Abstract
Objective
To clarify whether recurrence risk for intracerebral hemorrhage (ICH) is higher among black and Hispanic individuals and whether this disparity is attributable to differences in blood pressure (BP) measurements and their variability.
Methods
We analyzed data from survivors of primary ICH enrolled in 2 separate studies: (1) the longitudinal study conducted at Massachusetts General Hospital (n = 759), and (2) the ERICH (Ethnic/Racial Variations of Intracerebral Hemorrhage) study (n = 1,532). Participants underwent structured interview at enrollment (including self-report of race/ethnicity) and were followed longitudinally via phone calls and review of medical records. We captured systolic BP (SBP) and diastolic BP measurements, and quantified variability as SBP and diastolic BP variation coefficients. We used multivariable (Cox regression) survival analysis to identify risk factors for ICH recurrence.
Results
We followed 2,291 ICH survivors (1,121 white, 529 black, 605 Hispanic, and 36 of other race/ethnicity). Both black and Hispanic patients displayed higher SBP during follow-up (p < 0.05). Black participants also displayed greater SBP variability during follow-up (p = 0.032). In univariable analyses, black and Hispanic patients were at higher ICH recurrence risk (p < 0.05). After adjusting for BP measurements and their variability, both Hispanic (hazard ratio = 1.51, 95% confidence interval 1.14–2.00, p = 0.004) and black (hazard ratio = 1.98, 95% confidence interval 1.36–2.86, p < 0.001) patients remained at higher risk of ICH recurrence.
Conclusion
Black and Hispanic patients are at higher risk of ICH recurrence; hypertension severity (average BP and its variability) does not fully account for this finding. Additional studies will be required to further elucidate determinants for this health disparity.
Primary intracerebral hemorrhage (ICH) accounts for approximately 10% to 15% of all acute cerebrovascular events worldwide, and it is responsible for the majority of stroke-related morbidity and mortality.1–3 ICH survivors are at extremely high risk of recurrent ICH, which is often fatal.4–7 Treatment of hypertension, in the form of blood pressure (BP) control, represents the cornerstone of secondary ICH prevention.8,9 Current practice guidelines focus primarily on achieving prespecified goals for systolic BP (SBP) and diastolic BP (DBP) values. Whereas several studies have highlighted the potential role of elevated long-term BP variability in increasing stroke risk in general, dedicated studies investigating the association between long-term BP variability and ICH recurrence risk are lacking.10–12
Previous studies highlighted that black and Hispanic individuals are at higher risk of first-ever ICH compared to white individuals.13–15 While the mechanisms underlying this health disparity are poorly understood, racial and ethnic differences in hypertension prevalence, severity, and biology are likely to have a major role.16 Ethnic and racial differences in ICH recurrence risk, however, have never been the subject of dedicated investigations. Specifically, the potential roles of BP and its variability in determining ICH recurrence risk among nonwhite ICH survivors have never been explored. Greater insight into these modifiable risk factors for rebleeding would advance our understanding of ICH biology. Perhaps more importantly, this information may also reshape guidelines for secondary ICH prevention to account for race/ethnicity and potentially incorporate reducing BP variability as an additional treatment goal.
We therefore leveraged existing data from 2 large studies of ICH—the longitudinal study conducted at Massachusetts General Hospital (MGH), and the Ethnic/Racial Variations of Intracerebral Hemorrhage (ERICH) study4,17—to clarify whether (1) minority ICH survivors (namely, black and Hispanic) are at higher risk of ICH recurrence and (2) systematic differences in hypertension control and/or BP variability account for these disparities.
Methods
Participating studies
We analyzed longitudinal follow-up data of primary ICH survivors enrolled in 2 separate studies. The ICH study conducted at MGH is a single-center, prospective study of ICH.4 For the purposes of the analyses presented below, we included data for individuals admitted to MGH with a new diagnosis of primary ICH from January 2006 to December 2013. The ERICH study is a large multicenter study focused on recruitment of minority individuals for the identification of genetic and epidemiologic risk factors for ICH and associated outcomes.17 Enrollment in ERICH was conducted between August 2010 and September 2016. Of note, since MGH was one of the participating sites for the ERICH study, all ERICH participants enrolled at MGH were included only in the ERICH analyses to avoid data duplication.
Study eligibility and patient recruitment
We recruited participants among patients meeting the following eligibility criteria: (1) aged 18 years or older, and (2) diagnosed with acute primary (i.e., spontaneous) ICH, including intraparenchymal bleeding in the setting of oral therapeutic anticoagulation. Initial ICH was confirmed by CT scan, obtained within 24 hours of symptom onset. Patients with intracranial hemorrhage resulting from trauma, conversion of an ischemic infarct, rupture of a vascular malformation or aneurysm, or brain tumor were not considered eligible.4,18 Since we sought to investigate ethnic/racial disparities in long-term ICH recurrence rates, we excluded patients who survived <3 months after hemorrhage, in agreement with a previously published methodology.4 We also excluded patients who did not meet criteria for BP data availability during follow-up (see below for details).
