Short abstract
Objective
The aim of this study was to determine whether an expanded newborn screening programme, which is not yet available in Slovenia, would have detected the first two patients with medium-chain acyl-CoA dehydrogenase (MCAD) deficiency in the country. Two novel ACADM mutations are also described.
Methods
Both patients were diagnosed clinically; follow-up involved analysis of organic acids in urine, acylcarnitines in dried blood spots, and genetic analysis of ACADM. Cut-off values of acylcarnitines in newborns were established using analysis of 10,000 newborns in a pilot screening study.
Results
In both patients, analysis of the organic acids in urine showed a possible β-oxidation defect, while the specific elevation of acylcarnitines confirmed MCAD deficiency. Subsequent genetic analysis confirmed the diagnosis; both patients were compound heterozygotes, each with one novel mutation (c.861 + 2T > C and c.527_533del). The results from a retrospective analysis of newborn screening cards clearly showed major elevations of MCAD-specific acylcarnitines in the patients.
Conclusions
An expanded newborn screening programme would be beneficial because it would have detected MCAD deficiency in both patients before the development of clinical signs. Our study also provides one of the first descriptions of ACADM mutations in Southeast Europe.
Keywords: MCAD deficiency, neonatal screening, ACADM, acylcarnitines, mutations, dried blood spot
Introduction
Medium-chain acyl-CoA dehydrogenase (MCAD) deficiency is the most common inborn error of fatty acid metabolism; its incidence is about 1 in 15,000 live births.1,2 MCAD deficiency is caused by a deficient enzyme in the mitochondrial β-oxidation of medium-length fatty acids. 3 Clinical presentations range from asymptomatic to fatal. 4 Patients who cannot manage catabolic stress (i.e., vomiting and other gastrointestinal symptoms) are often the first to exhibit acute signs. Severe metabolic decompensation with hypoketotic hypoglycaemia can occur during illness and catabolic stress. The disease may progress to coma or even sudden death. 5 The major strategy for prevention of clinical manifestations is avoidance of catabolic stress using a regular feeding regimen. 4 When a child with MCAD deficiency is clinically well, the maximum permissible fasting interval increases with the child’s age. No universal recommendations have been established in Europe. In general, all guidelines state that the maximum fasting duration is 4 hours for the first 4 months, 8 hours for the second 4 months, and 10 hours from 8 to 12 months of age. At 1 year of age, children can fast for up to 12 hours, and this fasting interval must be maintained for the rest of their lives.6–9 A daily intake of 1 to 2 g/kg of slow-absorption carbohydrates is recommended from 8 months of age. During any acute intercurrent illness, the treatment protocol includes careful management with feeding every 2 to 3 hours with glucose polymer feeds until the patient improves. In case of vomiting or clinical deterioration, urgent hospital admission for intravenous glucose infusion is recommended.7,10 Supplementation with carnitine should also be considered in patients with MCAD deficiency. 10
MCAD deficiency can be detected by the accumulation of characteristic acylcarnitines in the blood just a few days after birth. 11 The specific acylcarnitine profile can be accurately measured in dried blood spots from newborns using tandem mass spectrometry (MS/MS). 11 Patients not diagnosed as newborns but whose illness is detected when clinical signs develop have an approximately 25% mortality rate because of acute manifestations of the disease.12–15 Early detection of MCAD deficiency can improve the outcome of affected patients and decrease mortality.12,16,17 MCAD deficiency is included in all newborn screening programmes that use MS/MS. 2
The first two unrelated patients with MCAD deficiency were diagnosed in Slovenia after the development of clinical signs. We aimed to determine whether expanded newborn screening would detect MCAD deficiency in such patients. We also herein present two novel ACADM mutations. Little is known about the mutation spectra of patients with MCAD deficiency in Southeast Europe; one reason for this could be that newborn screening, which detects more patients, is not performed in these countries.18,19 Patients often present with clinical features that are not characteristic of MCAD deficiency, and their condition is only diagnosed after biochemical testing has been performed. In the present study, we also compared the acylcarnitine concentrations obtained by newborns’ dried blood spot card with those obtained from a pilot study on expanded newborn screening.
