Abstract
Objective
To compare the functional outcomes and health-related quality of life metrics of restarting vs not restarting antiplatelet therapy (APT) in patients presenting with intracerebral hemorrhage (ICH) in the ERICH (Ethnic/Racial Variations of Intracerebral Hemorrhage) study.
Methods
Adult patients aged 18 years and older who were on APT before ICH and were alive at hospital discharge were included. Patients were dichotomized based on whether or not APT was restarted after hospital discharge. The primary outcome was a modified Rankin Scale score of 0–2 at 90 days. Secondary outcomes were excellent outcome (modified Rankin Scale score 0–1), mortality, Barthel Index, and health status (EuroQol–5 dimensions [EQ-5D] and EQ-5D visual analog scale scores) at 90 days.
Results
The APT and no APT cohorts comprised 127 and 732 patients, respectively. Restarting APT was associated with lower rates of good functional outcome (36.5% vs 40.8%; p = 0.021) and lower Barthel Index scores at 90 days (p = 0.041). The 2 cohorts were then matched in a 1:1 ratio, and the matched cohorts each comprised 107 patients. No difference in primary outcome was observed between restarting vs not restarting APT (35.5% vs 43.9%; p = 0.105). There were also no differences between the secondary outcomes of the 2 cohorts.
Conclusion
Restarting APT in patients with ICH of mild to moderate severity after acute hospitalization is not associated with worse functional outcomes or health-related quality of life at 90 days. In patients with significant cardiovascular risk factors who experience an ICH, restarting APT remains the decision of the treating practitioner.
Despite its effectiveness in primary and secondary myocardial infarction (MI) and stroke prevention, antiplatelet therapy (APT) is associated with an increased risk of spontaneous intracerebral hemorrhage (ICH).1,2 Patients with ICH taking APT, particularly dual APT (DAPT), have higher rates of in-hospital mortality.3,4 In patients with ICH who were on APT and remain alive at the time of hospital discharge, clinicians are frequently faced with the decision of whether or not to restart APT. APT after ICH has been found to be associated with a decreased incidence of ischemic cardiovascular events, without an elevated risk of recurrent ICH.5–7 The balance of ischemic cardiovascular events and recurrent ICH, among other bleeding complications, may affect functional outcomes and health-related quality of life (HRQoL) measures after hospital discharge. In addition, the effect of recurrent ICH in patients taking APT on these measures may not be accurately portrayed by the incidence of recurrent ICH alone. Therefore, the aim of this multicenter, retrospective matched cohort study was to compare the functional outcomes and HRQoL metrics of restarting vs not restarting APT in patients with ICH.
Methods
Standard protocol approvals, registrations, and patient consents
This study was approved by the institutional review board at each respective site, and written informed consent was obtained from all patients (or guardians of patients) participating in the study. Patient data were deidentified and then pooled for analysis. In this study, we followed the guidelines set forth by the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) statement.
Data sources and restarting APT
The Ethnic/Racial Variations of Intracerebral Hemorrhage (ERICH) study methods have been previously described in detail.8 Briefly, ERICH is a multicenter, prospective, case-control study designed to recruit 1,000 non-Hispanic white, 1,000 non-Hispanic black, and 1,000 Hispanic participants with spontaneous ICH, along with matched ICH-free controls for the identification of genetic and epidemiologic risk factors for ICH and outcomes after ICH. Participants were recruited from 19 US sites comprising 42 hospitals. All participants or designated proxies underwent a standardized data collection protocol, including a personal interview and medical chart abstraction.
Patients included in the present study were derived from the spontaneous ICH case cohort of the ERICH study. The inclusion criteria for this study were (1) age 18 years or older, (2) patients taking APT before presentation with ICH, and (3) alive at the time of hospital discharge. The exclusion criteria were (1) thrombocytopenia (platelet count <150,000/μL) on admission, (2) patients on anticoagulation (heparin, low-molecular-weight heparin, warfarin, or non-vitamin K antagonist oral anticoagulants) before presentation with ICH, and (3) patients who were designated comfort care at hospital discharge.
Baseline demographic medical history data included age, sex, smoking status, TIA, ischemic stroke, hypertension, diabetes mellitus, hyperlipidemia, coronary artery disease (CAD), MI, atrial fibrillation, angina, congestive heart failure, carotid artery disease, peripheral vascular disease, heart valve replacement, coronary artery angioplasty or stent placement, carotid artery angioplasty or stent placement, carotid endarterectomy, and coronary artery bypass grafting. Laboratory data obtained on admission included platelet count, total cholesterol, high-density lipoprotein, low-density lipoprotein, and triglycerides. Data regarding APT or DAPT before ICH were derived from directed chart review and comprehensive interviews.
Clinical, radiologic, and treatment data included Glasgow Coma Scale (GCS) score on admission, ICH volume, presence of intraventricular hemorrhage, ICH location (categorized as lobar, deep, and infratentorial), surgical evacuation of ICH, external ventricular drain (EVD) placement, intracranial pressure monitor placement, CSF shunt placement, intubation, platelet transfusion, ICH score on admission, and initiation of anticoagulation at hospital discharge.9,10 The resumption of APT, or lack thereof, at hospital discharge was determined from directed chart review, and APT status at 90-day follow-up was derived from interviews. For patients who were alive at follow-up, resumption of APT after ICH was defined as being on APT at 90 days. For patients who died prior to 90-day follow-up, resumption of APT was defined as inclusion of APT on the discharge medications list.
