Short abstract
Objectives
The serum concentration of brain-derived neurotrophic factor (BDNF) was compared among patients with Parkinson’s disease (PD), patients with essential tremor (ET), and healthy participants, and its association with clinical features of PD and ET was assessed.
Methods
Demographic and clinical data were collected from 60 patients with PD at different clinical stages, 60 patients with ET, and 60 controls. All participants’ serum BDNF concentrations were measured. Their motor abilities and activity were assessed by the Unified PD Rating Scale and the Hoehn and Yahr (H-Y) staging scale.
Results
Serum BDNF was significantly lower in patients with PD than in patients with ET and controls. BDNF decreased only in the early disease stages (H-Y stages I and II), but increased markedly in the advanced stages (H-Y stages III–V). There was no significant difference between patients with ET and controls. The BDNF concentration was negatively correlated with age at PD onset and positively associated with disease duration, severity of PD symptoms, and treatment with L-DOPA.
Conclusions
A low serum BDNF concentration may serve as a biomarker in the early stages of PD, whereas a high concentration with PD progression may be due to treatment with L-DOPA in the advanced stages.
Keywords: Brain-derived neurotrophic factor, Parkinson’s disease, essential tremor, L-DOPA, Hoehn and Yahr staging scale, biomarker
Introduction
Parkinson’s disease (PD) is a very common neurodegenerative disease characterized by gradual, irreversible loss of nigral dopaminergic neurons and perturbation of striatal circuits involved in motor control. 1 The etiology of the dopaminergic neuron loss is not fully known. It is postulated that the degeneration of dopaminergic neurons may be attributed to an inadequate supply of neurotrophic factors. The neurotrophin family is a group of highly related secreted proteins that play important roles in proliferation, differentiation, and survival of specific neurons in the brain. 2 This family includes neurotrophins 3, 4/5, and 6; nerve growth factor; and brain-derived neurotrophic factor (BDNF). BDNF, as a key member of the neurotrophin family, is essential for normal development of the nervous system as well as synaptic plasticity and neuronal survival in the adult brain. 3 Additionally, BDNF prevents damage to dopaminergic neurons by restoring their functions, suggesting that it may serve as a potential therapeutic intervention for PD. 4
Essential tremor (ET) is a prevalent neurological disorder characterized by an action tremor that occurs during voluntary motion and that primarily affects the upper limbs.5,6 However, its etiology is also unclear. PD and ET are the two most common movement disorders. Tremor is defined as a rhythmic, involuntary oscillatory movement of the body that is associated with brain activity. 7 Most patients with PD exhibit the tremor-dominant type, which shows symptomatic overlap with ET. ET is generally regarded as a benign, symptomatic, motor tremor disorder without involvement of the sensory system. However, this concept has been challenged. Increasing data are showing that ET may be a heterogeneous neurodegenerative disease because of its progression across time and lack of remission.8,9 In patients with ET, the tremor may be asymmetric and associated with rigidity and bradykinesis. However, some patients with PD also show hyperkinetic and postural tremor. Therefore, the differential diagnosis between PD and ET is difficult in some patients. No specific physiological or biological markers have yet been identified, and no reports have described the serum BDNF concentration in patients with ET.
In patients with PD, decreased levels of BDNF mRNA and protein have been observed in several brain regions, such as the striatum, substantia nigra, cerebellum, and frontal cortex.10–12 In an organotypic culture model of PD, BDNF prevented oxidopamine-induced death of dopaminergic neurons. 13 Another study showed an in vivo protective role of BDNF in a primate parkinsonian model induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). In that study, BDNF was delivered simultaneously or before administration of MPTP into the cisterna magna by an osmotic pump. 14 As a result, BDNF decreased neuronal loss and delayed onset of parkinsonism, implicating an obvious protective role. Moreover, brain tissue in the nigrostriatal dopamine regions of patients with PD exhibited a markedly reduced BDNF concentration relative to control subjects. 15 However, there are some conflicting reports on the peripheral concentration of BDNF in patients with PD. While some studies have shown a reduction in the concentration of serum BDNF in patients with PD,11,15 others have shown an increased BDNF level in patients with advanced PD. 16 Several researchers have speculated that these discrepancies were due to possible compensatory effects of drug treatments in patients with PD. 17
Therefore, to further examine the involvement of BDNF in patients with PD and ET, we compared the serum concentration of BDNF among patients with PD, patients with ET, and healthy participants and assessed its association with clinical variables in patients with PD versus ET.
