Abstract
Background:
Malaria and dengue are the most widespread infectious diseases of tropical countries with an estimated 219 and 50 million cases globally. The aim of the proposed study was to find out discriminating clinical features of falciparum malaria and dengue.
Method:
Falciparum malaria was diagnosed by looking at the ring and gametocyte stages by microscopic examination in Giemsa stained slides. Dengue was diagnosed by ELISA for dengue-specific IgM and IgG. Liver enzymes (AST and ALT) and kidney markers (creatinine and urea) were estimated by standard biochemical techniques.
Result:
AST and ALT showed similar rise in both, severe malaria and dengue patients but it was much pronounced in dengue haemorrhagic fever where it attained 3–4 folds increase. Creatinine and urea showed higher levels in dengue compared to malaria. Thrombocytopenia (76.27%), convulsions (18.64%) and hepatic dysfunction (5.08%) were more prominent in dengue than that in malaria where these parameters were 50.89, 7.14 and 2.67%, respectively. Conversely, cases with anaemia, splenomegaly and jaundice were three times more in falciparum malaria. Acute renal failures and neurological sequelae were noticed in slightly higher number of dengue patients.
Conclusion:
Thrombocytopenia and hepatic dysfunction were more common in dengue, while anaemia, splenomegaly, jaundice and convulsions were more frequent in falciparum malaria. Neurological sequelae and cases of acute renal failure were almost equal in both the infections.
Keywords: Malaria, Dengue, Clinical features, Liver enzymes, Kidney markers
Introduction
Dengue and malaria are the most common mosquito-borne diseases which emerged as a global public health problem. Single stranded RNA Flavivirus (DENV 1–4) is the causative agent of dengue and is generally transmitted by Aedes aegypti to around 50 million people of 2.5 billion population at risk throughout the world (1). Disease caused by dengue virus manifests as relatively minor febrile illness called dengue fever (DF) to a life-threatening dengue haemorrhagic fever (DHF)/ Dengue shock syndrome (DSS) (2). Malaria is another vector-borne disease caused by different species of Plasmodium and is transmitted by anopheline vectors in tropical countries to an estimated number of 219 million people resulting in 660,000 deaths every year throughout the world (3). Patients suffering from these diseases show somewhat similar clinical and biological presentation with variable pathological conditions (4). In malaria as well as dengue, patients suffer from high fever, headache, vomiting and severe body pain.
Majority of the dengue patients infected with flavivirus are asymptomatic but in a small proportion of cases the disease develops into the life-threatening DHF/ DSS, resulting in blood plasma leakage, bleeding and low levels of blood platelets with mortality rate of around 5% (5). Elevated values of liver enzymes were also observed in more than half of the patients in Brazil (6). Like dengue, hepatic dysfunction and renal failures were observed in patients who suffered from Plasmodium falciparum (7–9).
Since many symptoms and clinical features are common in falciparum malaria and dengue, the proposed study entitled “Discriminating clinical and biological features in malaria and dengue patients” was carried out to ascertain the response of biological markers in vital organs which could be of help in differential diagnosis of dengue and malaria.
Materials and Methods
The present study was carried out during 2014–15 from Makkah region of Saudi Arabia and Northern zone of India based on the confirmed dengue and malaria cases. A total of 380 and 183 suspected cases were examined for the positivity of malaria and dengue, respectively.
We have used the leftover blood samples taken for diagnosis by the doctors after taking consent of the patients and ethical approval of the respective institutes.
Clinical profiles of the febrile patients were recorded at the time of their admission in the hospital. Thick and thin blood smears were prepared on glass slides by pricking the finger of the patients complaining for high fever, vomiting and headache. Thin blood films were fixed in methanol, stained with Giemsa and examined under Nikon Eclipse E-600 research microscope at x1000. Typical ring and gametocyte stages were observed for the specific diagnosis of P. falciparum.
Patients with fever, headache, convulsions, body ache and haemorrhagic manifestations were tested for dengue fever (DF) and dengue haemorrhagic fever (DHF). Clinical symptoms such as high fever, headache, thrombocytopenia, splenomegaly, convulsions and neurological sequelae were observed and compared in the confirmed cases of falciparum malaria, dengue and dengue haemorrhagic fever. For non-structural protein 1 (NS1) antigen detection, ELISA was performed in patients who had fever for 3–5d, while those who had high fever for more than 5 d after the onset of illness were tested for dengue specific IgM and IgG. Serological studies were performed with the qualitative dengue IgM capture ELISA and dengue indirect IgG ELISA (Pan Bio Ltd, Brisbane, Australia). Dengue patients were treated with acetaminophen and symptomatic therapy in the form of fluid and antipyretics whereas patients suffering from falciparum malaria were advised artesunate, as chloroquine and fansidar are not responding in a fairly good number of cases in our experimental areas.
