Short abstract
Objective
Postoperative cognitive dysfunction (POCD) is common after surgery in elderly patients and is associated with high morbidity. The molecular mechanisms responsible for POCD are unknown. Minocycline, an inhibitor of microglial activation, may be useful in treating and preventing POCD. We explored whether minocycline can inhibit microglial activation and prevent POCD in aged rats as a surgery model.
Methods
Rats aged 18 to 20 months were randomly allocated to the following groups: naïve, abdominal surgery alone, or minocycline injection before abdominal surgery. Hippocampal cytokine mRNA levels were measured at 3 hours, 1 day, 3 days, and 7 days after surgery, and microglial activation was measured at 3 hours and 7 days after surgery. Memory was assessed using the Morris water maze test.
Results
Surgery resulted in severe cognitive impairment in aged rats and induced a significant neuroinflammatory response and microglial activation. The use of minocycline can prevent microglial activation after surgery, but delayed microglial activation may occur. The use of minocycline may further impair memory after surgery.
Conclusion
Minocycline can restrain microglial activation and restrict the inflammatory response in the hippocampus early after surgery, but it may induce delayed microglial activation and cannot prevent POCD in aged rats.
Keywords: Minocycline, inflammation, microglia, aged rat, postoperative cognitive dysfunction, surgery model
Introduction
After surgery, aged patients often develop postoperative cognitive dysfunction (POCD), which can persist long after physical recovery. Many risk factors may exist for POCD, but age is certainly a major risk factor. 1 According to the International Study of Post-Operative Cognitive Dysfunction, 2 the prevalence of POCD in patients aged >60 years is 25.8% at 1 week after surgery. Cognitive dysfunction after noncardiac surgery is associated with increased mortality, a risk of leaving the labor market prematurely, and dependency on social transfer payment. 3
The molecular mechanisms responsible for POCD are unknown. The central inflammatory response, especially increased cytokines in the hippocampus, may be involved in the pathogenesis of many neurologic disorders.4–7 The central inflammatory response has also been reported in a rat model of surgery.8,9 Microglia are the resident innate immune cells in the central nervous system. When active, they can produce proinflammatory cytokine factors [such as interleukin (IL)-1β and tumor necrosis factor-α (TNF-α)] that are toxic to neurons. Microglial activation has also been reported in a rat model of POCD. 10
Minocycline, a tetracycline derivative that can restrain microglial activity, is an established anti-inflammatory drug. This drug can cross the blood–brain barrier and improve the symptoms of neurodegenerative diseases such as Alzheimer's disease, Huntington’s disease, and Down syndrome.11,12 Previous research has suggested that minocycline may be useful to treat or prevent POCD after surgery in elderly patients. 1 Therefore, in this study, we used the Morris water maze (MWM) test to assess rats’ cognitive function and examined whether minocycline can reduce microglial activity.
Method
Experimental protocol
During the first experiment, we examined the effects of the surgical procedure and minocycline injection before surgery on proinflammatory cytokine mRNA expression and microglial activation in the hippocampus. Aged rats were divided into 3 experimental groups of 48 rats each: rats that underwent surgery alone, rats that received minocycline [45 mg/kg body weight (BW)] intraperitoneally 10 min before surgery, and rats that were intraperitoneally injected with sterile saline to control for the potential effects of handling and injection stress. The rats in each group were killed by carbon dioxide asphyxiation 3 hours, 1 day, 3 days, and 7 days after treatment (n = 12 per time point). The proinflammatory cytokine mRNA expression and microglial activation in the hippocampus were determined using real-time polymerase chain reaction (PCR) or immunohistochemistry.
Next, the effects of surgery and preoperative minocycline injection on the cognitive function of the aged rats were determined by the MWM test. The rats were divided into three experimental groups of 12 rats each: rats that underwent surgery alone, rats that received minocycline (45 mg/kg BW) intraperitoneally 10 min before surgery, and rats that were intraperitoneally injected with sterile saline to control for the potential effects of handling and injection stress. All rats were submitted to the MWM test from 1 to 7 days after surgery. The first 3 days were considered training, and the following 4 days were considered testing.