Standard protocol approvals, registrations, and patient consents
The study protocols were approved by institutional review boards at all participating institutions. Written informed consent was obtained from all study participants or their surrogates.
Baseline data collection
Pre-ICH baseline data (including demographic information and medical history) were collected by trained study staff in both studies, by means of in-person interview of patients and/or reliable informants, conducted at time of enrollment.4,17 We subsequently corroborated and expanded on information obtained in person via review of medical records. Participants and/or informants were also asked at enrollment to self-identify race and ethnicity, choosing from the option recommended by the NIH for use in research studies. For the purpose of this study, this information was used to determine self-reported race/ethnicity as white (i.e., non-Hispanic white), black (i.e., non-Hispanic black), Hispanic (i.e., self-identifying as white-Hispanic or black-Hispanic), and other (not meeting criteria for inclusion in white, black, or Hispanic groups). We analyzed admission CT scans to determine ICH location, hematoma volume, and presence of intraventricular blood according to a previously validated methodology.17,19 All neuroimaging analyses for both studies were conducted by study personnel at MGH blinded to clinical and follow-up information.
Longitudinal follow-up
ICH survivors and their caregivers were contacted and interviewed by dedicated study staff at 3, 6, and 12 months after first ICH for both studies, based on established protocols.4,17 For the MGH-ICH study, individuals and caregivers enrolled were also contacted every 6 months after the first year from index ICH, as previously described.4,18 We inquired about ICH recurrence, ischemic stroke, death, and medication use and dosing. We also queried the Social Security Death Index national database as an alternative way of identifying deaths among ICH survivors.
BP capture
Study staff acquired information on baseline and ambulatory BP measurements, based on previously published methods.4,17 Briefly, study investigators inquired about most recent BP measurement obtained in a medical setting by medical personnel (no inquiry was made about home or self-obtained BP measurements). If patients were unable to provide exact BP measurements, medical records for reported encounters falling within the follow-up period were obtained via (1) manual review of longitudinal electronic medical records and (2) patient-provided external medical records. We prespecified data capture targets of one or more BP measurements every 6 months. For all BP variables (see below), patients were assigned a single value within each 6-month follow-up interval, according to a previously validated methodology.4 Briefly, we utilized a single pair of SBP/DBP measurements per each follow-up interval. When multiple BP values were available in a single interval, the average value was used for analysis purposes. Study participants lost to follow-up contributed all BP measurements predating loss to follow-up to the analysis. Of note, study staff involved in BP data capture was blinded to clinical and neuroimaging data.
Statistical methods
Overall analysis plan
We initially conducted separate univariable and multivariable analyses (as described below) for the MGH-ICH and ERICH studies. Results from multivariable regression analyses in each study were then meta-analyzed using a conservative inverse variance-based random-effects pooling method (DerSimonian-Laird).20 We then calculated I2 (percentage of effect size attributable to heterogeneity)21; heterogeneity was considered significant for I2 > 0.20. To account for multiple testing burden, we used the false discovery rate (FDR) adjustment method as developed by Benjamini and Hochberg.22 All p values presented were adjusted using the FDR method. All p values <0.05 (2-tailed) after FDR adjustment were considered to be significant. All analyses were performed with R software version 3.3.1 (R Foundation for Statistical Computing).
Definition of variables
Age at index ICH was analyzed as a continuous variable. Race/ethnicity was analyzed as a categorical variable using white patients as reference (because of their numerical preponderance). To ascertain the association between BP and risk of ICH recurrence, we generated and analyzed 4 exposures: (1) SBP as a continuous, time-varying variable, (2) DBP as a continuous, time-varying variable, (3) SBP variability, defined as the variation coefficient for SBP measurements (SBP-VC), and (4) DBP variability, defined as the variation coefficient for DBP (DBP-VC). Variation coefficient values were calculated for each participant as the ratio of SD/mean BP value. We subdivided both SBP-VC and DBP-VC values into quartiles for all planned analyses.
Univariable and multivariable analyses
Categorical variables were compared using Fisher exact test (2-tailed) and continuous variables using the Mann-Whitney rank sum or unpaired t test as appropriate. We determined factors associated with ICH recurrence in univariable analyses using Kaplan-Meier plots with significance testing by the log-rank test. Patient data were censored only in case of death or loss to follow-up. Given the limited number of patients experiencing multiple ICH recurrences (n = 5), only the first recurrent event contributed to our analyses (and data were censored thereafter). We performed multivariable analyses using Cox regression models. We initially included in multivariable modeling all factors associated with ICH recurrence in univariable analyses with p < 0.20. Because of potential associations between ICH recurrence and exposure to antiplatelet agents and/or oral anticoagulants, we prespecified adjustment for these variables in our multivariable models (regardless of p value). Because of the potential effects of antihypertensive treatment on ICH recurrence via BP control, we repeated all multivariable analyses after adjustment for number and nature of antihypertensive agents. We subsequently used backward elimination procedures to arrive at a minimal model including only variables associated with ICH at p < 0.05. Multicollinearity was assessed by computing variance inflation factors (VIF) for all variables. The proportional hazard assumption was tested via graphical inspection and calculation of Schoenfeld residuals.
