13) and in cockroaches from LCB (P= 0.005), LC2-S (P= 3.45e-07) and MCF (P= 0.0001).
Mean parasite burdens/counts in P. americana (Table 2) and B. germanica (Table 3) varied with cockroach body region, parasite group (protozoa or helminth) and housing type. Highest parasite burdens on cockroach body surfaces and in gut were with the protozoans, E. histolytica/dispar and E. coli and the helminths, A. lumbricoides and T. trichiura, overall and in each housing type (Tables 2, 3).
Table 2.
Mean parasite burdens in Periplaneta americana cockroaches from houses in Somolu, Lagos, Nigeria
| Parasite species | LCB (N=207) | LC2-S (N=202) | MCF (N=100) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Body surface | Gut | Total | Body surface | Gut | Total | Body surface | Gut | Total | |
| Ascaris lumbricoides | 2.2±2.1 | 4.8±4.2 | 7.0±6.2 | 2.3±2.0 | 4.9±4.2 | 7.2±6.2 | 0.9±1.2 | 1.5±2.0 | 2.3±3.2 |
| Cryptosporidium sp | 0.3±0.8 | 0.6±1.6 | 0.9±2.3 | 0.5±1.1 | 0.9±2.1 | 1.3±3.1 | 0.1±0.3 | 0.2±0.6 | 0.2±1.0 |
| Entamoeba coli | 1.3±1.7 | 2.9±3.7 | 4.2±5.4 | 1.1±1.6 | 2.3±3.4 | 3.3±4.9 | 0.4±0.9 | 0.9±1.7 | 1.3±2.6 |
| Entamoeba histolytica/dispar | 1.4±1.9 | 3.1±3.8 | 4.5±5.6 | 1.7±1.9 | 3.3±3.8 | 5.0±5.6 | 0.5±1.0 | 0.9±1.8 | 1.3±2.8 |
| Enterobius vermicularis | 0.5±1.1 | 1.0±2.2 | 1.5±3.3 | 0.5±1.2 | 1.0±2.1 | 1.5±3.3 | 0.2±0.6 | 0.3±1.0 | 0.4±1.6 |
| Giardia lamblia | 0.3±0.9 | 0.7±1.7 | 1.0±2.6 | 0.5±1.0 | 0.9±2.1 | 1.4±3.1 | 0.3±0.6 | 0.5±1.2 | 0.8±1.8 |
| Hookworms | 0.2±0.6 | 0.4±1.3 | 0.6±1.9 | 0.4±1.0 | 0.7±1.8 | 1.0±2.7 | 0.1±0.4 | 0.2±0.8 | 0.2±1.1 |
| Hymenolepis nana | 0.3±0.8 | 0.6±1.5 | 0.8±2.3 | 0.3±0.8 | 0.5±1.5 | 0.8±2.3 | 0.1±0.3 | 0.1±0.6 | 0.2±0.9 |
| Strongyloides stercoralis | 0.2±0.5 | 0.3±1.0 | 0.5±1.5 | 0.2±0.6 | 0.5±1.2 | 0.7±1.8 | 0.0±0.2 | 0.1±0.6 | 0.2±0.8 |
| Taenia/Echinococcus sp | 0.3±0.8 | 0.6±1.7 | 0.8±2.4 | 0.2±0.7 | 0.5±1.5 | 0.5±2.1 | 0.1±0.5 | 0.3±1.0 | 0.4±1.5 |
| Trichuris trichiura | 1.9±1.9 | 3.9±3.8 | 5.8±5.7 | 2.2±2.0 | 4.4±3.8 | 6.7±5.8 | 0.7±1.2 | 1.3±2.0 | 2.1±3.1 |
| Total | 8.8±4.1 | 18.8±7.5 | 27.6±11.4 | 9.7±3.8 | 19.7±7.0 | 29.3±10.5 | 3.3±2.6 | 6.2±4.6 | 9.4±7.1 |
Table 3.