Patients and Methods
Patients
Patient 1, a 15-month-old girl, was the second child of non-consanguineous parents born following the mother’s second pregnancy. Her family history was not clinically significant. After an uneventful pregnancy, birth was induced vaginally at 41 weeks of gestation. Her birth weight was 3400 g (50th–75th percentile). She reached the expected developmental milestones. On the morning of the day before she was admitted to our hospital, she sustained minor head trauma while running at home but showed no concerning clinical signs. She ate dinner normally that day, but her parents noticed that she was somnolent around 2:00 to 3:00 AM; only after the second visit by a local general physician that night was she finally referred to our hospital. Upon admission, she was somnolent and less responsive than normal, her blood glucose concentration was 0.8 mmol/L (immediately corrected to 6.3 mmol/L by glucose infusion), and her blood ketone concentration was 0.3 µmol/L. The reanimation team decided not to intubate her, and she gradually regained complete consciousness. A computed tomography scan of the head was normal. Laboratory tests showed evidence of a mild systemic infection. She had moderately elevated creatine kinase, lactate dehydrogenase, and transaminase concentrations (aspartate transaminase of 13.96 µkat/L [837.6 U/L] and alanine transaminase of 11.81 µkat/L [708.6 U/L]), all of which gradually decreased later. The neurologist reported hypotonia, loss of proprioceptive reflexes, ataxia, and macrocephaly (98th percentile), but her electroencephalography findings were normal. Echocardiography findings were also normal. Urine organic acid analysis showed a possible disorder of fatty acid metabolism. Finally, acylcarnitine analysis revealed a typical MCAD deficiency pattern (Table 1). The diagnosis was later confirmed by mutation analysis of ACADM.
Table 1.
Values of C6, C8, C10, and C10:1 acylcarnitines for both patients with MCAD deficiency
| C6 (µmol/L) | C8 (µmol/L) | C10 (µmol/L) | C10:1 (µmol/L) | C8/C2 ratio | C8/C10 ratio | |
|---|---|---|---|---|---|---|
| Cut-off | 0.15 | 0.27 | 0.32 | 0.25 | 0.02 | 2.30 |
| Patient 1 | 0.75 | 12.47 | 1.08 | 0.70 | 2.23 | 11.53 |
| Patient 2 | 1.24 | 6.68 | 0.50 | 0.44 | 0.60 | 13.28 |
The cut-off values are those used in the laboratory for patients older than 1 month. C2, acetylcarnitine; C6, hexanoylcarnitine; C8, octanoylcarnitine; C10, decanoylcarnitine; C10:1, decenoylcarnitine.
Patient 2, a 20-month-old boy, was the first child of non-consanguineous parents born following the mother’s first pregnancy. The mother had epilepsy as a child but had not undergone therapy for 8 years. After an uneventful pregnancy, birth was induced vaginally at 40 weeks of gestation because of pathological cardiotocography results; the amniotic fluid was meconium-stained, and the umbilical cord was twisted around the infant’s neck twice. His birth weight and head circumference were 3150 g (10th–50th percentile) and 37 cm (95th percentile), respectively. The mother was febrile (38.2°C) during labour. Additionally, the boy’s markers of inflammation subsequently became mildly elevated, and he received antibiotic therapy for early sepsis. Severe multiple arthrogryposes of the distal joints and muscular hypotonia were also observed immediately after birth. Most of the extended diagnostic examinations performed in the neonatal period (i.e., electroencephalography, electromyography, echocardiography, and ultrasound of the abdomen and hips) showed normal results; however, an ultrasound of the head was mildly abnormal. The patient was managed with regular neurophysiotherapy, bracelets, and several surgical corrections of the arthrogryposes during the first year. At 18 months of age, he was admitted to our hospital after a short history of upper respiratory infection and a single occurrence of vomiting the day before. He was woken up after 16 hours of sleep and was less responsive than normal, flaccid, and unable to hold his head up. When the emergency physician arrived, he had a blood glucose concentration of 0.7 mmol/L, which was immediately corrected with a glucose infusion and glucagon administration, and the glucose concentration had increased to 3.4 mmol/L upon admission. Laboratory tests indicated a moderate systemic infection (rhinoviruses and enteroviruses were later confirmed in the nasopharynx), dehydration (blood urea nitrogen, 11.7 mmol/L), and ketosis (acetoacetate, 574 µmol/L; beta-OH-butyrate, 1157 µmol/L). Urine organic acid analysis showed ketosis and a possible disorder in fatty acid metabolism. Acylcarnitine analysis demonstrated a typical MCAD deficiency pattern (Table 1). The diagnosis was later confirmed by mutation analysis of ACADM.
