Short abstract
Autoimmune pancreatitis (AP) is a rare autoimmune pancreatic manifestation of systemic immunoglobulin G4 (IgG4)-related sclerosing disease. Distinguishing between AP and pancreatic cancer is crucial because the clinical courses, treatments, and prognoses of these two disease entities are quite different. We herein report a case involving a 52-year-old man with subacute epigastralgia who visited our hospital for evaluation of a suspicious pancreatic mass found during esophagogastroduodenoscopy. Enhanced computed tomography (CT) revealed an enlarged lesion in the pancreatic head with encasement of hepatic vessels. The lesion also exhibited increased 18F-fluorodeoxyglucose accumulation on positron emission tomography/CT imaging, which was highly suggestive of pancreatic cancer. After open biopsy, morphologic examination showed an inflammatory infiltrate in the pancreas, which was compatible with chronic sclerotic pancreatitis. Further laboratory tests revealed an elevated serum IgG4 level, and the diagnosis of sclerotic pancreatitis was then confirmed. After corticosteroid treatment, the pancreatic lesion showed shrinkage on follow-up CT, and the serum IgG4 titer decreased to the normal range. This case suggests that clinicians should be familiar with the clinical presentations and diagnostic criteria of AP versus pancreatic cancer. An awareness of the differences between these diseases may avoid misdiagnosis and unnecessary surgical intervention.
Keywords: Autoimmune pancreatitis, pancreatic cancer, IgG4-related sclerosing disease, FDG PET/CT, epigastralgia, inflammatory infiltration
Introduction
Autoimmune pancreatitis (AP) is a rare autoimmune pancreatic manifestation of systemic immunoglobulin G4 (IgG4)-related sclerosing disease. AP is clinically characterized by irregular narrowing of the main pancreatic duct, lymphoplasmacytic inflammation of the pancreas, and hypergammaglobulinemia. The presence of a hypermetabolic pancreatic mass allows AP to clinically mimic pancreatic cancer. Differentiation between AP and pancreatic cancer is crucial because the clinical courses, treatments, and prognoses of these two disease entities are quite different. AP responds well to glucocorticoid therapy, while pancreatic cancer may require surgical intervention with or without adjuvant chemotherapy. Although the diagnostic criteria for AP have been revised,1–4 clinical or radiological differentiation between AP and pancreatic cancer remains difficult.
We herein report a case involving a patient with AP who presented with a hypermetabolic pancreatic mass that mimicked pancreatic cancer.
Case report
A 52-year-old man with medically controlled hypertension visited a local medical clinic because of a 1-month history of intermittent epigastralgia. The symptom was not associated with food intake or daily exercise, and there were no aggravating factors. However, the epigastralgia improved to a certain degree when the patient assumed a bent body position. His laboratory tests revealed mildly elevated liver enzymes, including glutamate oxaloacetate transaminase of 64 IU/L (reference range, 10–42 IU/L), glutamate pyruvate transaminase of 80 IU/L (reference range, 10–40 IU/L), alkaline phosphate of 178 IU/L (reference range, 32–92 IU/L), total bilirubin of 2.26 mg/dL (reference range, 0.2–1.0 mg/dL), direct bilirubin of 1.46 mg/dL (reference range, 0.0–0.2 mg/dL), and gamma glutamyl transpeptidase of 391 IU/L (reference range, 7–64 IU/L). The results of other laboratory tests, including a complete blood count/differential count, cholesterol profile, electrolytes, renal function parameters, and tumor markers (including α-fetoprotein, carbohydrate antigen 19-9, and carcinoembryonic antigen) were within the normal range.
Esophagogastroduodenoscopy revealed gastritis and external compression of the duodenum, which raised suspicion for a pancreatic tumor. Abdominal ultrasonography showed a tumor in the uncinate process of the pancreas. The patient then presented to Kaohsiung Medical University Hospital, a tertiary medical center in southern Taiwan, for further medical evaluation. Abdominal computed tomography (CT) with contrast enhancement (Figure 1) revealed a lobulated mass lesion with a cystic component in the pancreatic head. Encasement of the common hepatic artery and main portal vein was also depicted. A malignant pancreatic tumor was suspected, and CT-guided biopsy of the lesion was performed. The tiny specimen showed fibrous tissue with focal sclerotic stroma and focal lymphoid cell aggregation. Cytokeratin and synaptophysin immunostaining revealed no epithelial cancerous cells, and CD20 and CD3 immunostaining revealed no monoclonal lymphocyte proliferation. However, the diagnosis remained uncertain because the possibility of lymphoma could not be totally excluded.