Outcomes
The primary outcome was a modified Rankin Scale (mRS) score of 0–2 (i.e., functional independence) at 90 days.11,12 The secondary outcomes were excellent outcome (mRS score 0–1), mortality, Barthel Index (on a scale of 0–100, with higher scores indicating less disability), and HRQoL, as measured by the EuroQol–5 dimensions (EQ-5D) (on a scale of −0.11 to 1, with higher values indicating better HRQoL), and EQ-5D visual analog scale (VAS) (on a scale of 0–100, with higher values indicating better HRQoL) self-report questionnaires.13
Statistical analysis
All statistical analyses were performed using Stata version 14.2 (StataCorp LP, College Station, TX). Patients who satisfied the inclusion and exclusion criteria were dichotomized into 2 cohorts (no APT and APT) based on resumption of APT after ICH, or lack thereof. Baseline, clinical, radiologic, and treatment characteristics were compared between the 2 cohorts. Continuous variables were compared using Student t or Mann-Whitney U tests, as appropriate. Categorical variables were compared using Pearson χ2 or Fisher exact tests, as appropriate. Univariable logistic and linear regression analyses were performed on the cohorts to assess the relationship between the resumption of APT and the primary and secondary outcomes. The findings from the logistic and linear regression analyses were adjusted for covariates of the cohorts with p < 0.10. In the subsequent analysis, to adjust for baseline differences, the 2 cohorts were matched, without replacement, in a 1:1 ratio with a caliper of 0.2 using propensity scores derived from baseline characteristics comparisons with p < 0.10. The matching was performed using the PSMATCH2 package developed for Stata.14 Reduction in standardized absolute bias for each covariate is provided in table e-1 (links.lww.com/WNL/A550) and figure e-1 (links.lww.com/WNL/A549). Univariable logistic and linear regression analyses were performed on the matched cohorts to assess the relationship between the resumption of APT and the primary and secondary outcomes. The findings from the logistic and linear regression analyses were adjusted for covariates of the matched cohorts with p < 0.10. The covariates were tested for multicollinearity using tolerance and variance inflation factor. Statistical significance was defined as p < 0.05, and all tests were 2-tailed. Missing data were not imputed.
Data availability
The data from the ERICH study, including interview, chart abstraction, and imaging findings for cases and controls are now made publicly available both through direct request from the ERICH study principal investigator (D.W. at daniel.woo@uc.edu) or the National Institute of Neurological Disorders and Stroke.
Results
Characteristics of matched cohorts
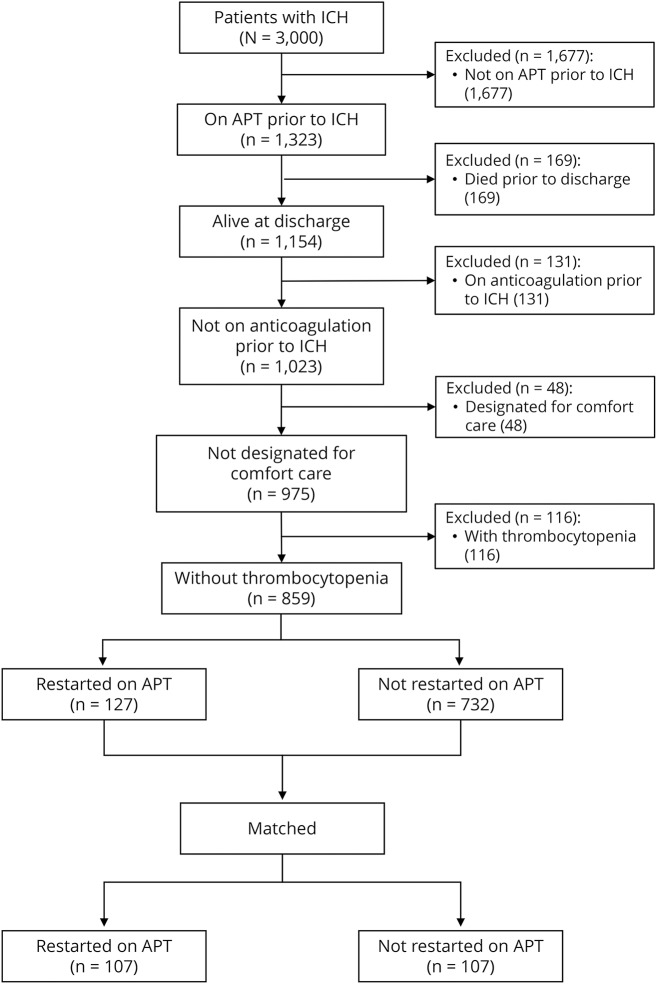
Of the 3,000 patients with spontaneous ICH enrolled in the ERICH study, 2,141 patients were excluded from the present study based on the inclusion and exclusion criteria. The remaining 859 patients comprised 127 patients who were restarted on APT and 732 patients who were not restarted on APT after ICH (figure 1).
Figure 1. Flow diagram showing the patient selection process.
APT = antiplatelet therapy; ICH = intracerebral hemorrhage.