Thrombocyte counts were recorded in both malaria and dengue patients. For the estimation of liver and kidney markers, blood was collected in sterile glass test tubes by vein puncture of the malaria and dengue patients after taking their consent. These samples were allowed to clot at room temperature and then transferred to refrigerator at 5 °C for 12h to squeeze the clot. Serum was collected and centrifuged at 2000rpm for 5min to remove probable contaminations and finally stored in sterilized vials at −80 °C until analyzed for liver enzymes and kidney markers. Aspartate transaminase (AST) and alanine transaminase (ALT) were estimated (10), while creatinine and urea were estimated as already reported (11, 12), respectively.
Follow up of all the patients was done till they were discharged from the hospital. All the dengue and malaria cases were monitored for their clinical, biochemical and haematological profiles. Clinical parameters of dengue and malaria positive patients were studied and compared to observe prominent discriminating features of these infections.
Statistical analysis
All clinical parameters were examined by chi-square test using GraphPad InStat 3.06 software (GraphPad Software, Inc., California, USA), and the level of significance was set at P< 0.05. The results were expressed as mean ± standard deviation (SD) using SPSS (version 17.0, SPSS inc., Chicago, IL, USA). Graphs were plotted on Sigma plot 12.0.
Results
Patient characteristics
During peak season, 380 suspected cases with high fever and headache were examined for malaria from Aligarh district during 2014–15, out of which 232 (61%) were positive for malaria. Out of 232 positive cases 112 (48.27%) patients were of P. falciparum, while 120 (51.72%) of P. vivax. As for dengue is concerned, 183 suspected cases having high fever, headache and body ache were examined during its active transmission season in 2014–15 from the northeastern region of India and Makkah region of Saudi Arabia, out of which 59 (32.77%) were found positive for dengue. Majority of the patients 47 (80%) were of classical dengue, while 12 (20%) patients showed the symptoms of dengue haemorrhagic fever. Overall, 40 (67.79%) patients were found positive for IgM and 12 (20.33%) were positive for IgG. A total of 26 (44.06%) patients showed both IgM and IgG. During illness, gum bleeding and skin petechiae were seen in 3.39 and 6.80% patients, respectively.
Symptoms in the patients
All the malaria and dengue patients had fever which ranged from 101 to 105 °C. Headache, nausea, vomiting, dizziness and joint pain were the other prominent symptoms in both falciparum malaria and dengue patients (Fig. 1). In this study prescribed dose of artemisinin was given to treat thirty two falciparum malaria patients. Parasitaemia was cleared in all the cases except 1 (3.1%), in which parasites reappeared after 23 days, indicating resistance as recrudescence occurred. Symptoms in patients infected with P. falciparum and dengue showed significant differences in skin rash, sweating, vomiting and body ache, but the values relating fever, headache, nausea, dizziness and abdominal pain were very close in both the diseases and did not show significant differences (Fig. 1).
Fig. 1.
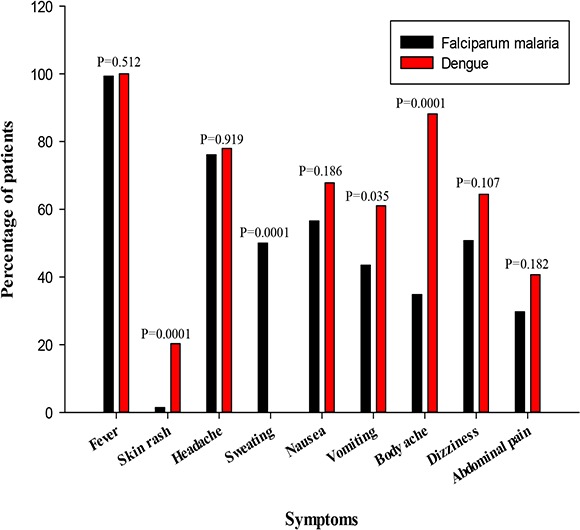
Symptoms in the patients of falciparum malaria and dengue
Haematological and systemic disorders
There was contrasting difference as for splenomegaly is concerned, it was 43.75% in P. falciparum infection compared to 10.17% of dengue showing P-value 0.0001. Thrombocytopenia in dengue patients was much higher compared to falciparum malaria (76.27: 50.89, P= 0.002). Convulsions and hepatic dysfunction were more common in dengue (18.64 and 5.08%) than in malaria (7.17 and 2.67%). In contrast, jaundice was more common in malaria patients (6.25%), than in dengue (1.70%). Acute renal failures and neurological sequelae were also slightly more pronounced in falciparum malaria compared to dengue in terms of percentage but the difference was not significant (Fig. 2). Anaemia was rarely observed in healthy individuals or patients having low or mild infections in both the diseases.
