Abstract
Chronic kidney disease (CKD) is a common, deadly, and expensive threat to public health. Patients susceptible to the development of CKD are difficult to identify, as there are few noninvasive clinical techniques and markers to assess early kidney dysfunction. Noninvasive imaging techniques are being developed to quantitatively measure renal morphology and function, both in preclinical research and in clinical trials. Magnetic resonance imaging (MRI) techniques in particular have the potential to provide both structural and functional information in the kidney. Novel molecular imaging techniques, using targeted magnetic nanoparticles that exploit the characteristics of the endogenous protein, ferritin, have been developed in conjunction with MRI to count every perfused glomerulus in the kidney and measure their individual volumes. This technique could open the door to the possibility of prospectively assessing and eventually reducing a patient’s risk for progression to CKD. This review highlights the potential clinical benefits of early detection in patients predisposed to CKD and discusses technologic and regulatory hurdles to the translation of these molecular MRI techniques to provide early diagnosis of CKD.
Keywords: MRI, magnetic resonance imaging, kidney, glomerular counting, glomerular sizing, glomerular filtration rate, stereology
Overview of Chronic Kidney Disease
Chronic kidney disease (CKD) is a progressive, expensive public health epidemic 1 affecting 26 million American adults 2. Although most clinicians recognize the increased morbidity and mortality associated with CKD, they have little ability to identify patients at risk for CKD who will late progress, predict an individual’s time course to end stage renal disease (ESRD), or halt the progression of CKD after diagnosis. CKD is defined by the presence of kidney damage and decreased kidney function 3, resulting from too few nephrons to maintain a patient’s necessary fluid, electrolyte and hormonal balance. Several human studies link clinical evidence of CKD and hypertension with lower nephron number 4–8,9, but current technology is limited to only post mortem assessment of nephron number 10,11. The only clinically available assessments of nephron number and kidney function are from a gross or macroscopic level using surrogates such as renal ultrasound or glomerular filtration rate (GFR). Despite estimates of several morphological and functional parameters 12 and a clinical trial underway in Japan combining renal biopsy and imaging techniques 13, there is currently no way to measure absolute nephron number or individual nephron function in living individuals. This review focuses on the clinical relevance of newly developed MRI contrast agents to measure kidney morphology and function at the micro-scale, in the whole kidney. This review will also discuss the current barriers to the translation of this technology from the laboratory to the clinic, where patients would benefit from the early detection of CKD.
Nephron development, demise and adaptation
Human nephrogenesis ends at 36 weeks gestation 14, meaning a human’s final nephron number is determined at the time of birth for most individuals. Although nephron number is fixed at birth, it is highly variable within the population (from 210,000 to 2.7 million nephrons per kidney 15). The prenatal cessation of nephrogenesis in humans is in contrast to other animals that can either continue nephrogenesis postnatally, like rodents 16, or for the duration of their life, like many fish 17. In humans, nephron loss naturally occurs over time 18. Adults aged 18–70 years have 4500 fewer total nephrons each year on average 19 and as a result of this natural nephron loss, both CKD and ESRD are prevalent in the elderly 20.
Besides the natural loss of nephrons with age, there are many risk factors that may cause a more rapid loss of nephrons and lead to CKD. Diabetes, hypertension, autoimmune disease, urinary tract infections, family history of CKD, recovery from acute kidney injury (AKI), exposure to nephrotoxic drugs, low birth weight, and exposure to certain chemicals and environment conditions are some of the clinical and sociodemographic factors associated with susceptibility to and initiation of CKD 3. Although nephrons do not have the ability to regenerate 16, they can adapt to an absolute lower number. Glomerular hypertrophy can occur to compensate for a reduction of nephrons 21. Glomerular hypertrophy is initially an adaptive response to continue the same amount of total renal work while utilizing fewer nephrons. Over time this response results in a maladaptive cyclical process, whereby glomerular hypertrophy and hyperfiltration result in glomerular sclerosis and death and further hyperfiltration 22–25
Challenges related to the clinical measurement of nephron number and function
The best clinical marker for kidney function is GFR, which is a combined metric of the total number of nephrons across both kidneys and their filtration rates. GFR declines when either nephron number or the single nephron filtration rate is reduced. Currently, the only way to clinically assess nephron number is through surrogates or by manual enumeration after the death of the patient. Renal volume, most often obtained by a renal ultrasound, has been utilized as a surrogate for nephron number as renal weight correlates with nephron number 19,26. Unfortunately, there are other variables such as glomerular and tubular hypertrophy, a patient’s size, and interobserver variability that may obscure the relationship, making renal volume an inconsistent surrogate for nephron number.
GFR is used clinically to stage the severity of CKD in patients. The stages of CKD are utilized for the development of practice guidelines, measurement of clinical performance, assessment of drug toxicities and outcomes in clinical trials. The stages of CKD are not based on nephron number and the cut offs between stages have been determined arbitrarily. GFR as an endpoint in clinical studies is limited by the fact that substantial GFR reductions are only realized late in the stages of CKD 27. Current clinical tools do not allow for the correlation between CKD stage and nephron number, nor the ability to predict the time course for an individual patient’s CKD progression. A method to detect glomerular loss in the early stages of CKD is imperative to reducing the morbidity and mortality associated with CKD.
MRI and CKD
Recent work has focused on developing noninvasive diagnostic tools to detect chronic diseases, such as CKD, at an early stage. Medical imaging technologies, such as magnetic resonance imaging (MRI) and positron emission tomography/single photon emission computed tomography (PET/SPECT), have begun to enable early diagnostics in patients susceptible to chronic disease. The development of imaging biomarkers often involves the rational design and implementation of nanometer-scale materials that can “report” on the local environment through the imaging technique, allowing for individual nephron assessments instead of whole organ assessments. While many of these nano-technological imaging tools are in a preclinical stage of development, they have the potential to make a long-term impact on clinical diagnosis.