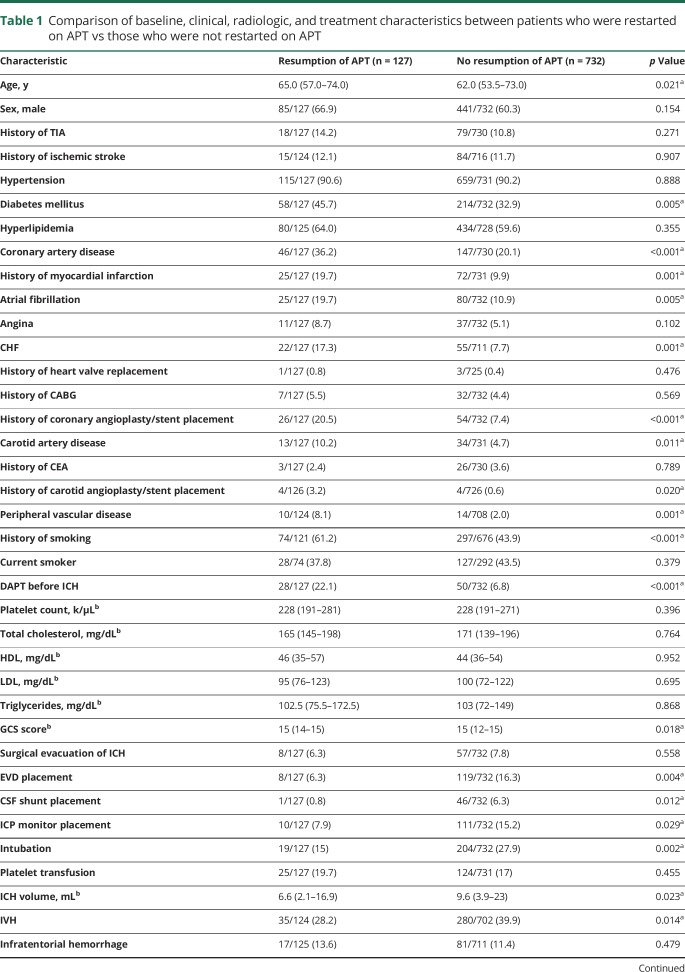
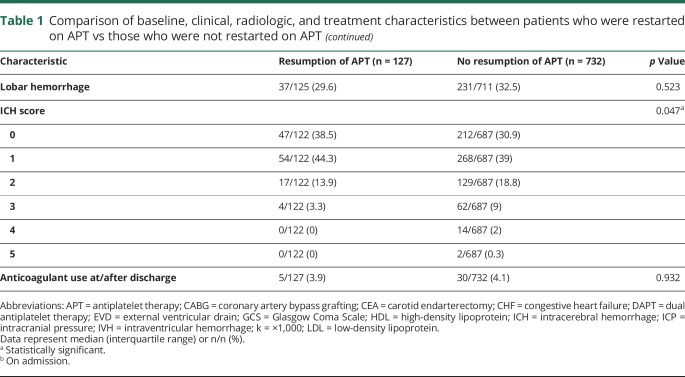
Table 1 shows a comparison of the baseline, clinical, radiologic, and treatment characteristics between patients who were restarted on APT vs those who were not restarted on APT, prior to matching. Patients who were restarted on APT were older (p = 0.021) and were more likely to have diabetes mellitus (p = 0.005), CAD (p < 0.001), previous MI (p = 0.001), atrial fibrillation (p = 0.005), congestive heart failure (p = 0.001), previous coronary angioplasty or stent placement (p < 0.001), carotid artery disease (p = 0.011), previous carotid angioplasty or stent placement (p = 0.020), peripheral vascular disease (p = 0.001), and a history of smoking (p < 0.001); they were also more likely to be on DAPT (22.1% vs 6.8%; p < 0.001). Patients who were restarted on APT had higher GCS scores (p = 0.018) and were less likely to have intraventricular hemorrhage (p = 0.014), had EVD (p = 0.004), CSF shunt (p = 0.012), intracranial pressure monitor placement (p = 0.029), or intubation (p = 0.002). Patients who were restarted on APT also had smaller ICH volume (p = 0.023) and lower ICH scores (p = 0.047).
Table 1.
