Short abstract
Objective
This study was performed to investigate the effects of age and sex on 10 salivary steroid hormones and analyze the correlations between salivary and plasma hormones.
Methods
The concentrations of 10 salivary steroid hormones in 1090 Chinese adult volunteers were examined using liquid chromatography–tandem mass spectrometry, and a related investigation was performed on the concentrations of salivary hormones in this population.
Results
The concentrations of androstenedione (A4), 17α-hydroxyprogesterone (17-OHP), aldosterone (ALD), cortisone (COR), corticosterone (CORT), cortisol (F), progesterone (P), and testosterone were significantly different between men and women (Student’s t-test). Differences in 17-OHP and ALD concentrations were highly significant between women in the follicular and luteal phases of their menstrual cycle (Student’s t-test). Five salivary steroid hormones (17-OHP, A4, CORT, COR, and F) significantly decreased with increasing age (Kruskal–Wallis test). A high linear correlation between salivary and plasma 17-OHP, P, A4, and F were observed with obvious sex-related differences (Pearson’s correlation, r > 0.7).
Conclusions
Our results provide important knowledge regarding the descriptive characteristics of salivary hormones in relation to age and sex and their correlations with plasma hormones.
Keywords: Saliva, plasma, steroid hormones, LC-MS/MS, Chinese, age, sex
Introduction
Salivary steroids are important compounds of saliva. Their use in clinical applications and medical research has recently attracted increasing attention because measurement of salivary hormones offers a noninvasive and stress-free alternative to measurement of plasma and serum hormones.1,2 Hormone concentrations are affected by various factors including sex, women’s menstrual cycle, and age. Many hormones, especially sex hormones, have significantly different salivary concentrations between the two sexes. 3 Women’s menstrual cycle can affect the concentrations of some hormones, including progesterone (P) and aldosterone (ALD).4–6 During the menstrual cycle of healthy women, changes in the P concentration are well documented in both serum and saliva. 5 Additionally, it has been demonstrated that plasma ALD shows cyclicity during the course of a normal menstrual cycle and that urinary and serum ALD concentrations are significantly higher during the luteal phase (Lp). 6 Many hormone concentrations may change with age,7–9 and understanding age-related endocrine changes is important because of the role that hormones play in maintenance of reproductive function, physiological responses to the environment, and diagnosis and management of endocrine and metabolic diseases.10,11 Previous studies have shown that serum and plasma concentrations of corticosterone (CORT) and androstenedione (A4) significantly decrease with increasing age. 12
Previous studies have clearly demonstrated that the quantification of salivary steroid hormones can be used to monitor concentrations of unbound free steroid hormones in blood.13,14 Measurements of salivary hormone concentrations have clinical value if they reflect the plasma hormone concentrations. The relationship between the salivary and plasma concentrations of many hormones has been evaluated in several studies.1,13–15 One review showed a strong correlation between salivary and plasma P concentrations in women. 1
The overall aim of the present study was to reveal the factors that affect human salivary hormone levels. Using liquid chromatography–tandem mass spectrometry (LC-MS/MS), we quantitated 10 steroid hormones in salivary samples from Chinese adult volunteers and investigated the effects of sex and age on the concentrations of these 10 salivary steroid hormones. The correlation of salivary and plasma steroid concentrations was also analyzed.
Materials and Methods
Sample collection and hormone quantification
Saliva samples were collected from employees of a company in Yantian District, Shenzhen, Guangdong Province, China. Plasma hormones were tested using an AbsoluteIDQ Stero17 Kit (Biocrates Life Sciences AG, Innsbruck, Austria).
Written informed consent was obtained from all participants in accordance with the Declaration of Helsinki. This study was approved by the Institutional Review Board on Bioethics and Biosafety of BGI in Shenzhen, China (approval number FT15222).
All saliva samples were collected from 7:00 to 9:00 AM. The participants were prohibited from eating food or smoking before saliva collection. The saliva samples were collected by natural secretion without external stimuli and placed in a 50-mL centrifuge tube wrapped in silver paper. Immediately after collecting 3 mL of saliva, blood samples for hormone quantification were collected. The blood samples were drawn in EDTA anticoagulant tubes to avoid hemolysis, and the whole blood samples were then separated to obtain plasma. The saliva and plasma samples were stored at −80°C.
Sample pretreatment
Before LC-MS/MS analysis, the saliva samples were left to thaw at 4°C for approximately 30 min; 900 µL of saliva was then centrifuged at 20,000 × g for 10 min, and 800 µL of supernatant was sent for further preparation. An equal volume of blank, double blank, quality control, and calibration standard solutions were prepared in parallel; the solvent of all solutions was water (Optima™ LC/MS Grade; Fisher Chemical, Loughborough, UK). Solid-phase extraction was carried out by adding 10 µL of mixed internal standard (IS) and 800 µL deionized water (Optima™ LC/MS Grade; Fisher Chemical) into each tube except for the double blank and transferring all solvent to the corresponding well of the pre-activated solid-phase extraction plate using a Waters Positive Pressure-96 Processor (Waters, Milford, MA, USA). Each well was then washed with 900 µL of water and eluted with 600 µL of acetonitrile. The eluate was dried under vacuum and redissolved in 50 µL of 25% methanol and 75% water for further LC-MS/MS analysis.