Mean parasite burdens in Blattella germanica cockroaches from houses in Somolu, Lagos, Nigeria
| Parasite species | LCB (N=91) | LC2-S (N=105) | MCF (N=44) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Body surface | Gut | Total | Body surface | Gut | Total | Body surface | Gut | Total | |
| Ascaris lumbricoides | 2.7±2.2 | 5.7±4.7 | 8.4±6.9 | 2.6±2.0 | 5.6±4.1 | 8.3±6.0 | 1.2±1.5 | 2.1±2.5 | 3.3±4.0 |
| Cryptosporidium sp | 0.2±0.6 | 0.3±1.1 | 0.5±1.7 | 0.4±1.0 | 0.8±2.1 | 1.2±3.0 | 0.2±0.5 | 0.3±0.9 | 0.5±1.4 |
| Entamoeba coli | 1.6±1.9 | 3.4±4.0 | 4.9±5.8 | 1.0±1.6 | 2.1±3.2 | 3.1±4.7 | 0.8±1.2 | 1.2±2.0 | 2.0±3.2 |
| Entamoeba histolytica/dispar | 1.6±1.8 | 3.4±4.0 | 5.0±5.8 | 1.7±1.8 | 3.4±3.7 | 5.0±5.4 | 0.5±1.0 | 0.7±1.5 | 1.2±2.5 |
| Enterobius vermicularis | 0.5±1.0 | 1.0±2.3 | 1.5±3.3 | 0.6±1.3 | 1.1±2.3 | 1.8±3.5 | 0.1±0.5 | 0.2±0.8 | 0.3±1.2 |
| Giardia lamblia | 0.7±1.3 | 1.4±2.7 | 2.2±4.0 | 0.4±1.1 | 0.9±2.4 | 1.4±3.5 | 0.5±0.9 | 0.8±1.4 | 1.2±2.2 |
| Hookworms | 0.3±0.9 | 0.5±1.9 | 0.8±2.8 | 0.4±1.0 | 0.9±2.0 | 1.3±3.0 | 0.1±0.5 | 0.2±0.8 | 0.3±1.2 |
| Hymenolepis nana | 0.2±0.7 | 0.5±1.4 | 0.7±2.2 | 0.3±0.9 | 0.5±1.6 | 0.8±2.4 | 0.1±0.3 | 0.2±0.8 | 0.3±1.1 |
| Strongyloides stercoralis | 0.2±0.6 | 0.4±1.1 | 0.6±1.6 | 0.3±0.7 | 0.5±1.3 | 0.8±1.9 | 0.1±0.4 | 0.3±1.0 | 0.4±1.5 |
| Taenia/Echinococcus sp | 0.3±0.8 | 0.7±1.8 | 1.0±2.6 | 0.1±0.4 | 0.2±1.0 | 0.3±1.4 | 0.2±0.5 | 0.4±1.2 | 0.6±1.6 |
| Trichuris trichiura | 1.6±1.9 | 3.5±3.8 | 5.1±5.6 | 2.0±2.0 | 4.2±3.9 | 6.3±5.9 | 0.9±1.2 | 1.6±2.0 | 2.5±3.1 |
| Total | 9.8±4.8 | 20.9±9.7 | 30.7±14.3 | 9.9±3.2 | 20.3±6.4 | 30.2±9.4 | 4.4±2.6 | 8.0±4.1 | 12.4±6.6 |
Overall mean parasite burdens in gut were significantly higher statistically than on body surfaces in P. americana (F= 330, P< 2e-16), B. germanica (F= 166.3, P< 2e-16), and both (F= 496, P< 2e-16). Similarly, overall burdens of helminth parasites were significantly higher statistically than those of protozoan parasites in P. americana (F= 156, P< 2e-16), B. germanica (F =74.89, P< 2e-16), both (F= 229.8, P< 2e-16) and in LCB (P= 0.0001), LC2-S (P= 0.0001) and MCF (P= 0.044). Differences in overall parasite burdens were statistically significant between LCB and MCF (P= 0.0001) and LC2-S and MCF (P= 0.0001), but not statistically significant between LCB and LC2-S (P= 0.996).