Data availability
The authors certify they have documented all data, methods, and materials used to conduct the research presented. Anonymized data pertaining to the research presented will be made available by request from qualified investigators.
Results
Study participants
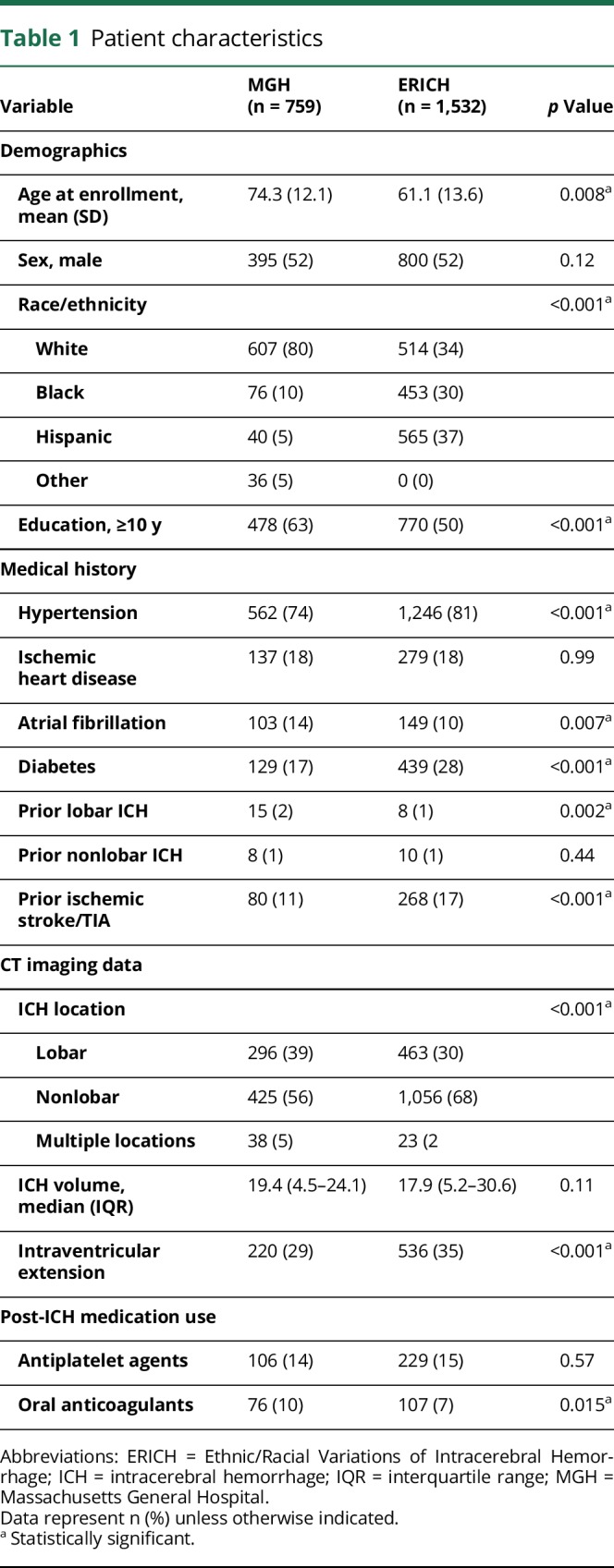
A total of 2,291 ICH survivors (1,121 white, 529 black, 605 Hispanic, and 36 of other race/ethnicity) fulfilled initial eligibility criteria. Detailed information on characteristics of participants enrolled in the MGH-ICH (n = 759) and ERICH (n = 1,532) studies is presented in table 1. Because of systematic differences in study design and setting, we identified several differences between ICH survivors enrolled in MGH-ICH vs ERICH (see table 1 for comparison p values). MGH-ICH participants were older, more likely to be white and highly educated, and more frequently presented with lobar ICH. They were also more likely to report a medical history of atrial fibrillation and to be exposed to oral anticoagulation after ICH. ERICH participants were more likely to report medical history of hypertension, diabetes, prior ischemic stroke, or TIA. They were also more likely to present with nonlobar ICH and with evidence of intraventricular hemorrhage on initial CT scans. We followed ERICH study participants up to 12 months (based on study protocol).17 Thirty-nine of 1,532 participants were lost to follow-up, corresponding to a rate of 2.5% per year. We identified 23 recurrent ICH events among ERICH study participants, for an estimated annual recurrence rate of 1.5% (95% confidence interval [CI] 0.8%–2.7%). We followed MGH-ICH study participants for a median follow-up time of 49.8 months (interquartile range [IQR] 38.5–66.2). We identified 55 of 759 participants who were lost to follow-up, corresponding to a rate of 1.5% per year. We identified 75 ICH recurrence events among MGH-ICH study participants, corresponding to an estimated annual recurrence rate of 3.9% (95% CI 2.5%–5.9%).