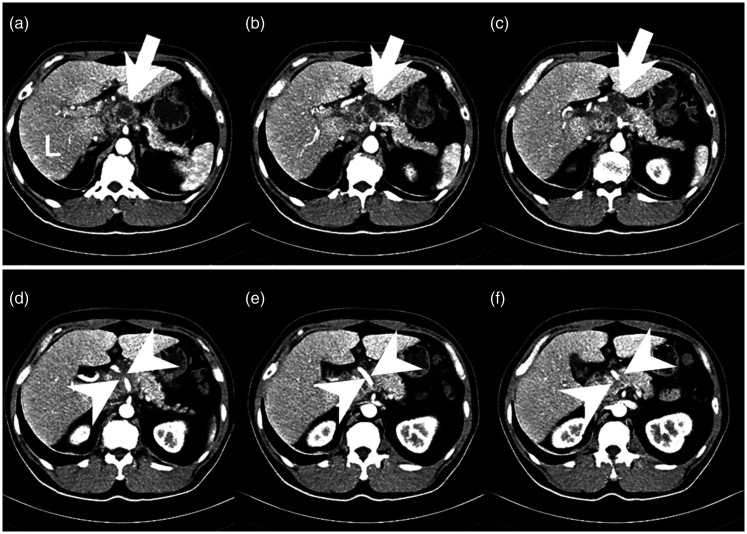
Figure 1.
A sequential axial abdominal computed tomography scan shows a lobulated mass lesion with a cystic component and peripheral enhancement in the head and proximal body of the pancreas with exophytic growth (white arrows in a–c). Encasement of the common hepatic artery and main portal vein is also depicted (white arrowheads in d–ƒ).
After the CT-guided biopsy, the patient developed more severe abdominal pain and an intermittent fever. Fluid accumulation was noted on abdominal ultrasonography. Empiric antibiotics (piperacillin/tazobactam) were prescribed. A subsequent blood culture showed no growth of aerobic or anaerobic bacteria.
After the fever subsided, 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET)/CT was performed and showed an FDG-avid, hypermetabolic, swollen soft tissue mass in the pancreatic head with a maximum standardized uptake value of 8.2 (Figure 2). Adjacent low-grade FDG-avid lymph nodes with a maximum standardized uptake value of 3.1 were also noted. These findings were highly suggestive of a malignant pancreatic tumor with metastatic lymphadenopathy. No FDG-avid lesions were present in the bilateral salivary glands, retroperitoneal spaces, or biliary tracts.
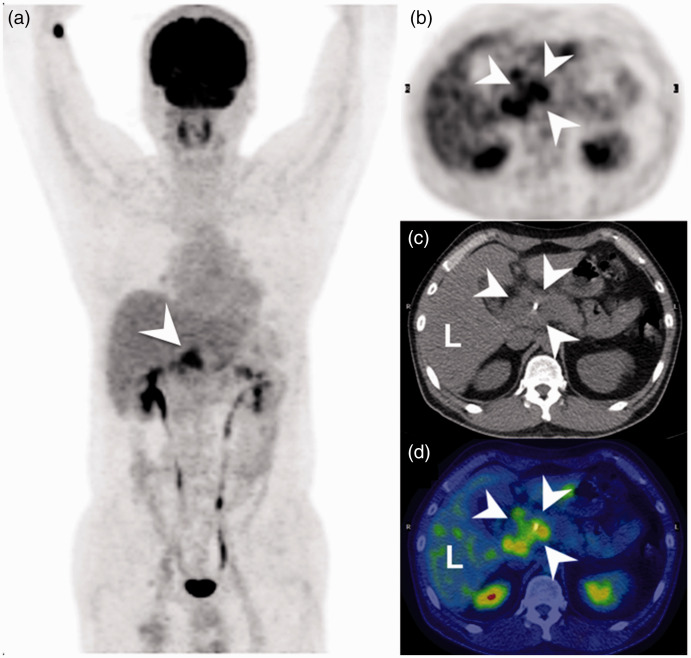
Figure 2.
