Abstract
The Merkel disc is a main type of tactile end organ consisting of Merkel cells and Aβ-afferent endings that responds to tactile stimulation with slowly adapting type 1 (SA1) afferent impulses. Our recent study has shown that Merkel discs in whisker hair follicles are serotonergic synapses using endogenous serotonin to transmit tactile signals from Merkel cells to Aβ-afferent endings. In the present study, we hypothesize that tactile sensitivity of Merkel discs can be modulated by chemical messengers. We tested this hypothesis by determining whether and how SA1 responses of mouse whisker hair follicles may be affected by exogenously applied chemical messengers. We found that SA1 responses were potentiated by serotonin at low concentration (10 μM) but almost completely occluded by serotonin at high concentration (2 mM). In contrast, SA1 responses were not significantly affected by ATP and its metabolically stable analog α,β-methylene-ATP, glutamate, GABA, and histamine. SA1 responses were also not affected by antagonists for P2X receptors, ionotropic glutamate receptors, and ionotropic GABA and glycine receptors. Whole-cell patch-clamp recordings reconfirm the presence of both ionotropic and metabotropic 5-HT receptors on afferent neurons and their terminals innervating whisker hair follicles. All whisker afferent neurons expressed hyperpolarization-activated inward currents (Ih), which are potentiated by serotonin through the activation of metabotropic 5-HT receptors. Taken together, the findings substantiate the serotonergic mechanism of tactile transmission at Merkel discs and identify the involvement of Ih currents in postsynaptic excitatory actions of serotonin. In addition, the findings do not favor any significant involvement of ATP, glutamate, histamine, GABA, or glycine in tactile transmission at the Merkel discs of whisker hair follicles.
Keywords: Merkel cells, touch, serotonin, 5-HT receptors, whisker hair follicles
Introduction
Tactile end organs, including Merkel discs, Meissner’s corpuscles, Pacinian corpuscles, and Ruffini endings are highly specialized sensory transducers located in the skin of mammals (Johnson, 2001, Zimmerman et al., 2014). They are not only essential for passive reflex responses to touch stimuli but also crucial for active performance of sophisticated sensory tasks such as environmental explorations, social interactions, and tactile discrimination (Johnson, 2001). The Merkel disc is a main type of tactile end organ highly abundant in human fingertips, whisker hair follicles, touch domes and other tactile-sensitive spots throughout mammalian bodies (Woo et al., 2015). They consist of Merkel cells and their associated Aβ-afferent nerve endings to form a disc-shaped complex, and are highly sensitive to skin indentation, pressure, hair movement and other gentle touch stimuli (Iggo and Muir, 1969, Halata et al., 2003, Woo et al., 2015). Merkel discs respond to tactile stimuli by generating slowly adapting type 1 (SA1) impulses on Aβ-afferent fibers, which are essential for fingertips and whisker hair follicles to discriminate the shape, texture, and other physical properties of an object. (Iggo and Muir, 1969, Johnson, 2001).
Cellular and molecular mechanisms underlying the tactile sensory functions of Merkel discs have been a scientific mystery for over a century. Only recently has a mechanotransduction mechanism at Merkel discs been uncovered with the identification of Piezo2 channels as tactile transducers in Merkel cells (Ikeda et al., 2014, Maksimovic et al., 2014, Woo et al., 2014). More recently, we have elucidated that the mechanotransduction in Merkel cells is transmitted synaptically to Aβ-afferent terminals via serotonin to excite Aβ-afferent terminals, evoke SA1 impulses, and lead to tactile behavioral responses (Chang et al., 2016). However, it has long been known that glutamate is a principal neurotransmitter for conveying somatosensory signals (Yoshimura and Jessell, 1990, Basbaum et al., 2009). In addition, ATP has also been indicated to be involved in sensory transmission and modulation (Bardoni et al., 1997, Gu and MacDermott, 1997). Therefore, there is a possibility that chemical messengers including glutamate, ATP, or other endogenous ligands may be co-transmitters with serotonin for tactile signaling at Merkel discs. The co-transmitter hypothesis is also suggested from findings showing the presence of vesicular glutamate transporter 2 in Merkel cells (Haeberle et al., 2004) and the possible presence of the chemical messengers including glutamate, ATP, substance P, enkephalin and others in dense-core vesicles of Merkel cells (Iggo and Muir, 1969, Halata et al., 2003). There is also another hypothesis, rather than being co-transmitters these chemical messengers may have autocrine and/or paracrine functions to modify Merkel disc’s functions in Merkel discs (Tachibana and Nawa, 2002, Halata et al., 2003, Maksimovic et al., 2013).