Methods
Participants
Baseline characteristic and clinical data were gathered from patients with PD and patients with ET at the Department of Neurology of Changzhou No. 2 People’s Hospital affiliated with Nanjing Medical University from May 2013 to October 2015. Additionally, healthy individuals were recruited as controls. This study was approved by the ethics committee of both hospitals. Written informed consent was obtained from all participants.
All patients were diagnosed with PD according to the United Kingdom Parkinson’s Disease Society Brain Bank Criteria and with ET according to the NIH Essential Tremor Consortium.18,19 The exclusion criteria were delirium, dementia, comorbid neurological or psychiatric disease, and a history of neurosurgery or brain injury.
Instruments
All patients with PD were assessed using the Unified Parkinson’s Disease Rating Scale (UPDRS) and the Modified Hoehn and Yahr (HY) Staging Scale. The UPDRS is widely used to assess different PD components. 20 It includes three subscales: UPDRS Section I (mentation, behavior, and mood, with a score ranging from 0 to 16), UPDRS Section II (activities of daily living, with a score ranging from 0 to 52), and UPDRS Section III (motor examination, with a score ranging from 0 to 108). A total score of 176 indicates maximal disability and 0 indicates no disability. Patients with PD were classified into five stages according to the HY scale for body distribution of symptoms and life dependence. Patients with stage I PD are slightly influenced, but those with stage V PD are completely bedridden. 21 Based on these H-Y staging criteria, patients with PD in the present study were classified into three clinical subgroups: the mild group (H-Y stages 1 and II), moderate group (H-Y stage III), and severe group (H-Y stages IV and V).
Blood sampling and biochemical examination
In total, 5 ml of peripheral blood was collected between 8:00 and 9:00 AM the morning prior to clinical assessment and following an overnight fast. Blood samples were transferred into non-anticoagulated tubes and then set aside for clot formation at room temperature for 1 hour. The serum was obtained by centrifugation at 1620 relative centrifugal force for 15 minutes and then stored at −80°C until assay.
The serum concentration of BDNF in patients and controls was detected using sandwich BDNF enzyme-linked immunosorbent assay (ELISA) kits (DuoSet; R&D Systems, Minneapolis, MN, USA) following the manufacturer’s instructions. The concentration of BDNF is presented as the equivalent of human recombinant protein. The limit of detection was set at 20 pg/ml. Data are presented as pg of protein per ml of serum. All measurements were repeated twice. Samples from patients and controls were simultaneously analyzed in the same ELISA templates. The variability among measures was monitored by utilizing four samples with a known concentration of BDNF. The mean inter-assay precision, which is presented as the variation coefficient, was approximately 8%.
Statistical analysis
All data are expressed as mean ± standard deviation or mean ± standard error of the mean. Student’s t test or the Mann–Whitney U test was employed to analyze continuous variables with a normal or non-normal distribution, respectively. The χ2 test was utilized to analyze categorical variables. Pearson’s correlation coefficient and Spearman’s rank correlation coefficient were used for correlation analysis. Variables that were markedly associated with the serum BDNF concentration in the univariate analysis were further used for multiple regression analysis. Indices indicating the goodness of fit for the estimated parameters were obtained following establishment of the regression model. A p value of <0.05 was considered statistically significant. The software SPSS 17.0 (SPSS Inc., Chicago, IL, USA) was utilized for all statistical analyses.