Fig. 2.
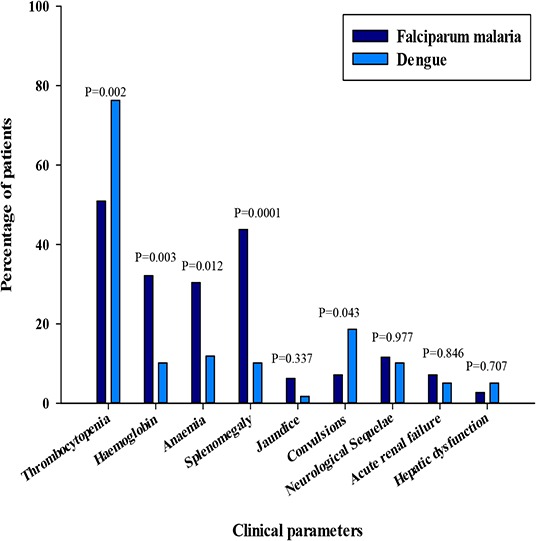
Haematological and systemic disorders in falciparum malaria and dengue patients
Liver and Kidney markers
Liver damage was observed as a common complication in both malaria as well as dengue infections. Liver function test (LFT) was performed by estimating serum aspartate transaminase (AST) and alanine transaminase (ALT). The normal values of these enzymes in applied technique were 0–45IU/L and 3–60IU/L for AST and ALT, respectively. Both liver enzymes and kidney markers were almost within normal range in falciparum malaria and dengue fever (DF) having low to moderate infections. Slightly raised values of AST (64.70± 4.38, 98.83±22.15IU/L) and ALT (79.23±5.70, 93.83±21.15IU/L) were recorded in severe cases of falciparum malaria and dengue fever, respectively. Almost five times increase in the level of AST (215.50±41.71IU/L) and three times in ALT (176.00±19.79IU/L) were recorded in severe dengue haemorrhagic fever followed by mild cases of DHF where AST and ALT were recorded as 108.00±32.35 and 75.33±12.66IU/L, respectively. However, these enzymes remained within normal range in light infections.
Impairments of renal function in P. falciparum and dengue infections were assessed by measuring elevated creatinine and urea in serum. Patients suffering from falciparum malaria with high parasitemia, severe dengue and dengue haemorrhagic fever showed an increase in the level of creatinine to 158.76±6.99, 186±11.50 and 187.00±5.65μmoles/L, respectively compared to the normal range of 72–126 μmoles/L, while it was within range in low and mild infections with the exception of DHF, which showed a slightly raised value (135.33±25.38μmoles/L) in mild DHF patients. Serum urea too showed slightly higher values in severe cases of malaria, DF and DHF, which were recorded as 9.60±1.32, 8.10±0.91 and 10.20±1.97mmoles/L against the normal range of 3.0–6.0mmoles/L (Fig. 3). It was slightly raised (6.90±0.36mmoles/L) in mild cases of DHF and was within normal range in falciparum malaria and dengue fever.
Fig. 3.
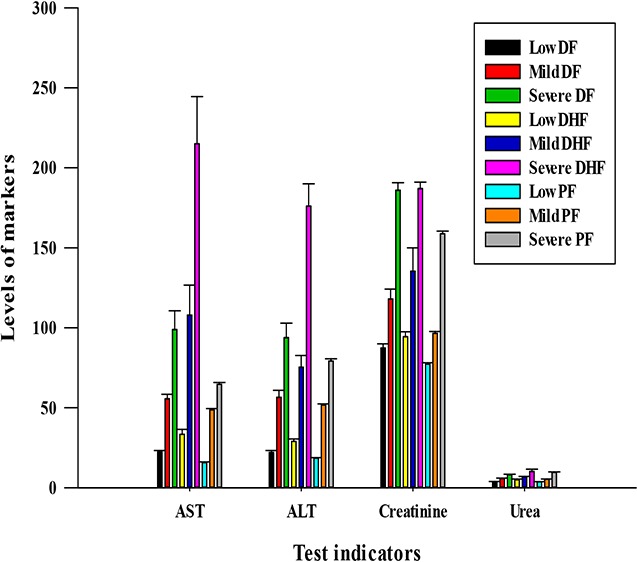
Liver and kidney markers in falciparum malaria, dengue and dengue haemorrhagic fever
Discussion
Malaria and dengue are true haematological infectious diseases as they affect most of the blood components of patients. Anaemia, leucocytosis and thrombocytopenia are the commonly associated haematological complications in falciparum malaria, and have received much attention due to their associated mortality (13–15). In dengue and dengue haemorrhagic fever abnormalities manifest as bone marrow suppression, leucopenia and thrombocytopenia (16–18).