The parents of both above-described patients provided written informed consent.
In a pilot study of expanded newborn screening, we evaluated 10,048 newborns born in 2013 and 2014. Blood spots from the newborns were taken 48 to 72 hours after birth to perform routine neonatal screening for phenylketonuria and congenital hypothyroidism and retrospectively analysed. The age of the samples at the time of analysis was 1 year. The Slovenian National Medical Ethics Committee approved the study.
Methods
Urine organic acids were measured using an in-house method. 20 We added 20 to 30 mg of O-ethylhydroxylamine (Sigma-Aldrich, St. Louis, MO, USA) to 1 mL of urine in a glass tube and incubated the solution for 15 minutes at room temperature. Next, we added 100 mmol of 2-phenylbutyric acid (Sigma-Aldrich) per mol of creatinine followed by 100 µL of 4N HCl (prepared from ≥37% hydrochloric acid; Sigma-Aldrich ), and the solution was saturated with NaCl (Sigma-Aldrich). After the addition of 2 mL of ethyl acetate (Sigma-Aldrich), the solution was vortexed and centrifuged for 5 minutes at 3500 revolutions per minute. Next, 1 mL of supernatant was transferred to a clean glass tube and the ethyl acetate was evaporated under a stream of nitrogen. Finally, 50 µL of pyridine (Sigma-Aldrich) and 200 µL of N,O-bis(trimethylsilyl)trifluoroacetamine (Sigma-Aldrich) were added, and the solution was mixed and analysed using an Agilent 5975C Series GC/MSD (Agilent Technologies, Santa Clara, CA, USA) on an Agilent Ultra 2 column (Agilent Technologies).
Acylcarnitines were analysed from the dried blood spots and derivatised with the Chromsystems kit Amino Acids and Acylcarnitines from Dried Blood (Chromsystems, Grüfelfing, Germany). They were quantified using a PerkinElmer 200 HPLC system (PerkinElmer, Waltham, MA, USA) coupled to an AB Sciex 3200 QTRAP (AB Sciex, Framingham, MA, USA).
For genetic analysis, whole blood samples were used for the isolation of genomic DNA using established laboratory protocols based on the FlexiGene DNA isolation kit (Qiagen, Hilden, Germany). Isolated DNA from both patients was sent to Centogene AG (Rostock, Germany), where genetic testing of ACADM (OMIM: 201450) was performed. Both pairs of parents were tested for the presence of causative variants. Primers were designed in-house and used for polymerase chain reaction amplification. The amplicons were Sanger sequenced using a BigDye Terminator v3.1 sequencing kit and an ABI Genetic Analyzer 3500 (both Applied Biosystems, Foster City, CA, USA).
Enzyme activity analysis by octanoyl-CoA oxidation in lymphocytes was performed at the University Medical Center in Freiburg, Germany.