Results
Clinical characteristics of participants
The study sample comprised 180 participants: 60 patients with PD (32 male, 28 female), 60 patients with ET (31 male, 29 female), and 60 healthy controls (29 male, 31 female). The patients’ demographic characteristics are shown in Tables 1 and 2. The age (mean ± standard deviation) of the controls was 59.5 ± 14.9 years (range, 25–85 years), that of patients with ET was 58.8 ± 14.3 years (range, 20–84 years), and that of patients with PD was 62.5 ± 9.9 years (range, 45–83 years). The age of onset in patients with PD was 55.9 ± 10.8 years (range, 37–80 years), and that in patients with ET was 50.9 ± 15.3 years (range, 8–78 years). Statistical analysis revealed no difference in sex (p = 0.856) or age (F = 1.310, p = 0.273).
Table 1.
Clinical and demographic details of patients with Parkinson’s disease
| Variables | |
|---|---|
| Number of patients (male/female) | 60 (32/28) |
| Age, years | 62.5 ± 9.9 |
| Age at onset, years | 55.9 ± 10.8 |
| Disease duration, years | 6.6 ± 4.0 |
| L-DOPA medication, n (%) | 32 (53.3) |
| UPDRS score | 44.0 ± 31.1 |
| Hoehn and Yahr stage | 1.9 ± 1.1 |
Data are presented as mean ± standard deviation unless otherwise indicated.
UPDRS = Unified Parkinson’s Disease Rating Scale.
Table 2.
Clinical and demographic details of patients with essential tremor
| Variables | |
|---|---|
| Number of patients (male/female) | 60 (31/29) |
| Age, years | 58.8 ± 14.3 |
| Age of onset, years | 50.9 ± 15.3 |
| Disease duration, years | 7.8 ± 4.0 |
| Family history of essential tremor, n (%) | 32 (53.3) |
| Tremor | |
| Aggravation by stress, n (%) | 12 (20.0) |
| Alcohol response, n (%) | 44 (73.3) |
Data are presented as mean ± standard deviation unless otherwise indicated.
Based on the UPDRS scores, the severity of symptoms was moderate in most patients with PD. The mean H-Y stage was 1.9 ± 1.1, suggesting a mild to moderate degree of PD. The number of patients with PD taking L-DOPAwas 32 (53.3%). The numbers of patients with ET with a family history, tremor aggravation, or alcohol response were 32 (53.3%), 12 (20.0%), and 44 (73.3%), respectively.
Serum BDNF concentration in patients with PD and ET and its association with clinical features
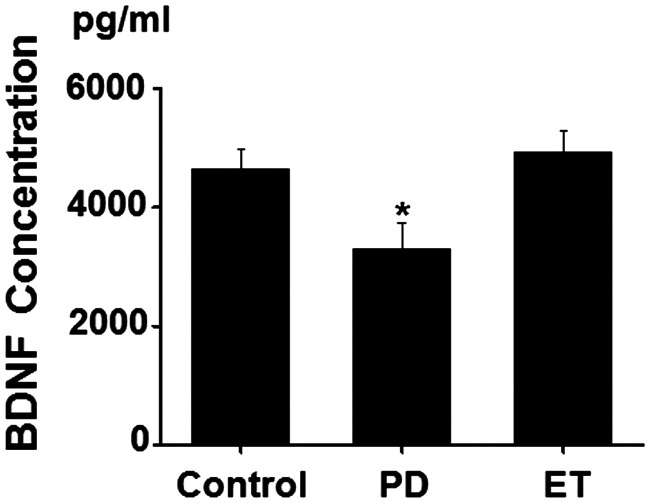
As shown in Figure 1, the serum BDNF concentration in patients with PD was markedly lower than that in patients with ET and controls (F = 4.201, p = 0.008; PD, 3305.7 ± 432.8 pg/ml; ET, 4923.9 ± 373.9 pg/ml; controls, 4649.8 ± 315.9 pg/ml). No difference in the BDNF concentration was observed between patients with ET and healthy controls. Further subgroup analysis based on the severity of PD revealed that a reduction in the BDNF concentration only occurred in the early stages (H-Y stages I and II) (1924.83 ± 331.24 pg/ml). In contrast, the BDNF concentration markedly increased in H-Y stages III (8000.84 ± 389.83 pg/ml) and IV and V (8644.65 ± 382.08 pg/ml) (Table 3).