Positron emission tomography/computed tomography (PET/CT) using 18F-fluorodeoxyglucose shows a hypermetabolic tumor in the head of the pancreas with a maximum standardized uptake value of 8.2 (arrowheads). (a) Maximal intensity projection. (b) PET image. (c, d) Non-enhanced CT and fused PET and CT images at corresponding levels, respectively.
An open biopsy was performed to obtain a larger specimen of the pancreatic lesion and confirm the diagnosis. Pathological examination showed chronic inflammatory infiltration of lymphocytes and plasma cells around the ductules and acini with sclerotic stroma (Figure 3). Cytokeratin immunostaining failed to disclose any epithelial malignancy. CD3 and CD20 immunostaining revealed a mixed population of B and T cells. Further immunohistochemical studies were negative for CD5, cyclin D1, Bcl-2, and CD10. Taken together, the morphology and immunohistochemical studies suggested a diagnosis of chronic sclerotic pancreatitis.
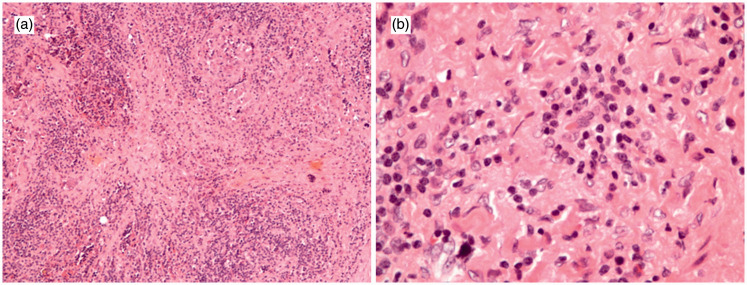
Figure 3.
Representative histological pictures (hematoxylin and eosin stain). (a) 100×, (b) 400×. These images show chronic inflammatory infiltration of lymphocytes and plasma cells around ductules and acini with sclerotic stroma in the pancreas.
We further checked the patient’s serum immunoglobulin titers. Laboratory examination showed elevation of IgG (2390 mg/dL; reference range, 751–1560 mg/dL) and IgG4 (1170 mg/dL; reference range, 39.2–864 mg/L), which was consistent with the clinical presentation of chronic sclerosing pancreatitis. IgA and IgM were within the reference ranges (310 mg/L and 142 mg/dL, respectively). The patient thereafter began corticosteroid treatment.
One month after the corticosteroid treatment, his IgG and IgG4 levels decreased to 1920 mg/dL and 1110 mg/L, respectively. Three months after the corticosteroid treatment, follow-up abdominal CT showed shrinkage of the lesion (Figure 4), and the IgG and IgG4 levels decreased to 1090 mg/dL and 532 mg/L, respectively. After 48 months of follow-up, the patient was in good condition without symptoms or signs of relapse.
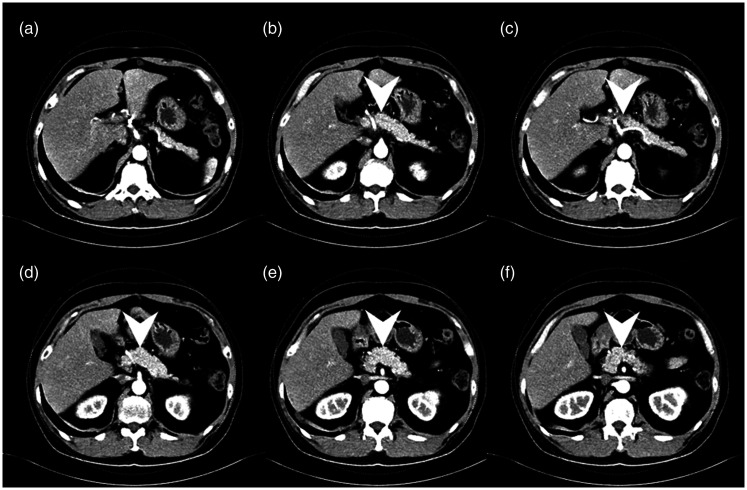
Figure 4.