Results
Organic acids
Organic acids in the urine samples of Patients 1 and 2 were significantly abnormal; both patients had high elevations of adipic acid, suberic acid, sebacic acid, decendioic acid, 5-hydroxyhexanoic acid, hydroxydecanoic acid, and octanoic acid. Patient 2 also had significantly elevated 3-hydroxybutyric acid, 3-hydroxyisovaleric acid, and decadienoic acid.
Acylcarnitines in dried blood spots
The acylcarnitines C6 (hexanoylcarnitine), C8 (octanoylcarnitine), C10 (decanoylcarnitine), and C10:1 (decenoylcarnitine), as well as the disease-specific ratios C8/C2 and C8/C10, were significantly elevated in both patients (Table 1).
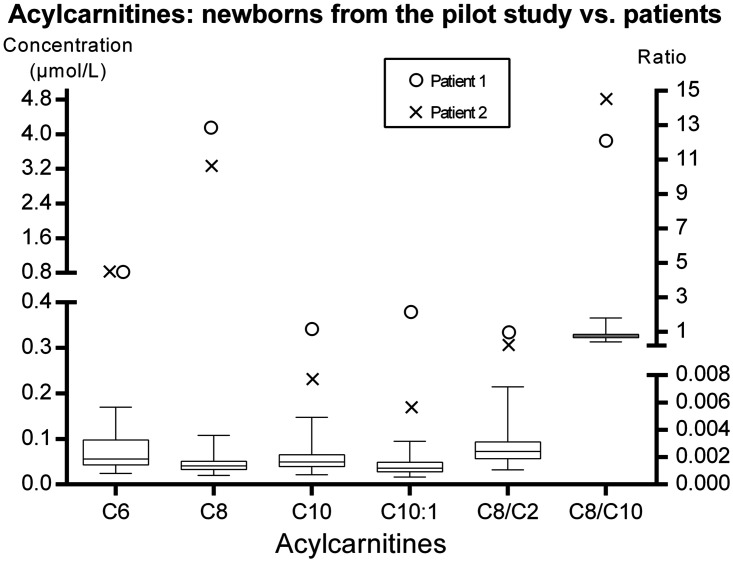
The acylcarnitine concentrations on the newborn screening cards of the patients were higher than those on the screening cards of the healthy newborns who were part of the pilot newborn screening study (Figure 1). The disease-specific acylcarnitines ratios C8/C2 (99th percentile in the normal population = 0.007) and C8/C10 (99th percentile in the normal population = 1.8) were significantly elevated in Patient 1 (0.588 and 12.1, respectively) and Patient 2 (0.393 and 14.5, respectively).
Figure 1.
Comparison of acylcarnitine concentrations between healthy newborns and patients. The patients’ values for all chosen acylcarnitines were higher than those in healthy controls. The box represents the 25th and 75th percentiles, the line in the box represents the median, and the whiskers are the 1st and 99th percentiles. C6, hexanoylcarnitine; C8, octanoylcarnitine; C10, decanoylcarnitine; C10:1, decenoylcarnitine; C2, acetylcarnitine.
Genetics and enzyme activity
Previously unreported genetic variants were found in both patients. The sequencing of ACADM in Patient 1 revealed two heterozygous mutations: a known variant in exon 4 of ACADM (NM_001127328.1: c.256dupT, p.Trp86Leufs*23) and a previously unreported and probably disease-causing variant in intron 9 of ACADM (NM_001127328.1: c.861 + 2T>C). Patient 2 had two heterozygous ACADM mutations: a known variant in exon 11 of ACADM (NM_001127328.1: c.997A>G p.Lys333Glu) and a previously unreported and probably disease-causing variant in exon 7 of ACADM (NM_001127328.1: c.527_533del p.Ile176Thrfs*19). The parents were also tested, and each parent had one of the variants.
There was no measurable enzyme activity in Patient 2. Enzyme activity studies were not performed in Patient 1.