Chemicals and reagents
Cortisone (COR) and 17α-hydroxyprogesterone (17-OHP) were purchased from Sigma–Aldrich (St. Louis, MO, USA). Aldosterone (ALD) and 11-deoxycortisol were purchased from The Technology Registrations Canada (Toronto, Canada). 11-Deoxycorticosterone, A4, CORT, cortisol (F), P, and testosterone (T) were purchased from Dr. Ehrenstorfer GmbH (Augsburg, Germany). Cortisone-d8, 11-deoxycorticosterone-d7, aldosterone-d7, corticosterone-d8, and testosterone-2,2,4,6,6-d5 were purchased from The Technology Registrations Canada. 11-Deoxycortisol-d5 (2,2,4,6,6-d5) was obtained from Cerilliant Corporation (Round Rock, TX, USA). 5α-Androstan-3α-ol-17-one-2,2,3,4,4-d5 and cortisol-9,11,12,12-d4 were purchased from C/D/N Isotopes Inc. (Pointe-Claire, Quebec, Canada), and progesterone-2,3,4-13C3 and 17α-hydroxyprogesterone-2,3,4-13C3 were purchased from Sigma–Aldrich. Acetonitrile and reagent grade ammonium acetate were obtained from Fisher Chemical.
Preparation of stock and standard solutions
Stock solutions of each compound and IS were prepared separately in methanol to produce a concentration of 1 mg/mL. The stock solutions were further individually diluted with methanol to obtain working standard solutions of all agents. IS mixtures were prepared in methanol at the final concentrations (ALD: 15.6250 ng/mL, A4: 4.6875 ng/mL, CORT: 18.7500 ng/mL, F: 46.875 ng/mL, COR: 46.875 ng/mL, 11-corticosterone [S]: 15.6250 ng/mL, 11-deoxycorticosterone [DOC]: 6.2500 ng/mL, 17-OHP: 15.6250 ng/mL, P: 3.1250 ng/mL). All stock solutions and working standard solutions were stored at −80°C when not in use.
Chromatographic conditions
A 20-µL mixture was injected into a high-performance liquid chromatography system (LC-20AD; Shimadzu, Kyoto, Japan) equipped with a C18 2- × 150-mm, 4-µm Hydro-RP column (Phenomenex, Torrance, CA, USA). The mobile phase consisted of 2 mM deionized water (Optima™ LC/MS Grade, Fisher Chemical) with ammonium acetate (A) and methanol with 2 mM ammonium acetate (B). A linear gradient was run from 5% to 100% B and maintained at 100% methanol for 2.6 min, followed by 1.7 min of equilibration at 5% methanol, resulting in a total run time of 10 min. The flow rate was 0.65 mL/min and the temperature of the auto sampler and column oven was 8°C and 55°C, respectively.
MS detection and data processing
A mass spectrometer (QTRAP 5500; AB SCIEX, Framingham, MA, USA/Concord, Ontario, Canada) equipped with an atmospheric pressure chemical ionization ion source was operated in the positive ion mode. The curtain gas (N2), collision gas (N2), nebulizer current, source temperature, and ion source gas 1 (N2) were set to 35.0 psi, medium, 3.0 µA, 500°C, and 60 psi, respectively. Quantification was achieved using the mass spectrometer in multiple reaction monitoring mode. The transitions are listed in Table 1.
Table 1.
Tandem mass spectrometry conditions for the steroid hormone compounds
| Compound | MRM (Da) | DP (V) | EP (V) | CE (eV) | CXP (V) |
|---|---|---|---|---|---|
| CORT* | 347.101→121.1 | 80 | 10 | 32 | 12 |
| CORT | 347.3→329.2 | 80 | 10 | 22 | 12 |
| CORT IS | 355.2→337.1 | 71 | 10 | 23 | 8 |
| T* | 289.135→97 | 100 | 10 | 30 | 16 |
| T | 289.135→109 | 100 | 10 | 34 | 18 |
| T C3 | 292.2→100.1 | 90 | 10 | 29.6 | 10 |
| P* | 315.112→97 | 100 | 10 | 30 | 12 |
| P | 315.112→109 | 100 | 10 | 38 | 12 |
| P_C3 | 318.2→100.1 | 95 | 10 | 50.4 | 12 |
| A2* | 287.112→97.1 | 100 | 10 | 28 | 14 |
| A2 | 287.112→109 | 100 | 10 | 36 | 20 |
| A2_IS | 290.2→100.1 | 71 | 10 | 35 | 8 |
| F* | 363.13→121 | 141 | 10 | 36 | 20 |
| F | 363.13→115.1 | 141 | 10 | 114 | 18 |
| F IS | 367.2→121.1 | 66 | 10 | 35 | 8 |
| ALD* | 361.1→343.1 | 130 | 10 | 26 | 26 |
| ALD | 361.1→115.1 | 130 | 10 | 112 | 18 |
| ALD_IS | 368.2→350.2 | 76 | 10 | 25 | 13 |
| 11DOC* | 347.11→109 | 115 | 10 | 40 | 20 |
| 11DOC | 347.11→97 | 115 | 10 | 38 | 10 |
| 11DOC IS | 352.2→113.1 | 76 | 10 | 37 | 15 |
| COR | 361→163 | 130 | 10 | 30 | 13 |
| COR IS | 368.2→169.1 | 76 | 10 | 35 | 12 |
| 17-OHP* | 331.3→97.1 | 100 | 10 | 30 | 12 |
| 17-OHP | 331.3→109.1 | 100 | 10 | 34 | 12 |
| 17-OHP_C3 | 334.2→100 | 270 | 10 | 30 | 12 |
| DOC* | 331.3→97.2 | 100 | 10 | 29 | 12 |
| DOC | 331.3→109.11 | 100 | 10 | 31 | 12 |
| DOC IS | 339.2→113.15 | 71 | 10 | 43 | 6 |
For each compound, the most sensitive transition (marked with an asterisk) was used for quantitation (quantifier), and the second one was used for confirmation (qualifier). MRM, multiple reaction monitoring; DP, declustering potential; EP, entrance potential; CE, collision energies; CXP, collision cell exit potential; T, testosterone; A4, androstenedione; F, cortisol; COR, cortisone; P, progesterone; 17-OHP, 17α-hydroxyprogesterone; CORT, corticosterone; ALD, aldosterone; DOC, 11-deoxycorticosterone; S, 11-corticosterone.