(a–f) Axial abdominal computed tomography scan 3 months after corticosteroid treatment shows marked shrinkage of the previous lobulated mass lesion (arrowheads).
The patient provided informed consent for all medical procedures performed. The institutional review board of our hospital waived the need for ethics approval for publication of this report because of the study design (retrospective chart review).
Discussion
AP is a type of rare chronic pancreatitis characterized by an autoimmune inflammatory mechanism. It occurs in both sexes; however, it is predominant in men (male:female ratio of 3:1). 5 With respect to age distribution, AP occurs mostly in elderly patients. 6 Clinically, patients with AP may present with obstructive jaundice and abdominal pain, and imaging examination may reveal a swelling mass. Impairment of pancreatic endocrine or exocrine function may also be present. Up to half of patients may have glucose intolerance. Some patients are diagnosed with diabetes mellitus and AP simultaneously, while some exhibit progression of pre-existing diabetes mellitus at the onset of AP. The clinical symptoms of AP, especially localized AP, mimic those of pancreatic cancer. However, the clinical course, treatment, and prognosis are quite different between AP and pancreatic cancer; thus, correct differentiation between these two diseases is clinically very important.
AP is classified into type 1 or type 2 depending on its histopathological findings. Type 1 AP, also termed lymphoplasmacytic sclerosing pancreatitis, is microscopically characterized by dense infiltration of T lymphocytes and IgG4-postive plasma cells, storiform fibrosis, and obliterative phlebitis. The main histological feature of type 2 AP (idiopathic duct-centric pancreatitis) is neutrophilic infiltration in the epithelium of the pancreatic ducts.
No diagnostic serologic markers for AP are currently available. Therefore, the diagnosis is mainly based upon the presence of abnormal signs unique to AP. Diagnostic criteria were recently developed in Asia1,2 and the United States. 3 In 2008, Korean and Japanese pancreatologists revised the diagnostic consensus on AP. 4 In June 2017, Chari 7 wrote an article on the evolution of the diagnostic criteria for AP while celebrating the 15th anniversary of clinical gastroenterology and hepatology. Five cardinal features are used to diagnose AP: imaging findings of the pancreatic ducts and parenchyma, serology, involvement of other organs, pancreatic histology, and clinical responsiveness to steroid therapy.
The typical imaging finding of the pancreatic ducts and parenchyma is diffuse enlargement with delayed enhancement, sometimes associated with rim-like enhancement. This enhancement pattern is caused by fibroinflammatory change of the peripancreatic adipose tissue, which is rarely seen in pancreatic cancer. Corresponding patterns of perfusion abnormalities are seen on magnetic resonance imaging (MRI). Diffusion-weighted MRI shows lower apparent diffusion coefficients, playing an important role in differentiating AP from pancreatic cancer. 8 Endoscopic retrograde cholangiopancreatography may show irregular narrowing of the main pancreatic duct. Other less characteristic findings include focal to multifocal enlargement of the pancreatic glandular tissues. The patient in the present case did not undergo MRI examination. Contrast-enhanced CT showed a lobulated mass lesion with a cystic component, and heterogeneous enhancement was noted in the pancreatic head. The imaging features of enhanced CT were not typical for localized AP. Certain pancreatic neoplasms, such as mucinous cystadenocarcinoma of the pancreas, may present characteristic imaging findings. Furthermore, because of the localized entity and encasement of the common hepatic artery and main portal vein in the present case, it was difficult to differentiate the lesion from pancreatic cancer. Elevated Hounsfield units during the delayed phase of the CT scan may help distinguish localized AP from pancreatic cancer 9 ; however, the clinician and radiologist in the present case suggested tissue biopsy because a malignant pancreatic tumor could not be completely excluded.