Our recent study has shown that serotonin released from Merkel cells can mediate tactile transmission by activating both ionotropic and metabotropic 5-HT receptors that are expressed at whisker afferent terminals in Merkel discs (Chang et al., 2016). Activation of ionotropic 5-HT receptors could directly excite whisker Aβ-afferent endings because activation of ionotropic 5-HT receptors (i.e., 5-HT3 receptors) results in cation influx and membrane depolarization. However, it remains unclear how the activation of metabotropic serotonin receptors can excite whisker afferent neurons to contribute to the generation of SA1 impulses in whisker afferent fibers.
In the present study we determined whether SA1 responses in whisker hair follicles were modulated by exogenously applied chemical messengers, and we also explored mechanisms underlying excitatory actions by serotonin following its activation of metabotropic 5-HT receptors in whisker afferent neurons.
Materials and Methods
Animals.
Animal care and use conformed to NIH guidelines for care and use of experimental animals. Experimental protocols were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Alabama at Birmingham. Wild type C57BL/6 mice were obtained from Harlan Laboratories. 5-HT3A-/- mice were transferred from the Rocky Mountain Taste and Smell Center at the University of Colorado Medical School, which were originally obtained from Jackson Laboratory (B6.129X1-Htr3atm1Jul/J; RRID:IMSR_JAX:005251). Male mice at the age of 4–8 weeks were used.
Whisker afferent fiber recordings.
Whisker hair follicle preparations and whisker afferent fiber recordings were performed using our previously described method (Ikeda et al., 2014, Chang et al., 2016). In brief, whisker hair follicles with attached afferent fiber bundles were dissected out and anchored in a recording chamber. The whisker hair follicles were submerged and perfused in oxygenated Krebs solution that contained (in mM): 117 NaCl, 3.5 KCl, 2.5 CaCl2, 1.2 MgCl2, 1.2 NaH2PO4, 25 NaHCO3, and 11 glucose bubbled with 95% O2 and 5% CO2, had pH of 7.3 and osmolarity of 325 mOsm, and was maintained at 24oC. Unless otherwise indicated, the end of the follicle capsule was cut open to facilitate drug diffusion into Merkel disc region of the whisker hair follicle. To record whisker afferent impulses elicited by whisker deflections (SA1 responses) or induced by focal puff-applications of testing chemicals, compound action potentials conducted on whisker afferent fibers were recorded using a suction electrode. Signals were amplified using a Multiclamp 700A amplifier and sampled at 10 KHz with low pass filter set at 1 KHz.
Hair deflection was used as a tactile stimulus to elicit whisker afferent SA1 impulses. In the present study, we anchored the whisker hair follicles in a recording chamber by affixing whisker hair shaft onto the bottom of the recording chamber and perfused them with Krebs solution. A fire-polished blunted glass probe was used for delivering mechanical stimuli. The probe was attached onto the capsule surface at the whisker hair follicle center and controlled by a piezo device. When the mechanical probe displaced the whisker hair follicle, it generated a whisker hair shaft deflection. This modified tactile stimulation method improved consistency of whisker afferent SA1 responses. Unless otherwise indicated, hair deflection was induced by a 38-µm forward step to push the hair follicle for the duration of 2.62 s; the step had a 56-ms ramp at the speed of 0.68 µm/ms (dynamic phase) before reaching the 38 µm step (static phase).
The effects of exogenously applied chemical messengers on SA1 responses were examined. In this set of experiments, after recording SA1 responses evoked by whisker hair deflection in normal bath solution as a control, serotonin (10 μM or 2 mM), ATP (1 mM), α,β-methylene-ATP (100 μM), glutamate (1 mM), GABA (1 mM), or histamine (1 mM) was bath applied for 30 min and SA1 responses were then evoked again by the whisker hair deflection.
Effects of several 5-HT receptor agonists were tested for their ability to directly elicit afferent impulses. In this set of experiments, the capsule of whisker hair follicle was cut to open a small hole at the ring sinus segment of the whisker hair follicle. Under the visual guidance with a 40X objective, a puff-application electrode filled with a testing compound was inserted through the opening into the Merkel disc region underneath the follicle ring sinus. Testing compounds were focally puff-applied to the Merkel disc region with duration of 400 ms and puff pressure of 5 mmHg and their actions to elicit whisker afferent impulses were determined by whisker afferent fiber recordings. Testing compounds include the 5-HT2A receptor agonist TCB-2 (1 mM), the 5-HT2B receptor agonist BW 723C86 (1 mM), and the 5-HT3 receptor agonist SR57227 (1 mM).
Patch-clamp recordings from retrogradely-labeled whisker afferent neurons in whole-mount trigeminal ganglia.
Mice were anesthetized by isoflurane and anesthesia maintained by continuous administration of isoflurane via a nose cone. Whisker hairs were lifted by forceps to expose the top part of the hair follicle. A sharp glass microelectrode with a tip of ~5 µm was filled with 2.5% DiI and then attached to a 25-µm microsyringe. The microsyringe was mounted on the injection holder of a stereotaxic apparatus. The glass microelectrode was inserted vertically along the whisker hair into the top part of the hair follicle. DiI solution (1 µl) was then injected into the hair follicle. 5–10 hair follicles were injected in each mouse.