Table 1.
Patient characteristics
Hypertension severity and BP variability based on race/ethnicity
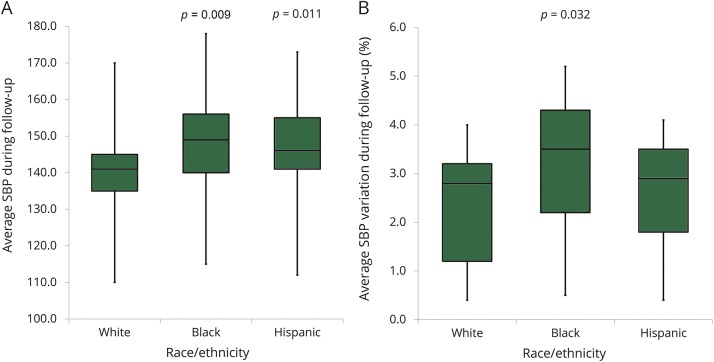
We initially sought to determine whether systematic differences in hypertension severity (average SBP/DBP, SBP-VC, and DBP-VC) existed between white, black, and Hispanic ICH survivors. Average SBP during follow-up was higher for black ICH survivors (median 149 mm Hg, IQR 140–165 mm Hg, p = 0.009) as well as for Hispanic ICH survivors (median 146 mm Hg, IQR 141–155 mm Hg, p = 0.011) when compared with white individuals (median 141 mm Hg, IQR 135–145 mm Hg). Furthermore, SBP variability (SBP-VC) was also higher for black participants (median 3.5%, IQR 2.2%–4.3%, p = 0.032) when compared with white individuals (median 2.8%, IQR 1.2%–3.2%). We provide a graphic representation of differences in average SBP and SBP-VC by race/ethnicity in figures 1 and 2.
Figure 1. SBP following ICH by race and ethnicity.
(A) Average SBP during follow-up after ICH, by self-reported race/ethnicity. (B) Average SBP variation (SD/mean) during follow-up, by self-reported race/ethnicity. Boxplot upper and lower margins indicate 25th and 75th percentiles of average SBP during follow-up (A) and average SBP variation (%) during follow-up (B). Horizontal lines in boxes indicate corresponding median values. ICH = intracerebral hemorrhage; SBP = systolic blood pressure.
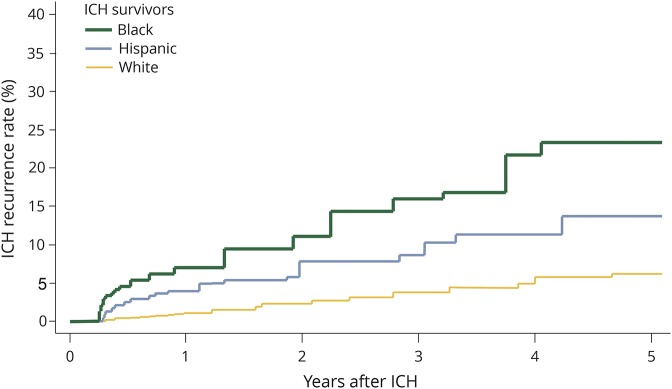
Figure 2. ICH recurrence by race and ethnicity.
Kaplan-Meier plot of ICH recurrence rates for white, black, and Hispanic study participants. ICH = intracerebral hemorrhage.