Comparison of baseline, clinical, radiologic, and treatment characteristics between patients who were restarted on APT vs those who were not restarted on APT
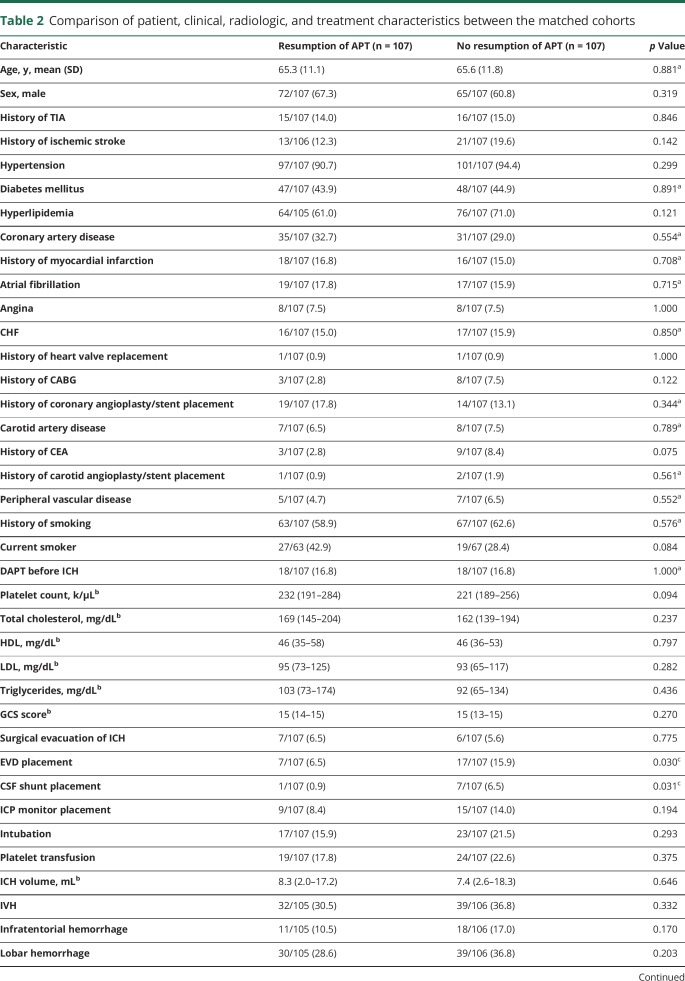
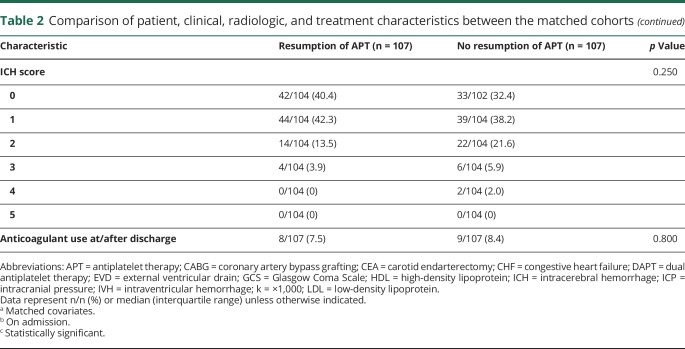
The 2 cohorts were matched in a 1:1 ratio using propensity scores based on significant baseline characteristics. The matched APT and no APT cohorts each comprised 107 patients. Table 2 shows a comparison of the patient, clinical, radiologic, and treatment characteristics between the matched cohorts. EVD (15.9% vs 6.5%) and CSF shunt (6.5% vs 0.9%) placement were more common in the no APT cohort. There were no differences between the matched cohorts in the remainder of the variables.
Table 2.
Comparison of patient, clinical, radiologic, and treatment characteristics between the matched cohorts
Primary outcome
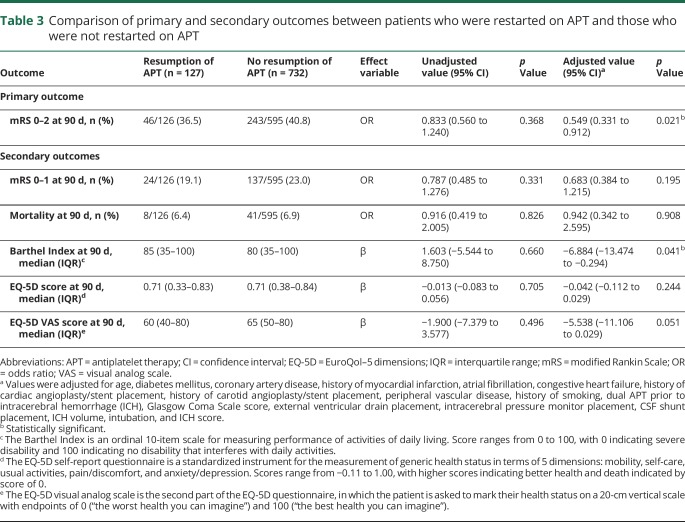
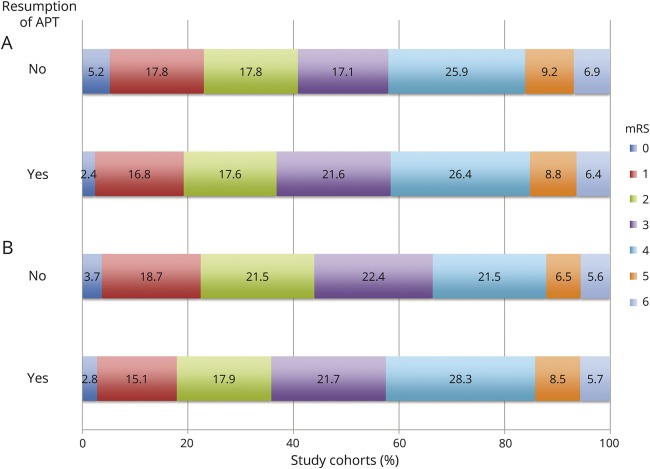
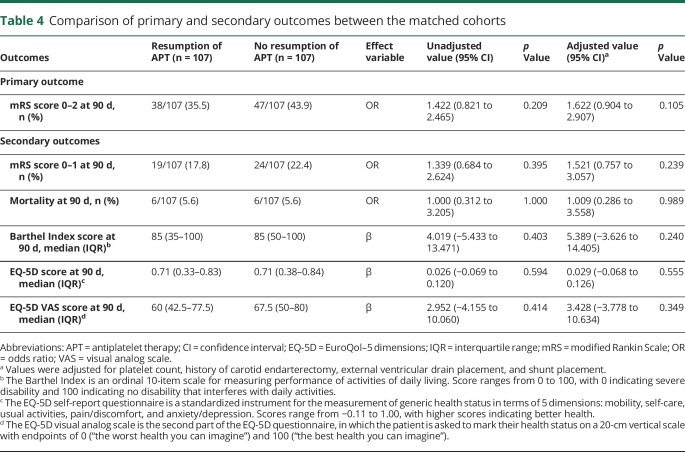
The primary outcome was observed in 36.5% vs 40.8% of the APT vs no APT cohorts, respectively (table 3 and figure 2). Restarting APT was associated with lower rates of good functional outcome at 90 days on multivariable analysis (adjusted odds ratio [OR] = 0.55 [95% confidence interval, CI 0.33–0.91]; p = 0.021). In the matched cohort analysis, the primary outcome was observed in 35.5% vs 43.9% of the APT vs no APT cohorts, respectively (table 4 and figure 2). The difference in the rate of the primary outcome was not significant between the matched cohorts in the univariate analysis (OR = 1.42 [95% CI 0.82–2.47]; p = 0.209) and remained nonsignificant after adjustments for platelet count, history of carotid endarterectomy, EVD placement, and CSF shunt placement (adjusted OR = 1.62 [95% CI 0.90–2.91]; p = 0.105).