Seven days after DiI microinjection, animals were sacrificed and trigeminal ganglia (TG) were dissected out. Under a dissection microscope, connective tissues on the surface of TG were removed carefully using a pair of forceps. The TG were then affixed in a recording chamber with a tissue anchor and perfused in normal Krebs solution saturated with 95% O2 and 5% CO2 and at 24oC. The recording chamber was mounted on the stage of an Olympus IX50 microscope equipped with IR-DIC and fluorescent imaging systems. The ganglia were exposed to 0.05% dispase II plus 0.05% collagenase in Krebs solution for 5–10 min, and the enzymes were then washed off with Krebs solution. DiI-labeled cells were identified under the fluorescent microscope.
Whole-cell patch-clamp recordings were performed on randomly selected DiI-labeled neurons. Electrodes for patch-clamp recordings were fabricated from thin-walled capillary glass. The resistance of recording electrodes was ~6 MΩ when filled with internal solution containing (in mM) 105 K-Gluconate, 35 KCl, 2.4 MgCl2, 0.5 CaCl2, 5 EGTA, 10.0 HEPES, 5.0 Na2ATP, 0.33 GTP-Tris salt; pH 7.35 (adjusted with NaOH) and 320 mOsm (adjusted with sucrose). Signals were amplified using an Axopatch 200B amplifier (Axon Instruments) with a low-pass filter set at 2 kHz and digitized at 10 kHz. Junction potential between bath and electrode solution was −13 mV and it was corrected off-line. Unless otherwise stated, the junction potential was corrected for in the data analysis.
After gaining whole-cell access and in voltage-clamp mode, whole-cell currents evoked by serotonin were recorded with neurons being held at −73 mV (voltage command at −60 mV). Serotonin (100 μM) was bath applied for 1 min, and currents were recorded for 10 min. To determine Ih currents, whole-cell currents were evoked by a series of voltage steps ranging from −83 to −163 mV (voltage command −70 mV to −150 mV) in 10 mV increments and at 250 ms duration. Ih currents were recorded before (control) and following the application of serotonin (100 μM) to examine the effects of serotonin on Ih currents.
Data Analysis.
Electrophysiological results were analyzed using Clampfit software. Sample sizes were 6 to 11 hair follicles or cells that were randomly chosen for each experimental group. The sample sizes were calculated using power analysis based on our previous recording data to provide adequate power of more than 80% at p < 0.05 to test null hypotheses. Data are presented as mean ± SEM. Statistical significance was evaluated using Student’s t-test; one-way or two-way ANOVA with Bonferroni post-hoc tests for multiple groups, * p<0.05, ** p<0.01, and *** p<0.001.
Results
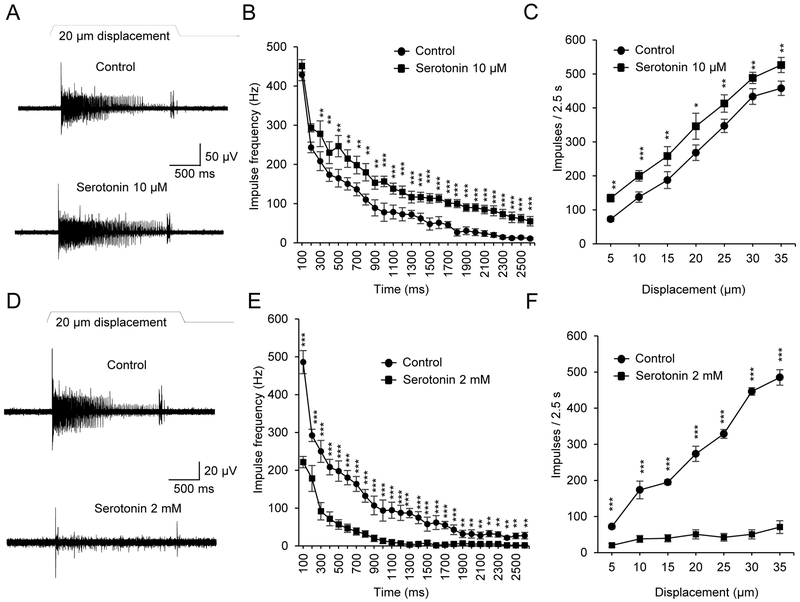
Afferent impulses were recorded by using a suction electrode tightly attached to a whisker afferent fiber bundle of a whisker hair follicle. Slowly adapting type 1 whisker afferent impulses (SA1 responses) were evoked by a 20-μm whisker hair deflection (Figure 1A&B). Under control conditions, in normal bath solution, the SA1 impulses induced by the 20-μm hair deflection showed nearly complete adaptation before the end of hair deflection. In contrast, in the presence of 10 μM serotonin applied through bath solution, SA1 responses were significantly increased at almost all time points and the SA1 impulses continued until the termination of hair deflection (Figure 1A&B). With different magnitudes (5 to 35 μm) of whisker hair displacements, SA1 impulses were consistently potentiated by 10 μM serotonin (Figure 1C). In sharp contrast, the SA1 impulses evoked by the whisker hair deflection were almost completely abolished following bath application of a high concentration of serotonin at 2 mM (Figure 1D&E). The inhibitory effects by the high concentration of serotonin were consistently shown in SA1 impulses elicited by different magnitudes (5 to 35 μm) of whisker hair displacements (Figure 1F).