Variations in ICH recurrence risk by race/ethnicity
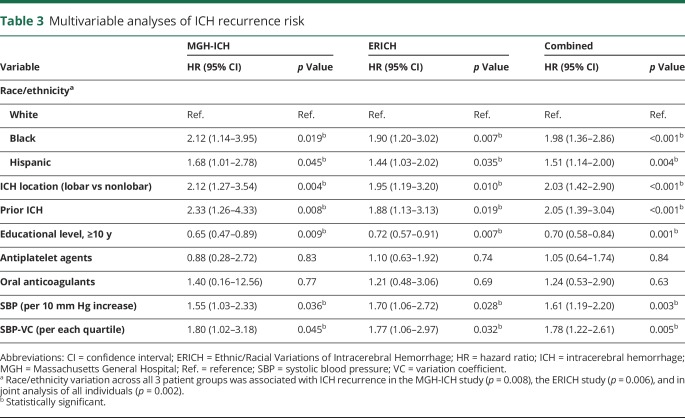
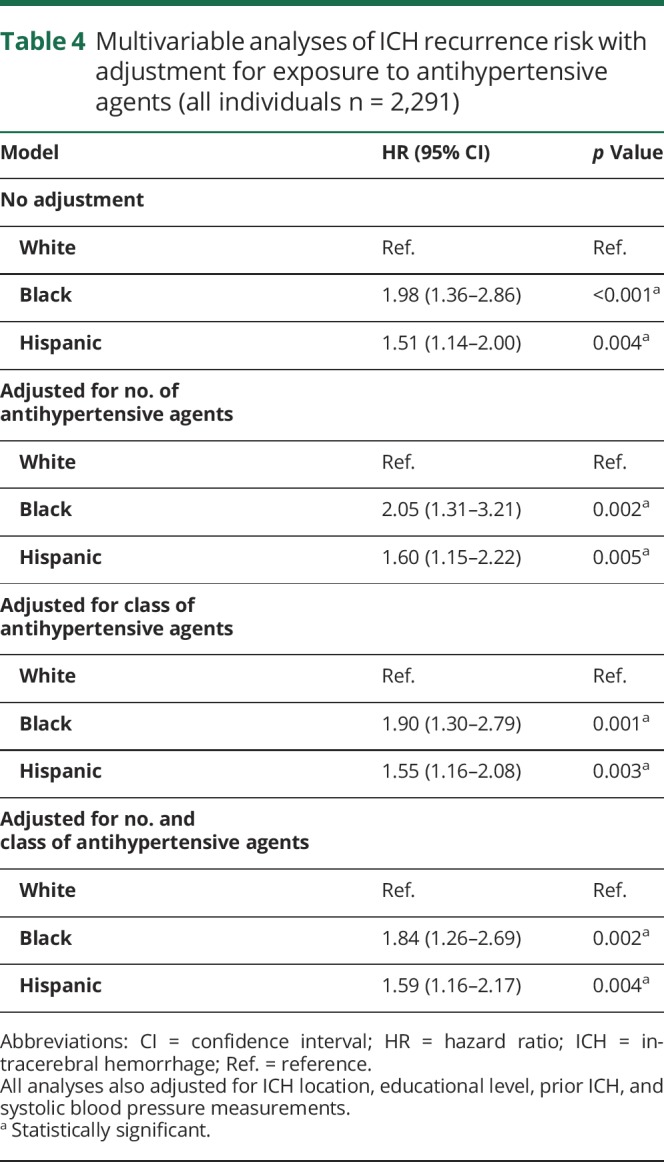
Across both participating studies, we observed 26 ICH recurrences among 1,121 white ICH survivors (1.7%) compared to 35 recurrences among 529 black participants (6.6%) and 37 recurrences among 605 Hispanic participants (6.1%). Yearly estimated ICH recurrence rates were 1.1% (IQR 0.7%–2.1%) for white ICH survivors, 3.9% (IQR 3.2%–4.4%) for black ICH survivors, and 3.5% (IQR 2.8%–4.2%) for Hispanic ICH survivors. In univariable analyses, race/ethnicity, educational level, prior ICH (before index event), ICH location, and ICH volume were associated with ICH recurrence (table 2). Higher SBP measurements were associated with increased ICH recurrence risk (hazard ratio for 10 mm Hg increase 1.27, 95% CI 1.02–1.59, p = 0.039). Greater SBP variability, defined as SBP-VC, was also associated with increased ICH recurrence risk (hazard ratio for each SBP-VC quartile increase 1.69, 95% CI 1.04–2.74, p = 0.034). We found no association between DBP or DBP-VC and ICH recurrence risk in univariable analyses (both p > 0.20). In multivariable analyses (table 3), black and Hispanic race/ethnicity remained independently associated with increased ICH recurrence risk in both studies, even after adjustment for SBP and SBP-VC (both of which were also independently associated with rebleeding events). Of note, exposure to antihypertensive agents during follow-up was not associated with ICH recurrence (all p > 0.20) and did not substantially modify observed results (table 4). Combination of findings from each study revealed consistent associations without evidence of between-study heterogeneity (all I2 < 0.05).
Table 2.