Figure 1.
Serum brain-derived neurotrophic factor (BDNF) concentrations (pg/ml) (mean ± SEM) in control subjects, patients with Parkinson’s disease (PD) and patients with essential tremor (ET). *p < 0.05, PD versus Control or ET. Control = control subjects, PD = Parkinson’s disease, ET = essential tremor.
Table 3.
BDNF levels in controls and patients with PD with different H-Y stages
| H-Y stage | Patients (n) | BDNF concentration, pg/ml |
|---|---|---|
| I and II | 47 | 1924.83 ± 331.24 a |
| III | 7 | 8000.84 ± 389.83 b |
| IV and V | 6 | 8644.65 ± 382.08 c |
| Control | 60 | 4649.84 ± 315.86 |
Data are presented as mean ± standard error of the mean.
p values of <0.05 are given in bold.
BDNF = brain-derived neurotrophic factor, H-Y stage = Hoehn and Yahr stage.
Compared with the control, at = 5.90, p < 0.001; bt = −3.58, p = 0.001; ct = −3.95, p < 0.001
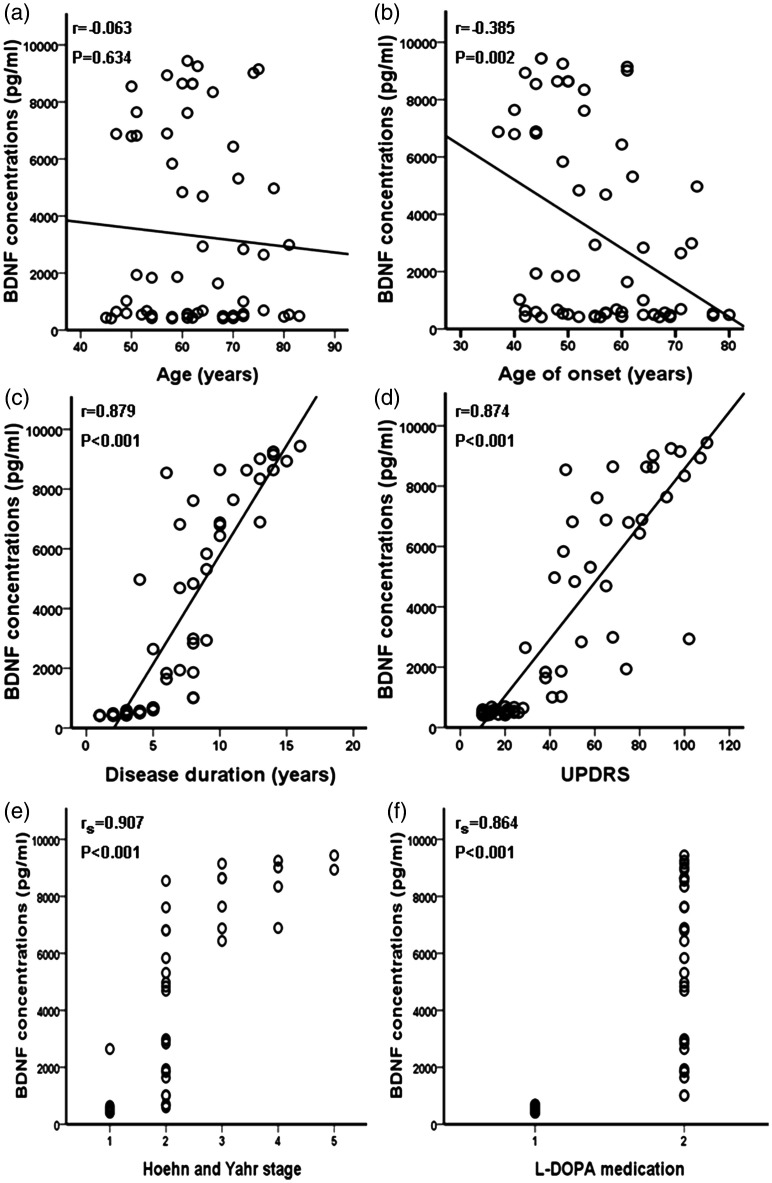
Next, we carried out an association analysis between the serum BDNF concentration and the clinical features of PD. Interestingly, we found that the serum BDNF concentration was associated with the age at onset, disease duration, severity of impairment as assessed by the UPDRS score, H-Y stage, and treatment with L-DOPA, but not with age (Table 4, Figure 2). Considering controls as H-Y stage 0, we further analyzed the correlations between the serum BDNF concentration and different H-Y stages. As shown in Table 5, the serum BDNF concentration was not correlated with H-Y stages 0 to V or III to V, but instead with H-Y stages I to V and 0 to II. Additionally, our data suggested that the serum BDNF concentration in patients with ET was not associated with disease duration or age (data not shown).