Discussion
Besides phenylketonuria, MCAD deficiency is one of the most common inborn disorders of metabolism, 12 yet the two patients described herein were the first to be identified in Slovenia. The clinical signs of both patients were typical of MCAD deficiency with an acute onset due to hypoglycaemia and its clinical consequences. 21 The other manifestations seen in our patients, such as arthrogryposis, were concomitant but probably not due to MCAD deficiency. Elevations of organic acids in the urine indicated a possible fatty acid oxidation disorder, and elevations of specific acylcarnitines and acylcarnitine ratios in the dried blood spots confirmed MCAD deficiency. In the urine of both patients, adipic acid, suberic acid, sebacic acid, decendioic acid, 5-hydroxyhexanoic acid, hydroxydecandioic acid, and octanoic acid were significantly elevated. Various disorders cause these elevations, including β-oxidation defects. 22 Patient 2 also had elevations of 3-hydroxybutyric acid, 3-hydroxyisovaleric acid, and decadienoic acid in the urine; such elevations are normally seen in patients with ketosis. This condition is not typical of MCAD deficiency because fatty acid oxidation disorders typically prevent the formation of ketones. 23 However, several cases of ketosis in patients with MCAD deficiency have been described and can be explained by some residual enzyme function.24–27 Interestingly, Patient 2 was in ketosis despite the absence of MCAD activity.
MCAD deficiency can usually be definitively diagnosed by the presence of hexanoylglycine in the urine.12,28,29 No such elevations were present in the urine of our two patients. Quantification of acylcarnitines was necessary to determine the type of fatty acid oxidation disorder. An MCAD deficiency is readily detected by the quantification of acylcarnitines in dried blood spots, where an increased level of C8 acylcarnitine is most characteristic of the disorder; C6, C10, C10:1 and the ratios C8/C2 and C8/C10 are also elevated in some patients. 30 All of these analytes were significantly elevated in both patients at the time of the clinical signs, which facilitated the early aetiological clarification and the start of targeted treatment.
No universal newborn screening for MCAD is currently available in Southeast Europe; 19 however, such screening would detect more patients than are diagnosed clinically. The difference in diagnostic rates arises because some cases do not manifest clinically even without prevention strategies, 12 and sudden deaths in infants with MCAD deficiency have been described.5,12 Thus, it is possible that many such cases remain undiagnosed. Neonatal screening with MS/MS offers a high level of sensitivity that prevents most cases from being missed.29,31,32 Little is known about the MCAD mutation spectra in Southeast Europe; the two patients described herein were the first reported patients with MCAD deficiency in Slovenia. Both patients were compound heterozygotes; interestingly, however, neither had the common mutation c.958A > G, which causes a change from lysine to glutamate at position 329 of the MCAD protein (p.Lys329Glu, legacy p.Lys304Glu). 21 This is the most common mutation in patients with MCAD deficiency and occurs at a frequency of up to 90% of disease alleles in clinically detected patients; 21 however, the mutation is present in only half of patients identified through newborn screening.4,29
Several mutations are associated with MCAD deficiency; the Public Human Gene Mutation Database® (Cardiff, UK) lists 160 different mutations. Both patients had one previously known disease-causing mutation. Patient 1 had a heterozygous mutation in exon 4 of ACADM (c.256dupT p.Trp86Leufs*23), which creates a shift in the reading frame starting at codon Trp86. The new reading frame ends in a stop codon 22 positions downstream. 33 The previously known mutation in Patient 2 was a mutation in exon 11 of ACADM (c.997A- > G p.Lys333Glu). This mutation was previously described as the disease-causing mutation for MCAD deficiency. 34 The National Center for Biotechnology Information Single Nucleotide Polymorphism database (NCBI dbSNP) reported this mutation with a frequency of 0.002 (rs77931234),35 and the Exome Sequencing Project described it with a frequency of 0.0074 in the European American population and 0.0014 in the African-American population (Alamut v.2.4; Interactive Biosoftware, Rouen, France).36
The two other variants in our patients were previously unreported. In Patient 1, the variant was in intron 9 of ACADM (c.861 + 2T > C). This variant is located in the highly conserved donor splice site of intron 9 and is probably disease-causing because software analyses using Alamut v.2.4 predicted an aberrant effect on splicing as very probable. The other previously unreported heterozygous variant in Patient 2 was very likely a disease-causing variant in exon 7 of ACADM (c.527_533del, p.Ile176Thrfs*19). This variant creates a shift in the reading frame starting at codon Ile176. The new reading frame ends in a stop codon 18 positions downstream. To date, this variant has not been described in the NCBI dbSNP or Exome Sequencing Project. The absence of enzyme activity in this patient confirmed the pathogenicity of the variant.