Discussion
Cockroaches are nuisance pests whose activities impact negatively on humans. Of great concern to human and public health, is their capability as potential mechanical vectors of pathogens, including parasites. Previous studies from other parts of Nigeria (13, 24, 25) and elsewhere (6, 12, 26) had reported that cockroaches captured from homes, hostels, hospitals, and markets carry an array of human intestinal parasites. Results of the present study, which show clearly that the two cockroach species (P. americana and B. germanica) from residential buildings in Somolu, Lagos, southwest Nigeria, carry human intestinal parasites on their body surfaces and/or in the gut indicate that concerns over their potential and/or role as mechanical vectors cannot be overlooked.
The species of human intestinal parasites recovered from cockroaches in the present study, (E. histolytica/dispar, E. coli, G. lamblia, Cryptosporidium sp. A. lumbricoides, T. trichiura, hookworms, S. stercoralis, Taenia/Echinococcus spp., H. nana, and E. vermicularis) are responsible for a number of disease conditions in man, some of which could be life-threatening. The three major soil-transmitted helminths (A. lumbricoides, T. trichiura, and hookworms) account for a high burden of disease globally and are intimately related with malnutrition, growth stunting and cognitive deficits in children (27). Strongyloides stercoralis may cause complicated infections with high case fatality rates due to hyper-infection or dissemination, especially in immunocompromised individuals (28). Cryptosporidium sp. and G. lamblia are nowadays, major causes of diarrhoea, especially in children (29). Entamoeba histolytica causes amoebiasis, a potentially severe and life-threatening disease and the second most common cause of death from parasitic diseases, after malaria (30). Accidental ingestion of Taenia (particularly, T. solium) eggs often results in human neurocysticercosis, the leading cause of preventable epilepsy worldwide and also, a leading cause of deaths from food-borne diseases (31). Species of Echinococcus cause life-threatening chronic diseases with poor prognosis and high fatality rates, if not carefully managed clinically (32).
Parasite species reported in the present study are consistent with those documented in similar studies (13, 24, 25, 33, 34). However, the disparities could be due to differences in the levels of household and environmental hygiene, transmission dynamics between study localities, and in the diagnostic procedure employed. Predominance of A. lumbricoides on body surfaces and/or in the gut of cockroaches across all different housing types in the present study is in consonance with findings from similar studies in other parts of the country (13, 25, 33). This could be due to its predominance in the human population and/or the persistence of its eggs in the environment for months to years (35).
Identity of parasite species in cockroaches from all three housing types indicates that these parasites have equal chances of being acquired by cockroaches, probably because they are endemic in the study area. Differences in individual parasite species burdens between cockroaches from low-cost (LCB or LC2-S) and MCF could then be explicated by the varying levels of hygiene and sanitation in each housing type. Poorer housing and sanitary conditions, which could predispose to higher parasite contamination, characterize low-cost households.
Overwhelming and widespread prevalence (96.4%) of human intestinal parasites in domiciliary cockroaches in the present study is singular in Nigeria and is a cause for public health concern. Other studies from Nigeria had reported prevalences of 58.6% in Calabar, Southsouth (25), 67.1% in Owerri, Southeast (33) and 77.5% in Sokoto, Northwest (24). Al-Mayali and Al-Yaqoobi (36) and El-Sherbini and El-Sherbini (34) reported prevalences of 83.3% and 98% respectively, in Iraq and Egypt. Disparities in prevalences between different studies may be explained by differences in the levels of hygiene and sanitation between study localities.
Identity in the diversity of parasites between P. americana and B. germanica as well as the insignificant statistical differences in their respective prevalences in the cockroach species indicate a uniform distribution of parasite species between the two cockroach species in the same environment. They also suggest that the two cockroach species have equal potential for mechanical transport and possibly, consequent dissemination of parasites in the environment.