All the malaria, dengue and dengue haemorrhagic fever patients had high fever as earlier observed for these infections in Jazan and Makkah region of Saudi Arabia, Pakistan and India (18–21). In our study headache, nausea and vomiting were slightly more pronounced in dengue (77.96, 67.79 and 61.01%) compared to falciparum malaria (76.08, 56.52 and 43.47%). Headache and nausea in dengue recorded in 75 and 69% patients in Makkah region (22) were almost the same as recorded in our study. We observed vomiting in 61.01% dengue patients which was slightly less (43.47 %) in malaria patients. Earlier workers noticed vomiting in slightly less number (56%) of patients of dengue in Pakistan. Compared to malaria (34.78%), dengue patients were more frequently associated (88.13%) with body ache in the present study. In contrast body ache was recorded in only 34.44% dengue patients in Pakistan (18) which was less than half of what we observed in India and Saudi Arabia. Abdominal pain and dizziness were slightly higher in dengue patients (64.40 and 42.85%) as compared to falciparum malaria (50.72 and 29.71 %) in our study.
In our study, thrombocytopenia was recorded in 76.27% cases which have similarity with the earlier findings of Pakistan and India where thrombocytopenia was recorded in 60 to 79.49% dengue patients (16, 17, 20). Thrombocytopenia was less frequently associated with falciparum malaria and was noted in 50.89% patients in our study which is almost same as earlier observed in Pakistan where 54.5% cases of P. falciparum had thrombocytopenia (23). In contrast its higher value (77%) was recorded in Madhya Paradesh, India (24). Moderate degree of thrombocytopenia was observed in 26 to 50% DF cases (25). Nevertheless, deep and rapid decrease in platelet count is one of the main criteria for severe dengue (1). Thrombocytopenia was observed in DF and DHF in our study where thrombocyte counts varied from 20,000/mm3 to 140,000/mm3. Forty four percent of dengue patients had low thrombocyte counts of around 50,000/mm3. Continuous drop in thrombocyte count was observed by Withana et al. (26) who reported a low count of 36,000/mm3 on 7th day of illness. It may be due to the suppression of bone marrow at an early stage which resulted in immune-mediated platelet destruction, vasculopathy and coagulopathy as earlier hypothesized (27). In fact there is an increased utilization of platelets during coagulopathy which play a major role in the induction of thrombocytopenia. Unlike dengue, thrombocytopenia was noticed in comparatively lesser number of falciparum malaria patients ranging between 80,000/mm3 and 145,000/mm3. It might be due to the invasion of large number of P. falciparum parasites which suppress bone marrow and spleen which might have resulted in immune-mediated platelet destruction which generally reflects in chronic cases.
Anaemia is an inevitable consequence in malaria due to hemolysis. We observed anaemia in 30.35% P. falciparum patients. In contrast, higher numbers of anaemia cases (60%) in P. falciparum infection were recorded in Saudi Arabia (28). Low counts for anaemia of around 13% for P. falciparum was also recorded in Mumbai (29). This variation might be due to severity of infection and level of immunity against the parasite in patients of falciparum malaria in different countries having endemic and non-endemic pockets. Plasmodium falciparum within the RBC modifies the erythrocytes in several ways and the cell membranes become less deformable, rigid and fragile, resulting in hemolysis and anaemia. Alteration in membranes of uninfected red blood cells by addition of glycosylphosphatidylinositol causes increased clearance of cells especially in P. falciparum infections that contributes to anaemia (30). Anaemia was reported only in 11.86% cases of dengue. Low rate of anaemia in dengue was probably because of a few systemic hemorrhages due to endothelial rupture as dengue virus targets monocytes or macrophages which are responsible for dissemination of virus (31).