In developed countries, MCAD deficiency is mostly detected through expanded newborn screening of newborns’ dried blood spots with MS/MS.12,29,31 Because newborn screening for MCAD is not currently available in Slovenia, the cut-offs for acylcarnitines in dried blood spots from newborn children have not yet been determined. The 99th percentile of the acylcarnitine concentrations and ratios was used for the approximate cut-off point in the present study. Figure 1 clearly shows that the concentrations of all relevant acylcarnitines were significantly elevated. These patients would be easily detected in a newborn screening programme, preventing the development of clinical signs. Additionally, false-negative results are rare or non-existent in newborn screening programmes, 12 and after implementing NBS metabolic screening centres, no new cases have been clinically diagnosed. 37 Sudden death of patients with MCAD deficiency is still being reported, 5 yet the number of such deaths after the initiation of newborn screening is now significantly smaller than in the past. 12 Some cases of sudden death can happen before the screening results are available and this cannot be prevented with newborn screening.5,12
Conclusion
In the present study, acylcarnitine measurements in blood spots from patients both at the time of presentation of clinical signs and at the time of birth clearly showed elevations of acylcarnitines specific for MCAD deficiency. These results indicate that the MCAD deficiency in these patients would have been clearly detected if they had been part of a newborn screening programme, thus preventing the symptoms. Our study presents one of the first descriptions of ACADM mutations in Southeast Europe. Neither of the patients had the common 958A- > G mutation, and each had a previously unreported, probably disease-causing variant.
Declaration of conflicting interests
The authors declare that there is no conflict of interest.
Funding
This work was partially supported by Slovenian Research Agency Grants V3-1505 and J3-6798.
References
- 1.Frazier DM, Millington DS, McCandless SE, et al. The tandem mass spectrometry newborn screening experience in North Carolina: 1997-2005. J Inherit Metab Dis 2006; 29: 76–85. [DOI] [PubMed] [Google Scholar]
- 2.Lindner M Hoffmann GF andMatern D.. Newborn screening for disorders of fatty-acid oxidation: experience and recommendations from an expert meeting. J Inherit Metab Dis 2010; 33: 521–526. [DOI] [PubMed] [Google Scholar]
- 3.Matern D andRinaldo P. Medium-Chain Acyl-Coenzyme A Dehydrogenase Deficiency. GeneReviews, http://www.ncbi.nlm.nih.gov/books/NBK1424/ (2015, accessed 5 August 2016).