Animals
Aged (18–19 months old) Sprague–Dawley male rats were housed in a temperature-controlled room (20°C–23°C) on a 12-hour light and 12-hour dark cycle with ad libitum access to food and water. All rats were allowed to adapt to their environment for 7 to 10 days before experimentation. All experimental procedures were performed in accordance with the Declaration of the National Institutes of Health Guide for Care and Use of Laboratory Animals. This study was approved by the Ethical Committee of the University of Electronic Science and Technology of China.
Surgical procedure
The rats were deeply anesthetized using intraperitoneal chloral hydrate (3 mg/10 g BW). After shaving and sterilizing the surgical site, a 0.5-cm incision was made through the skin and muscle wall of the ventral midline. A sterile probe was then inserted into the body cavity to gently manipulate the internal organs for 1 min, and the body cavity was probed again after 10 min. The animals were probed a total of three times. The surgery lasted 40 min. Three dissolvable sutures were used to close the muscle wall, and four silk thread sutures were used to close the skin. The animals that received postoperative analgesics were injected subcutaneously with buprenorphine (1.0 mg/10 g BW) upon recovery from anesthesia. The animals in the minocycline group received intraperitoneal minocycline (45 mg/kg BW) 10 min before surgery. The animals in the control group received saline to control for the effects of injection stress.
Spatial working memory: MWM
The symptoms of POCD are similar to those of Alzheimer’s disease and mainly involve anterograde amnesia. 13 To test cognitive function, the rats were submitted to the MWM beginning on postoperative day 1 and ending on postoperative day 7. 14
The first 3 days were considered training, and the following 4 days were considered testing. The MWM is a 1.5-m-diameter black pool filled with water (26°C) and containing a fixed submerged platform in one quadrant with various visual clues on the surrounding walls. This test has two sections as described below.
Place navigation test
The place navigation test (PNT) was performed to measure the time required for the rat to locate the hidden platform after having been placed in the pool (defined as the latency). The animals underwent daily testing with four trials per day during the testing period. At each trial, the animals were placed in the pool in different randomly assigned quadrants. If they were unable to find the platform, the maximum swimming time in the pool was limited to 60 s. After the animal had stayed on the platform for 4 s, it was removed from the pool and allowed to rest for 30 min before the next quadrant training.
Spatial probe test
The spatial probe test (SPT) was performed to evaluate the rats’ memory on postoperative day 7. In this test, the platform was moved while all distal visual cues remained the same. The number of times that the rat passed the previous platform location was calculated. The number of times that the rat passed the previous location of the platform was positively associated with the rat’s memory.
mRNA expression of hippocampal tissue as measured by quantitative real-time PCR
Total RNA was extracted by homogenization of 200-mg hippocampal tissue samples according to the manufacturer’s instructions for the Trizol Reagent (Invitrogen, Carlsbad, CA, USA). Briefly, the RNA samples were mixed with RNase-free water and cDNA Wipeout Buffer and incubated at 42°C for 2 min. Quantiscript RT Buffer, Quantiscript Reverse Transcriptase, and RT Primer Mix (Qiagen, Hilden, Germany) were added to the samples, which were then incubated at 42°C for 15 min followed by incubation at 95°C for 3 min to inactivate the Quantiscript Reverse Transcriptase. The Assays-on-Demand™ Gene Expression Protocol (Applied Biosystems; Foster, CA, USA) was applied to perform quantitative real-time PCR. Briefly, PCR was performed to amplify the cDNA. A target cDNA (IL-1β, Mm00434228_ml; TNF-α, m00443258_ml) and a reference cDNA (glucose-3 phosphate dehydrogenase, Mn99999915_gl) were simultaneously amplified through an oligonucleotide probe with a 3′ nonfluorescent quencher dye and a 5′ fluorescent reporter dye (6-carboxyfluorescein). The PCR reactions were conducted under the following conditions: 50°C for 2 min, 95°C for 10 min, 40 cycles of 95°C for 15 s, and 60°C for 1 min. The fluorescence was determined on an ABI PRISM 7900HT-sequence detection system (Perkin Elmer, Forest City, CA, USA). The comparative threshold cycle method was used to analyze the data, and the results are expressed as the fold change.