Quantitation was completed using MultiQuant software, Version 1.1.1296.0 (AB SCIEX). The calibration curves were determined by the area ratio of target ion transition and its corresponding IS transition as the y axis against the concentration of the target molecules as the x axis. The saliva hormone concentrations of the unknown sample were determined by a regression equation.
Standard calibration curves and method validation
Calibration standards were prepared by spiking blank saliva samples with standard solutions to the final concentrations of 0, 0.001, 0.01, 0.05, 0.1, 0.5, 1, 5, and 10 ng/mL for all steroids. Linearity was assessed by the correlation coefficient (r) on the calibrators with seven different concentrations. Calibration curves were built using the formula y = ax + b, where x represents the compound concentration and y represents the ratio of the compound peak area to the IS peak area.
Inter- and intra-assay variability was determined by calculating precision and accuracy estimates for saliva samples with seven replicates each on three separate days. Accuracy and precision were obtained as the percentage of relative error and the percentage coefficient of variation, respectively. Accuracy and precision batches comprised seven replicates of quality control samples at three different concentrations (low, middle, and high quality control) for all tested compounds.
To evaluate the matrix effect and method recovery, three blank saliva samples with seven replicates each were spiked with pure standard samples at three different concentrations (i.e., the low, middle, and high quality control values specified above). The response of the analyte was compared to the response of a neat standard solution. The matrix effect value for each sample was calculated as follows: ((B/A) − 1) × 100%, where A is the analyte peak area of the pure standard sample and B is the analyte peak area of a saliva spiked with the standard sample. The method recovery value was calculated as follows: C/D × 100%, where C is the concentration value quantitated from blank saliva spiked with the standard sample using our LC-MS/MS method, while D is the concentration value of the respective standard sample.
The linearity, limit of detection, and limit of quantification of the method were determined and are summarized in Table 2. The positive ion mode was used for all steroids. Near-perfect linear correlation coefficients (r > 0.99) were found for each analyte, indicating adequate linearity of the analytical procedure. The limit of detection for each compound is shown in Table 2 and ranges from 0.595 to 14.286 ng/L. The matrix effect, recovery, and inter- and intra-assay coefficients of variation are shown in Table 3.
Table 2.
Linearity, detection limit, and retention time for the examined steroid hormones
| Hormone | Calibration curve | r | LOQ (ng/L) | LOD (ng/L) | R-time (min) |
|---|---|---|---|---|---|
| CORT | y = 0.03338x + 0.00211 | 0.98706 | 47.619 | 14.286 | 5.08 |
| T | y = 0.29413x + 0.00570 | 0.98891 | 4.762 | 0.794 | 5.75 |
| P | y = 0.39121x + 0.00782 | 0.99437 | 9.921 | 5.952 | 6.65 |
| A4 | y = 3.44495x + 0.06099 | 0.98407 | 3.571 | 0.595 | 5.52 |
| F | y =0.01516x + 0.00770 | 0.98686 | 23.810 | 2.381 | 4.66 |
| ALD | y =0.60511x + 0.00667 | 0.98938 | 8.929 | 2.976 | 4.42 |
| S | y =0.55426x + 0.01445 | 0.98781 | 7.143 | 1.19 | 5.12 |
| COR | y =3.10874x + 2.44755 | 0.98172 | 19.841 | 1.984 | 4.53 |
| 17-OHP | y =0.07371x + 0.00312 | 0.98709 | 5.952 | 0.992 | 5.74 |
| DOC | y =0.44927x + 0.01018 | 0.98721 | 5.952 | 0.992 | 5.58 |
LOQ, limit of quantification; LOD, limit of detection; R-time, retention time; T, testosterone; A4, androstenedione; F, cortisol; COR, cortisone; P, progesterone; 17-OHP, 17α-hydroxyprogesterone; CORT, corticosterone; ALD, aldosterone; DOC, 11-deoxycorticosterone; S, 11-corticosterone.
Table 3.