Splenomegaly was more pronounced in P. falciparum infections (43.75%) compared to dengue where it was observed only in 10.17 % cases. Higher rate of splenomegaly in falciparum malaria was probably due to phagocytosis of parasitized R.B.Cs which got accumulated in the spleen for clearance. Almost similar enlargement in around 39–45% P. falciparum malaria patients was observed in Saudi Arabia (19, 32) However, much higher rate of splenomegaly (71%) was recorded in falciparum malaria from Rajasthan, India (33). This variation might be due to the difference in the immune status of the patients in different malaria transmission regions.
In our study, 6.25% P. falciparum patients had jaundice which is much less than that recorded in Sudan and Pakistan where it was reported in 14 and 43% patients (34, 35). We noticed jaundice in only 1.70% dengue patients from our study area which is much less than that in falciparum malaria. Slightly higher rate of jaundice (3.1%) was reported in Vietnam (36). It appears that jaundice is an outcome of the combined effects of hemolysis and liver dysfunction and probably this is the reason why jaundice was more abundant in severe cases in P. falciparum infections where destruction of RBC is quite high. Liver dysfunctions have been documented in earlier findings for both P. falciparum and dengue infections (26, 37, 38).
In our study, mean AST and ALT values were 64.70±4.38 and 79.23±5.70 in severe P. falciaprum cases, but the level of enzymes were within the normal range in those who had mild and low parasitaemia. Comparatively lower levels of AST and ALT (33.76–37.6 and 34.8–39.74) were recorded for mild P. falciparum infections in Nigeria (9, 39). In contrast, higher levels of AST and ALT (98.83±28.79 and 93.83±22.15IU/L) were recorded in the patients of dengue fever, which was still high in dengue haemorrhagic fever and was observed as 215±41.71 and 176±19.79 in our study area. Similar increase in AST and ALT was observed in dengue patients of Brazil (26). Significant rise was recorded in the level of liver enzymes in the severely infected patients as compared to mild and low infections. Liver damage in dengue infection ranges from mild lesion to fulminant hepatitis (6). It appears that the parasites during their hepatic phase destroy membrane of parasitized cells leading to leakage of the liver enzyme into the blood circulation. However, damage to hepatocytes and enzyme leakage due to toxicity of antimalarial administered to treat malaria cannot be ruled out. Increased liver enzymes may result in liver dysfunction in patients having high parasitaemia in malaria. Like malaria in dengue infections, inflammatory process in the liver parenchyma is provoked by the virus, reducing the lumen of biliary canaliculi, causing obstruction which may lead to bilirubinemia or even jaundice in a few cases (40). In dengue haemorrhagic fever hepatic injury leading to coagulopathy might have caused hemorrhage (41).
Acute renal failure (ARF) is a common cause of morbidity and mortality in severe P. falciparum and dengue infections. We estimated kidney markers such as creatinine and urea in both malaria and dengue patients and recorded an elevated levels of these markers in both the diseases in our experimental areas. In dengue and malaria, levels of creatinine were raised to 187±5.65 and 158.76±6.99 μmoles/L, against normal range of 72–126 μmoles/L. In severe P. falciparum, DF and DHF patients, urea was raised to 9.6±1.32, 8.10±0.91 and 10.20±1.97mmoles/L, respectively. We observed acute renal failures (ARF) in 7.14% P. falciparum. Comparatively, much higher rate of ARF of 19% was recorded from the patients in Pakistan (42). Low rate of ARF (5.08%) was observed in dengue patients of our study area in India and Saudi Arabia while earlier workers reported ARF ranging from 3.3–10.8% in Taiwan, Pakistan and India (17, 43, 44). Usually ARF occurs in non-immune individuals especially in P. falciparum infections with high parasitaemia, but we noticed renal failures in dengue infections as well which warrants immediate attention and change in treatment strategy especially in resistant P. falciparum infections. Physiological changes associated with P. falciparum infection make erythrocytes adherent to each other as well as to the walls of capillary vessels which cause mechanical obstructions. In addition to this, Plasmodium encroach and cause inflammation of glomerular capillaries resulting in altered renal microcirculation by adherent RBCs and increased accumulation of creatinine which damage the kidney tissue. This might be the probable cause of renal failures as indicated earlier (7).