There are several available noninvasive anatomical imaging techniques to measure basic physiological parameters in the kidney. Many of these techniques are readily translated to the clinic, and are being developed as surrogate markers for traditional clinical measurements such as bulk glomerular filtration rate and proteinuria. Other measurements are fairly new, such as the detection of pH through MRI spectroscopy and the measurement of local renal perfusion using both contrast-enhanced and anatomical or functional magnetic resonance imaging. Several groups have developed sophisticated techniques to directly measure blood flow though ultrasound techniques and MR arterial spin labeling, helping to better define hyper- or hypo-perfused areas of the kidney and detect hypertrophy. Blood oxygen level dependent MRI (BOLD-MRI), an imaging technique that exploits the magnetic differences between oxy- and deoxyhemoglobin 28 has been used since the early 1990’s to assess oxygen utilization in the brain as a surrogate for brain function. BOLD-MRI has been adapted in the kidney 29 and has proven quite effective in detecting renal ischemia 30 but has been shown to be insensitive to renal function in patients with CKD 31. Diffusion-weighted MRI has also shown promise in assessing renal health, with recent studies suggesting a positive correlation between apparent diffusion coefficient (ADC) and GFR 32,33. The mechanisms of ADC variations in the kidney are a topic of great interest.
Efforts have also been made to measure kidney perfusion directly. Dynamic contrast enhanced MRI (DCE-MRI) has proven to be a powerful tool in the measurement of single kidney GFR. DCE-MRI is based on the dynamic renal uptake and clearance of gadolinium (Gd)-based contrast agents 34–36. The utility of DCE-MRI is enormous; unfortunately the involvement of Gd-based contrast agents in the development of nephrogenic systemic fibrosis (NSF) in patients with reduced renal function 37,38 renders this technique an unlikely candidate for assessing renal function in potential CKD patients. Arterial spin labeling (ASL) MRI is an imaging technique to measure perfusion without administration of contrast agent 39. ASL presents a promising direction for non-invasive imaging of renal perfusion. These MRI techniques to estimate renal function have been thoroughly reviewed by Zhang, et al. 40.
While MRI has been used to evaluate renal function, true surrogate imaging markers of glomerular number and function are still absent. Each of these advanced MRI techniques presented above requires some form of mathematical modeling to estimate physiologically relevant parameters. Gross kidney morphology has been aided immensely by the development of MRI-based volumetric analysis, which can be used to measure changes in organ size associated with disease. However, an imaging technique that specifically detects nephrons would prove to be a robust, unambiguous clinical tool.
Glomerulus-specific, MRI-detectable nanoparticles
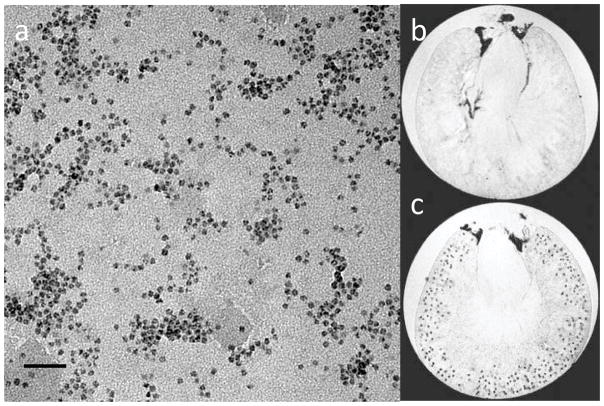
Recently, an MRI-based technique has been developed to quantitatively assess kidney glomerular morphology based on the systemic injection of targeted (cationic), superparamagnetic nanoparticles and subsequent 3D imaging. The MRI-visible nanoparticles used to detect changes in kidney microstructure are based on a cationized version of the iron storage protein ferritin. Ferritin is a highly conserved 24-mer protein expressed in some form in mammalian and non-mammalian cells designed to protect cells from oxidant injury caused by iron. Because of ferritin’s ability to oxidize and incorporate iron in a crystalline form, as shown in the transmission electron micrographs of Figure 1a, endogenous, or native, ferritin is superparamagnetic. Superparamagnetic nanoparticles such as ferritin create a local perturbation in an applied magnetic field, which in turn reduces the transverse relaxation times (t2 and t2*) of nearby proton populations. This reduction in transverse relaxation time creates a local darkening in a typical MRI image. When administered as a targeted MRI contrast agent, the accumulation of ferritin at a target site can be detected with T2- and T2*-weighted MRI, each glomerulus is detectable as a black “dot”.
Figure 1.
Cationic ferritin nanoparticles as intravenous MRI contrast agents. a) Individual ferritin molecules observed in transmission electron microscopy, showing the magnetic ferritin nanoparticle core (Scale bar- 50 nm). Image courtesy of V. Clavijo Jordan. b–c) Intravenously injected cationized ferritin accumulates in the glomerular basement membrane, allowing detection with MRI. b) Excised, perfused rat kidney (with no injected cationized ferritin) in 3D gradient-recalled echo MRI, showing gross kidney structure. c) Perfused, excised rat kidney after intravenous injection of cationized ferritin in 3D MRI (~50 μm voxel resolution) with individual glomeruli visible. b–c reprinted with permission from MI Gateway (Vol. 5, No. 3). Copyright 2011, Society of Nuclear Medicine and Molecular Imaging.
The effect of intravenous injection of cationized ferritin (CF) on T2*-weighted gradient echo MR images is illustrated in the images of Figure 1b–c. Cationized ferritin (with an isoelectric point ~9) reversibly binds to anionic proteoglycans in the glomerular basement membrane (GBM). Once bound, the ferritin nanoparticles accumulate transiently in the lamina rara externa. The hypointense punctate spots in the MRI images, taken ex vivo in ~50-μm resolution in 3D, are individual labeled glomeruli. The contrast between the CF-labeled glomeruli and the surrounding cortex is adjustable by the parameters of MRI acquisition (e.g. resolution, echo time, etc), the dosage of CF, and the “strength” of the effect that the ferritin nanoparticle has on the MRI signal, described by its relaxivity. While cationization represents a relatively simple surface functionalization of the ferritin nanoparticle, the use of CF and similar molecules has the potential to enable a wide range of studies that were previously impossible in the kidney 41–46.
The MRI relaxivity of a magnetic nanoparticle is determined by the magnetic moments of the component atoms, coupling between electrons in the nanoparticle crystal core, and the interaction between the magnetic moments of the nanoparticle crystal and the surrounding water protons. It is the surrounding water molecules that are often directly imaged with MRI, making the effects of the MRI contrast agent indirect. Improving the sensitivity of MRI contrast agents is a major goal of ongoing research. While there are many completely noninvasive MRI techniques that give insight into parenchymal structure and function in the kidney, the benefit of targeted contrast agents is a high level of target specificity and the potential to increase sensitivity to the target molecule.