Figure 1. Effects of exogenously applied serotonin on slowly adapting type 1 responses evoked by whisker hair deflection.
(A) Sample traces show slowly adapting type 1 (SA1) impulses elicited by a 20-µm whisker hair deflection in normal bath solution (control, upper panel) and in the presence of 10 μM serotonin. (B) Summary data of the SA1 frequency at different time points during whisker hair deflection under the following conditions, in normal bath solution before serotonin application (control, circles, n = 6) and following the bath application of 10 μM serotonin (squares, n = 6). (C) Summary data show that bath application of 10 μM serotonin (squares, n = 6) significantly increases SA1 impulses when compared with control (circles, n = 6) at each displacement step. Displacement steps were from 5 to 35 μm at an increment of 5 μm. (D) Sample traces show SA1 impulses elicited by a 20-µm whisker hair deflection in normal bath solution (control, upper panel) and in the presence of 2 mM serotonin (lower panel). (E) Summary data of the SA1 frequency at different time points during whisker hair deflection under the following conditions, in normal bath solution before serotonin application (control, circles, n = 6) and following the bath application of 10 μM serotonin (squares, n = 6). (F) Summary data show that bath application of 2 mM serotonin (squares, n = 6) significantly decreases SA1 impulses when compared with control (circles, n = 6) at each displacement step. Data represent the mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001; two-way ANOVA with Bonferroni post-hoc tests.
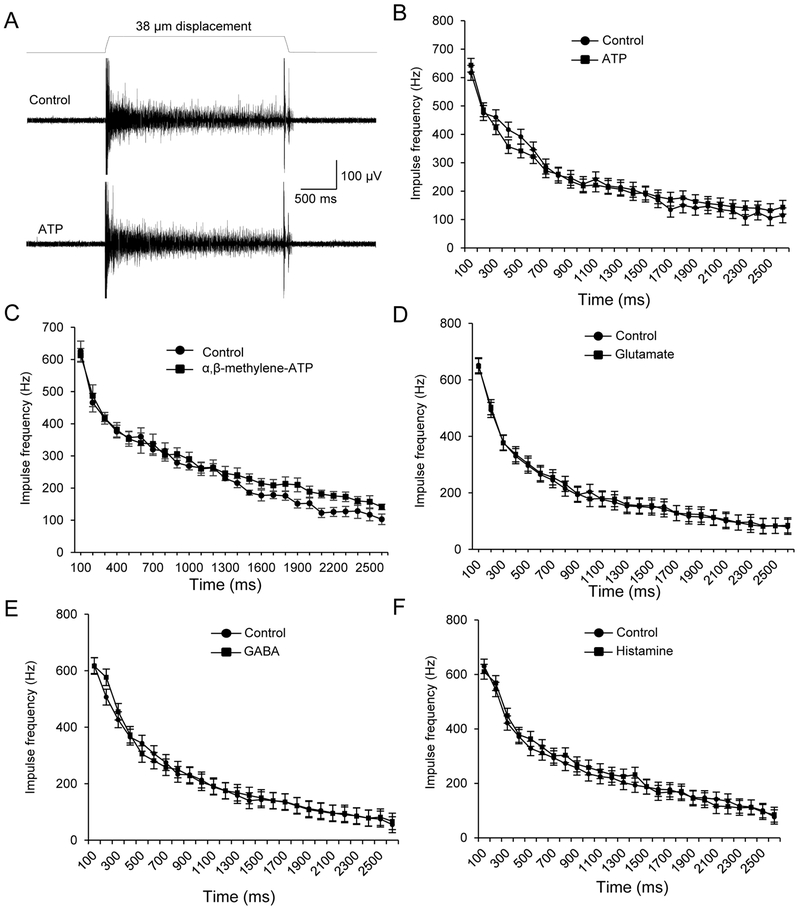
Our previous study (Chang et al., 2016) has failed to show that whisker afferent impulses could be directly elicited by ATP, glutamate and other chemical messengers when they were focally applied to whisker afferent endings. We determined whether some of these chemical messengers may have a modulatory role in tuning SA1 responses that are elicited by tactile stimulation. In this set of experiments, SA1 responses were evoked by the 38-μm hair deflection. Each of these chemical messengers was applied through bath solution. SA1 responses were not found to be significantly different between controls in normal bath solution and following the exogenously applied chemical messengers including ATP, glutamate, BAGA, and histamine (Figure 2A-F). Since ATP might be substantially metabolized in the tissues by ecto-ATPase before it reached Merkel discs, we tested αβ-Me-ATP, a metabolically stable ATP analogue, and also did not observe a significant change of SA1 responses (Figure 2C).