Neurological sequelae are associated with damaged neurons which manifest as impaired consciousness, convulsions which often lead to coma. Cases of convulsions were more frequent in dengue (18.64%) than in malaria (7.14%) indicating more neurological damage in dengue than in falciparum malaria. Immune mediated mechanism and direct tropic effect of virus are implicated in neurological manifestations, as dengue antigen has been demonstrated in the brain of few patients with dengue encephalitis (45). We observed neurological sequelae in 11.60% P. falciparum patients. Other workers observed convulsions and other neurological deficits in 12–21% falciparum malaria patients of Sudan, Thailand and India (35, 46–48). Still higher rate (28%) of neurological sequelae was reported in P. falciparum infections in Mali (49). Like dengue, in P. falciparum infection too, parasites invade brain tissue and cause tropic effect on neurons which probably results in neurological deficits. We observed neurological sequelae in 10.17% dengue patients which is almost same as that of P. falciparum
Conclusion
Thrombocytopenia and hepatic dysfunction were more common in dengue, whereas anaemia, splenomegaly, jaundice and convulsions were more frequent in falciparum malaria. Neurological sequelae and cases of acute renal failure were almost equal in both the types of infections.
Acknowledgments
This work was funded by the Deanship of Scientific Research (DSR), King Abdulaziz University, Jeddah, under grant No. (142-279-D1435). The authors, therefore, acknowledge with thanks DSR technical and financial support. The authors have no conflict of interest related to this work.
References
- 1.World Health Organization (2009) Dengue guidelines for diagnosis, treatment, prevention and control. TDR. Available at: https://bit.ly/2aQ2sZi [PubMed]
- 2.Whitehorn J, Farrar J. (2010) Dengue. Br Med Bull. 95: 161–173. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organisation (2012) World malaria report (2012) summary. Available at: https://bit.ly/1i5xZBT
- 4.Epelboin L, Boulle C, Ouar-Epelboin S, Hanf M, Dussart P, Djossou F, Nacher M, Carme B. (2013) Discriminating malaria from dengue fever in endemic areas: clinical and biological criteria, prognostic score and utility of the C-reactive proteins: retrospective matched- pair study in French Guiana. PLoS Negl Trop Dis. 7(9): e2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Noisakran S, Perng GC. (2008) Alternate hypothesis on the pathogenesis of dengue hemorrhagic fever (DHF)/dengue shock syndrome (DSS) in dengue virus infection. Exp Biol Med. (Maywood). 233(4): 401–408. [DOI] [PubMed] [Google Scholar]
- 6.de Souza LJ, Nogueira RM, Soares LC, Soares CE, Ribas BF, Alves FP, Vieira FR, Pessanha FE. (2007) The impact of dengue on liver function as evaluated by aminotransferase levels. Braz J Infect Dis. 11(4): 407–410. [DOI] [PubMed] [Google Scholar]
- 7.Das BS. (2008) Renal failure in malaria. J Vector Borne Dis. 45: 83–97. [PubMed] [Google Scholar]
- 8.Ogbadoyi EO, Tsado RD. (2009) Renal and hepatic dysfunction in malaria patients in Minna, North Central Nigeria. OJHAS. 8(3): 1–6. [Google Scholar]
- 9.Onyesom I, Onyemakonor N. (2011) Level of parasitaemia and changes in some liver enzymes among malarial infected patients in Edo-Delta region of Nigeria. Curr Res J Biol Sci. 3: 78–81. [Google Scholar]
- 10.Wilkinson JH, Baron DN, Moss DW, Walker PG. (1972) Standardization of clinical enzyme assays: a reference method for aspartate and alanine transaminase. J Clin Path. 25: 940–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thomas L. (1998) Clinical laboratory diagnostics. TH-Books Verlagsgesellschaft, Frankfurt, pp. 366–374. [Google Scholar]
- 12.Kaplan A. (1965) Standard methods of clinical chemistry. Academic press, New York, USA, pp. 245–256. [Google Scholar]
- 13.Wickramasinghe SN, Abdalla SH. (2000) Blood and bone marrow changes in malaria. Baillieres Best Pract Res Clin Haematol. 13: 277–299. [DOI] [PubMed] [Google Scholar]