Inter- and intra-assay variability, matrix effect, and extraction recovery for 10 salivary steroids
| Hormone | Nominal (ng/mL) |
Intra-assay |
Inter-assay |
R ± SD (%) compound | ME ± SD (%) compound | |||
|---|---|---|---|---|---|---|---|---|
| %RE | %CV | %RE | %CV | |||||
| CORT | Low | 0.0714 | −31.4 | 19.5 | −37.5 | 31.3 | 62 ± 20 | 10 ± 5 |
| Median | 1.4286 | 6.7 | 4.6 | 9.4 | 6.0 | 109 ± 7 | ||
| High | 2.1429 | 14.6 | 7.5 | 22.1 | 8.6 | 122 ± 10 | ||
| T | Low | 0.0238 | −43.6 | 9.7 | −35.5 | 13.4 | 65 ± 9 | 6 ± 5 |
| Median | 0.4762 | 13.6 | 6.2 | 14.6 | 6.0 | 115 ± 7 | ||
| High | 0.7143 | 17.8 | 5.2 | 22.4 | 4.9 | 122 ± 6 | ||
| P | Low | 0.0298 | −50.0 | 11.1 | −41.8 | 41.2 | 58 ± 24 | 28 ± 14 |
| Median | 0.5952 | 5.3 | 4.0 | 17.1 | 9.0 | 117 ± 11 | ||
| High | 0.8929 | 15.8 | 3.5 | 33.9 | 10.4 | 134 ± 14 | ||
| A4 | Low | 0.0179 | −54.6 | 8.8 | −49.0 | 13.1 | 51 ± 7 | 3 ± 13 |
| Median | 0.3571 | 21.5 | 3.2 | 17.1 | 4.6 | 117 ± 5 | ||
| High | 0.5357 | 33.0 | 2.9 | 27.7 | 4.6 | 128 ± 6 | ||
| F | Low | 0.7143 | −43.0 | 7.9 | −45.4 | 17.2 | 55 ± 9 | 7 ± 5 |
| Median | 14.2857 | 9.9 | 6.1 | 8.0 | 7.4 | 115 ± 7 | ||
| High | 21.4286 | 14.7 | 7.5 | 13.9 | 6.5 | 114 ± 7 | ||
| ALD | Low | 0.0089 | −29.0 | 25.0 | −19.2 | 38.1 | 81 ± 31 | 13 ± 7 |
| Median | 0.1786 | 21.7 | 6.2 | 24.5 | 9.5 | 124 ± 12 | ||
| High | 0.2679 | 28.0 | 7.7 | 28.8 | 7.4 | 129 ± 10 | ||
| S | Low | 0.0357 | −27.7 | 7.0 | −35.1 | 18.4 | 65 ± 12 | 14 ± 7 |
| Median | 0.7143 | 13.6 | 7.4 | 13.2 | 7.5 | 113 ± 8 | ||
| High | 1.0714 | 23.5 | 2.8 | 21.6 | 6.1 | 122 ± 7 | ||
| COR | Low | 0.5952 | −48.7 | 27.3 | −56.2 | 31.3 | 44 ± 19 | 0 ± 9 |
| Median | 11.9048 | 14.7 | 10.2 | 18.9 | 6.0 | 119 ± 11 | ||
| High | 17.8571 | 29.0 | 5.3 | 14.1 | 8.6 | 114 ± 19 | ||
| 17-OHP | Low | 0.0744 | −42.2 | 8.7 | −34.4 | 14.4 | 66 ± 9 | 14 ± 6 |
| Median | 1.4881 | 12.3 | 6.1 | 12.7 | 7.5 | 113 ± 8 | ||
| High | 2.2321 | 17.7 | 3.8 | 23.3 | 4.8 | 123 ± 6 | ||
| DOC | Low | 0.0298 | −40.0 | 18.3 | −37.6 | 18.1 | 62 ± 11 | 18 ± 7 |
| Median | 0.5952 | 16.1 | 6.7 | 14.8 | 5.2 | 115 ± 6 | ||
| High | 0.8929 | 16.9 | 6.9 | 22.4 | 7.0 | 122 ± 9 | ||
%RE: percentage of relative error; %CV: percentage coefficient of variation; ME: matrix effect; R: method recovery; SD, standard deviation; T, testosterone; A4, androstenedione; F, cortisol; COR, cortisone; P, progesterone; 17-OHP, 17α-hydroxyprogesterone; CORT, corticosterone; ALD, aldosterone; DOC, 11-deoxycorticosterone; S, 11-corticosterone.
Statistical analysis
Samples were divided into three groups [men, women in the Lp, and women in the follicular phase (Fp)] to investigate the relationships among sex, the menstrual cycle, and salivary hormones. The differences between the men and the women in different stages of the menstrual cycle were statistically analyzed by one-way analysis of variance (ANOVA) and Student’s t-test. Before one-way ANOVA, the normality and homogeneity of variance all of variables were checked by the Shapiro–Wilk test and Bartlett test. If any of the variables failed the requirements for normality or homogeneity of variance (p-value < 0.05), the data were converted by normal conversion and rechecked until all variables satisfied the requirements of one-way ANOVA.
The samples were divided into three age groups and then further divided into groups according to sex and menstrual phases. The Kruskal–Wallis test was used among the groups to analyze the effects of increasing age on salivary hormone concentrations.
Metabolites in saliva and blood were analyzed using Pearson’s correlation with calculation of r and the p-value. The associations between steroid hormones in saliva were measured using Pearson’s correlation, and heat maps were drawn using the heatmap package based on the R language. The strength of correlation was interpreted following the method established by Mukaka. 16
Results
Participants
In total, 1090 saliva samples were collected from 1090 Chinese adult volunteers. The volunteers comprised 421 men, 471 women, and 198 individuals of unspecified sex. Data on the women’s menstrual cycles were collected by questionnaires: 310 women were in the Fp and 153 were in the Lp, 7 women were pregnant, and 1 woman was in menopause. The data of the pregnant and menopausal women were excluded from the sex- and menstruation-related analyses.
Sex-related differences
According to the density plot of the 10 hormones, different levels of heteroscedasticity were present in the distribution of the 10 salivary steroid hormones, indicating that sex, age, and other factors may have important effects on the concentrations of salivary metabolites. Consequently, we investigated the differences in the concentration distributions of the 10 salivary steroid hormones between the two sexes, among the three age groups, and between women in the different stages of the menstrual cycle.
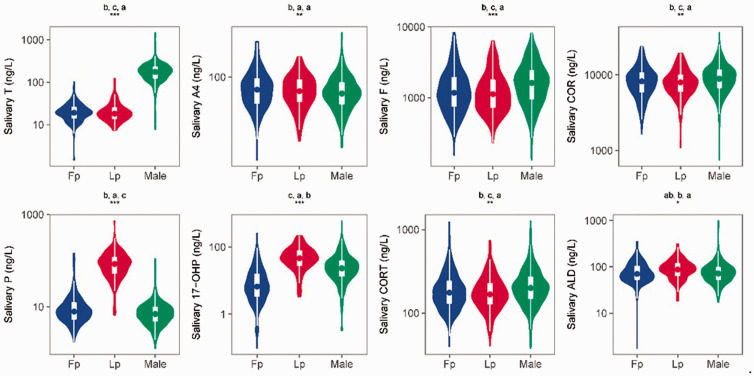
After logarithmic conversion, all 10 variables satisfied the requirements for normality (Shapiro–Wilk test) and homogeneity of variance (Bartlett test). Next, we compared the concentrations of the 10 salivary steroid hormones among women in the Fp, women in the Lp, and men. Eight metabolites (17-OHP, ALD, A4, COR, CORT, F, T, and P) showed significant differences between women in different menstrual stages and men (Student’s t-test test, p < 0.05). The concentrations of 17-OHP, ALD, COR, CORT, F, T, and P showed extremely significant differences between women in different menstrual stages and men (Student’s t-test, p < 0.01). The violin plot of these eight metabolites is shown in Figure 1. The difference in the salivary DOC and S concentrations between women in different menstrual stages and men was not statistically significant (Student’s t-test).