Figure 2. Lack of effects on SA1 responses by ATP, glutamate, GABA and histamine.
(A) Sample traces show SA1 impulses elicited by a 38-µm whisker hair deflection in normal bath solution (control, upper panel) and in the presence of 1 mM ATP (lower panel). (B) Summary data of the SA1 frequency at different time points during whisker hair deflection before (control, circles) and following the bath application of 1 mM ATP (squares). (C-F) Similar to B except 100 µM α,β-methylene-ATP (C), 1 mM glutamate (D), 1 mM GABA (E), or 1 mM histamine (F) was tested (n = 6 in each test). Data represent the mean ± SEM. No significant difference was found in each test, two-way ANOVA with Bonferroni post-hoc tests.
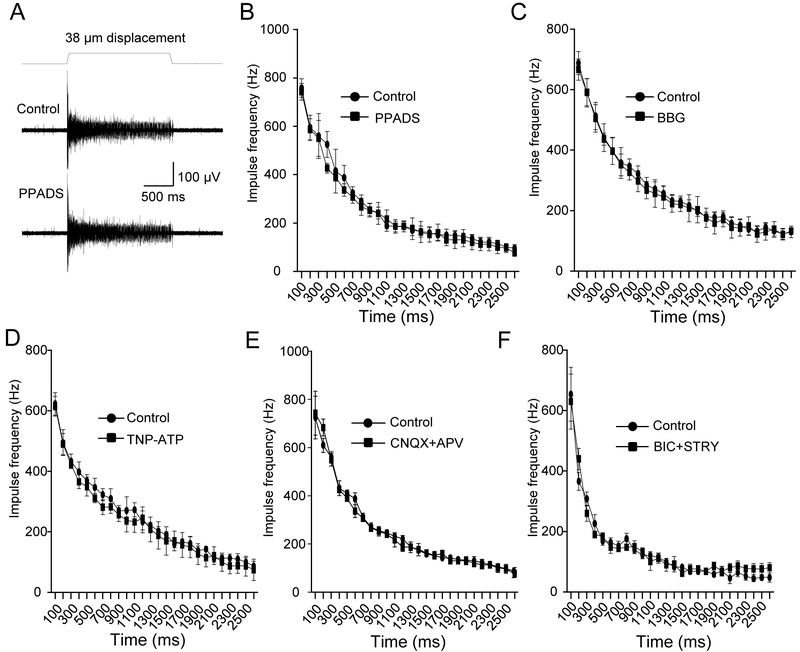
Since whole-mount tissue preparations were used in the present study, there was a possibility that an exogenously applied chemical messenger may not reach the target that is deep inside the tissues due to diffusion barriers, metabolism by enzymes, and/or clearance by re-uptake systems. Therefore, we performed antagonist experiments to validate the results of agonist experiments. Consistent with the lack of effects on SA1 responses by the above chemical messengers applied exogenously, SA1 responses were not significantly affected (Figure 3A-F) by the P2X receptor antagonists pyridoxalphosphate-6-azophenyl-2’,4’-disulfonic acid (PPADS, 100 μM), brilliant blue G (BBG, 100 μM), and 2’,3’-O-(2,4,6-Trinitrophenyl) adenosine-5’-triphosphate (TNP-ATP, 10 μM). The SA1 responses were also not affected by the ionotropic glutamate receptor antagonists 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX, 10 μM) plus D-(−)-2-Amino-5-phosphonopentanoic acid (APV, 50 μM), or by the GABAA and glycine receptor antagonists bicuculline (BIC, 20 μM) plus strychnine (STRY, 50 μM).
Figure 3. Lack of effects on SA1 responses by the antagonists to P2X receptors, ionotropic glutamate receptors, GABAA receptors and glycine receptors.
(A) Sample traces show slowly adapting type 1 (SA1) impulses elicited by a 38-µm whisker hair deflection in normal bath solution (control, upper panel) and in the presence of 100 μM PPADS (lower panel). (B) Summary data of the SA1 frequency at different time points during whisker hair deflection before (control, circles) and following the bath application of 100 μM PPADS (squares). (C-F) Similar to B except 100 μM BBG (C), 10 μM TNP-ATP (D), 10 μM CNQX plus 50 μM APV (E), or 20 μM BIC plus 50 μM STRY (F) was tested (n = 6 in each test). Data represent the mean ± SEM. No significant difference was found in each test, two-way ANOVA with Bonferroni post-hoc tests.