Because pathogens carried by cockroaches are acquired from their immediate environments (37), the human intestinal parasites reported herein, were acquired through contact with unhygienic environments. Most of the parasites whose cysts, oocysts, eggs and/or larvae (hookworm: A. duodenale only) were isolated from cockroaches in the present study are transmitted to humans via consumption of food and/or water so contaminated. Since cockroaches travel indiscriminately between filth and human food, they may be capable of disseminating parasite stages (on their body surfaces and/or in the gut) through physical dislodgement, vomitus and/or feces onto any substrate in the environment, including human food and food preparation surfaces. The medical and public health implications of this are better imagined.
Cysts of E. histolytica and E. dispar are morphologically indistinguishable microscopically, so also are the eggs of Echinococcus and Taenia species. Since molecular techniques, which differentiate reliably, cysts of E. histolytica from E. dispar (38) and eggs of Echinococcus from Taenia species (39, 40) were not employed in the present study, the former was simply identified morphologically as E. histolytica/dispar and the latter as Taenia/Echinococcus spp.
Conclusion
Cockroaches (P. americana and B. germanica) across different housing types in Somolu, Lagos metropolis, Nigeria, transport on their body surfaces and/or in the gut, transmissive stages of human intestinal parasites and thus, may serve as reservoirs and potential mechanical vectors for disease transmission. The exceptionally high prevalence of parasites in cockroaches (96.4%) justifies the need for improvements in existing standards of household hygiene and environmental sanitation in order to minimize cockroach contact with unhygienic sites/substrates from which parasites are acquired.
Acknowledgements
We are grateful to all residents of the different houses selected for participation in the study, for their cooperation and understanding in the execution of the study.
The authors declare no conflict of interests.
References
- 1.Cochran DG. (1999) Cockroaches: Their Biology, Distribution and Control. WHO/CDS/CPC/WHOPES/99.3. World Health Organization, Geneva. [Google Scholar]
- 2.Kramer RD, Brenner RJ. (2009) Cockroaches (Blattaria). In: Mullen GR, Durden LA. (Eds) Medical and Veterinary Entomology, 2nd Edition Academic Press/Elsevier Inc, New York, USA, pp. 41–55. [Google Scholar]
- 3.Beccaloni GW. (2014) Cockroach Species File Online. Version 5.0/5.0. World Wide Web electronic publication. Available at: http://Cockroach.SpeciesFile.org
- 4.Rust MK, Reierson DA. (2007) Cockroaches. Integrated Pest Management for Home Gardeners and Landscape Professionals. Pest Notes, Publication 7467, University of California Agriculture and Natural Resources, California, USA. [Google Scholar]
- 5.Anikwe JC, Adetoro FA, Anogwih JA, Makanjuola WA, Kemabonta KA, Akinwande KL. (2014) Laboratory and field evaluation of an Indoxacarb Gel bait against two cockroach species (Dictyoptera: Blattellidae, Blattidae) in Lagos, Nigeria. J Econ Entomol. 107 (4): 1639–1642. [DOI] [PubMed] [Google Scholar]
- 6.Hamu H, Debalke S, Zemene E, Birlie B, Mekonnen Z, Yewhalaw D. (2014) Isolation of intestinal parasites of public health importance from cockroaches (Blattella germanica) in Jimma Town, Southwestern Ethiopia. J Parasitol Res. 2014: 186240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hashemi-Aghdam SS, Oshaghi MA. (2015) A checklist of Iranian cockroaches (Blattodea) with description of Polyphaga sp. as a new species in Iran. J Arthropod Borne Dis. 9: 161–175. [PMC free article] [PubMed] [Google Scholar]