AP appears to be a pancreatic manifestation of IgG4-related sclerosing disease based on histological and immunohistochemical examination of various organs in patients with AP. Such disease, which may clinically overlap or include AP, sclerosing cholecystitis/cholangitis, sclerosing sialadenitis/dacryoadenitis, inflammatory pseudotumors, and retroperitoneal fibrosis, is a systemic disorder affecting multiple organs with clinically apparent tissue fibrosis and obliterative phlebitis. 10 The disease extent and degree vary clinically depending on the size and numbers of involved organs. 11 The affected organs, including the pancreas, may be synchronous or metachronous. 12 An FDG PET/CT scan can help differentiate AP from pancreatic malignancy, especially in patients with increased uptake in the salivary glands, lacrimal glands, kidneys, and sometimes in other extrapancreatic organs. 13 Although some reports have described clinical detection of AP by FDG PET/CT,14,15 improvement is needed in the use of FDG PET/CT for differential diagnosis between AP and pancreatic cancer. 16 Differentiation is difficult because both AP and pancreatic cancer present a hypermetabolic status and increased FDG accumulation, as shown in the present case.
AP is the pancreatic manifestation of IgG4-related sclerosing disease; approximately two-thirds of patients with AP have an elevated serum IgG4 level. 17 However, elevation of serum IgG4 is not specific to AP. Mild elevation (1–2 times the upper limit of the reference range) is seen in 10% to 15% of patients with cholangiocarcinoma, 18 primary sclerosing cholangitis, 19 and pancreatic malignancy. 20 In a study by Hamano et al., 21 a significant difference in the IgG4 concentration was observed between patients with and without AP (median, 663 vs. 51 mg/dL, respectively). The use of a cut-off value for a higher serum IgG4 concentration (>135 mg/dL) resulted in high accuracy (97%), sensitivity (95%), and specificity (97%) for the differentiation of AP from pancreatic cancer. In our patient, the serum IgG4 level was 1170 mg/dL at the time of the initial diagnosis. This was a clue to the diagnosis of AP. However, the IgG4 level was about 1.4 times the upper limit of the reference range. Because of different reference ranges, we could not apply the above-mentioned cut-off value to our patient. Confirmative diagnosis in our patient was based on pathological examination.
The patient in this case underwent an open biopsy to obtain a confirmative pathological specimen. This procedure was relatively invasive. The use of endoscopic ultrasonography-guided fine needle aspiration (EUS-FNA) to evaluate solid pancreatic masses has been described in previous reports.22,23 EUS-FNA is less invasive and has a high accuracy rate (>90%) for distinguishing benign from malignant pancreatic masses. 24 EUS-FNA may be suggested to confirm the diagnosis when esophagogastroduodenoscopy reveals external compression of the duodenum, which raises suspicion for a pancreatic tumor.
AP is a fibroinflammatory disease in which severe inflammation may be present in the early phase. Effective treatment should involve anti-inflammatory therapy to achieve relief from symptoms and perhaps decrease or delay the progression to fibrosis. Furthermore, when it is difficult to obtain a definitive diagnosis clinically, preceding corticosteroid therapy may help the diagnosis. A poor response to therapy may raise the possibility of a malignant pancreatic tumor, and reevaluation of the diagnosis may be needed. According to established guidelines,25,26 steroid treatment is the standard therapy for AP. Initially, oral prednisolone (30–40 mg/day or 0.6 mg/kg per day) is administered for 2 to 4 weeks; thereafter, the dose is tapered by 5 mg every 1 to 2 weeks. This treatment may be continued for 3 to 6 months while carefully monitoring the patient’s symptoms and biochemical, serological, and imaging findings until a maintenance dose is reached. Morphological and serological evaluation should be performed to assess the disease responsiveness. Our patient’s IgG and IgG4 levels decreased within 1 month and continued to decrease to the normal range at 3 months after the initial steroid therapy was begun. This response helped to confirm the disease entity. On the follow-up CT scan 3 months later, the tumor in the pancreatic head had shrunk. To prevent relapse, maintenance therapy at 2.5 to 5.0 mg/day is recommended for almost all patients for at least 6 months. Maintenance therapy may be withdrawn for patients who have achieved complete remission 1 year after the initial steroid therapy. The patient remained in good condition without symptoms or signs of relapse during a follow-up period of 48 months. The results of a multicenter trial on the best approach to long-term management of AP are being eagerly awaited.
In summary, AP is an IgG4-related sclerosing disease of the pancreas that may also involve other organs. The prevalence of AP is relatively low compared with pancreatic cancer. It is a benign disease with different therapy and prognosis compared with malignant pancreatic cancer; however, misdiagnosis between these two diseases readily occurs, especially in patients with localized AP. Clinicians should be aware of this disease and be familiar with the diagnostic criteria to avoid misdiagnosis and unnecessary surgical intervention.