Table 4.
Correlation between serum BDNF concentration and demographic and clinical variables in patients with Parkinson’s disease (n = 60)
| Variable | BDNF (correlation coefficient) | p value |
|---|---|---|
| Age, years* | r = −0.063 | 0.634 |
| Age of onset (years)* | r = −0.385 | 0.002 |
| Disease duration (years)* | r = 0.879 | <0.001 |
| UPDRS score* | r = 0.874 | <0.001 |
| H-Y stage (I–V) # | rs = 0.907 | <0.001 |
| Treatment with L-DOPA # | rs = 0.864 | <0.001 |
p values of <0.05 are given in bold.
BDNF = brain-derived neurotrophic factor, UPDRS = Unified Parkinson’s Disease Rating Scale
*Pearson’s correlation coefficient (r)
#Spearman’s rank correlation coefficient (rs)
Figure 2.
Correlation analysis of serum brain-derived neurotrophic factor (BDNF) concentration according to age, age at onset, disease duration, UPDRS score, Hoehn and Yahr stage, and treatment with L-DOPA. UPDRS = Unified Parkinson’s Disease Rating Scale.
Table 5.
Correlation between serum BDNF concentration and H-Y stage in patients with Parkinson’s disease controls (n = 120)
| Variable | BDNF (correlation coefficient) | p value |
|---|---|---|
| H-Y stages I–V # | rs = 0.907 | <0.001 |
| H-Y stages 0–V # | rs = 0.008 | 0.927 |
| H-Y stages 0–II # | rs = −0.387 | <0.001 |
| H-Y stages III–V # | rs = 0.481 | 0.096 |
Note: Controls were considered H-Y stage 0. The serum BDNF concentration was not correlated with stages 0–V, consistent with the following clinical condition: the serum BDNF concentration was higher in controls (H-Y stage 0), relatively lower in patients with the early stages of PD (stages I and II), and higher in patients with the advanced stages of PD (stages III–V).
BDNF = brain-derived neurotrophic factor, H-Y stage = Hoehn and Yahr stage
#Spearman’s rank correlation coefficient (rs)
Discussion
BDNF is involved in the modulation of neuronal survival and differentiation in the brain. There is evidence that BDNF exerts trophic effects on dopaminergic neurons and that a decrease in the BDNF concentration is associated with dopaminergic neuronal loss in patients with PD. 3 Moreover, BDNF prevents damage to dopaminergic neurons by stimulating their function. Collectively, our results suggest that a low serum BDNF concentration may serve as a potential blood biomarker in the early stages of PD, whereas a high serum BDNF concentration with PD progression may be due to treatment with L-DOPA in the advanced stages of PD.
In our study, the serum BDNF concentration in patients with PD was lower than that in patients with ET and control individuals. Consistent with our data, low serum BDNF concentrations in patients with PD have also been observed in several other reports. 11 However, some studies have also shown an increased BDNF concentration. 16 These conflicting results might be attributed, at least in part, to differences in the severity of PD. Moreover, no significant alterations in the BDNF concentration were found in patients with ET compared with control participants, suggesting that the pathophysiology of ET is less involved in the insufficient supply of neurotrophic factors.