Table 3.
Comparison of primary and secondary outcomes between patients who were restarted on APT and those who were not restarted on APT
Figure 2. Comparisons of functional outcomes at 90 days.
Bar chart comparing the functional outcomes at 90 days for (A) the resumption of APT vs no resumption of APT cohorts, and (B) the matched resumption of APT vs no resumption of APT cohorts, as measured by the mRS. APT = antiplatelet therapy; mRS = modified Rankin Scale.
Table 4.
Comparison of primary and secondary outcomes between the matched cohorts
Secondary outcomes
Excellent outcome at 90 days was observed in 19.1% and 23% of the APT and no APT cohorts, respectively (OR = 0.79 [95% CI 0.49–1.28]; p = 0.331; table 3 and figure 2). No difference was found between the cohorts in the rate of excellent outcome, even after adjustment (adjusted OR = 0.68 [95% CI 0.38–1.22]; p = 0.195). There were also no differences, either before or after adjustment, between the APT and no APT cohorts in mortality (6.4% vs 6.9%; adjusted OR = 0.94 [95% CI 0.34–2.60]; p = 0.908), EQ-5D scores (median 0.71 vs 0.71; adjusted β = −0.04 [95% CI −0.11 to 0.03]; p = 0.244), or EQ-5D VAS scores (median 60 vs 65; adjusted β = −5.54 [95% CI −11.11 to 0.03]; p = 0.051). Restarting APT was associated with lower Barthel Index scores at 90 days after adjustments (adjusted β = −6.88 [95% CI −13.47 to 0.29]; p = 0.041). In the matched cohort analysis, excellent outcome at 90 days was observed in 17.8% and 22.4% of the APT and no APT cohorts, respectively (OR = 1.34 [95% CI 0.68–2.62]; p = 0.395; table 4 and figure 2). No difference was found between the matched cohorts in the rate of excellent outcome, even after adjustment (adjusted OR = 1.52 [95% CI 0.76–3.06]; p = 0.239). There were also no differences, either before or after adjustment, between the APT and no APT cohorts in mortality (5.6% vs 5.6%; adjusted OR = 1.01 [95% CI 0.29–3.56]; p = 0.989), Barthel Index scores (median 85 vs 85; adjusted β = 5.39 [95% CI −3.63 to 14.41]; p = 0.240), EQ-5D scores (median 0.71 vs 0.71; adjusted β = 0.029 [95% CI −0.068 to 0.126]; p = 0.555), or EQ-5D VAS scores (median 60 vs 67.5; adjusted β = 3.43 [95% CI −3.78 to 10.63]; p = 0.349).
Discussion
Although platelets are essential to achieving hemostasis, platelet aggregation in the setting of atherosclerosis can lead to thromboembolic complications.15 The effectiveness of APT in both primary and secondary prevention of ischemic CAD and stroke has resulted in an increase in the number of patients receiving APT.16–21 Despite the overall benefit of APT in preventing the thromboembolic sequelae of atherosclerosis, its use may elevate the risk of spontaneous ICH.1,2,22,23 In addition, APT use before ICH is associated with hematoma expansion, increased mortality, and worse outcomes.3,24–26 For patients who survive the initial ICH, recurrent ICH incurs the risk of further morbidity and mortality, and its incidence has been reported to range between 2.0 and 4.0 per 100 patient-years.6,27–31 Risk factors for recurrent ICH include lobar ICH location, advanced age, hypertension, and previous ischemic stroke.27,31
Despite the increased cardiovascular risks associated with APT withdrawal, some clinicians are reluctant to restart APT after ICH because of a perception that doing so will subject the patient to an elevated risk of recurrent ICH. Since thrombotic diseases and ICH share many common risk factors, ICH survivors are frequently at risk of both ischemic cardiovascular disease and recurrent ICH. The decision to restart APT after ICH in the posthospitalization setting represents a major challenge, not only for clinicians who manage these patients during the acute hospitalization but also for primary care physicians who encounter these patients after discharge. Prior studies have not shown that restarting APT increases the risk of recurrent ICH.5–7,31,32 However, the effect of APT resumption in patients with ICH on functional outcomes and HRQoL has not been previously assessed. In this multicenter, retrospective matched cohort study, we report the first analysis of functional outcomes and HRQoL after restarting vs withholding APT in patients with ICH following acute hospitalization. In the initial analysis using the entire cohort, we found restarting APT was associated with lower rates of good functional outcome and lower Barthel Index scores at 90 days. However, after adjusting for differences in baseline characteristics using matched cohort analysis, we did not find a difference in functional outcomes between patients with ICH who restarted APT vs those who did not, including comparable rates of good (mRS score 0–2) and excellent (mRS score 0–1) outcomes at 90 days. Furthermore, the HRQoL metrics and mortality rates were not different between the 2 matched cohorts.