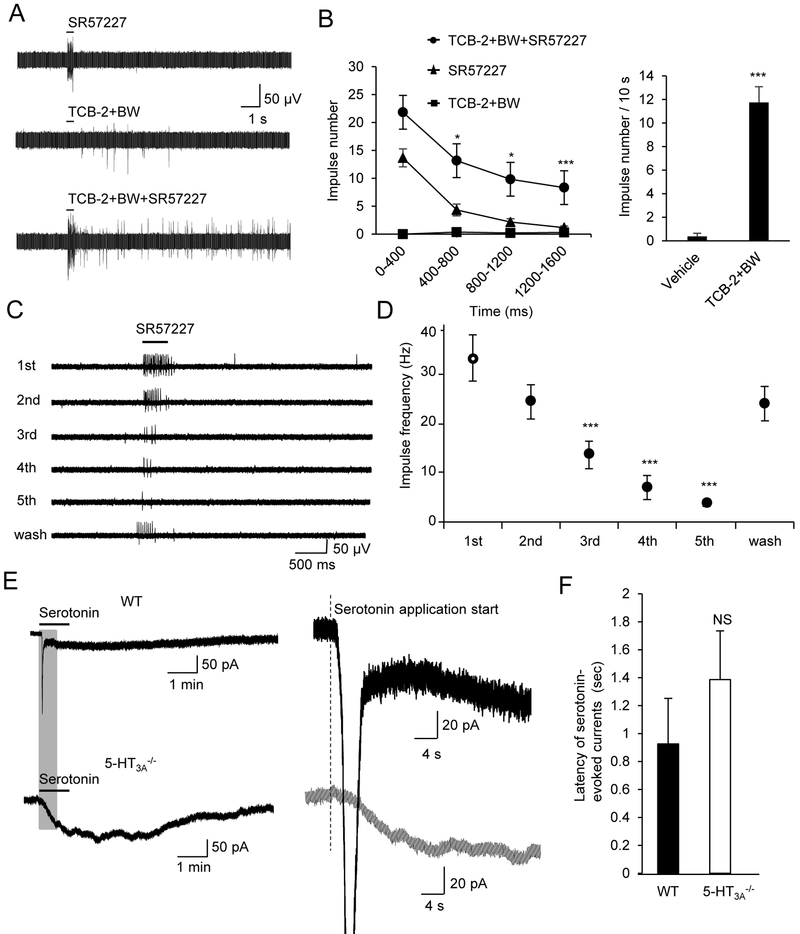
We recorded afferent impulses from whisker afferent fiber bundles following focal puff-application of 5-HT receptor agonists to Merkel Disc regions in whisker hair follicles. Whisker afferent impulses could be evoked by the focal puff-application of the 5-HT3 receptor agonist SR57227 (1 mM) and the responses were immediate but brief (Figure 4A&B). Afferent impulses could also be evoked by the focal pull-application of a mixture of 5-HT2A and 5-HT2B receptor agonists TCB-2 (1 mM) and BW (1 mM), respectively; the responses were very weak and had a delayed onset (Figure 4A&B). Robust responses were evoked when all three agonists were co-applied focally (Figure 4A&B). SR57227-evoked whisker afferent impulse showed strong adaptation when repeatedly puff-applied (Figure 4C&D). No attempt was made to examine the adaptation following the applications of TCB-2 (1 mM) and BW (1 mM) because their effects were very weak.
Figure 4. Direct effects of 5-HT receptor agonists on whisker afferent neurons.