This is the first study to date to assess the association of the serum BDNF concentration with the clinical features of PD and ET. Our results indicate that a higher serum BDNF concentration in patients with PD is correlated with a longer disease history and more severe symptoms. Our correlation analysis showed that the serum BDNF concentration is associated with early but not advanced disease stages. This appears paradoxical because we expected a low BDNF concentration to occur with the progression of PD. The mechanisms underlying this condition are unclear. In one study, the levels of c-Fos and BDNF were increased in the dopamine-depleted subthalamic nucleus of L-DOPA-treated rats. 22 Moreover, several lines of evidence suggest higher serum BDNF concentrations in patients with PD exhibiting severe motor impairment at advanced stages. 16 The increase in the BDNF concentration as PD progresses might be correlated with L-DOPA treatment, which promotes the function of the dopamine system in the advanced stages of PD. Thus, such an increase might be regarded as a mechanism of neuronal protection. 16 Additionally, there is evidence that L-DOPA administration causes the release of BDNF from corticostriatal fibers and upregulates the expression of dopamine D3 receptors, eventually exerting beneficial symptomatic effects.17,23 These results support the notion that the low serum BDNF concentration in the early stages of PD is associated with the disease itself, whereas the increased concentration in the advanced stages may due to treatment with L-DOPA.
This study has several limitations. First, the small sample size (especially for patients in advanced stages of PD) limits the generalizability of our findings. Other limitations include the lack of ethnic diversity and the measurement of the serum BDNF concentration at one time point rather than at multiple time points.
Conclusions
In conclusion, we found that the serum BDNF concentration was lower in patients with PD than ET and controls. The serum BDNF concentration decreased only in the early stages of PD, but increased markedly in its advanced stages. The BDNF concentration was positively associated with the disease duration, severity of PD symptoms, and treatment with L-DOPA. Collectively, our results suggest that a low serum BDNF concentration may serve as a potential blood biomarker in the early stages of PD, whereas a high serum BDNF concentration with PD progression may be due to treatment with L-DOPA in the advanced stages of PD. The relationship between the serum BDNF concentration and other factors influencing PD awaits further investigation.
Declaration of conflicting interests
The authors declare that there is no conflict of interest.
Funding
This work was supported by the National Natural Science Foundation of China (no. 81200970 to Y.H. and No. 81471338 to X.Z.), a Changzhou Sci & Tech Program Grant (CJ20130046 to Y.H. and CE20145045 to X.Z.), and the Changzhou High-Level Medical Talents Training Project (No. 2016CZBJ023 to Y.H. and No. 2016CZLJ018 to X.Z.).