Molecular MRI for glomerular structure
Labeling and imaging of glomeruli with CF and MRI has been used to develop whole-kidney maps of glomerular number and an estimate of the distribution of the volume of glomeruli 42,47. This preclinical application of CF has opened the door to rapid longitudinal studies of rodent models of human disease and the potential to clinically detect many structural abnormalities on the pathway to CKD (Figure 2). These techniques rely on advanced image processing techniques to locally segment and quantitatively measure changes in contrast. Accuracy is a concern with image-based processing. Specifically, the boundary between the glomerulus and the surrounding kidney cortex is typically defined by a local threshold, which can vary depending on the difference in MRI signal magnitude between the structures. This signal magnitude is strongly affected by the presence of local magnetic field inhomogeneities created by variations in the applied radiofrequency field and also by magnetic susceptibility differences between tissues. These measurements are also confounded by the dark susceptibility artifact created by the CF. While these confounding artifacts likely represent systematic factors that can be corrected, the development of professional software represents an area where research/energy can focused. This effort parallels the work that has been done to establish histology-based renal morphology.
Figure 2.
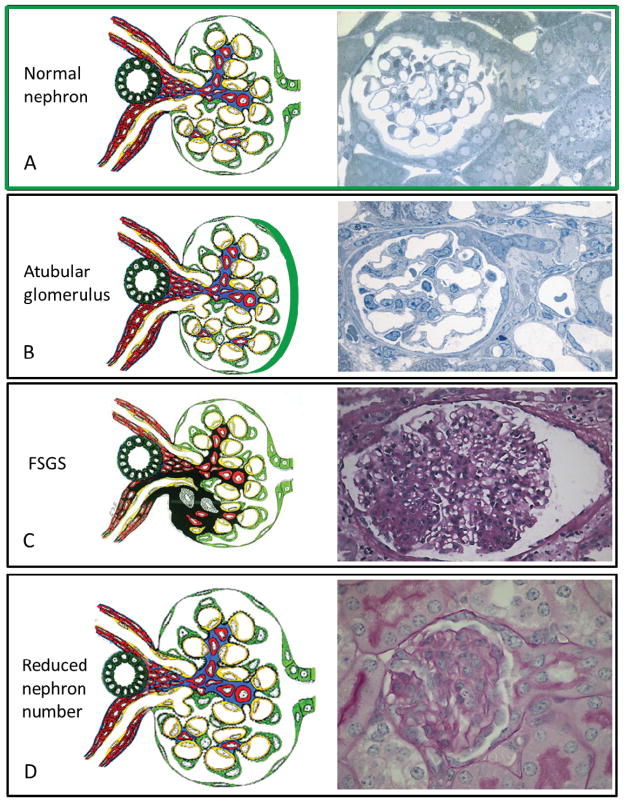
Structural changes in CKD that have the potential to be detected by CF enhanced MRI technology (diagrams adapted from J. Charles Jennette, MD, www.unckidneycenter.org and micrographs donated by Michael Forbes from Dr. Robert Chevalier’s laboratory). A-Normal structure of a nephron with an intact glomerulotubular junction. B-Atubular glomeruli have been detected in a variety of human diseases and animal models of these diseases. The tubule on the right is closing, as it progresses to a complete disconnection between the glomerulus and tubule. It is felt that glomerulotubular disconnection is an underappreciated mechanism in the progression of chronic kidney disease 68,69. C-FSGS is human renal disease with various lesions that each portends a different clinical course. But due the small sample size obtained on a renal biopsy it is not possible to determine how many glomeruli are sclerotic in a living individual. D–A reduction of nephron number can occur from a congenital etiology or after any renal injury. Although the glomerular structure is normal, an initial and adaptive sign of a nephron reduction is with glomerular hypertrophy. Individual glomerular volume can be determined utilizing CF enhanced MRI.
Molecular MRI for glomerular function
The dynamic uptake of CF may provide insight into kidney macromolecular filtration. After intravenous injection, the blood half-life of CF is short (~45 minutes) compared to that of un-functionalized ferritin. This is likely due to rapid binding to the extracellular matrix of several organs, including the liver, kidney, and spleen 48. CF is generally cleared from the kidney after 2 days and from the liver after 7 days, though the mechanisms of clearance have yet to be investigated. Dynamic contrast enhancement has become important for investigating the macromolecular extravasation from the vasculature, vascular microstructure, and parenchymal transport. Similarly, dynamic uptake of CF after injection in vivo or in isolated perfused organs may give new insight into the relationship between glomerular and tubule structure and function within the kidney.
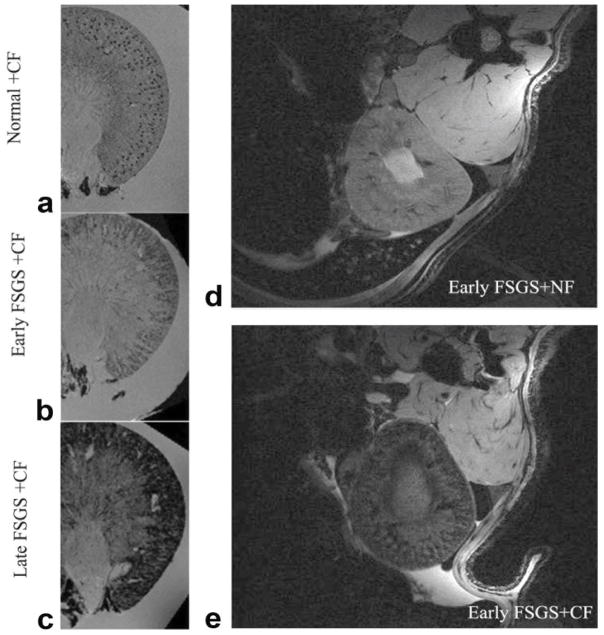
One of the most important potential uses of CF is in tracking structural changes with the development of chronic kidney disease. At the surface many of these alterations will present as either an increase in glomerular permeability and increased traversal of CF through the GBM and into the tubule. In reality, these changes may be followed by a decrease in CF labeling in the glomerulus due to sclerosis, as observed in a puromycin model of focal and segmental glomerular sclerosis in rats 49. Similar to what is expected in many of the morphological changes associated with CKD (Figure 2), focal leakage was observed even in early-stage FSGS, as shown in Figure 3. This indicates the possibility that many morphological changes with CKD can also be detected during monitoring. Furthermore, moderate leakage of CF through the GBM has been observed in healthy animals, raising the possibility that the ratio of glomerular to tubular labeling of CF may be a marker for GBM function 46.