Figure 1.
Comparison of the concentrations of 10 salivary steroid hormones between women in the follicular phase (Fp, n = 310), women in the luteal phase (Lp, n = 153), and men (Male, n = 421) (one-way analysis of variance; *p < 0.05, **p < 0.01, ***p < 0.001). The letters above each plot represent the comparison result. For all groups with the same letter, the difference between the means was not statistically significant (Student’s t-test, p < 0.05). The groups that do not share the same letters are significantly different. T, testosterone; A4, androstenedione; F, cortisol; COR, cortisone; P, progesterone; 17-OHP, 17α-hydroxyprogesterone; CORT, corticosterone; ALD, aldosterone.
Some hormones in women are affected by the menstrual cycle. The salivary concentrations of P, 17-OHP, and ALD were significantly different between women in the different menstrual stages (Student’s t-test, p < 0.05). The concentration of P in saliva was significantly higher in women in the Lp than Fp, while the salivary P concentration was not significantly different between women in the Fp and men (Student’s t-test). Like the serum T, S, F, COR, and CORT concentrations, the salivary T, S, F, COR, and CORT concentrations in women did not vary between different stages of the menstrual cycle (Student’s t-test test); however, they showed significant differences between men and women (Student’s t-test, p < 0.05).
Age-related differences
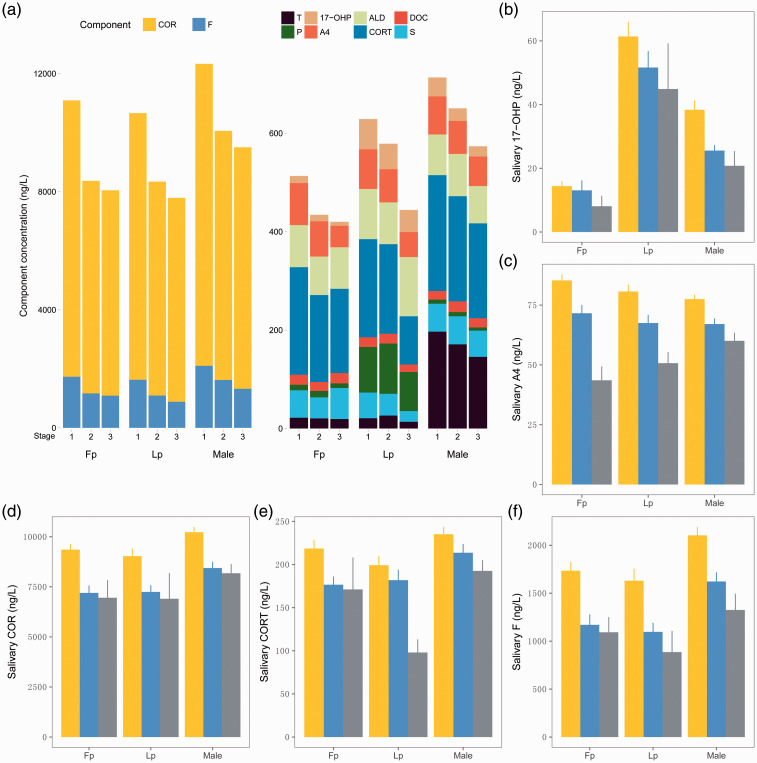
To investigate the effect of age on the concentrations of the 10 salivary steroid hormones, we divided the samples into three age groups: 20–29 years (n = 600), 30–39 years (n = 252), and ≥40 years (n = 40). We grouped the samples by age group, sex, and female menstrual stage, then compared the mean value of each group. The mean concentrations of the 10 salivary steroid hormones by age group, sex, and menstrual stage were compared using the Kruskal–Wallis test and are shown in Figure 2.
Figure 2.
Bar plot of salivary steroid hormones in different age groups, sexes, and female menstrual cycle stages. The number of patients in each of the 9 groups from left to right is 220, 81, 7, 101, 48, 4, 272, 121, and 28, respectively. (a) Bar plot of 10 salivary steroid hormones in different age groups, sexes, and female menstrual cycle stages. On the x-axis, 1 corresponds to the 20- to 29-year age group, 2 corresponds to the 30- to 39-year age group, and 3 corresponds to the ≥40-year age group. (b)–(f) Bar plots of salivary 17-OHP, A4, F, CORT, and COR levels in different age groups, sexes, and menstrual phases. The yellow, blue, and gray columns represent the 20- to 29-year, 30- to 39-year, and ≥40-year age groups, respectively. T, testosterone; A4, androstenedione; F, cortisol; COR, cortisone; P, progesterone; 17-OHP, 17α-hydroxyprogesterone; CORT, corticosterone; ALD, aldosterone; DOC, 11-deoxycorticosterone; S, 11-corticosterone.