- 14.Jadhav UM, Patkar VS, Kadam NN. (2004) Thrombocytopaenia in malaria-correlated with type and severity of malaria. J Assoc Physicians India. 52: 615–618. [PubMed] [Google Scholar]
- 15.Rodriguez-Morales AJ, Sanchez E, Vargas M, Piccolo C, Colina R, Arria M. (2006) Anaemia and thrombocytopenia in children with Plasmodium vivax malaria. J Trop Pediatr. 52(1): 49–51. [DOI] [PubMed] [Google Scholar]
- 16.Riaz MM, Mumtaz K, Khan MS, Patel J, Tariq M, Hilal H, Siddiqui SA, Shezad F. (2009) Outbreak of dengue fever in Karachi 2006: a clinical perspective. J Pak Med Assoc. 59(6): 339–344. [PubMed] [Google Scholar]
- 17.Mehra N, Patel A, Abraham G, Reddy YN, Reddy YN. (2012) Acute kidney injury in dengue fever using acute kidney injury network criteria: incidence and risk factors. Trop Doct. 42(3): 160–162. [DOI] [PubMed] [Google Scholar]
- 18.Hasan SR, Riaz M, Jafri FA. (2013) Characteristics and outcome of dengue infection; clinical perspective from a secondary care hospital of Karachi. Pak J Med Sci. 29(1): 115–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Banzal S, Ayoola FA, El Sammani FE, Rahim SI, Subramanium P, Gadour MOE, Jain AK. (1999) The clinical pattern and complications of severe malaria in the Jazan region of Saudi Arabia. Ann Saudi Med. 1: 378–380. [DOI] [PubMed] [Google Scholar]
- 20.Ayyub M, Khazindar AM, Lubbad EH, Barlas S, Alfi AY, Al-Ukayli S. (2006) Characteristics of dengue fever in a large public hospital, Jeddah, Saudi Arabia. J Ayub Med Coll Abbottabad. 18: 9–13. [PubMed] [Google Scholar]
- 21.Gurumurthy R, Gayathri KB, Seethamma R, Bhargav PRK. (2014) Clinical spectrum and course of dengue fever during pregnancy: Institutional experience from South India. IOSR-JDMS. 13(3): 93–95. [Google Scholar]
- 22.Khan NA, Azhar EI, El-Fiky S, Madani HH, Abuljadial MA, Ashshi AM, Turkistani AM, Hamouh EA. (2008) Clinical profile and outcome of hospitalized patients during first outbreak of dengue in Makkah, Saudi Arabia. Acta Trop. 105(1): 39–44. [DOI] [PubMed] [Google Scholar]
- 23.Rasheed A, Saeed S, Khan SA. (2009) Clinical and laboratory findings in acute malaria cases by various Plasmodium species. J Pak Med Assoc. 54: 220–223. [PubMed] [Google Scholar]
- 24.Gupta NK, Bansal SB, Jain UC, Sahare K. (2013) Study of thrombocytopenia in patients of malaria. Trop Parasitol. 3(1): 58–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eapen CE, Eliasi E, Goel A, John TJ. (2015) Hypothesis of mechanism of thrombocytopenia in severe dengue, providing clues to better therapy to save lives. Curr Sci. 108(2): 168–169. [Google Scholar]
- 26.Withana M, Rodrigo C, Chang T, Karunanayake P, Rajapakse S. (2014) Dengue fever presenting with acute cerebellitis: a case report. BMC Res Notes. 7: 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clémente-Bartoli A, Epelboin L, Daval S, Fabre I, Lamaury I, Beaucaire G. (2012) Deep thrombocytopenia due to dengue fever in a patient splenectomized for immune thrombocytopenia. Presse med. 41(11): 1151–1153. [DOI] [PubMed] [Google Scholar]
- 28.Bashawri LA, Mandil AA, Bahnassy AA, Ahmed MA, Al-Shamsi MA, Bukhari HA. (2001) Epidemiological profile of malaria in a university hospital in eastern region of Saudi Arabia. Saudi Med J. 22: 133–138. [PubMed] [Google Scholar]
- 29.Limaye SL, Londhey VA, Nabar ST. (2012) The study of complications of vivax malaria in comparision with falciparum malaria in Mumbai. J Assoc Physicians India. 60: 15–18. [PubMed] [Google Scholar]
- 30.Brattig NW, Kowalsky K, Liu X. (2008) Plasmodium falciparum glycosylphosphatidylin-ositol toxin interacts with the membrane of non-parasitized red blood cells: a putative mechanism contributing to malaria anemia. Microbes Infect. 10: 885–891. [DOI] [PubMed] [Google Scholar]