Acknowledgments
We thank Dr. Chun-Chieh Wu at the Department of Pathology, Kaohsiung Medical University Hospital, Kaohsiung, Taiwan, for his review of the pathological slides and help with the diagnosis.
Declaration of conflicting interests
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Kim KP, Kim MH, Kim JC, et al. Diagnostic criteria for autoimmune chronic pancreatitis revisited. World J Gastroenterol 2006; 12: 2487–2496. 2006/05/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Okazaki K, Kawa S, Kamisawa T, et al. Clinical diagnostic criteria of autoimmune pancreatitis: revised proposal. J Gastroenterol 2006; 41: 626–631. 2006/08/26. DOI: 10.1007/s00535-006-1868-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chari ST, Smyrk TC, Levy MJ, et al. Diagnosis of autoimmune pancreatitis: the Mayo Clinic experience. Clin Gastroenterol Hepatol 2006; 4: 1010–1016. quiz 1934. 2006/07/18. DOI: 10.1016/j.cgh.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 4.Otsuki M, Chung JB, Okazaki K, et al. Asian diagnostic criteria for autoimmune pancreatitis: consensus of the Japan-Korea Symposium on Autoimmune Pancreatitis. J Gastroenterol 2008; 43: 403–408. 2008/07/05. DOI: 10.1007/s00535-008-2205-6. [DOI] [PubMed] [Google Scholar]
- 5.Hart PA, Kamisawa T, Brugge WR, et al. Long-term outcomes of autoimmune pancreatitis: a multicentre, international analysis. Gut 2013; 62: 1771–1776. 2012/12/13. DOI: 10.1136/gutjnl-2012-303617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kamisawa T, Yoshiike M, Egawa N, et al. Chronic pancreatitis in the elderly in Japan. Pancreatology 2004; 4: 223–227; discussion 227–228. 2004/05/19. DOI: 10.1159/000078433. [DOI] [PubMed] [Google Scholar]
- 7.Chari ST. Diagnosis of autoimmune pancreatitis: the evolution of diagnostic criteria for a rare disease. Clin Gastroenterol Hepatol 2017; 15: 1485–1488. 2017/06/22. DOI: 10.1016/j.cgh.2017.06.023. [DOI] [PubMed] [Google Scholar]
- 8.Kamisawa T, Takuma K, Anjiki H, et al. Differentiation of autoimmune pancreatitis from pancreatic cancer by diffusion-weighted MRI. Am J Gastroenterol 2010; 105: 1870–1875. 2010/03/11. DOI: 10.1038/ajg.2010.87. [DOI] [PubMed] [Google Scholar]
- 9.Sun GF, Zuo CJ, Shao CW, et al. Focal autoimmune pancreatitis: radiological characteristics help to distinguish from pancreatic cancer. World J Gastroenterol 2013; 19: 3634–3641. 2013/06/27. DOI: 10.3748/wjg.v19.i23.3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takuma K, Kamisawa T, Gopalakrishna R, et al. Strategy to differentiate autoimmune pancreatitis from pancreas cancer. World J Gastroenterol 2012; 18: 1015–1020. 2012/03/15. DOI: 10.3748/wjg.v18.i10.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kamisawa T, Takuma K, Egawa N, et al. Autoimmune pancreatitis and IgG4-related sclerosing disease. Nat Rev Gastroenterol Hepatol 2010; 7: 401–409. 2010/06/16. DOI: 10.1038/nrgastro.2010.81. [DOI] [PubMed] [Google Scholar]
- 12.Takuma K, Kamisawa T, Anjiki H, et al. Metachronous extrapancreatic lesions in autoimmune pancreatitis. Intern Med 2010; 49: 529–533. 2010/03/17. [DOI] [PubMed] [Google Scholar]
- 13.Kamisawa T, Takum K, Anjiki H, et al. FDG-PET/CT findings of autoimmune pancreatitis. Hepatogastroenterology 2010; 57: 447–450. 2010/08/12. [PubMed] [Google Scholar]