- 4.Gregersen N, Andresen BS, Pedersen CB, et al. Mitochondrial fatty acid oxidation defects–remaining challenges. J Inherit Metab Dis 2008; 31: 643–657. [DOI] [PubMed] [Google Scholar]
- 5.Yusupov R, Finegold DN, Naylor EW, et al. Sudden death in medium chain acyl-coenzyme a dehydrogenase deficiency (MCADD) despite newborn screening. Mol Genet Metab 2010; 101: 33–39. [DOI] [PubMed] [Google Scholar]
- 6.Derks TGJ, Van Spronsen FJ, Rake JP, et al. Safe and unsafe duration of fasting for children with MCAD deficiency. Eur J Pediatr 2007; 166: 5–11. [DOI] [PubMed] [Google Scholar]
- 7.British Inherited Metabolic Diseases Group. MCADD dietetic management guidelines, http://www.bimdg.org.uk/site/guidelines-enbs.asp?t=6 (2015, accessed 25 July 2017)
- 8.Spiekerkoetter U, Lindner M, Santer R, et al. Treatment recommendations in long-chain fatty acid oxidation defects: Consensus from a workshop. J Inherit Metab Dis 2009; 32: 498–505. [DOI] [PubMed] [Google Scholar]
- 9.Spiekerkoetter U, Haussmann U, Mueller M, et al. Tandem mass spectrometry screening for very long-chain acyl-CoA dehydrogenase deficiency: the value of second-tier enzyme testing. J Pediatr 2010; 157:668–673. [DOI] [PubMed] [Google Scholar]
- 10.Couce ML, Sánchez-Pintos P, Diogo L, et al. Newborn screening for medium-chain acyl-CoA dehydrogenase deficiency: regional experience and high incidence of carnitine deficiency. Orphanet J Rare Dis 2013; 8:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Hove JL, Zhang W, Kahler SG, et al. Medium-Chain Acyl-CoA Dehydrogenase (MCAD) Deficiency: Diagnosis by Acylcarnitine Analysis in Blood. Am J Hum Genet 1993; 52: 958–966. [PMC free article] [PubMed] [Google Scholar]
- 12.Wilcken B, Haas M, Joy P, et al. Outcome of neonatal screening for medium-chain acyl-CoA dehydrogenase deficiency in Australia: a cohort study. Lancet 2007; 369: 37–42. [DOI] [PubMed] [Google Scholar]
- 13.Pollitt RJ andLeonard JV.. Prospective surveillance study of medium chain acyl-CoA dehydrogenase deficiency in the UK. Arch Dis Child 1998; 79: 116–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilcken B Hammond J andSilink M.. Morbidity and mortality in medium chain acyl coenzyme A dehydrogenase deficiency. Arch Dis Child 1994; 70: 410–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meng M andZhang YP.. Impact of inborn errors of metabolism on admission in a neonatal intensive care unit: A 4-year report. J Pediatr Endocrinol Metab 2013; 26: 689–693. [DOI] [PubMed] [Google Scholar]
- 16.Nennstiel-Ratzel U, Arenz S, Maier EM, et al. Reduced incidence of severe metabolic crisis or death in children with medium chain acyl-CoA dehydrogenase deficiency homozygous for c.985A>G identified by neonatal screening. Mol Genet Metab 2005; 85: 157–159. [DOI] [PubMed] [Google Scholar]
- 17.Lindner M, Gramer G, Haege G, et al. Efficacy and outcome of expanded newborn screening for metabolic diseases - Report of 10 years from South-West Germany. Orphanet J Rare Dis 2011; 6: 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smon A, Groselj U, Tansek MZ, et al. Newborn screening in Slovenia. Zdr Var 2015; 54: 86–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Groselj U, Tansek MZ, Smon A, et al. Newborn screening in southeastern Europe. Mol Genet Metab 2014; 113: 42–45. [DOI] [PubMed] [Google Scholar]
- 20.Šmon A, Murko S, Repič Lampret B, et al. Pilot research on expanding Slovenian newborn screening programme for inherited metabolic disorders detectable by tandem mass spectrometry. Chem List 2014; 108: s183–s186. [Google Scholar]