Figure 3.
Detection of glomerular injury in puromycin-induced focal segmental glomerulosclerosis (FSGS) by GRE MRI with cationized ferritin (CF). The accumulation of CF showed a clear spotted distribution in glomeruli from normal kidney (a), the spots were surrounded by areas of signal hypointensity but still visible in cortex of kidney from early FSGS (b) at 13 days after PAN injection, and then cortical signal was darkened without visible spots in kidneys of late FSGS (c) 13 weeks after PAN administration in an ex vivo study. A similar image was seen in kidney from early FSGS rat after CF injection (e) compared with NF administration (d) in vivo. Reproduced with permission from 49, John Wiley and Sons, Magn. Reson Med 60:564–574 (2008).
It is important to consider whether MRI with CF will eventually be capable of distinguishing the types of histological changes shown in Figure 2. Currently only a reduced nephron number can be determined using contrast enhanced MRI, both by complete enumeration of the glomeruli in the kidney and by increased glomerular volume. The atubular glomeruli continue to maintain their blood supply and could be mistaken for intact glomeruli. The tubular flow of CF can potentially differentiate the glomeruli that are connected from those that are not. FSGS is the most subtle and hardest to resolve clearly on MRI of structural lesions, but for clinical purposes even a concept of how many glomeruli are affected is helpful in this disease. It is also possible to filter the 3D MR images based on CF label density, and on shape of the accumulation. This type of feature analysis may become crucial to distinguishing the various types of pathologies common to CKD.
The presence of proteinuria, especially in severe cases as shown in Figure 3c, can confound glomerular counts and volume measurements. This may prove to be a limitation in severe cases of CKD. One possible approach around this problem may be the use of dynamic studies, where the diffusion of CF into the kidney (through the glomerulus) is measured. If the dynamics of CF uptake can be established through dynamic MRI acquisition, it may be possible to distinguish between glomerular labeling and proteinuria. Furthermore, the presence of focal proteinuria, rather than the more severe global case, should only affect local measurements and may allow for quantification of glomerular permeability. Studies to this effect are ongoing.
In vivo visualization of CF
The uptake and binding of CF to the GBM has been observed in vivo. However, there remain several important technical challenges to making in vivo detection of CF practical in animal models and eventually in humans. First, the relaxivity of CF formed from native horse spleen ferritin is relatively low. This low relaxivity requires the delivery of a larger amount in vivo. For example, in the 2008 demonstration of in vivo CF detection 41, the delivered in vivo dose was ~50% higher than required for ex vivo detection in perfused kidneys. A second problem is that the baseline T2* shortening from blood, conferred mainly by high concentrations of paramagnetic oxyhemoglobin, reduces the detectable concentrations of the CF. To overcome this, the ferritin nanoparticle can be chemically modified to have an increased payload or to have a higher relaxivity 50–53. Image acquisition time is another current limitation to sensitive in vivo detection of CF labeling in the glomeruli. Because the scan acquisition time is linked to image resolution in MRI, in vivo studies are still unable to detect individual glomeruli in the entire organ. However, several notable studies have overcome this barrier in images of rats through the use of implantable resonators that locally amplify the MRI signal and make it possible to detect CF uptake in individual glomeruli in real time 45.
There are several technical challenges to imaging in live patients, including limits to image acquisition time and the presence of patient motion. In principle, while precise glomerular measurements are important, it may be possible to acquire lower-resolution images in vivo and estimate uptake over a larger voxel volume. This approach can reduce image acquisition time (by an order of magnitude) for typical clinical signal-to-noise ratio. Motion artifacts can be reduced through patient placement and through respiratory gating. We have used these gating techniques to acquire images in the rat kidney in vivo with ~200 micron resolution (Figure 3d–e) and detect CF uptake in both healthy and sclerotic kidneys 41. Thus, the technical challenges to glomerular morphology do not appear insurmountable for future clinical use, though the end result may be an estimate of glomerular number or volume rather than a precise count.
Potential Toxicity of CF
Toxicity is a major concern for injected MRI contrast agents. There has been a great deal of effort in understanding the effects of environmental and therapeutic exposure to nanoparticles, and these efforts will continue to develop with the maturation of nanotechnology. The recent observation of NSF in some patients due to injected gadolinium chelates underscores the importance in fully understanding the action, distribution, and toxicity of injected agents, particularly of those targeted to the kidney 37. While diagnostic drugs, such as nanoparticle contrast agents, are a relatively recent invention, the potential clinical impact of these drugs has driven a strong interest in systematic toxicology in these agents 54,55. “Natural” contrast agents, such as ferritin, based on biological molecules, may have advantages in toxicity as compared to fully synthetic agents, because natural contrast agents may be more immunologically inert. Furthermore, it is possible to create these agents from recombinant versions of the protein that are specific to the species being imaged. While studies are ongoing, ferritin has been proposed to have limited toxicity in MRI-detectable doses. For example, rats given systemic injections of CF in MRI-detectable (~50 mg/kg over several hours) showed no acute or chronic increase in liver (AST, ALT) or kidney function (BUN, creatinine) or peripheral white blood cells 48. While studies of CF toxicity are still preliminary, an earlier study of a potential immune response in rabbits given intravenous CF from horse spleen necessitates caution 56. Apart from these functional and immune tests, further studies, including ones to look at oxidant injury, are ongoing. In addition, future work will focus on the possibility of the development of a local immune response in the kidney at these doses, and recombinant ferritin specific to the species being imaged may prove useful to reduce any adverse immune response.
The reduction in detectable CF concentrations through increased ferritin relaxivity is likely to allow detection in lower doses 51,53,57,58.
Clinically applicability
The ability to noninvasively determine nephron number and function in a living individual could revolutionize how clinicians diagnosis and manage patients with and at risk for CKD. A few examples of situations where a noninvasive, safe, and repeatable method to determine nephron number and function could improve clinical care include (1) the early detection of patients at risk for CKD, (2) an objective assessment of renal health in both kidney transplant recipients and donors, and (3) shorter term endpoints for the testing of renal toxicity in new drug development.
In translation to humans, we must factor in the likely morphological changes with CKD as compared to the more gross changes detected in preclinical models. In most cases the glomerular labeling by CF can be distinguished from less intense labeling of the tubules 59. This can be used to map both the distribution of the glomeruli and the tubules in 3D. While we have shown severe cases of proteinuria, such as in the FSGS model of Figure 3, result in gross differences in CF uptake, it is important to distinguish the more subtle changes likely in humans. The technique of image processing to distinguish normal glomerular and tubule labeling intensity can be used to track these subtle changes with disease progression.