Immunohistochemistry
Tissue sections (8 µm) were paraffinized, rehydrated, incubated with 3% hydrogen peroxide in methanol at room temperature for 10 min to block endogenous peroxidase, washed with phosphate-buffered saline three times, and blocked in 1% normal goat serum at room temperature for 30 min. The primary antibody was the mouse anti-rat CD11b antibody (1:50; Santa Cruz Biotechnology, Dallas, TX, USA). Incubation with the primary antibody was carried out at 4°C overnight, followed by rinsing several times. A goat anti-rat antibody (1:200; BosterBio, Pleasanton, CA, USA) was used as the secondary antibody, and incubation was performed at 37°C for 30 min. The diaminobenzidine method was used to visualize the reaction product after thorough washing. The sections were counterstained with hematoxylin, dehydrated, and mounted. Samples in the control group were prepared in parallel, which omitted the primary antibody. Every fifth section was chosen, and 5 to 10 sections were generated per reference space in a systematic random manner. For quantification, the positive cells in three of the randomly selected fields of the hippocampus were estimated through the mean integrated optical density, which was counted at 400× magnification. The MetaMorph software system (Molecular Devices, Sunnyvale, CA, USA) was applied for image analysis.
Statistical analysis
The cytokine expression data obtained from real-time PCR and the microglia activation data were assessed with two-way analysis of variance (ANOVA), in which the treatment was the dependent variable. For the MWM test, the mean was calculated for each test session, and the behavioral data were analyzed by repeated-measures ANOVA for group comparison during acquisition training (days 4–6). A separate two-way ANOVA was applied to evaluate the effects of treatment on the MWM performance during reversal testing (day 7). If the ANOVA indicated an interaction or significant main effect between the main factors, a post hoc Student’s t-test using Fisher’s least significant differences was applied to determine whether a significant difference was present in the treatment means among the groups (P < 0.05). All data are presented as mean ± standard error of the mean.
Results
Effects of preoperative treatment with minocycline on expression of IL-1β and TNF-α mRNA in the hippocampus
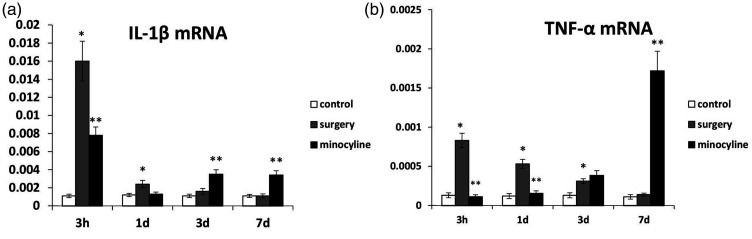
We sought to determine whether surgery induces cytokine production postoperatively and whether the use of minocycline can reduce cytokine production in the hippocampus. Hippocampal IL-1β and TNF-α mRNA levels were measured at 3 hours, 1 day, 3 days, and 7 days after surgery. Surgery induced significant upregulation of IL-1β and TNF-α mRNA at 3 hours in aged rats (P < 0.05) compared with naïve rats. Minocycline prevented postoperative upregulation of IL-1β and TNF-α mRNA, but the levels of these mRNAs were elevated again at 7 days after surgery (P < 0.05) (Figure 1).
Figure 1.
IL-1β and TNF-α mRNA levels in the hippocampus measured by quantitative real-time polymerase chain reaction. (a) IL-1β mRNA levels in the hippocampus of aged rats 3 hours, 1 day, 3 days, and 7 days after the intervention. (b) TNF-α mRNA levels in the hippocampus of aged rats 3 hours, 1 day, 3 days, and 7 days after the intervention. *Mean values are significantly different than controls (P < 0.05). **Mean values are significantly different from surgery group (P < 0.05). IL, interleukin; TNF, tumor necrosis factor.