- 21.Wang SS, Fernhoff PM, Hannon WH, et al. Medium chain acyl-CoA dehydrogenase deficiency human genome epidemiology review. Genet Med 1999; 1: 332–339. [DOI] [PubMed] [Google Scholar]
- 22.Kumps A Duez P andMardens Y.. Metabolic, nutritional, iatrogenic, and artifactual sources of urinary organic acids: A comprehensive table. Clin Chem 2002; 48: 708–717. [PubMed] [Google Scholar]
- 23.Morris AM andSpiekerkoetter U.. Disorders of mitochondrial fatty acid oxidation and related metabolic pathways. In: Saudubray JM van den Berghe G andWalter J (eds) Inborn metabolic diseases 5th ed. Berlin-Heidelberg: Springer-Verlag, 2012, pp.201–216. [Google Scholar]
- 24.Iafolla AK Thompson RJJ andRoe CR.. Medium-chain acyl-coenzyme A dehydrogenase deficiency: clinical course in 120 affected children. J Pediatr 1994; 124: 409–415. [DOI] [PubMed] [Google Scholar]
- 25.Hsu H-W, Zytkovicz TH, Comeau AM, et al. Spectrum of Medium-Chain Acyl-CoA Dehydrogenase Deficiency Detected by Newborn Screening. Pediatrics 2008; 121:e1108–e1114. [DOI] [PubMed] [Google Scholar]
- 26.Gramer G, Haege G, Fang-Hoffmann J, et al. Medium-Chain Acyl-CoA Dehydrogenase Deficiency: Evaluation of Genotype-Phenotype Correlation in Patients Detected by Newborn Screening. JIMD Rep 2015; 23: 101–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fletcher JM andPitt JJ.. Fasting medium chain acyl-coenzyme A dehydrogenase-deficient children can make ketones. Metabolism 2001; 50: 161–165. [DOI] [PubMed] [Google Scholar]
- 28.Ventura F V, Leandro P, Luz A, et al. Retrospective study of the medium-chain acyl-CoA dehydrogenase deficiency in Portugal. Clin Genet 2014; 85: 555–561. [DOI] [PubMed] [Google Scholar]
- 29.Andresen BS, Lund AM, Hougaard DM, et al. MCAD deficiency in Denmark. Mol Genet Metab 2012; 106: 175–188. [DOI] [PubMed] [Google Scholar]
- 30.Spiekerkoetter U andDuran M.. Mitochondrial fatty acid oxidation defects. In: Blau N, Duran M, Gibson KM, et al. (eds) Physician’s guide to the diagnosis, treatment, and follow-up of inherited metabolic diseases. Berlin Heidelberg: Springer-Verlag, 2014, pp.247–264. [Google Scholar]
- 31.Insinga RP Laessig RH andHoffman GL.. Newborn screening with tandem mass spectrometry: Examining its cost-effectiveness in the Wisconsin newborn screening panel. J Pediatr 2002; 141: 524–531. [DOI] [PubMed] [Google Scholar]
- 32.Hall PL Wittenauer A andHagar A.. Newborn screening for medium chain acyl-CoA dehydrogenase deficiency: performance improvement by monitoring a new ratio. Mol Genet Metab 2014; 113: 274–277. [DOI] [PubMed] [Google Scholar]
- 33.Andresen BS, Dobrowolski SF, Reilly LO, et al. Medium-Chain Acyl-CoA Dehydrogenase (MCAD) Mutations Identified by MS/MS-Based Prospective Screening of Newborns Differ from Those Observed in Patients with Clinical Symptoms: Identification and Characterization of a New, Prevalent Mutation That Res. Am J Hum Gen 2001; 1408–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matsubara Y, Narisawa K, Miyabayashi S, et al. Identification of a common mutation in patients with medium-chain acyl-CoA dehydrogenase deficiency. Biochem Biophys Res Commun 1990; 171: 498–505. [DOI] [PubMed] [Google Scholar]
- 35.Bethesda (MD): National Center for Biotechnology Information, National Library of Medicine.
- 36.Database of Single Nucleotide Polymorphisms (dbSNP), (dbSNP Build ID: 141). http://www.ncbi.nlm.nih.gov/SNP/ (2014, accessed 18 January 2017)
- 37.McCandless SE, Chandrasekar R, Linard S, et al. Sequencing from dried blood spots in infants with ‘false positive’ newborn screen for MCAD deficiency. Mol Genet Metab 2013; 108: 51–55. [DOI] [PMC free article] [PubMed] [Google Scholar]