Acute kidney injury is an assortment of diseases that can directly affect the glomerulus (acute glomerulonephritis), the tubules (acute tubular necrosis), and the vasculature (hemolytic uremic syndrome). In diseases that affect the glomerulus and vasculature, the only way to determine if an individual glomeruli can recover or not is to repeat the imaging study. These studies in AKI have the potential to shed a great deal of light on the progression of AKI to CKD.
There are several technical hurdles associated with the development of targeted MRI-visible nanoparticles to measure kidney morphology in the clinic. Sensitivity is a concern with all MRI contrast agents, and the development of contrast agents of higher relaxivity must also be accompanied by better MRI hardware development, particularly in translating these techniques to in vivo imaging. Some progress has been made in developing novel radiofrequency coils, for example, that may enable highly sensitive detection in some circumstances in vivo 45.
Early detection
CKD affects 26 million adults, and the ESRD program in the US was $32.9 billion in 20102. Even in the population of nondialysis patients, the cost of healthcare for patients with CKD is double those of a patient without CKD 60. In addition to higher healthcare costs, significant morbidity occurs in patients diagnosed with CKD. Cardiovascular disease is the most common cause of death in patients on dialysis. Death due to cardiac etiology is 100 time greater in young patients requiring dialysis than in the general population 61. Even at the earliest stages of CKD, risk of cardiovascular disease is increased 62. Similar to diseases such as diabetes or hypertension, an objective definition for “pre-CKD” could be clinically useful to identify individuals at risk of developing CKD.
There are many risk factors for the development of CKD, but the wide range for normal nephron number and the variable genetic susceptibility of renal disease in humans make it difficult to determine without further knowledge of the connections between these factors, which at risk patients will progress to CKD and ESRD. Patients with a solitary kidney or a unilateral disease such as ureteropelvic junction obstruction are at risk for progressive CKD and ESRD 63, but it is nearly impossible to determine which patients do not have enough nephrons in the remaining or healthy kidney to avoid CKD. An imaging technique like the CF-based MRI technique reviewed in this manuscript would allow, for the first time, an assessment of the viability of the remaining kidney in these patients. Additionally, the assessment of all glomeruli provided by this technique could aid in the diagnosis and categorization of diseases such as focal segmental glomerulosclerosis where biopsy information is limited by sample size 64.
With nephron endowment determined at birth, children with chronic medical issues are another group that could benefit from early detection of CKD. Today, more than 90% of children born with a chronic health condition are expected to live for an average of 20 years 65. Children born with complex congenital heart disease, sickle cell anemia, cystic fibrosis and other childhood diseases, who have suffered from years of kidney insults, are now able to transition their medical care to adult practitioners. Another group of “survivors” includes premature and low birth weight infants. In response to improved neonatal medical management, 90% of infants <1500 grams survive 66. Unfortunately, there is little known about the long term effects on kidney health for these infants forced to develop a majority of their nephrons while exposed to nephrotoxins, hemodynamic alterations, and infections, all of which are routine exposures in a neonatal intensive care unit67.With nephron number determination being fixed at birth, the risk for CKD may be unrecognized in these populations, underscoring the potential impact for an individualized diagnostic technique for early CKD.
Renal transplant
Inevitably, patients with progressive CKD will require renal replacement and in most, a kidney transplant is the ultimate goal. As the age of patients needing a kidney transplant and individuals wishing to donate a kidney increases, an improved method for matching kidney between recipients and donors is necessary. Currently, the deceased donor registry provides children requiring a kidney transplant a priority to kidneys from younger donors. This imprecise pairing has occurred to match the number of nephrons in a donor kidney to the recipient’s potential lifespan. MRI technology to assess nephron endowment within a kidney could provide a more accurate way to pair donors and recipients. Additionally, it could provide an objective means to expand the pool of potential donors, protect living donors from a future personal risk of CKD, and monitor kidney health over time in recipients.
Regulatory issues
The FDA has struggled with objective, meaningful endpoints to evaluate medications designed to halt the progression of CKD. Currently, studies utilizing endpoints such as a 50% decline in GFR have to be very long to assess the beneficial effects of drugs 27. These limitations also factor into the enormous cost of nephrotoxicity screening required during pre-clinical and clinical trials of all novel drugs. A repeatable imaging technique that detects the early stages of CKD might reduce the time and cost required to evaluate the efficacy of CKD therapeutics and the nephrotoxicity of all drugs bound for the market.
Regulatory hurdles are a major concern for the development of all drugs, either therapeutic or diagnostic. There are several federally funded mechanisms for the translation of therapeutic drugs, but fewer mechanisms to support clinical translation of diagnostic drugs. One charge to researchers working to establish increased funding and infrastructure for diagnostic drugs is to articulate how these drugs serve a major public health benefit. Another charge is to clearly identify the toxicology of proposed diagnostic drugs and to collaborate to avoid any impact to patients using them in the early stages of clinical trials. In nanoparticles such as CF, there is the potential for the early recognition of risks associated with low nephron number, including the risk of development of cardiovascular and chronic kidney disease. Early patient education and lifestyle management based on this information may lead to improved prognosis and reduced need for therapy later in life. Furthermore, the abundance of novel preclinical contrast agents and the dearth of clinically approved diagnostic drugs highlights the importance of a sustained effort to develop public-private partnerships and partnerships between scientists and clinicians to identify a clear pathway to clinical translation for these drugs.
Conclusion
Novel molecular MRI techniques have been used in preclinical studies to quantitatively detect renal morphology. The extracted parameters could lead to early detection of total numbers of kidney glomeruli, glomerular and nephron function, and glomerular volume distribution in the clinic, which could potentially enable early assessment of patient risk for CKD. While the technical and regulatory challenges to translating magnetic nanoparticles and MRI protocols to the clinic are daunting, the potential benefits to patient of early detection of CKD are significant.
Acknowledgments
J. Charleton’s work is supported by a grant from the Hartwell Foundation. K. Bennett’s work is supported by the American Heart Association 12BGIA9840020, NIH DK091722, and a grant from the NIH Diabetic Complications Consortium.