Surgery induces microglial activation in the hippocampus
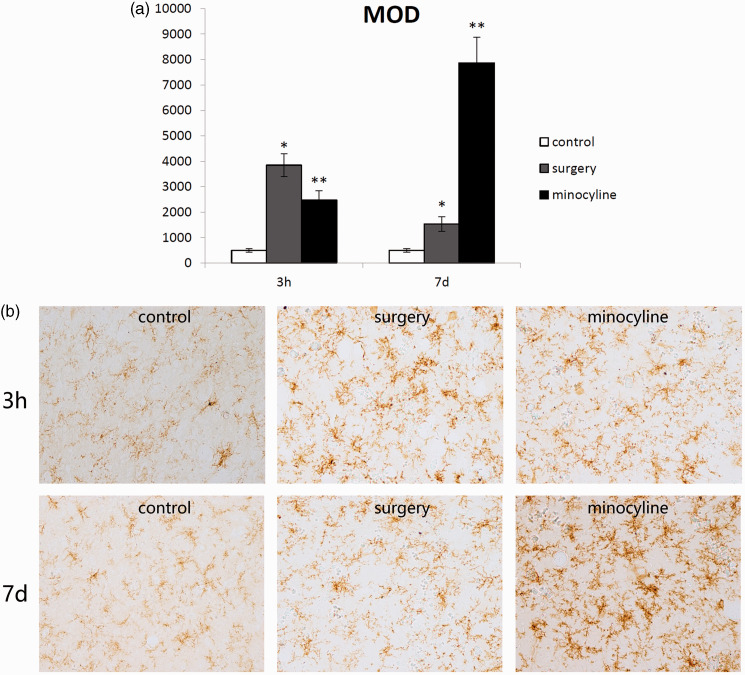
We sought to determine whether surgery induces microglial activation in the hippocampus postoperatively and whether the use of minocycline can reduce microglial activation in the hippocampus. The rats’ fixed brains were collected for immunohistochemical staining of CD11b and scored as described above at 3 hours and 7 days after surgery. Surgery induced significant morphological changes in microglial reactivity at 3 hours in aged rats compared with naïve rats (P < 0.05). The surgery-induced microglial activation was still higher than that at baseline in the aged rats at day 7 (P < 0.05). Minocycline prevented microglial activation at 3 hours postoperatively (P < 0.05), but it induced higher microglial activation than surgery alone at 7 days postoperatively (P < 0.05) (Figure 2).
Figure 2.
(a, b) Immunohistochemistry of microglia using anti-CD11b. The hippocampi were harvested 3 hours or 7 days after treatment. Integrated optical density of microglial immunostaining with CD11b. *Mean values are significantly different from controls (P < 0.05). **Mean values are significantly different from the surgery group (P < 0.05). MOD, mean optical density.
Spatial working memory: MWM
PNT
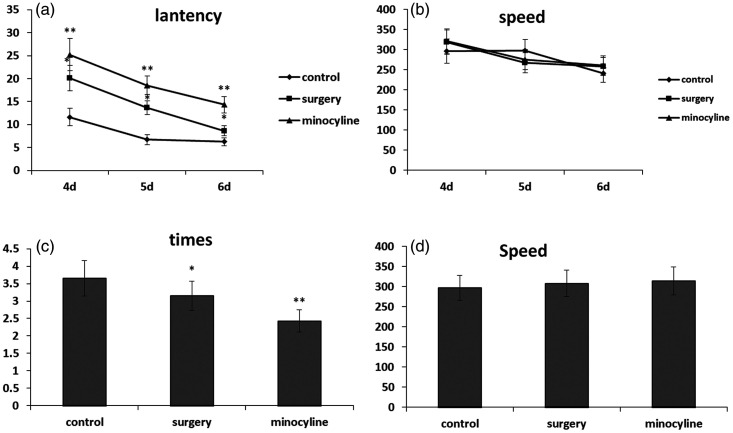
Repeat-measure ANOVA revealed a main effect of surgery for latency during the acquisition phase, indicating that the spatial memory in aged rats was impaired after surgery. The aged rats swam further and longer to locate the platform during the acquisition phase (P < 0.05) (Figure 3(a)). The reduced performance of aged rats did not appear to be due to a lack of general motor ability or motivation because the swim speed did not significantly differ across the test sessions of the acquisition phase (Figure 3(b)).