Univariable analyses of ICH recurrence risk in all individuals (n = 2,291)
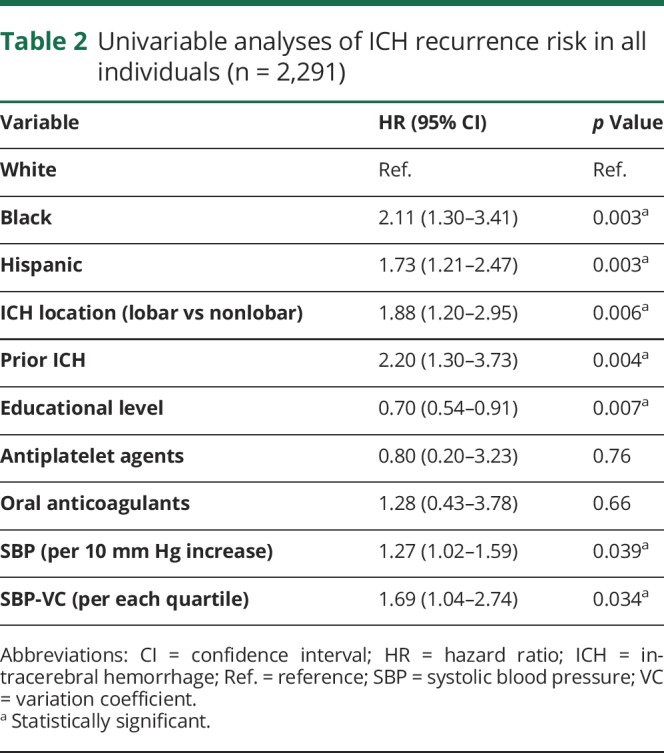
Table 3.
Multivariable analyses of ICH recurrence risk
Table 4.
Multivariable analyses of ICH recurrence risk with adjustment for exposure to antihypertensive agents (all individuals n = 2,291)
Discussion
We present evidence that minority ICH survivors of black and Hispanic racial/ethnic background are at substantially increased risk of recurrent ICH. While minority ICH survivors demonstrated greater hypertension severity (specifically, higher average SBP) and BP variability, these findings did not account for the entirety of excess ICH recurrence risk.
Hypertension is the most potent risk factor for ICH incidence and recurrence, thus warranting specific emphasis from both a clinical management and research standpoint.8,9,16 Our findings expand on this published evidence in a number of ways. Despite that ICH survivors are a high-risk group, multiple research groups (including our own) reported that less than 50% of them achieve adequate BP control according to published guidelines.4,23 In the present study, we demonstrated that black and Hispanic ICH survivors are disproportionately less likely to achieve recommended BP goals, with expected consequences of ICH recurrence risk. The mechanisms underlying limited success in BP control among ICH survivors at large remain unclear. However, it is likely that socioeconomic factors, chiefly availability and access to health care,24 represent major obstacles toward achieving optimal hypertension control for minority ICH survivors. Cultural factors associated with racial/ethnic background are also likely to influence risk of ICH recurrence. For example, cultural beliefs may determine personal behavior toward healing and disease prevention, thus affecting adherence with traditional prescribed medications.25
Our findings suggesting greater long-term BP variability among black participants may also reflect less “stable” control of underlying hypertension. While long-term BP variability has been demonstrated to act as a major risk factor for stroke in general, we provide the first proof of association specific to ICH.10–12 As mentioned above, these findings may reflect variations in quality/stability in hypertension control over time. Alternatively, specific and separate biological mechanisms may account for the effect of long-term BP variability on rebleeding. We are unfortunately unable to provide additional insights in support of either hypothesis with currently available data.
When compared to ICH survivors in most previously published reports,26 individuals included in our analyses (particularly those enrolled in the ERICH study) differ in terms of earlier age at time of ICH. This discrepancy reflects known disparities in ICH epidemiology among minority patients. Several studies reported that nonwhite individuals are more likely to present with ICH at a younger age.13,27 The prespecified enrollment targets based on race/ethnicity therefore account for the younger age of ICH survivors in ERICH.17
Our study has a number of limitations. First, despite the overall sample size of our study, the number of recurrent ICH events was limited, thus preventing us from conducting dedicated recurrence risk analyses within the black and Hispanic patient subgroups. Second, because of our study design, we focused only on long-term BP variability. Circadian and day-to-day variation in BP may also have a role in determining ICH recurrence risk, but cannot be explored using our data. Third, the nonstandardized nature of BP capture in participating studies implies that excess rebleeding risk among minority individuals (after adjusting for BP and its variability) may reflect limited data density rather than a specific biological effect. However, these considerations do highlight the challenges associated with clinical and research approaches to BP management following ICH. We currently captured data at a temporal frequency (approximately every 6 months) matching investigative and health care practices in all but the highest resource settings. More frequent capture of BP data on a large scale among ICH survivors may still provide additional guidance in achieving maximal reduction of recurrence risk, but will also likely result in substantial logistical challenges. We also utilized self-reported race/ethnicity to capture the complex network of biological, social, and cultural determinants of the identified health disparity in ICH recurrence. We are unable to further dissect and study these individual aspects of personal racial/ethnic background. However, recent studies in the field of genetic epidemiology identified strong correlations between self-reported race/ethnicity, cultural perception, and genetic ancestry.28 Our approach also exhibits several strengths. Although participants in the MGH-ICH and ERICH studies differed substantially in demographics, medical history, and ICH characteristics, we observed highly consistent associations between race/ethnicity and ICH recurrence risk. Of note, reported associations reached our prespecified threshold for significance in each study individually and were independently replicated in the other. Our findings are therefore likely to be generalizable to minority ICH patients at large.