- 31.Al-hoot MA, Wang SM, Sekaran SD. (2011) Inhibition of dengue virus entry and multiplication into monocytes using RNA interference. PLoS Negl Trop Dis. 5(11): e1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Malik GM, Seidi O, El-Taher AM, Mohammad AS. (1998) Clinical aspect of malaria in the Asir region, Saudi Arabia. Ann Saudi Med. 18: 15–17. [DOI] [PubMed] [Google Scholar]
- 33.Kochar DK, Kaswan K, Kochar SK, Sirohi P, Pal M, Kochar A. (2006) A comparative study of regression of jaundice in patients of malaria and acute viral hepatitis. J Vector Borne Dis. 43: 123–129. [PubMed] [Google Scholar]
- 34.Ahsan T, Ali H, Bakht SF, Ahmad N, Farooq MU, Shaheer A, Mahmood T. (2008) Jaundice and falciparum malaria: Changing trends in clinical presentation-A need for awareness. J Pak Med Assoc. 58: 616–621. [PubMed] [Google Scholar]
- 35.Abdallah TH, Abeen MT, Ahmad IS, Hamdan ZH, Magzoub M, Adam I. (2013) Plasmodium falciparum and Plasmodium vivax malaria among adults at Kassala hospital, Eastern Sudan. Malar J. 12: 148–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Phuong HL, de Vries PJ, Nga TTT, Giao PT, Hung LQ, Binh TQ, Nam NV, Nagelkerke N, Kager PA. (2006) Dengue as a cause of acute undifferentiated fever in Veitnam. BMC Infect Dis. 6: 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Premaratna R, Gunatilake AKE, de Silva NR, Tilakaratne Y, Fonseka MM, de Silva HJ. (2001) Severe hepatic dysfunction associated with falciparum malaria. Southeast Asian J Trop Med Public Health. 32(1): 70–72. [PubMed] [Google Scholar]
- 38.Abro AH, Ustadi AM, Abro HA, Abdau AS, Yunis NJ, Akaila SI. (2009) Jaundice with hepatic dysfunction in P. falciparum malaria. J Coll Physicians Surg Pak. 19: 363–366. [PubMed] [Google Scholar]
- 39.Uzuegbu UE, Emeka CB. (2011) Changes in liver function markers among malaria infected patients in Ikeja Lagos State, Nigeria. Curr Res J Biol Sci. 3: 172–174. [Google Scholar]
- 40.Lum LC, Lam SK, George R, Devi S. (1993) Fulminant hepatitis in dengue infection. Southeast Asian J Trop Med Public Health. 24(3): 467–471. [PubMed] [Google Scholar]
- 41.Rothman AL, Ennis FA. (1999) Immunopathogenesis of dengue hemorrhagic fever. Virology. 257: 1–6. [DOI] [PubMed] [Google Scholar]
- 42.Mannan JA, Ali H, Lal M. (2006) Acute renal failure associated with malaria. J Ayub Med Coll Abbottabad. 18(4): 47–52. [PubMed] [Google Scholar]
- 43.Lee IK, Liu JW, Yang KD. (2009) Clinical characteristics, risk factors, and outcomes in adults experiencing dengue hemorrhagic fever complicated with acute renal failure. Am J Trop Med Hyg. 80(4): 651–655. [PubMed] [Google Scholar]
- 44.Khalil MAM, Sarwar S, Chaudry MA, Maqbool B, Khalil Z, Tan J, Yaqub S, Hussain SA. (2012) Acute kidney injury in dengue virus infection. Clin Kidney J. 5(5): 390–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lum LC, Lam SK, Choy YS, George R, Harun F. (1996) Dengue encephalitis: A true entity? Am J Trop Med Hyg. 54: 256–259. [DOI] [PubMed] [Google Scholar]
- 46.Giha HA, Elghazali G, A-Elgadir TM, A-Elbasit IE, Eltahir EM, Baraka OZ, Khier MM, Adam I, Troye-Blomberg M, Theander TG, Elbashir MI. (2005) Clinical pattern of severe Plasmodium falciparum malaria in Sudan in an area characterized by seasonal and unstable malaria transmission. Trans R Soc Trop Med Hyg. 99: 243–251. [DOI] [PubMed] [Google Scholar]
- 47.Thanachartwet V, Desacorn V, Sahassananda D, Win KYKK, Supaporn T. (2013) Acute renal failure in patients with severe falciparum malaria: Using the WHO 2006 and RIFLE Criteria. Int J Nephrol. 2013: 841518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Khan W, Zakai HA, Asma U. (2014) Clinico-pathological studies of Plasmodium falciparum and Plasmodium vivax-malaria in India and Saudi Arabia. Acta Parasitol. 59(2): 206–212. [DOI] [PubMed] [Google Scholar]
- 49.Ngoungou EB, Poudiougou B, Dulac O, Dicko A, Boncoeur MP, Traoré AM, Coulibaly D, Keita MM, Preux PM, Doumbo OK, Druet-Canbanac M. (2007) Persistent neurological sequelae due to cerebral malaria in a cohort children from Mali. Rev Neurol. (Paris). 163: 583–588. [DOI] [PubMed] [Google Scholar]