The salivary concentrations of five steroid hormones (17-OHP, A4, COR, CORT, and F) significantly decreased with age (Kruskal Wallis test; p < 0.05) (Figure 2(b)–(f)). Differences in the effects of age on some hormones were also observed in the different sex and menstrual cycle stage groups. For instance, a significant decrease in the salivary 17-OHP concentration was only observed in men, while there was no significant difference among the different age groups in women in either the Fp or Lp.. The difference in the salivary concentration of DOC across all groups was not significant, indicating that the concentration of salivary DOC is not affected by age or sex (Kruskal–Wallis test, Benjamini–Hochberg correction).
Correlations between salivary and blood concentrations of metabolites
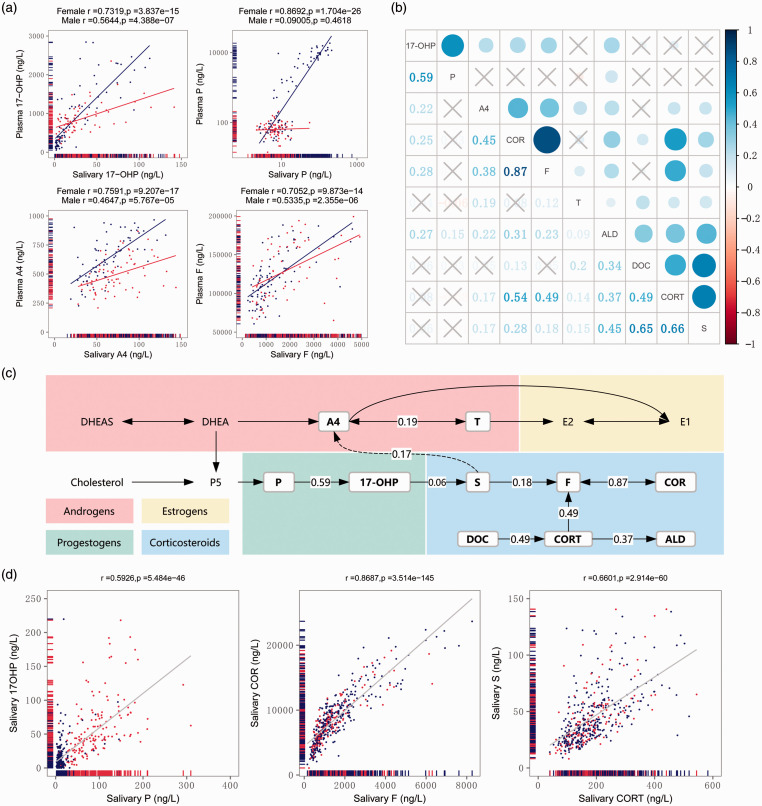
The correlations between salivary and plasma steroid hormone concentrations in men and women were analyzed by Pearson’s correlation coefficient. The top four metabolites (17-OHP, P, A4, and F) with the highest correlation (Pearson’s correlation, r > 0.7, p < 0.001) 16 were chosen for plotting in Figure 3(a). Each salivary concentration of 17-OHP, P, A4, and F obtained in this study was plotted against the corresponding plasma concentration of 17-OHP, P, A4, and F. As shown in Figure 3(a), an excellent linear relationship was present between the salivary and plasma concentrations of 17-OHP, P, A4, and F in women, with correlation coefficients of 0.7319, 0.8692, 0.7591, and 0.7052, respectively (Pearson’s correlation, p < 0.001). However, the corresponding correlation coefficients of 17-OHP, A4, and F in men were 0.5644, 0.4647, and 0.5335, respectively (p < 0.001), and that of salivary P and plasma P was 0.09005. The correlations of the salivary and plasma concentrations of steroid hormones varied between men and women, and the correlations tended to be stronger in women.
Figure 3.
(a) Correlations between salivary and plasma 17-OHP, P, A4, and F (Pearson’s correlation). The red and blue points represent male and female samples. (b) Concentration correlations between salivary steroid hormones in women (Pearson’s correlation). The cross symbols represent no statistical significance. (c) Metabolic pathway of steroid hormones. The hormones surrounded by a box are hormones evaluated in the present study, and the numbers represent the salivary concentration of the Pearson’s correlations. (d) Correlations between salivary 17-OHP and P, COR and F, and S and CORT in women (Pearson’s correlation). The red and blue points represent samples from women in the luteal phase and follicular phase. T, testosterone; A4, androstenedione; F, cortisol; COR, cortisone; P, progesterone; 17-OHP, 17α-hydroxyprogesterone; CORT, corticosterone; ALD, aldosterone; DOC, 11-deoxycorticosterone; S, 11-corticosterone.
Steroid hormones follow the same metabolic pathway and therefore have a certain degree of correlation with respect to their salivary concentrations. Linear correlations were calculated to study the relationships among the steroids measured. The correlations among the salivary concentrations of these hormones are presented in Figure 3(b). As shown in Figure 3(c), the correlations among hormones in the direct upstream and downstream metabolic pathway were relatively strong. The correlation coefficients between P and 17-OHP, COR and F, and S and CORT were much stronger (r = 0.683, r = 0.875, and r = 0.678, respectively) (Figure 3(d)).