(A) Sample traces of whisker afferent fiber recordings show impulses evoked by focal puff-application of the 5-HT3 receptor agonist SR57227 (1 mM, upper), a mixture of 5-HT2A agonist TCB-2 (1 mM) and the 5-HT2B receptor agonist BW (1 mM, middle), and a mixture of all three agonists (lower). (B) Left panel, summary data of impulse numbers over the initial 1.6 s time period of the experiments illustrated in A. Each data point was impulse numbers in the time bin of 400 ms. Comparison was made between TCB-2+BW+SR57227 group and SR57227 group (n = 8). Right panel, total impulses measured in 10 s following the focal puff-application of vehicle (n = 8) or TCB-2 + BW (n = 8). (C) Sample traces of whisker afferent impulses evoked by repeatedly focal puff-application of 1 mM SR57227. The interval of each application was 5 s. The time for the recovery was 10 min. (D) Summary data of the impulse frequency in the experiments illustrated in C. The response of the first application is the control (n = 7). (E) Sample traces show patch-clamp recordings of inward currents evoked by 100 μM serotonin from whisker afferent neurons in whole-mount trigeminal ganglion preparations. Whisker afferent neurons were retrogradely-labeled by DiI and pre-identified in the patch-clamp recording experiments. Upper left, serotonin-evoked a complexed inward current with a fast and a slow component in a whisker afferent neuron of a wild type mouse (WT). Lower left, serotonin application evokes only a slow inward current in a whisker afferent neuron of a 5-HT3A−/− mouse. Upper right and lower right, expanded time scale at the position highlighted on the traces on left to show the onset of serotonin-evoked currents in neurons of WT and 5-HT3A−/− mice. Vertical dashed line indicates the start time of serotonin application. (F) Summary data of the latency of serotonin-evoked currents in whisker afferent neurons of wild type (closed bar, n = 9). and 5-HT3A−/− (open bar, n = 11) mice. Data represent the mean ± SEM. NS, no significant difference, *P < 0.05, **P < 0.01, ***P < 0.001, two-way or one-way ANOVA with Bonferroni post-hoc tests, or unpaired Student t-test.
Patch-clamp recordings were made from DiI-labeled whisker afferent neurons in whole-mount trigeminal ganglion preparations. Similar to our previous study (Chang et al., 2016), bath application of serotonin (100 μM) evoked a biphasic current with a fast early component and a slow late component in wild type mice (Figure 4E, upper panel), but only a slow current in 5-HT3A−/− mice (Figure 4E, lower panel). Amplitudes of serotonin-evoked currents were analyzed in our previous study (Chang et al., 2016). Here we analyzed the latency of serotonin-evoked currents in whisker afferent neurons of WT mice and 5-HT3A−/− mice. We found that there was a tendency towards longer onset latency of serotonin-evoked currents in whisker afferent neurons of WT mice (n = 9) than that in 5-HT3A−/− mice (n = 11), but the difference was not statistically significant (Figure 4E&F).
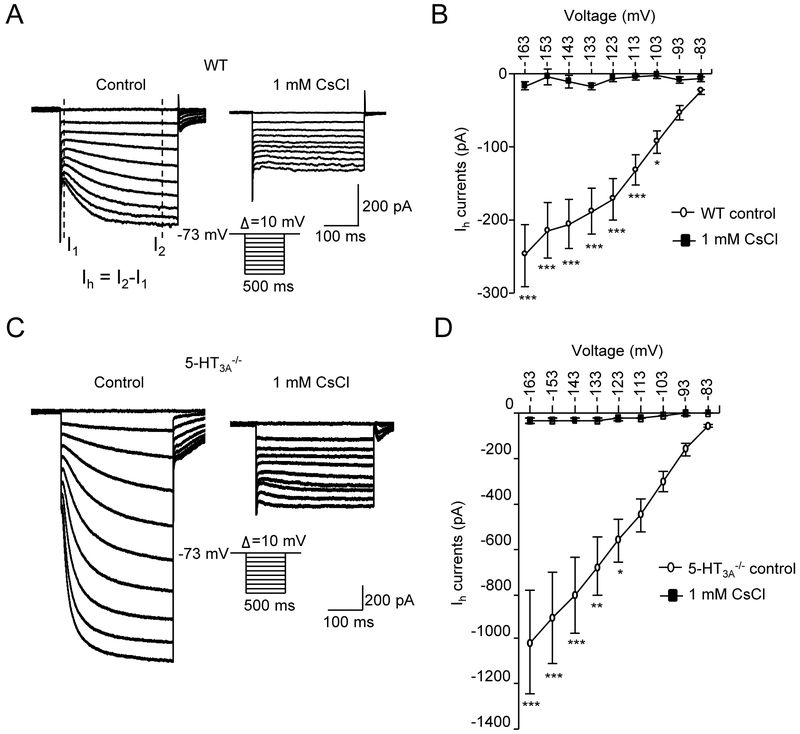
A common feature of the DiI-labeled whisker afferent neurons is the presence of hyperpolarization-activated inward currents (Ih) in all these neurons from both wild type (Figure 5A&B) and 5-HT3A−/− mice (Figure 5C&D). In the presence of extracellular 1 mM CsCl, the Ih of whisker afferent neurons were completely abolished. The inhibitory effect is consistent with the pharmacological profile of hyperpolarization-activated and cyclic nucleotide–gated (HCN) channels which mediate Ih currents (Tang and Trussell, 2015). Interestingly, the amplitude of Ih currents in whisker afferent neurons of 5-HT3A−/− mice (Figure 5C&D) was much higher than that of wild type mice (Figure 5A&B). For examine, at the hyperpolarization step of −113 mV, the amplitude of Ih currents was −452.3 ± 72.1 pA (n = 8) in whisker afferent neurons of 5-HT3A−/− mice, and were only −132.5 ± 20.8 pA (n = 7, P<0.001) in whisker afferent neurons of wide type mice.