Abbreviations
- AKI
acute kidney injury
- ADC
apparent diffusion coefficient
- ASL
arterial spin labeling
- BOLD-MRI
blood oxygen level dependent MRI
- CF
cationized ferritin
- CKD
chronic kidney disease
- DCE MRI
dynamic contrast MRI
- ESRD
end stage renal disease
- FSGS
focal segmental glomerulosclerosis
- Gd
gadolinium
- GBM
glomerular basement membrane
- GFR
glomerular filtration rate
- MRI
magnetic resonance image
- NSF
nephrogenic systemic fibrosis
- PET/SPECT
positron emission tomography/single photon emission computed tomography
Footnotes
Financial Disclosure: JC and SB have no financial disclosures. KB owns Nanodiagnostics, LLC.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jennifer Charlton, Department of Pediatrics, Division of Nephrology, University of Virginia Medical Center, Charlottesville, VA.
Scott C. Beeman, Department of Radiology, Washington University School of Medicine, St. Louis, MO
Kevin M. Bennett, Department of Biology, College of Natural Sciences, University of Hawaii at Manoa, Honolulu, HI, USA.
References
- 1.Rettig RA, Norris K, Nissenson AR. Chronic kidney disease in the United States: a public policy imperative. Clin J Am Soc Nephrol. 2008;3(6):1902–1910. doi: 10.2215/CJN.02330508. [DOI] [PubMed] [Google Scholar]
- 2.Collins AJ, Kasiske B, Herzog C, et al. Excerpts from the United States Renal Data System 2004 annual data report: atlas of end-stage renal disease in the United States. Am J Kidney Dis. 2005;45(1 Suppl 1):A5–7. S1–280. doi: 10.1053/j.ajkd.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 3.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 Suppl 1):S1–266. [PubMed] [Google Scholar]
- 4.Brenner BDL, GSA Glomeruli and blood pressure. Less of one, more of the other? Am J Hypertens. 1988;1:335–347. doi: 10.1093/ajh/1.4.335. [DOI] [PubMed] [Google Scholar]
- 5.Kett MM, Bertram JF. Nephron endowment and blood pressure: what do we really know? Curr Hypertens Rep. 2004;6(2):133–139. doi: 10.1007/s11906-004-0089-2. [DOI] [PubMed] [Google Scholar]
- 6.Cullen-McEwen LA, Kett MM, Dowling J, Anderson WP, Bertram JF. Nephron number, renal function, and arterial pressure in aged GDNF heterozygous mice. Hypertension. 2003;41(2):335–340. doi: 10.1161/01.hyp.0000050961.70182.56. [DOI] [PubMed] [Google Scholar]
- 7.Bertram JF, Douglas-Denton RN, Diouf B, Hughson MD, Hoy WE. Human nephron number: implications for health and disease. Pediatr Nephrol. 2011;26(9):1529–1533. doi: 10.1007/s00467-011-1843-8. [DOI] [PubMed] [Google Scholar]
- 8.Moritz AR, Hayman JM. The Disappearance of Glomeruli in Chronic Kidney Disease. The American Journal of Pathology. 1934;10(4):505–518.3. [PMC free article] [PubMed] [Google Scholar]
- 9.Keller G, Zimmer G, Mall G, Ritz E, Amann K. Nephron number in patients with primary hypertension. N Engl J Med. 2003;348(2):101–108. doi: 10.1056/NEJMoa020549. [DOI] [PubMed] [Google Scholar]
- 10.Bertram JF. Analyzing renal glomeruli with the new stereology. Int Rev Cytol. 1995;161:111–172. doi: 10.1016/s0074-7696(08)62497-3. [DOI] [PubMed] [Google Scholar]
- 11.Bertram JF. Counting in the kidney. Kidney Int. 2001;59(2):792–796. doi: 10.1046/j.1523-1755.2001.059002792.x. [DOI] [PubMed] [Google Scholar]
- 12.Tan JC, Busque S, Workeneh B, et al. Effects of aging on glomerular function and number in living kidney donors. Kidney Int. 2010;78(7):686–692. doi: 10.1038/ki.2010.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Imasawa T, Nakazato T, Ikehira H, et al. Predicting the outcome of chronic kidney disease by the estimated nephron number: the rationale and design of PRONEP, a prospective, multicenter, observational cohort study. BMC Nephrol. 2012;13:11. doi: 10.1186/1471-2369-13-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Osathanodh V, Potter EL. Development of human kidney as shown by microdissection. I. Preparation of tissue with reasons for possible misinterpretation of observations. Arch Pathol. 1063;76:271–276. [PubMed] [Google Scholar]
- 15.McNamara BJ, Diouf B, Douglas-Denton RN, Hughson MD, Hoy WE, Bertram JF. A comparison of nephron number, glomerular volume and kidney weight in Senegalese Africans and African Americans. Nephrol Dial Transplant. 2010;25(5):1514–1520. doi: 10.1093/ndt/gfq030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hartman HA, Lai HL, Patterson LT. Cessation of renal morphogenesis in mice. Dev Biol. 2007;310(2):379–387. doi: 10.1016/j.ydbio.2007.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davidson AJ. Uncharted waters: nephrogenesis and renal regeneration in fish and mammals. Pediatr Nephrol. 2011;26(9):1435–1443. doi: 10.1007/s00467-011-1795-z. [DOI] [PubMed] [Google Scholar]
- 18.McLachlan MS, Guthrie JC, Anderson CK, Fulker MJ. Vascular and glomerular changes in the ageing kidney. J Pathol. 1977;121(2):65–78. doi: 10.1002/path.1711210202. [DOI] [PubMed] [Google Scholar]
- 19.Nyengaard JR, Bendtsen TF. Glomerular number and size in relation to age, kidney weight, and body surface in normal man. Anat Rec. 1992;232(2):194–201. doi: 10.1002/ar.1092320205. [DOI] [PubMed] [Google Scholar]
- 20.Stevens LA, Viswanathan G, Weiner DE. Chronic kidney disease and end-stage renal disease in the elderly population: current prevalence, future projections, and clinical significance. Adv Chronic Kidney Dis. 2010;17(4):293–301. doi: 10.1053/j.ackd.2010.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luyckx VA, Brenner BM. The clinical importance of nephron mass. J Am Soc Nephrol. 2010;21(6):898–910. doi: 10.1681/ASN.2009121248. [DOI] [PubMed] [Google Scholar]
- 22.Brenner BM, Chertow GM. Congenital oligonephropathy and the etiology of adult hypertension and progressive renal injury. Am J Kidney Dis. 1994;23(2):171–175. [PubMed] [Google Scholar]