Figure 3.
(a, b) Performance of aged rats from 3 to 6 days after surgery in the PNT phase of the Morris water maze. (a) Latency to find the platform across testing days. (b) Swim speed across PNT days. There was no significant difference among the groups. (c, d) After 6 days of performing the PNT, the rats were evaluated in an SPT test 1 day after the last PNT. (c) Number of times that the aged rats passed the platform in the SPT test. (d) Swim speed across SPT days. There were no significant differences among the groups. *Mean values are significantly different from controls (P < 0.05). **Mean values are significantly different from the surgery group (P < 0.05). PNT, place navigation test; SPT, spatial probe test.
We administered minocycline before surgery to determine whether minocycline could reduce the rats’ surgery-induced memory impairment. We found that the use of minocycline prolonged the latency (P < 0.05) (Figure 3(a)), indicating that the use of minocycline further impaired the rats’ memory.
SPT
A separate two-way ANOVA was performed to examine the effects of treatment on working memory performance during the SPT. Analysis of the swim data revealed a significant main effect of surgery on the number of times that the aged rats passed the platform (P < 0.05) (Figure 3(c)). The results indicated that surgery impaired the rats’ memory. The reduced performance of the aged rats did not appear to be caused by a lack of general motor ability or motivation because the swim speed did not significantly differ by age across the test sessions during the acquisition phase (Figure 3(d)).
As mentioned above, we administered minocycline before surgery to determine whether minocycline could reduce the rats’ surgery-induced memory impairment. We found that the use of minocycline reduced the number of times that the aged rats passed the platform after surgery (P < 0.05) (Figure 3(c)), indicating that the use of minocycline further impaired the rats’ memory.
Discussion
This study has shown that abdominal surgery can cause microglial activation in aged rats, trigger the central inflammatory response, and damage rat memory but that the use of minocycline cannot reduce this damage.
Age, surgical trauma, and anesthesia may be associated with postoperative cognitive impairment. Previous studies performed in several laboratories,10,15 including ours, have suggested that surgical trauma, rather than anesthesia, results in cognitive function impairment potentiated by aging in the MWM test. Although we cannot rule out the role of blood pressure or blood gases, neither reduced blood pressure nor oxygenation are associated with POCD. 1 It is generally accepted that various stress factors such as perioperative surgery and environmental stimulation lead to the occurrence of POCD, not just a single factor such as anesthesia or surgery. Therefore, in the present experiment, we simulated the stress response during the anesthesia process. The aged rats were subjected to exploratory laparotomy, which induces moderate surgical trauma. Three days after this surgery, the rats showed memory impairment.
This study also showed that surgery causes central microglial activation and a central inflammatory response in elderly rats. Microglia are central inflammatory cells that play a dual role in the stress response. Moderate expression of microglia can resist the inflammatory response, but their overexpression may aggravate the inflammatory response and further damage tissues. 16 Overexpression of central inflammatory factors can damage the body’s memory.17–19 We found that surgery can induce an inflammatory response in the central nervous system in elderly rats but can also damage their memory, suggesting that this phenomenon may be associated with overexpression of the central inflammatory response. In normal brains, microglia remain in a resting state and are activated when traumatic stress occurs. 20 Activated microglia can release a large number of inflammatory factors, triggering a central inflammatory response, while the central inflammatory response further activates microglia. Microglia can release both anti-inflammatory and proinflammatory cytokines, playing a dual role.20,21 However, central inflammatory factors can also be derived from the periphery. 22 Whether the inflammatory factors that initially activate the central inflammatory response pathway are from the periphery, the center, or both remains unclear. Henry et al. 23 found that the microglial activation marker major histocompatibility complex (MHC) type II increased with age, while lipopolysaccharide can cause MHC-labeled microglia to release IL-1β in large quantities. Thus, they suggested that increased expression of age-related microglia was an important cause of a central inflammatory response induced by hyperglycemia. Control of microglial activation has thus become a hot topic in the field of senile dementia, POCD, and other age-related diseases. In the present study, we found obvious postoperative microglial activation in rats. Using a high dose of minocycline could inhibit early microglial activation, but we found that rebound activation was present in a large number of microglia on postoperative day 7. This phenomenon may have been related to further decline of the rats’ memory after using minocycline compared with the rats not treated with minocycline.