Flynn et al.7 found higher rates of ischemic stroke/MI compared to recurrent ICH (28.7 vs 9.7 per 1,000 patient-years) among the 417 ICH survivors in the Tayside Stroke Cohort. The authors reported recurrent ICH and ischemic stroke rates of 9.4 and 5.1 per 1,000 patient-years for the APT cohort, respectively. In the non-APT cohort, the recurrent ICH and ischemic stroke rates were 9.8 and 23.1 per 1,000 patient-years, respectively. No differences in recurrent ICH (hazard ratio [HR] = 1.07 [95% CI 0.24–4.84]) and ischemic stroke (HR 0.23 [95% CI 0.03–1.68]) rates between the APT and non-APT cohorts in the study were found. In addition, no differences in other endpoints, including MI (HR = 1.77 [95% CI 0.49–6.49]), ischemic stroke/MI (HR = 0.72 [95% CI 0.25–2.02]), and serious vascular events (HR = 0.73 [95% CI 0.42–1.28]), were observed. It should be noted that only 33% of patients who received APT after discharge were on APT before presenting with ICH. Viswanathan et al.31 studied 207 patients with ICH and found no association between APT and either lobar (HR = 0.8 [95% CI 0.3–2.3]; p = 0.73) or deep (HR = 1.2 [95% CI 0.1–14.3]; p = 0.88) ICH recurrence in the subgroup of 46 patients who were prescribed APT during the follow-up period. Chong et al.6 also reported comparable risks of recurrent ICH between patients who were prescribed aspirin (n = 56) vs those who were not (n = 384) after ICH (22.7 vs 22.4 per 1,000 patient-years; p = 0.70). However, in their subgroup analysis of patients with standard indications for aspirin, the incidence of combined vascular events (recurrent ICH, ischemic stroke, and acute coronary syndrome) was lower in patients prescribed aspirin (52.4 vs 112.8 per 1,000 patient-years; p = 0.04). In a recent study examining the effects of antithrombotic therapy use in Danish patients with a history of ICH, Ottosen et al.5 reported that APT in patients with appropriate indications was not associated with recurrent ICH (HR = 1.33 [95% CI 0.93–1.92]), mortality (HR = 0.96 [95% CI 0.80–1.15]), thromboembolic events (HR = 0.99 [95% CI 0.75–1.31]), or major bleeding events (HR = 0.89 [95% CI 0.68–1.16]). Although the data collected in the ERICH study does not include cardiovascular events or recurrent ICH in the posthospitalization period, the results of our analysis concur with prior studies, and they provide additional, clinically relevant findings that were not previously available.
We acknowledge that our study has several limitations. Our results are contingent on the accuracy and reliability of medication documentation on admission, at discharge, and during follow-up, which were based on patient self-report and family members of patients who were incapacitated. Therefore, this study is subject to reporting and recall biases. In addition, balancing of both measured and unmeasured variables between the cohorts may be limited by the small dataset available for propensity score matching. Hence, results from both the unmatched and matched analyses were presented in this study. The timing of restarting APT during the follow-up period could not be determined. Hence, no recommendation regarding the appropriate time interval between presentation with ICH and the resumption of APT can be derived from our findings. Although the major clinical indications for APT were included in the baseline variables, there may be other indications that were not accounted for. The specific indication for APT use in each patient could not be differentiated, since it was not a part of the ERICH questionnaire. We acknowledge that the results may be confounded by indication for restarting APT. Furthermore, detailed data regarding indications for DAPT rather than antiplatelet monotherapy, differences in specific APT medications, and APT dosage were not available. Since only 1.6% of patients with ICH were restarted on DAPT after hospitalization, a comparison among patients restarted on DAPT, single APT, and no APT could not be performed. Because the ERICH study was not specifically designed to evaluate outcomes associated with restarting APT in the post-ICH setting, data regarding recurrent ICH, bleeding complications, cardiovascular events, and thromboembolic complications after discharge were not recorded. Lastly, the findings of this study may not be generalizable to all patients with ICH, as most patients presented with relatively low ICH scores, high GCS scores, and small ICH volumes.
In patients who were taking APT before presenting with a spontaneous ICH of mild to moderate severity, restarting APT after acute hospitalization does not appear to be associated with worse functional outcomes or HRQoL. However, without detailed data regarding ischemic vs hemorrhagic cerebrovascular and cardiovascular events, we were unable to attribute our findings to restarting APT. In patients with significant cardiovascular risk factors, restarting APT in the posthospitalization setting remains the decision of the treating practitioner. Future prospective studies are necessary to ascertain the long-term benefits and risks of restarting APT after ICH.