- 23.Bidani AK, Griffin KA. Pathophysiology of hypertensive renal damage: implications for therapy. Hypertension. 2004;44(5):595–601. doi: 10.1161/01.HYP.0000145180.38707.84. [DOI] [PubMed] [Google Scholar]
- 24.Helal I, Fick-Brosnahan GM, Reed-Gitomer B, Schrier RW. Glomerular hyperfiltration: definitions, mechanisms and clinical implications. Nat Rev Nephrol. 2012;8(5):293–300. doi: 10.1038/nrneph.2012.19. [DOI] [PubMed] [Google Scholar]
- 25.Brenner BM, Garcia DL, Anderson S. Glomeruli and blood pressure. Less of one, more the other? Am J Hypertens. 1988;1(4 Pt 1):335–347. doi: 10.1093/ajh/1.4.335. [DOI] [PubMed] [Google Scholar]
- 26.Silver LE, Decamps PJ, Korst LM, Platt LD, Castro LC. Intrauterine growth restriction is accompanied by decreased renal volume in the human fetus. Am J Obstet Gynecol. 2003;188(5):1320–1325. doi: 10.1067/mob.2003.270. [DOI] [PubMed] [Google Scholar]
- 27.Stevens LA, Greene T, Levey AS. Surrogate end points for clinical trials of kidney disease progression. Clin J Am Soc Nephrol. 2006;1(4):874–884. doi: 10.2215/CJN.00600206. [DOI] [PubMed] [Google Scholar]
- 28.Ogawa S, Lee TM, Kay AR, Tank DW. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci US A. 1990;87(24):9868–9872. doi: 10.1073/pnas.87.24.9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prasad PV, Edelman RR, Epstein FH. Noninvasive evaluation of intrarenal oxygenation with BOLD MRI. Circulation. 1996;94(12):3271–3275. doi: 10.1161/01.cir.94.12.3271. [DOI] [PubMed] [Google Scholar]
- 30.Juillard L, Lerman LO, Kruger DG, et al. Blood oxygen level-dependent measurement of acute intra-renal ischemia. Kidney Int. 2004;65(3):944–950. doi: 10.1111/j.1523-1755.2004.00469.x. [DOI] [PubMed] [Google Scholar]
- 31.Michaely HJ, Metzger L, Haneder S, Hansmann J, Schoenberg SO, Attenberger UI. Renal BOLD-MRI does not reflect renal function in chronic kidney disease. Kidney Int. 2012;81(7):684–689. doi: 10.1038/ki.2011.455. [DOI] [PubMed] [Google Scholar]
- 32.Thoeny HC, Zumstein D, Simon-Zoula S, et al. Functional evaluation of transplanted kidneys with diffusion-weighted and BOLD MR imaging: initial experience. Radiology. 2006;241:812–821. doi: 10.1148/radiol.2413060103. [DOI] [PubMed] [Google Scholar]
- 33.Zhang JL, Sigmund EE, Chandarana H, et al. Variability of renal apparent diffusion coefficients: limitations of the monoexponential model for diffusion quantification. Radiology. 2010;254(3):783–792. doi: 10.1148/radiol.09090891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buckley DL, Shurrab AE, Cheung CM, Jones AP, Mamtora H, Kalra PA. Measurement of single kidney function using dynamic contrast-enhanced MRI: comparison of two models in human subjects. J Magn Reson Imaging. 2006;24(5):1117–1123. doi: 10.1002/jmri.20699. [DOI] [PubMed] [Google Scholar]
- 35.Tofts PS, Cutajar M, Mendichovszky IA, Peters AM, Gordon I. Precise measurement of renal filtration and vascular parameters using a two-compartment model for dynamic contrast-enhanced MRI of the kidney gives realistic normal values. Eur Radiol. 2012 doi: 10.1007/s00330-012-2382-9. [DOI] [PubMed] [Google Scholar]
- 36.Lee CU, Glockner JF. Vascular staging of renal and adrenal malignancies with a noncontrast enhanced steady state free precession technique. J Magn Reson Imaging. 2011;33(6):1406–1413. doi: 10.1002/jmri.22568. [DOI] [PubMed] [Google Scholar]
- 37.Grobner T. Gadolinium--a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol Dial Transplant. 2006;21(4):1104–1108. doi: 10.1093/ndt/gfk062. [DOI] [PubMed] [Google Scholar]
- 38.Marckmann P, Skov L, Rossen K, et al. Nephrogenic systemic fibrosis: suspected causative role of gadodiamide used for contrast-enhanced magnetic resonance imaging. J Am Soc Nephrol. 2006;17(9):2359–2362. doi: 10.1681/ASN.2006060601. [DOI] [PubMed] [Google Scholar]
- 39.Detre JA, Zhang W, Roberts DA, et al. Tissue specific perfusion imaging using arterial spin labeling. NMR Biomed. 1994;7(1–2):75–82. doi: 10.1002/nbm.1940070112. [DOI] [PubMed] [Google Scholar]
- 40.Zhang JL, Rusinek H, Chandarana H, Lee VS. Functional MRI of the kidneys. J Magn Reson Imaging. 2013;37(2):282–293. doi: 10.1002/jmri.23717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bennett KM, Zhou H, Sumner JP, et al. MRI of the basement membrane using charged nanoparticles as contrast agents. Magn Reson Med. 2008;60(3):564–574. doi: 10.1002/mrm.21684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beeman SC, Zhang M, Gubhaju L, et al. Measuring glomerular number and size in perfused kidneys using MRI. Am J Physiol Renal Physiol. 300(6):F1454–7. doi: 10.1152/ajprenal.00044.2011. Available at: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=21411479. [DOI] [PubMed] [Google Scholar]
- 43.Heilmann M, Neudecker S, Wolf I, et al. Quantification of glomerular number and size distribution in normal rat kidneys using magnetic resonance imaging. Nephrol Dial Transplant. 2012;27(1):100–107. doi: 10.1093/ndt/gfr273. Available at: http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=21642513&retmode=ref&cmd=prlinks. [DOI] [PubMed] [Google Scholar]
- 44.Xie L, Cianciolo RE, Hulette B, et al. Magnetic resonance histology of age-related nephropathy in the Sprague Dawley rat. Toxicol Pathol. 2012;40(5):764–778. doi: 10.1177/0192623312441408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qian C, Yu X, Chen D-Y, et al. Wireless Amplified Nuclear MR Detector (WAND) for High-Spatial-Resolution MR Imaging of Internal Organs: Preclinical Demonstration in a Rodent Model. Radiology. 2013 doi: 10.1148/radiol.13121352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.The emerging role of MRI in quantitative renal glomerular morphology.