We detected two proinflammatory factors, IL-1β and TNF-α, and both were upregulated. IL-1β was previously shown to contribute to regulation of memory processes, 24 and IL-1β upregulation can impair memory. 25 Surgical trauma resulted in an exaggerated neuroinflammatory response, especially the upregulation of IL-1β in the hippocampus, and this may have accounted for the greater cognitive impairment in aged rats. TNF-α is also upregulated in many nervous system diseases, such as Alzheimer’s disease. 26 In the present study, we found that the expression of IL-1β and TNF-α in rats was significantly higher after the operation. Although the expression of these proinflammatory factors was decreased in the early stage in rats treated with minocycline, expression was increased on day 7. Because central inflammatory cytokines are mainly derived from microglia, we speculate that this phenomenon is associated with decreased microglial expression followed by increased expression.
In this study, we used minocycline to reduce microglial activation during surgery, but we found that the use of minocycline did not improve the rats’ memory in the MWM test. Furthermore, while the use of minocycline did reduce microglial activation and the neuroinflammatory response early after surgery, by 7 days after surgery microglial activation was detected in rats that had received minocycline. This may be why minocycline did not improve the rats’ memory after surgery. However, the mechanism by which microglia are reactivated after inhibition of their activation is unclear. Importantly, the main limitation of this study is that we only used one dose of minocycline. Other doses of the drug should be further studied. Whether minocycline can be used to prevent POCD and determination of its safe range and optimal application method remain to be extensively studied before clinical use.
Declaration of conflicting interests
The authors declare that there is no conflict of interest.
Funding
This study was supported by a grant from the National Natural Science Foundation of China [No. 81171034].
References
- 1.Fan L, Wang TL, Xu YC, et al. Minocycline may be useful to prevent/treat postoperative cognitive decline in elderly patients. Med Hypotheses 2011; 76: 733–736. [DOI] [PubMed] [Google Scholar]
- 2.Moller JT, Cluitmans P, Rasmussen LS, et al. Long-term postoperative dysfunction in the elderly. ISPOCD 1 Study. ISPOCD investigators. International Study of Post-Operative Cognitive Dysfunction. Lancet 1998; 351: 857–861. [DOI] [PubMed] [Google Scholar]
- 3.Steinmetz J, Christensen KB, Lund T, et al. Long-term consequences of postoperative cognitive dysfunction. Anesthesiology 2009; 110: 548–555. [DOI] [PubMed] [Google Scholar]
- 4.Justin Y Thomas C andAndrew H.. The anti-inflammatory actions of noradrenergic agents as a target to prevent neurodegeneration in Parkinson's disease. J. Neuroimmunol 2014; 275: 122–123. [Google Scholar]
- 5.Koudriavtseva T andMainero C.. Neuroinflammation, neurodegeneration and regeneration in multiple sclerosis: intercorrelated manifestations of the immune response. Neural Regen Res 2016; 11: 1727–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bagyinszky E, Giau V, Shim K, et al. Role of inflammatory molecules in the Alzheimer's disease progression and diagnosis. J Neurol Sci 2017; 376: 242–254. [DOI] [PubMed] [Google Scholar]
- 7.Alam Q, Alam MZ, Mushtaq G, et al. Inflammatory process in Alzheimer and Parkinson's diseases: central role of cytokines. Curr Pharm Des 2016; 22: 541–548. [DOI] [PubMed] [Google Scholar]
- 8.Tan HY, Cao JB, Zhang JF, et al. Critical role of inflammatory cytokines in impairing biochemical processes for learning and memory after surgery in rats. J Neuroinflammation 2014; 11: 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gouweleeuw L, Hovens IB, van Leeuwen BL, et al. Neutrophil gelatinase-associated lipocalin and microglial activity are associated with distinct postoperative behavioral changes in rats. Behav Brain Res 2017; 319: 104–109. [DOI] [PubMed] [Google Scholar]