Glossary
- APT
antiplatelet therapy
- CAD
coronary artery disease
- CI
confidence interval
- DAPT
dual antiplatelet therapy
- EQ-5D
EuroQol–5 dimensions
- ERICH
Ethnic/Racial Variations of Intracerebral Hemorrhage
- EVD
external ventricular drain
- GCS
Glasgow Coma Scale
- HR
hazard ratio
- HRQoL
health-related quality of life
- ICH
intracerebral hemorrhage
- MI
myocardial infarction
- mRS
modified Rankin Scale
- OR
odds ratio
- VAS
visual analog scale
Contributor Information
Collaborators: ERICH Investigators, Daniel Woo, Jennifer Osborne, Jonathan Rosand, Chelsea Kidwell, Jacob L. McCauley, Mark W. Brown, Eric W. Rademacher, Salina Waddy, Jamie N. Roberts, Sebastian Koch, Nicole R. Gonzales, Gene Sung, Steven J. Kittner, Lee Birnbaum, Michael Frankel, Fernando Daniel Testai, Christiana E. Hall, Mitchell S.V. Elkind, Matthew Flaherty, Bruce Coull, Ji Y. Chong, Tanya Warwick, Marc Malkoff, Michael L. James, Latisha K. Ali, Bradford B. Worrall, Floyd Jones, Tiffany Watson, Anne Leonard, Rebecca Martinez, Ralph I. Sacco, Carl D. Langefeld, and Jennifer Osborne
Author contributions
Ching-Jen Chen: design of study, acquisition of data, data analysis and interpretation, drafting of manuscript, critical revision of the manuscript for important intellectual content. Dale Ding: design of study, data interpretation, drafting of manuscript, critical revision of the manuscript for important intellectual content. Thomas Buell: data interpretation, drafting of manuscript, critical revision of the manuscript for important intellectual content. Fernando Testai: data interpretation, critical revision of the manuscript for important intellectual content. Sebastian Koch: data interpretation, critical revision of the manuscript for important intellectual content, study supervision. Daniel Woo: data interpretation, critical revision of the manuscript for important intellectual content, study supervision. Bradford Worrall: data interpretation, critical revision of the manuscript for important intellectual content, study supervision.
Study funding
This study was supported by a grant from the National Institute of Neurological Disorders and Stroke (NINDS: U-01-NS069763).
Disclosure
C. Chen, D. Ding, and T. Buell report no disclosures relevant to the manuscript. F. Testai reports receiving NIH grant support, including NIH NS-069763. S. Koch reports receiving NIH grant support, including NIH NS-069763. D. Woo reports receiving NIH grant support, including NIH NS-069763, NS-36695, and NS-30678. B. Worrall reports receiving NIH grant support, including NIH NS-069763, and is the deputy editor for the journal of Neurology®. Go to Neurology.org/N for full disclosures.
References
- 1.He J, Whelton PK, Vu B, Klag MJ. Aspirin and risk of hemorrhagic stroke: a meta-analysis of randomized controlled trials. JAMA 1998;280:1930–1935. [DOI] [PubMed] [Google Scholar]
- 2.Ducrocq G, Amarenco P, Labreuche J, et al. A history of stroke/transient ischemic attack indicates high risks of cardiovascular event and hemorrhagic stroke in patients with coronary artery disease. Circulation 2013;127:730–738. [DOI] [PubMed] [Google Scholar]
- 3.Khan NI, Siddiqui FM, Goldstein JN, et al. Association between previous use of antiplatelet therapy and intracerebral hemorrhage outcomes. Stroke 2017;48:1810–1817. [DOI] [PubMed] [Google Scholar]
- 4.Thompson BB, Bejot Y, Caso V, et al. Prior antiplatelet therapy and outcome following intracerebral hemorrhage: a systematic review. Neurology 2010;75:1333–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ottosen TP, Grijota M, Hansen ML, et al. Use of antithrombotic therapy and long-term clinical outcome among patients surviving intracerebral hemorrhage. Stroke 2016;47:1837–1843. [DOI] [PubMed] [Google Scholar]
- 6.Chong BH, Chan KH, Pong V, et al. Use of aspirin in Chinese after recovery from primary intracranial haemorrhage. Thromb Haemost 2012;107:241–247. [DOI] [PubMed] [Google Scholar]
- 7.Flynn RW, MacDonald TM, Murray GD, MacWalter RS, Doney AS. Prescribing antiplatelet medicine and subsequent events after intracerebral hemorrhage. Stroke 2010;41:2606–2611. [DOI] [PubMed] [Google Scholar]
- 8.Woo D, Rosand J, Kidwell C, et al. The Ethnic/Racial Variations of Intracerebral Hemorrhage (ERICH) study protocol. Stroke 2013;44:e120–e125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Teasdale G, Jennett B. Assessment of coma and impaired consciousness: a practical scale. Lancet 1974;2:81–84. [DOI] [PubMed] [Google Scholar]
- 10.Hemphill JC III, Bonovich DC, Besmertis L, Manley GT, Johnston SC. The ICH score: a simple, reliable grading scale for intracerebral hemorrhage. Stroke 2001;32:891–897. [DOI] [PubMed] [Google Scholar]
- 11.Rankin J. Cerebral vascular accidents in patients over the age of 60. II. Prognosis. Scott Med J 1957;2:200–215. [DOI] [PubMed] [Google Scholar]
- 12.Farrell B, Godwin J, Richards S, Warlow C. The United Kingdom transient ischaemic attack (UK-TIA) aspirin trial: final results. J Neurol Neurosurg Psychiatry 1991;54:1044–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mahoney FI, Barthel DW. Functional evaluation: the Barthel Index. Md State Med J 1965;14:61–65. [PubMed] [Google Scholar]
- 14.Leuven E, Sianesi B. PSMATCH2: Stata module to perform full Mahalanobis and propensity score matching, common support graphing, and covariate imbalance testing [online]. 2003. Available at: ideas.repec.org/c/boc/bocode/s432001.html. Accessed September 3, 2017.