References
- 1.Calne D. A definition of Parkinson’s disease. Parkinsonism Relat Disord 2005; 11(Suppl 1): S39–S40. [DOI] [PubMed] [Google Scholar]
- 2.Balaratnasingam S andJanca A.. Brain derived neurotrophic factor: a novel neurotrophin involved in psychiatric and neurological disorders. Pharmacol Ther 2012; 134: 116–124. [DOI] [PubMed] [Google Scholar]
- 3.Arancio O andChao MV.. Neurotrophins, synaptic plasticity and dementia. Curr Opin Neurobiol 2007; 17: 325–330. [DOI] [PubMed] [Google Scholar]
- 4.Binder DK andScharfman HE.. Brain-derived neurotrophic factor. Growth Factors 2004; 22: 123–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deuschl G Bain P andBrin M.. Consensus statement of the Movement Disorder Society on Tremor Ad Hoc Scientific Committee. Mov. Disord 1998; 13(Suppl. 3): 2–23. [DOI] [PubMed] [Google Scholar]
- 6.Louis ED. Functional correlates of lower cognitive test scores in essential tremor. Mov Disord 2001; 25: 481–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cagnan H, Little S, Foltynie T, et al. The nature of tremor circuits in parkinsonian and essential tremor. Brain 2014; 137(Pt 12): 3223–3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonuccelli U. Essential tremor is a neurodegenerative disease. J Neural Transm (Vienna) 2012; 119: 1383–1387. [DOI] [PubMed] [Google Scholar]
- 9.Schmouth JF Dion PA andRouleau GA.. Genetics of essential tremor: from phenotype to genes, insights from both human and mouse studies. Prog Neurobiol 2014; 119–120: 1–19. [DOI] [PubMed] [Google Scholar]
- 10.Howells DW, Porritt MJ, Wong JY, et al. Reduced BDNF mRNA expression in the Parkinson’s disease substantia nigra. Exp Neurol 2000; 166: 127–135. [DOI] [PubMed] [Google Scholar]
- 11.Scalzo P, Kummer A, Bretas TL, et al. Serum levels of brain-derived neurotrophic factor correlate with motor impairment in Parkinson’s disease. J Neurol 2010; 257: 540–545. [DOI] [PubMed] [Google Scholar]
- 12.Parain K, Murer MG, Yan Q, et al. Reduced expression of brain-derived neurotrophic factor protein in Parkinson’s disease substantia nigra. Neuroreport 1999; 10: 557–561. [DOI] [PubMed] [Google Scholar]
- 13.Levivier M, Przedborski S, Bencsics C, et al. Intrastriatal implantation of fibroblasts genetically engineered to produce brain-derived neurotrophic factor prevents degeneration of dopaminergic neurons in a rat model of Parkinson’s disease. J Neurosci 1995; 15: 7810–7820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsukahara T, Takeda M, Shimohama S, et al. Effects of brain-derived neurotrophic factor on 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced parkinsonism in monkeys. Neurosurgery 1995; 37: 733–739, discussion 739–741. [DOI] [PubMed] [Google Scholar]
- 15.Mogi M, Togaria A, Kondo T, et al. Brain-derived growth factor and nerve growth factor concentrations are decreased in the substantia nigra in Parkinson’s disease. Neurosci Lett 1999; 270: 45–48. [DOI] [PubMed] [Google Scholar]
- 16.Ventriglia M, Zanardini R, Bonomini C, et al. Serum brain-derived neurotrophic factor levels in different neurological diseases. Biomed Res Int 2013; 2013: 901082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kostrzewa RM, Nowak P, Kostrzewa JP, et al. Peculiarities of L-DOPA treatment of Parkinson’s disease. Amino Acids 2005; 28: 157–164. [DOI] [PubMed] [Google Scholar]
- 18.Gelb DJ Oliver E andGilman S.. Diagnostic criteria for Parkinson disease. Arch Neurol 1999; 56: 33–39. [DOI] [PubMed] [Google Scholar]
- 19.Hughes AJ, Daniel SE, Kilford L, et al. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 1992; 55: 181–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fahn S andElton R.. Unified Parkinson’s Disease Rating Scale. In: Fahn S Marsden CD Caine DB andGoldstein M, (eds) Recent developments in Parkinson’s disease, vol2. Florham Park: Macmillan Health Care information, 1987, pp 153–163, 293–304. [Google Scholar]
- 21.Hoehn MM andYahr MD.. Parkinsonism: onset, progression and mortality. Neurology 1967; 17: 427–442. [DOI] [PubMed] [Google Scholar]
- 22.Zhang X andAndrenb PE andSvenningssona P.. Repeated L-DOPA treatment increases c-fos and BDNF mRNAs in the subthalamic nucleus in the 6-OHDA rat model of Parkinson's disease. Brain Res 2006; 1095: 207–210. [DOI] [PubMed] [Google Scholar]
- 23.Bezard E, Ferry S, Mach U, et al. Attenuation of levodopa-induced dyskinesia by normalizing dopamine D3 receptor function. Nature Med 2003; 6: 762–767. [DOI] [PubMed] [Google Scholar]