- 10.Cibelli M, Fidalgo AR, Terrando N, et al. Role of interleukin-1b in postoperative cognitive dysfunction. Ann Neurol 2010; 68: 360–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mattei D, Ivanov A, Ferrai C, et al. Maternal immune activation results in complex microglial transcriptome signature in the adult offspring that is reversed by minocycline treatment. Transl Psychiatry 2017; 7: e1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumar A, Aggrawal A, Pottabathini R, et al. Possible neuroprotective mechanisms of clove oil against icv-colchicine induced cognitive dysfunction. Pharmacol Rep 2016; 68: 764–772. [DOI] [PubMed] [Google Scholar]
- 13.Arora SS, Gooch JL, Garcia PS. Postoperative cognitive dysfunction, Alzheimer's disease, and anesthesia. Int J Neurosci 2014; 124: 236–242. [DOI] [PubMed] [Google Scholar]
- 14.Vorhees CV andWilliams MT.. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc 2006; 1: 848–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Z, Liu F, Ma H, et al. Age exacerbates surgery-induced cognitive impairment and neuroinflammation in Sprague-Dawley rats: the role of IL-4. Brain Res 2017; 1665: 65–73. [DOI] [PubMed] [Google Scholar]
- 16.Li YP, Pan k, Chen L, et al. Deferoxamine regulates neuroinflammation and iron homeostasis in a mouse model of postoperative cognitive dysfunction. J Neuroinflammation 2016; 13: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ren X Clair DK andButterfield DA.. Dysregulation of cytokine mediated chemotherapy induced cognitive impairment. Pharmacol Res 2017; 117: 267–273. [DOI] [PubMed] [Google Scholar]
- 18.Shields GS, Kuchenbecker SY, Pressman SD, et al. Better cognitive control of emotional information is associated with reduced pro-inflammatory cytokine reactivity to emotional stress. Stress 2016; 19: 63–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Cosmo G, Sessa F, Fiorini F, et al. Effect of remifentanil and fentanyl on postoperative cognitive function and cytokines level in elderly patients undergoing major abdominal surgery. J Clin Anesth 2016; 35: 40–46. [DOI] [PubMed] [Google Scholar]
- 20.Block ML andHong JS.. Microglia and inflammation-mediated neurodegeneration: Multiple triggers with a common mechanism. Prog Neurobiol 2005; 76: 77–98. [DOI] [PubMed] [Google Scholar]
- 21.Minagar A, Shapshak P, Fujimura R, et al. The role of macrophage/microglia and astrocytes in the pathogenesis of three neurologic disorders: HIV-associated dementia, Alzheimer disease, and multiple sclerosis. J Neurol Sci 2002; 202: 13–23. [DOI] [PubMed] [Google Scholar]
- 22.Fidalgo AR, Cibelli M, White JP, et al. Systemic inflammation enhances surgery-induced cognitive dysfunction in mice. Neurosci Lett 2011; 498: 63–66. [DOI] [PubMed] [Google Scholar]
- 23.Henry CJ, Huang Y, Wynne AM, et al. Peripheral lipopolysaccharide (LPS) challenge promotes microglial hyperactivity in aged mice that is associated with exaggerated induction of both pro-inflammatory IL-1beta and anti-inflammatory IL-10 cytokines. Brain Behav Immun 2009; 23: 309–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shi S, Liang D, Chen Y, et al. Gx-50 reduces β-amyloid-induced TNF-α, IL-1β, NO and PGE2 expression and inhibits NF- κB signaling in a mouse model of Alzheimer's disease. Eur J Immunol 2016; 46: 665–676. [DOI] [PubMed] [Google Scholar]
- 25.Lee YH, Choi SJ, Ji JD, et al. Association between TNF-α promoter -308 A/G polymorphism and Alzheimer's disease: a meta-analysis. Neurol Sci 2015; 36: 825–832. [DOI] [PubMed] [Google Scholar]
- 26.Hao W andFriedman A.. Mathematical model on Alzheimer's disease. BMC Syst Biol 2016; 10: 108. [DOI] [PMC free article] [PubMed] [Google Scholar]