- 8.Nazari M, Alipourian Motlagh B, Nasirian H. (2016) Toxicity of cypermethrin and chlorpyrifos against German cockroach [Blattella germanica (Blattaria: Blattellidae)] strains from Hamadan, Iran. Pak J Biol Sci. 19: 259–264. [DOI] [PubMed] [Google Scholar]
- 9.Jeong KY, Son M, Lee JH, Hong CS, Park JW. (2015) Allergenic characterization of a novel allergen, homologous to chymotrypsin, from German cockroach. Allergy, Asthma and Immunol Res. 7(3): 283–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rabito FA, Carlson JC, He H, Werthmann D, Schal C. (2017) A single intervention for cockroach control reduces cockroach exposure and asthma morbidity in children. J Allergy Clin Immunol. 140(2): 565–570. [DOI] [PubMed] [Google Scholar]
- 11.Salehzadeh A, Tavacol P, Mahjub H. (2007) Bacterial, fungal and parasitic contamination of cockroaches in public hospitals of Hamadan, Iran. J Vector Borne Dis. 44: 105–110. [PubMed] [Google Scholar]
- 12.Kinfu A, Erko B. (2008) Cockroaches as carriers of human intestinal parasites in two localities in Ethiopia. Trans R Soc Trop Med Hyg. 102: 1143–1147. [DOI] [PubMed] [Google Scholar]
- 13.Isaac C, Orue PO, Iyamu MI, Ehiaghe JI, Isaac O. (2014) Comparative analysis of pathogenic organisms in cockroaches from different community settings in Edo State, Nigeria. Korean J Parasitol. 52: 177–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sayyad S, Vahabi A, Vahabi B, Sayyadi M, Sahne SH. (2016) Investigation of bacteriological infections of the American cockroaches in Paveh City, Kermanshah Province. Mater Sociomed. 28(1): 17–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yones DA, Galal LA, Abdallah AM, Zaghlol KS. (2015) Effect of enteric parasitic infection on serum trace elements and nutritional status in upper Egyptian children. Trop Parasitol. 5(1): 29–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sinniah B, Hassan AKR, Sabaridah I, Soe MM, Ibrahim Z, Ali O. (2014) Prevalence of intestinal parasitic infections among communities living in different habitats and its comparison with one hundred and one studies conducted over the past 42 years (1970 to 2013) in Malaysia. Trop Biomed. 31(2): 190–206. [PubMed] [Google Scholar]
- 17.Ahmad A, Ghosh A, Schal C, Zurek L. (2011) Insects in confined swine operations carry a large antibiotic resistant and potentially virulent enterococcal community. BMC Microbiol. 11: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Federal Republic of Nigeria (2010) 2006 Population and Housing Census: Priority Table Volume III: Population Distribution by Sex, State, LGA and Senatorial District. National Population Commission, Abuja, Nigeria. [Google Scholar]
- 19.Burgess NRH. (1993) Cockroaches (Blattaria). In: Lane RP, Crosskey RW. (Eds) Medical Insects and Arachnids. Chapman and Hall, London, pp. 473–482. [Google Scholar]
- 20.WHO (1991) Basic Laboratory Methods in Medical Parasitology. World Health Organization, Geneva. [Google Scholar]
- 21.Adegbola RA, Demba E, DeVeer G, Todd J. (1994) Cryptosporidium infection in Gambian children less than five years of age. J Trop Med Hyg. 97: 103–107. [PubMed] [Google Scholar]
- 22.WHO (1994) Bench Aids for the Diagnosis of Intestinal Parasites. World Health Organization, Geneva. [Google Scholar]
- 23.R Core Team (2015) R: A language and environment for statistical computing. R Foundation for Statistical computing, Vienna, Austria. [Google Scholar]
- 24.Bala AY, Sule H. (2012) Vectorial potential of cockroaches in transmitting parasites of medical importance in Arkilla, Sokoto, Nigeria. Niger J Basic Appl Sci. 20(2): 111–115. [Google Scholar]
- 25.Etim SE, Okon OE, Akpan PA, Ukpong GI, Oku EE. (2013) Prevalence of cockroaches (Periplanata americana) in households in Calabar: Public health implications. J Public Health Epidemiol. 5(3): 149–152. [Google Scholar]