- 15.Weiss HJ. Platelets and ischemic heart disease. N Engl J Med 1980;302:225–226. [DOI] [PubMed] [Google Scholar]
- 16.Green A, Pottegard A, Broe A, et al. Initiation and persistence with dual antiplatelet therapy after acute myocardial infarction: a Danish nationwide population-based cohort study. BMJ Open 2016;6:e010880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Willard JE, Lange RA, Hillis LD. The use of aspirin in ischemic heart disease. N Engl J Med 1992;327:175–181. [DOI] [PubMed] [Google Scholar]
- 18.Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med 2001;345:494–502. [DOI] [PubMed] [Google Scholar]
- 19.Sherwood MW, Wiviott SD, Peng SA, et al. Early clopidogrel versus prasugrel use among contemporary STEMI and NSTEMI patients in the US: insights from the National Cardiovascular Data Registry. J Am Heart Assoc 2014;3:e000849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karve S, Levine D, Seiber E, Nahata M, Balkrishnan R. Trends in ambulatory prescribing of antiplatelet therapy among US ischemic stroke patients: 2000–2007. Adv Pharmacol Sci 2012;2012:846163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jernberg T, Johanson P, Held C, Svennblad B, Lindback J, Wallentin L. Association between adoption of evidence-based treatment and survival for patients with ST-elevation myocardial infarction. JAMA 2011;305:1677–1684. [DOI] [PubMed] [Google Scholar]
- 22.Swedish Aspirin Low-Dose Trial (SALT) of 75 mg aspirin as secondary prophylaxis after cerebrovascular ischaemic events. The SALT Collaborative Group. Lancet 1991;338:1345–1349. [PubMed] [Google Scholar]
- 23.Steering Committee of the Physicians' Health Study Research Group. Final report on the aspirin component of the ongoing Physicians' Health Study. N Engl J Med 1989;321:129–135. [DOI] [PubMed] [Google Scholar]
- 24.Roquer J, Rodriguez Campello A, Gomis M, Ois A, Puente V, Munteis E. Previous antiplatelet therapy is an independent predictor of 30-day mortality after spontaneous supratentorial intracerebral hemorrhage. J Neurol 2005;252:412–416. [DOI] [PubMed] [Google Scholar]
- 25.Toyoda K, Okada Y, Minematsu K, et al. Antiplatelet therapy contributes to acute deterioration of intracerebral hemorrhage. Neurology 2005;65:1000–1004. [DOI] [PubMed] [Google Scholar]
- 26.Saloheimo P, Ahonen M, Juvela S, Pyhtinen J, Savolainen ER, Hillbom M. Regular aspirin-use preceding the onset of primary intracerebral hemorrhage is an independent predictor for death. Stroke 2006;37:129–133. [DOI] [PubMed] [Google Scholar]
- 27.Chan KH, Ka-Kit Leung G, Lau KK, et al. Predictive value of the HAS-BLED score for the risk of recurrent intracranial hemorrhage after first spontaneous intracranial hemorrhage. World Neurosurg 2014;82:e219–e223. [DOI] [PubMed] [Google Scholar]
- 28.Bailey RD, Hart RG, Benavente O, Pearce LA. Recurrent brain hemorrhage is more frequent than ischemic stroke after intracranial hemorrhage. Neurology 2001;56:773–777. [DOI] [PubMed] [Google Scholar]
- 29.Vermeer SE, Algra A, Franke CL, Koudstaal PJ, Rinkel GJ. Long-term prognosis after recovery from primary intracerebral hemorrhage. Neurology 2002;59:205–209. [DOI] [PubMed] [Google Scholar]
- 30.Weimar C, Benemann J, Terborg C, Walter U, Weber R, Diener HC. Recurrent stroke after lobar and deep intracerebral hemorrhage: a hospital-based cohort study. Cerebrovasc Dis 2011;32:283–288. [DOI] [PubMed] [Google Scholar]
- 31.Viswanathan A, Rakich SM, Engel C, et al. Antiplatelet use after intracerebral hemorrhage. Neurology 2006;66:206–209. [DOI] [PubMed] [Google Scholar]
- 32.Wang DN, Hou XW, Yang BW, Lin Y, Shi JP, Wang N. Quantity of cerebral microbleeds, antiplatelet therapy, and intracerebral hemorrhage outcomes: a systematic review and meta-analysis. J Stroke Cerebrovasc Dis 2015;24:2728–2737. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data from the ERICH study, including interview, chart abstraction, and imaging findings for cases and controls are now made publicly available both through direct request from the ERICH study principal investigator (D.W. at daniel.woo@uc.edu) or the National Institute of Neurological Disorders and Stroke.