Discussion
Differences in the concentrations of P, 17-OHP, and ALD between the Fp and Lp in women were highly significant (p < 0.001). Changes in the salivary concentrations of P and ALD during the menstrual cycle of healthy women are well documented.4–6 The serum P concentration reaches its lowest level with the onset of menstruation and maintains a relatively low level until the theca granulosa cells are luteinized after ovulation; this pattern of changes in the serum P concentration is also reflected in saliva samples.5,17,18 Age-dependent variations of salivary P were not observed in the women and men of the present study. Our data showed a significant difference in salivary 17-OHP between the women in different menstrual cycle stages, which is consistent with previously reported findings. The significant linear correlations between salivary P and 17-OHP, between plasma P and 17-OHP, and between salivary P and 17-OHP suggest that changes in the salivary P and 17-OHP concentrations during the menstrual cycle predominantly reflect changes in the ovaries; therefore, the salivary 17-OHP concentration could be used as an additional endocrine parameter with which to assess ovarian function. Salivary P and 17-OHP can thus be considered additional endocrine parameters with which to assess ovarian function. 17 Previous studies have demonstrated that salivary ALD shows cyclicity during the course of a normal menstrual cycle and that the urinary and serum aldosterone concentrations are significantly higher during the Lp.18,19 Similar to the concentrations in plasma, serum, and urine, a significant increase in the salivary ALD concentration was observed in women in the Lp, indicating that changes in the salivary ALD concentration during the menstrual cycle can also be used as an additional endocrine parameter with which to assess ovarian function.18,19
Age is another important factor that can affect hormone concentrations. The concentrations of many hormones may change with age.12,20 Understanding age-related changes in endocrinology is important because of the role that hormones play in maintenance of reproductive function, physiological responses to the environment, and diagnosis and management of endocrine and metabolic diseases.10,11 In the present study, we examined the age-related trends of 10 salivary steroid hormones and observed that 5 hormones showed significant differences among the different age groups (17-OHP, A4, CORT, COR, and F). Consistent with previous reports of serum and plasma hormone concentrations, the salivary CORT and A4 concentrations also showed statistically significant decreases with increasing age. 12 The concentrations of salivary F and COR decreased as age increased. These results are consistent with previous reports of plasma F; however, the mechanism behind the phenomena remains unclear. The observed age-related changes in F and COR may be related to HPA activity, and the decreasing F concentration may be involved in the cognitive decline that is often observed with human aging.7,21,22
The present study clearly demonstrates that the quantification of salivary steroid hormones can be used to monitor the concentrations of unbound free steroid hormones in the blood.13,14 Measurements of salivary hormone concentrations have clinical value if they reflect the plasma hormone concentrations. The relationship between the saliva and plasma concentrations of many hormones has been studied by a number of research groups.14,23 We found that seven steroid hormones showed significant correlations between their salivary and plasma concentrations in women, and four of these steroid hormones showed high correlations (r > 0.6). The correlation coefficient between salivary F and plasma F (r = 0.705) was higher than previously reported (r = 0.646). 24 These results confirm the conclusion previously obtained by several authors: that only the free F in serum can be transferred into saliva through the epithelial cell membrane of the parotid glands. 1 Thus, there is an equilibration between these two compounds of salivary steroid hormones and blood unbound hormones.
In contrast to women, only three steroid hormones showed significant correlations between saliva and plasma in men. Several previous studies have also shown sex-related differences in the correlations between salivary and blood hormone concentrations. 25 One reason for the large sex-related differences in the salivary concentrationsof some hormones, such as 17-OHP and P, is the different degree of accuracy of quantification. 23 However, although the distribution of the concentrations of some hormones, such as F and A4, does not show substantial differences between the two sexes, there are large sex-related differences in the saliva and blood levels. This may be caused by differences in the concentration of corticosteroid-binding globulin and requires further study. 1 A high correlation coefficient was found between salivary P and 17-OHP and between COR and F, which may have been due to the close metabolic relationship of these steroids and their similar biological functions. Interestingly, although salivary S and CORT do not have a direct metabolic relationship, they also yielded a strong correlation of r = 0.678; the exact reason remains unclear.
Notably, the quantification method for the 10 salivary steroid hormones used in the present study still lacks sufficient accuracy for clinical practice. The method is limited by its sampling conditions, and the study results are limited to only the Chinese population. Salivary hormone testing is a very promising technology. Further optimization is needed to obtain more accurate and reliable data, carry out large-scale assessment in various populations, and facilitate the development of salivary hormone-related research and clinical applications.
Conclusions
The salivary concentrations of T, F, COR, P, 17-OHP, CORT, and ALD were significantly different between men and women (Student’s t-test, p < 0.001). The salivary concentrations of P, 17-OHP, and ALD were also significantly different between women in the Fp and Lp states of menstruation (Student’s t-test, p < 0.001). The salivary concentrations of 17-OHP, A4, CORT, COR, and F significantly declined with increasing age (Kruskal–Wallis test, p < 0.001). A highly linear correlation between salivary and plasma 17-OHP, P, A4, and F was observed with obvious sex-related differences. Our results therefore offer important information regarding the descriptive characteristics of salivary hormones in relation to age, sex, and correlations with plasma hormones.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This work was supported by BGI-Shenzhen, Shenzhen 518083, China.
References
- 1.Gatti R andDe Palo EF.. An update: salivary hormones and physical exercise. Scand J Med Sci Sport 2011; 21: 157–169. doi: 10.1111/j.1600-0838.2010.01252.x. [DOI] [PubMed] [Google Scholar]
- 2.Gröschl M. Current status of salivary hormone analysis. Clin Chem 2008; 54: 1759–1769. doi: 10.1373/clinchem.2008.108910. [DOI] [PubMed] [Google Scholar]
- 3.Takai N, Yamaguchi M, Aragaki T, et al. Gender-specific differences in salivary biomarker responses to acute psychological stress. Ann N Y Acad Sci 2007; 1098: 510–515. doi: 10.1196/annals.1384.014. [DOI] [PubMed] [Google Scholar]
- 4.Liening SH, Stanton SJ, Saini EK, et al. Salivary testosterone, cortisol, and progesterone: Two-week stability, interhormone correlations, and effects of time of day, menstrual cycle, and oral contraceptive use on steroid hormone levels. Physiol Behav 2010; 99: 8–16. doi: 10.1016/j.physbeh.2009. 10.001. [DOI] [PubMed] [Google Scholar]
- 5.Celec P, Ostaniková D, Skoknová M, et al. Salivary sex hormones during the menstrual cycle. Endocr J 2009; 56: 521–523. doi: 10.1507/endocrj.K09E-020. [DOI] [PubMed] [Google Scholar]
- 6.Hlavacova N, Kerlik J, Radikova Z, et al. Measurement of salivary aldosterone: validation by low-dose ACTH test and gender differences. Endocr Regul 2013; 47: 201–204. [DOI] [PubMed] [Google Scholar]
- 7.Larsson C a, Gullberg B, Råstam L, et al. Salivary cortisol differs with age and sex and shows inverse associations with WHR in Swedish women: a cross-sectional study. BMC Endocr Disord 2009; 9: 1–11. doi: 10.1186/1472-6823-9-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamana R, Shirai M, Suzuki T, et al. Corticosterone synthesis inhibitor-induced decrease in the age-dependant expression of nitric oxide synthase 3 in the preterm ductus arteriosus of rats. J Vet Med Sci 2010; 72: 555–560. doi:JST.JSTAGE/jvms/09-0440 [pii]. [DOI] [PubMed] [Google Scholar]
- 9.Hadlow N, Henley SCRWD. Variation of serum cortisol with age and gender. https://www.aacb.asn.au/documents/item/508. Accessed July 8, 2017.