In summary, we present evidence that black and Hispanic survivors of primary ICH are at higher risk of rebleeding than their white counterparts. We found that hypertension severity was greater among both black and Hispanic individuals; black participants also displayed greater SBP variability. These findings notwithstanding, adjustment for hypertension severity and BP variability did not fully account for the observed racial/ethnic disparities in ICH recurrence risk. Additional studies will be required in order to expand on our findings linking hypertension with ICH recurrence risk, as well as to explore novel biological, socioeconomic, and cultural factors accounting for the observed disparities.
Glossary
- BP
blood pressure
- CI
confidence interval
- DBP
diastolic blood pressure
- ERICH
Ethnic/Racial Variations of Intracerebral Hemorrhage
- FDR
false discovery rate
- ICH
intracerebral hemorrhage
- IQR
interquartile range
- MGH
Massachusetts General Hospital
- SBP
systolic blood pressure
- VC
variation coefficient
Footnotes
CME Course: NPub.org/cmelist
Author contributions
Study concept: D.W., C.L., J.R., A.B. Study Design: A.R.-T., D.W., C.L., J.R., A.B. Acquisition of data: A.R.-T., M.M., C. Kourkoulis, K.S., A.M.A., C.J.M., S.Y.K., J.V.B., M.E.G., S.M.G., A.V., C.D.A., M. Flaherty, M.L.J., L.B., G.Y.S., G.P., A.K.B., D.M., K.N.S., C. Kidwell, S.K., M. Frankel, C.D.L., F.T., D.W., J.R., A.B. Statistical analysis: A.R.-T., A.B. Manuscript preparation: A.R.-T., A.B. Manuscript review: A.R.-T., M.M., C. Kourkoulis, K.S., A.M.A., C.J.M., S.Y.K., J.V.B., M.E.G., S.M.G., A.V., C.D.A., M. Flaherty, M.L.J., L.B., G.Y.S., G.P., A.K.B., D.M., K.N.S., C. Kidwell, S.K., M. Frankel, C.D.L., F.T., D.W., J.R., A.B. Study supervision/coordination: C. Kourkoulis, K.S., C.J.M., D.W., F.T., J.R., A.B.
Study funding
The authors' work on this study was supported by funding from the US NIH (R25NS065743, K23NS100816, U01NS069763, R01NS093870, P50NS051343, and R01AG26484).
Disclosure
A. Rodriguez-Torres, M. Murphy, C. Kourkoulis, K. Schwab, A. Ayres, C. Moomaw, S. Young Kwon, and J. Berthaud report no disclosures relevant to the manuscript. M. Gurol is supported by K23NS083711. S. Greenberg is supported by R01AG26484. A. Viswanathan reports no disclosures relevant to the manuscript. C. Anderson is supported by K23086873. M. Flaherty, M. James, L. Birnbaum, G. Yong Sung, G. Parikh, A. Boehme, D. Mayson, K. Sheth, C. Kidwell, S. Koch, M. Frankel, C. Langefeld, F. Testai, and D. Woo report no disclosures relevant to the manuscript. J. Rosand is supported by R01NS036695, UM1HG008895, R01NS093870, and R24 NS092983. A. Biffi is supported by R25NS065743. Go to Neurology.org/N for full disclosures.