- 47.Heilmann M, Neudecker S, Wolf I, et al. Quantification of glomerular number and size distribution in normal rat kidneys using magnetic resonance imaging. Nephrol Dial Transplant. 2012;27(1):100–107. doi: 10.1093/ndt/gfr273. [DOI] [PubMed] [Google Scholar]
- 48.Beeman SC, Georges JF, Bennett KM. Toxicity, biodistribution, and ex vivo MRI detection of intravenously injected cationized ferritin. Magn Reson Med. 2012 doi: 10.1002/mrm.24301. [DOI] [PubMed] [Google Scholar]
- 49.Bennett KM, Zhou H, Sumner JP, et al. MRI of the basement membrane using charged nanoparticles as contrast agents. Magn Reson Med. 2008;60(3):564–574. doi: 10.1002/mrm.21684. Available at: http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=18727041&retmode=ref&cmd=prlinks. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Meldrum FCHBAMS. Magnetoferritin: in vitro synthesis of a novel magnetic protein. Science. 1992;257(5069):522–523. doi: 10.1126/science.1636086. [DOI] [PubMed] [Google Scholar]
- 51.Bulte JW, Douglas T, Mann S, et al. Magnetoferritin. Biomineralization as a novel molecular approach in the design of iron-oxide-based magnetic resonance contrast agents. Invest Radiol. 1994;29 (Suppl 2):S214–6. [PubMed] [Google Scholar]
- 52.Uchida M, Terashima M, Cunningham CH, et al. A human ferritin iron oxide nano-composite magnetic resonance contrast agent. Magn Reson Med. 2008;60(5):1073–1081. doi: 10.1002/mrm.21761. [DOI] [PubMed] [Google Scholar]
- 53.Clavijo-Jordan VMRC, KMB Simplified synthesis and relaxometry of magnetoferrin for magnetic resonance imaging. Magn Reson Med. 2010 doi: 10.1002/mrm.22526. In Press. [DOI] [PubMed] [Google Scholar]
- 54.Clavijo-Jordan V, Kodibagkar VD, Beeman SC, Hann BD, Bennett KM. Principles and emerging applications of nanomagnetic materials in medicine. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2012;4(4):345–365. doi: 10.1002/wnan.1169. [DOI] [PubMed] [Google Scholar]
- 55.Kaiser CR, Flenniken ML, Gillitzer E, et al. Biodistribution studies of protein cage nanoparticles demonstrate broad tissue distribution and rapid clearance in vivo. Int J Nanomedicine. 2007;2(4):715–733. [PMC free article] [PubMed] [Google Scholar]
- 56.Batsford SR, Sasaki M, Takamiya H, Vogt A. Cationic macromolecule-induced nephrotic syndrome in rabbits. Lack of immune complex involvement. Lab Invest. 1983;49(3):260–269. [PubMed] [Google Scholar]
- 57.Meldrum FCHBAMS. Magnetoferritin: in vitro synthesis of a novel magnetic protein. Science. 1992;257(5069):522–523. doi: 10.1126/science.1636086. [DOI] [PubMed] [Google Scholar]
- 58.Bulte JW, Douglas T, Mann S, Vymazal J, Laughlin PG, Frank JA. Initial assessment of magnetoferritin biokinetics and proton relaxation enhancement in rats. Acad Radiol. 1995;2(10):871–878. doi: 10.1016/s1076-6332(05)80064-9. [DOI] [PubMed] [Google Scholar]
- 59.Bennett KM, Bertram JF, Beeman SC, Gretz N. The emerging role of MRI in quantitative renal glomerular morphology. Am J Physiol Renal Physiol. 2013;304(10):F1252–7. doi: 10.1152/ajprenal.00714.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Smith DH, Gullion CM, Nichols G, Keith DS, Brown JB. Cost of medical care for chronic kidney disease and comorbidity among enrollees in a large HMO population. J Am Soc Nephrol. 2004;15(5):1300–1306. doi: 10.1097/01.asn.0000125670.64996.bb. [DOI] [PubMed] [Google Scholar]
- 61.Foley RN, Parfrey PS, Sarnak MJ. Epidemiology of cardiovascular disease in chronic renal disease. J Am Soc Nephrol. 1998;9(12 Suppl):S16–23. [PubMed] [Google Scholar]
- 62.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu C-Y. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 63.Sanna-Cherchi S, Ravani P, Corbani V, et al. Renal outcome in patients with congenital anomalies of the kidney and urinary tract. Kidney Int. 2009;76(5):528–533. doi: 10.1038/ki.2009.220. [DOI] [PubMed] [Google Scholar]
- 64.Sorof JM, Vartanian RK, Olson JL, Tomlanovich SJ, Vincenti FG, Amend WJ. Histopathological concordance of paired renal allograft biopsy cores. Effect on the diagnosis and management of acute rejection. Transplantation. 1995;60(11):1215–1219. [PubMed] [Google Scholar]
- 65.Gortmaker SL, Sappenfield W. Chronic childhood disorders: prevalence and impact. Pediatr Clin North Am. 1984;31(1):3–18. doi: 10.1016/s0031-3955(16)34532-1. [DOI] [PubMed] [Google Scholar]
- 66.Horbar JD, Carpenter JH, Badger GJ, et al. Mortality and neonatal morbidity among infants 501 to 1500 grams from 2000 to 2009. Pediatrics. 2012;129(6):1019–1026. doi: 10.1542/peds.2011-3028. [DOI] [PubMed] [Google Scholar]
- 67.Carmody JB, Charlton JR. Short-Term Gestation, Long-Term Risk: Prematurity and Chronic Kidney Disease. Pediatrics. 2013 doi: 10.1542/peds.2013-0009. [DOI] [PubMed] [Google Scholar]
- 68.Marcussen N. Atubular glomeruli and the structural basis for chronic renal failure. Lab Invest. 1992;66(3):265–284. [PubMed] [Google Scholar]
- 69.Chevalier RL, Forbes MS. Generation and evolution of atubular glomeruli in the progression of renal disorders. J Am Soc Nephrol. 2008;19(2):197–206. doi: 10.1681/ASN.2007080862. [DOI] [PubMed] [Google Scholar]