- 10.Pike CJ, Carroll JC, Rosario ER, et al. Protective actions of sex steroid hormones in Alzheimer’s disease. Front Neuroendocrinol 2009; 30: 239–258. doi:10.1016/j.yfrne.2009. 04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McKune AJ. Relationship between salivary androstenedione levels, body composition and physical activity levels in young girls. J Endocrinol Metab Diabetes South Africa 2012; 17: 44–50. doi:10.1080/22201009. 2012.10872273. [Google Scholar]
- 12.Davison SL, Bell R, Donath S, et al. Androgen levels in adult females: changes with age, menopause, and oophorectomy. J Clin Endocrinol Metab 2005; 90: 3847–3853. doi:10.1210/jc.2005-0212. [DOI] [PubMed] [Google Scholar]
- 13.Perogamvros I, Keevil BG, Ray DW, et al. Salivary cortisone is a potential biomarker for serum free cortisol. J Clin Endocrinol Metab 2010; 95: 4951–4958. doi:10.1210/jc.2010-1215. [DOI] [PubMed] [Google Scholar]
- 14.Cadore E, Lhullier F, Brentano M, et al. Correlations between serum and salivary hormonal concentrations in response to resistance exercise. J Sports Sci 2008; 26: 1067–1072. doi:10.1080/02640410801919526. [DOI] [PubMed] [Google Scholar]
- 15.Wood P. Salivary steroid assays - research or routine? Ann Clin Biochem 2009; 46(Pt 3): 183–196. doi:10.1258/acb.2008.008208. [DOI] [PubMed] [Google Scholar]
- 16.Mukaka MM. A guide to appropriate use of correlation coefficient in medical research. Malawi Med J 2012; 24: 69–71. doi:10. 1016/j.cmpb.2016.01.020. [PMC free article] [PubMed] [Google Scholar]
- 17.Gröschl M, Rauh M, Schmid P, et al. Relationship between salivary progesterone, 17-hydroxyprogesterone, and cortisol levels throughout the normal menstrual cycle of healthy postmenarcheal girls. Fertil Steril 2001; 76: 615–617. doi:10.1016/S0015-0282(01)01960-4. [DOI] [PubMed] [Google Scholar]
- 18.Ahmed AH, Gordon RD, Ward G, et al. Should aldosterone suppression tests be conducted during a particular phase of the menstrual cycle, and, if so, which phase? Results of a preliminary study. Clin Endocrinol (Oxf) 2015; 83: 303–307. doi:10.1111/cen.12705. [DOI] [PubMed] [Google Scholar]
- 19.Szmuilowicz ED, Adler GK, Williams JS, et al. Relationship between aldosterone and progesterone in the human menstrual cycle. J Clin Endocrinol Metab 2006; 91: 3981–3987. doi:10.1210/jc.2006-1154. [DOI] [PubMed] [Google Scholar]
- 20.Farage MA, Miller KW, Zouboulis CC, Piérard GE, Maibach HI. Gender differences in skin aging and the changing profile of the sex hormones with age. J Steroids Horm Sci 2012; 3(2): 109. doi: 10.4172/2157-7536.1000109. [Google Scholar]
- 21.Antonelli G, Ceccato F, Artusi C, Marinova M, Plebani M. Salivary cortisol and cortisone by LC-MS/MS: validation, reference intervals and diagnostic accuracy in Cushing’s syndrome. Clin Chim Acta 2015; 451: 247–251. doi:10.1016/j.cca.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 22.Nicolson N, Storms C, Ponds R, et al. Salivary cortisol levels and stress reactivity in human aging. Journals Gerontol Ser A Biol Sci Med Sci 1997; 52A: M68–M75. doi:10.1093/gerona/52A.2.M68. [DOI] [PubMed] [Google Scholar]
- 23.Lewis JG. Steroid analysis in saliva: an overview. Clin Biochem Rev 2006; 27: 139–146. http://www.ncbi.nlm.nih.gov/pubmed/17268582%5Cnhttp://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC1579286. [PMC free article] [PubMed] [Google Scholar]
- 24.Putignano P, Dubini A, Toja P, et al. Salivary cortisol measurement in normal-weight, obese and anorexic women: comparison with plasma cortisol. Eur J Endocrinol 2001; 145: 165–171. doi:10.1530/eje.0. 1450165. [DOI] [PubMed] [Google Scholar]
- 25.Granger DA, Shirtcliff EA, Booth A, et al. The “trouble” with salivary testosterone. Psychoneuroendocrinology 2004; 29: 1229–1240. doi:10.1016/j.psyneuen.2004. 02.005. [DOI] [PubMed] [Google Scholar]