- 26.Chamavit P, Sahaisook P, Niamnuy N. (2011) The majority of cockroaches from the Samutprakarn Province of Thailand are carriers of parasitic organisms. EXCLI J. 10: 218–222. [PMC free article] [PubMed] [Google Scholar]
- 27.Pullan RL, Smith JL, Jasrasaria R, Booker SJ. (2014) Global numbers of infection and disease burden of soil transmitted helminth infections in 2010. Parasit Vectors. 7: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Henriquez-Camacho C, Gotuzzo E, Echevarria J, White AC, Jr, Terashima A, Samalvides F, Pérez-Molina JA, Plana MN. (2016) Ivermectin versus albendazole or thiabendazole for Strongyloides stercoralis infection. Cochrane Database Syst Rev. (1): CD007745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Osman M, El Safadi D, Cian A, Benamrouz S, Nourrisson C, Poirier P, Pereira B, Razakandrainibe R, Pinon A, Lambert C, Wawrzyniak I, Dabboussi F, Delbac F, Favennec L, Hamze M, Viscogliosi E, Certad G. (2016) Prevalence and risk factors for intestinal protozoan infections with Cryptosporidium, Giardia, Blastocystis and Dientamoeba among schoolchildren in Tripoli, Lebanon. PLoS Negl Trop Dis. 10(3): e0004496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Skappak C, Akierman S, Belga S, Novak K, Chadee K, Urbanski SJ, Church D, Beck PL. (2014) Invasive amoebiasis: a review of Entamoeba infections highlighted with case reports. Can J Gastroenterol Hepatol. 28: 355–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.WHO (2016) Preventable epilepsy: Taenia solium infection burdens economies, societies and individuals: A rationale for investment and action. WHO/HTM/NTD/NZD/2016.1. World Health Organization, Geneva. [Google Scholar]
- 32.McManus DP, Gray DJ, Zhang W, Yang Y. (2012) Diagnosis, treatment, and management of echinococcosis. BMJ. 344: e3866. [DOI] [PubMed] [Google Scholar]
- 33.Ajero CMU, Ukaga CN, Ebirim C. (2011) The role of cockroaches (Blatta orientalis and Periplaneta americana) in mechanical transmission of parasites in households in Owerri, South East Nigeria. Niger J Parasitol. 32(2): 153–156. [Google Scholar]
- 34.El-Sherbini GT, El-Sherbini ET. (2011) The role of cockroaches and flies in mechanical transmission of medical important parasites. J Entomol Nematol. 3: 98–104. [Google Scholar]
- 35.WHO (2004) Integrated guide to sanitary parasitology. Regional Office for the Eastern Mediterranean. WHO Regional Centre for Environmental Health Activities, Document WHO-EM/CEH/121/E. Amman, Jordan. [Google Scholar]
- 36.Al-Mayali HH, Al-yaqoobi MSM. (2010) Parasites of cockroach, P. americana (L), in Al-Diwaniya Province, Iraq. J Thi-Qar Sci. 2(3): 93–104. [Google Scholar]
- 37.Menasria T, Tine S, Mahcene D, Benammar L, Megri R, Boukoucha M, Debabza M. (2015) External bacterial flora and antimicrobial susceptibility patterns of Staphylococcus spp. and Pseudomonas spp. isolated from two household cockroaches, Blattella germanica and Blatta orientalis. Biomed Environ Sci. 28 (4): 316–320. [DOI] [PubMed] [Google Scholar]
- 38.Lau YL, Anthony C, Fakhrurrazi SA, Ibrahim J, Ithoi R, Mahmud R. (2013) Real-time PCR assay in differentiating Entamoeba histolytica, Entamoeba dispar and Entamoeba moshkovskii infections in Orang Asli settlements in Malaysia. Parasit Vectors. 6(1): 250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jimenez JA, Rodriguez S, Moyano LM, Castillo Y, Garcia HH. (2010) Differentiating Taenia eggs found in human stools - does Ziehl Neelsen staining help? Trop Med Int Health. 15(9): 1077–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Salant H, Abbasi I, Hamburger J. (2012) The development of a loop-mediated isothermal amplification method (LAMP) for Echinococcus granulosus coprodetection. Am J Trop Med Hyg. 87: 883–887. [DOI] [PMC free article] [PubMed] [Google Scholar]