References
- 1.Feigin VL, Mensah GA, Norrving B, Murray CJ, Roth GA; GBD 2013 Stroke Panel Experts Group. Atlas of the Global Burden of Stroke 1990–2013: the GBD 2013 study. Neuroepidemiology 2015;45:230–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Poon MT, Fonville AF, Al-Shahi Salman R. Long-term prognosis after intracerebral haemorrhage: systematic review and meta-analysis. J Neurol Neurosurg Psychiatry 2014;85:660–667. [DOI] [PubMed] [Google Scholar]
- 3.Krishnamurthi RV, Feigin VL, Forouzanfar MH, et al. Global and regional burden of first-ever ischaemic and haemorrhagic stroke during 1990–2010: findings from the Global Burden of Disease Study 2010. Lancet Glob Health 2013;1:e259–e281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biffi A, Anderson C, Battey TW, et al. Association between blood pressure control and risk of recurrent intracerebral hemorrhage. JAMA 2015;9:904–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hanger HC, Wilkinson TJ, Fayez-Iskander N, Sainsbury R. The risk of recurrent stroke after intracerebral haemorrhage. J Neurol Neurosurg Psychiatry 2007;78:836–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bailey RD, Hart RG, Benavente O, Pearce LA. Recurrent brain hemorrhage is more frequent than ischemic stroke after intracranial hemorrhage. Neurology 2001;56:773–777. [DOI] [PubMed] [Google Scholar]
- 7.Bae H, Jeong D, Doh J, Lee K, Yun I, Byun B. Recurrence of bleeding in patients with hypertensive intracerebral hemorrhage. Cerebrovasc Dis 1999;9:102–108. [DOI] [PubMed] [Google Scholar]
- 8.Hemphill JC III, Greenberg SM, Anderson CS, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2015;46:2032–2060. [DOI] [PubMed] [Google Scholar]
- 9.Steiner T, Al-Shahi Salman R, Beer R, et al. European Stroke Organisation (ESO) guidelines for the management of spontaneous intracerebral hemorrhage. Int J Stroke 2014;9:840–855. [DOI] [PubMed] [Google Scholar]
- 10.Hussein WF, Chang TI. Visit-to-visit variability of systolic blood pressure and cardiovascular disease. Curr Hypertens Rep 2015;17:14. [DOI] [PubMed] [Google Scholar]
- 11.Stevens SL, Wood S, Koshiaris C, et al. Blood pressure variability and cardiovascular disease: systematic review and meta-analysis. BMJ 2016;354:i4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rothwell PM, Howard SC, Dolan E, et al. Effects of beta blockers and calcium-channel blockers on within-individual variability in blood pressure and risk of stroke. Lancet Neurol 2010;9:469–480. [DOI] [PubMed] [Google Scholar]
- 13.Labovitz DL, Halim A, Boden-Albala B, Hauser WA, Sacco RL. The incidence of deep and lobar intracerebral hemorrhage in whites, blacks, and Hispanics. Neurology 2005;65:518–522. [DOI] [PubMed] [Google Scholar]
- 14.Shen AYJ, Yao JF, Brar SS, Jorgensen MB, Chen W. Racial/ethnic differences in the risk of intracranial hemorrhage among patients with atrial fibrillation. J Am Coll Cardiol 2007;50:309–315. [DOI] [PubMed] [Google Scholar]
- 15.Bruno A, Carter S, Qualls C, Nolte KB. Incidence of spontaneous intracerebral hemorrhage among Hispanics and non-Hispanic whites in New Mexico. Neurology 1996;47:405–408. [DOI] [PubMed] [Google Scholar]
- 16.Walsh KB, Woo D, Sekar P, et al. Untreated hypertension: a powerful risk factor for lobar and nonlobar intracerebral hemorrhage in whites, blacks, and Hispanics. Circulation 2016;134:1444–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Woo D, Rosand J, Kidwell C, et al. The Ethnic/Racial Variations of Intracerebral Hemorrhage (ERICH) study protocol. Stroke 2013;44:e120–e125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Biffi A, Halpin A, Towfighi A, et al. Aspirin and recurrent intracerebral hemorrhage in cerebral amyloid angiopathy. Neurology 2010;75:693–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Biffi A, Bailey D, Anderson CD, et al. Risk factors associated with early vs. delayed dementia after intracerebral hemorrhage. JAMA Neurol 2016;73:969–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–188. [DOI] [PubMed] [Google Scholar]
- 21.Ioannidis JP. Interpretation of tests of heterogeneity and bias in meta-analysis. J Eval Clin Pract 2008;14:951–957. [DOI] [PubMed] [Google Scholar]
- 22.Hsueh HM, Chen JJ, Kodell RL. Comparison of methods for estimating the number of true null hypotheses in multiplicity testing. J Biopharm Stat 2003;13:675–689. [DOI] [PubMed] [Google Scholar]
- 23.Zahuranec DB, Wing JJ, Edwards DF, et al. Poor long-term blood pressure control after intracerebral hemorrhage. Stroke 2012;43:2580–2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Safford MM. Intracerebral hemorrhage, racial disparities, and access to care. Circulation 2016;134:1453–1455. [DOI] [PubMed] [Google Scholar]
- 25.Brady E. Healing Logics: Culture and Medicine in Modern Health Belief Systems. Logan: Utah State University Press; 2001. [Google Scholar]
- 26.Poon MT, Bell SM, Al-Shahi Salman R. Epidemiology of intracerebral haemorrhage. Front Neurol Neurosci 2015;37:1–12. [DOI] [PubMed] [Google Scholar]
- 27.Broderick JP, Brott T, Tomsick T, Huster G, Miller R. The risk of subarachnoid and intracerebral hemorrhages in blacks as compared with whites. New Engl J Med 1992;326:733–736. [DOI] [PubMed] [Google Scholar]
- 28.Banda Y, Kvale MN, Hoffmann TJ, et al. Characterizing race/ethnicity and genetic ancestry for 100,000 subjects in the Genetic Epidemiology Research on Adult Health and Aging (GERA) cohort. Genetics 2015;200:1285–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors certify they have documented all data, methods, and materials used to conduct the research presented. Anonymized data pertaining to the research presented will be made available by request from qualified investigators.