- 14.Han L, Jiang L, Tan H, et al. An atypical case of IgG4-related sclerosing pancreatitis on PET/CT imaging. Clin Nucl Med 2014; 39: e236–e238. 2013/04/13. DOI: 10.1097/RLU.0b013e3182816752. [DOI] [PubMed] [Google Scholar]
- 15.Cao Z, Tian R, Zhang T, et al. Localized autoimmune pancreatitis: report of a case clinically mimicking pancreatic cancer and a literature review. Medicine (Baltimore) 2015; 94: e1656. 2015/10/27. DOI: 10.1097/MD.0000000000001656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nanni C, Romagnoli R, Rambaldi I, et al. FDG PET/CT in autoimmune pancreatitis. Eur J Nucl Med Mol Imaging 2014; 41: 1264–1265. 2014/02/25. DOI: 10.1007/s00259-014-2699-5. [DOI] [PubMed] [Google Scholar]
- 17.Kamisawa T, Chari ST, Giday SA, et al. Clinical profile of autoimmune pancreatitis and its histological subtypes: an international multicenter survey . Pancreas 2011; 40: 809–814. 2011/07/13. DOI: 10.1097/MPA.0b013e3182258a15. [DOI] [PubMed] [Google Scholar]
- 18.Oseini AM, Chaiteerakij R, Shire AM, et al. Utility of serum immunoglobulin G4 in distinguishing immunoglobulin G4-associated cholangitis from cholangiocarcinoma. Hepatology 2011; 54: 940–948. 2011/06/16. DOI: 10.1002/hep.24487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bjornsson E, Chari S, Silveira M, et al. Primary sclerosing cholangitis associated with elevated immunoglobulin G4: clinical characteristics and response to therapy. Am J Ther 2011; 18: 198–205. 2010/03/17. DOI: 10.1097/MJT.0b013e3181c9dac6. [DOI] [PubMed] [Google Scholar]
- 20.Raina A, Krasinskas AM, Greer JB, et al. Serum immunoglobulin G fraction 4 levels in pancreatic cancer: elevations not associated with autoimmune pancreatitis. Arch Pathol Lab Med 2008; 132: 48–53. 2008/01/10. DOI: 10.1043/1543-2165(2008)132[48:SIGFLI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 21.Hamano H, Kawa S, Horiuchi A, et al. High serum IgG4 concentrations in patients with sclerosing pancreatitis. N Engl J Med 2001; 344: 732–738. 2001/03/10. DOI: 10.1056/NEJM200103083441005. [DOI] [PubMed] [Google Scholar]
- 22.Kanno A, Masamune A, Fujishima F, et al. Diagnosis of autoimmune pancreatitis by EUS-guided FNA using a 22-gauge needle: a prospective multicenter study. Gastrointest Endosc 2016; 84: 797–804.e1. 2016/04/14. DOI: 10.1016/j.gie.2016.03.1511. [DOI] [PubMed] [Google Scholar]
- 23.Kamisawa T, Anjiki H, Takuma K, et al. Endoscopic approach for diagnosing autoimmune pancreatitis. World J Gastrointest Endosc 2010; 2: 20–24. 2010/12/17. DOI: 10.4253/wjge.v2.i1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Imai K, Matsubayashi H, Fukutomi A, et al. Endoscopic ultrasonography-guided fine needle aspiration biopsy using 22-gauge needle in diagnosis of autoimmune pancreatitis. Dig Liver Dis 2011; 43: 869–874. 2011/07/08. DOI: 10.1016/j.dld.2011.05.021. [DOI] [PubMed] [Google Scholar]
- 25.Kamisawa T, Shimosegawa T, Okazaki K, et al. Standard steroid treatment for autoimmune pancreatitis. Gut 2009; 58: 1504–1507. 2009/04/29. DOI: 10.1136/gut.2008.172908. [DOI] [PubMed] [Google Scholar]
- 26.Kamisawa T, Okazaki K, Kawa S, et al. Japanese consensus guidelines for management of autoimmune pancreatitis: III. Treatment and prognosis of AIP. J Gastroenterol 2010; 45: 471–477. 2010/03/10. DOI: 10.1007/s00535-010-0221-9. [DOI] [PubMed] [Google Scholar]