Figure 5. Hyperpolarization-activated inward currents (Ih) in whisker afferent neurons of wild type and 5-HT3−/− mice.
(A) Two sets of sample traces show hyperpolarization-activated inward currents (Ih) recorded from a whisker afferent neuron of a wild type mouse in normal bath solution (control, left panel) and in the bath solution containing 1 mM CsCl (right panel). Inset, hyperpolarization voltage steps. Ih current measurement is indicated in the left panel. (B) Summary data of the Ih currents evoked at different hyperpolarization voltages in normal bath solution (open circles) and in the bath solution containing 1 mM CsCl (solid circles) (n = 8). (C&D) Similar to A&B except the recordings were made from whisker afferent neurons of 5-HT3a−/− mice (n = 9). Data represent the mean ± SEM. *P<0.05, **P<0.01, ***P<0.001, two-way ANOVA with Bonferroni post-hoc tests.
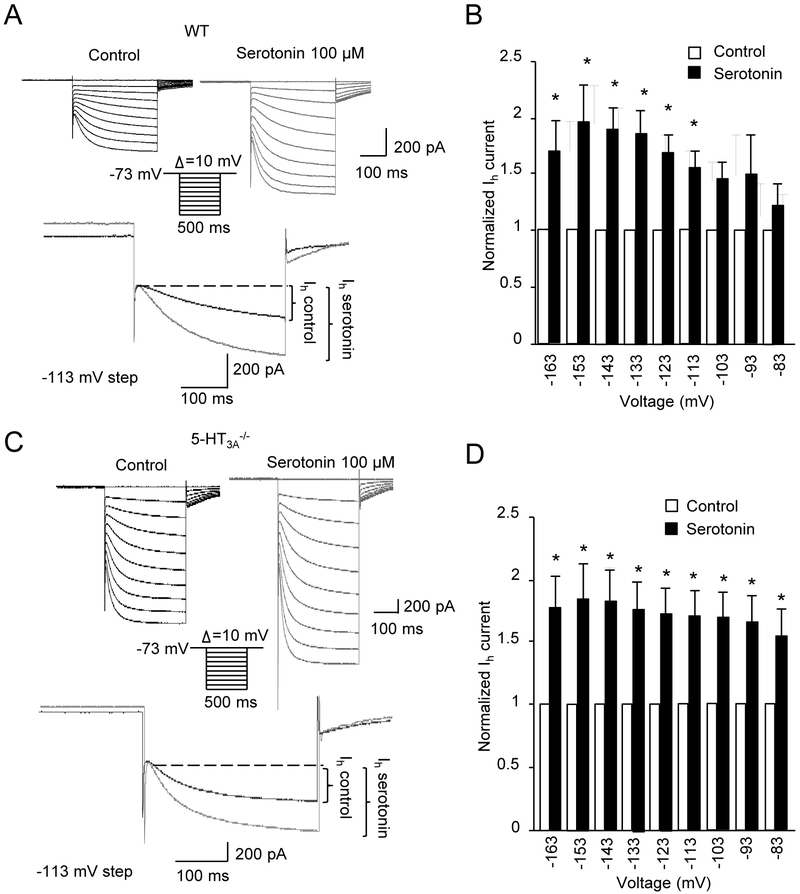
We determined whether serotonin might modulate Ih currents. As shown in Figure 6, Ih currents were increased in whisker afferent neurons following the bath application of serotonin (Figure 6A&B). To determine whether the potentiation of Ih currents was mediated by the activation of ionotropic 5-HT receptors (5-HT3 receptors) or through the activation of metabotropic 5-HT receptors, we examined Ih currents in whisker afferent neurons of 5-HT3A−/− mice (Figure 6C&D). We found that there is still a significant potentiation of Ih currents following serotonin bath application in the whisker afferent neurons of 5-HT3A−/− mice.
Figure 6. Potentiation of Ih currents by serotonin in whisker afferent neurons.
(A) Two sets of sample traces (upper panel) show Ih currents recorded from a whisker afferent neuron of a wild type mouse before (control, left panel) and following the application of 100 μM serotonin (right panel). Inset, hyperpolarization voltage steps. Lower panel at an expanded scale shows Ih currents evoked by the voltage step of −113 mV in control (black trace) and following 100 μM serotonin application (gray trace). (B) Summary data (n=7) of the Ih currents evoked at different hyperpolarization voltages before (open circles) and following the application of 100 μM serotonin (solid circles). (C&D) similar to A&B except the recordings were made from whisker afferent neurons of 5-HT3a−/− mice (n = 7). Data represent the mean ± SEM. *P<0.05, two-way ANOVA with Bonferroni post-hoc tests.
Discussion
In the present study we show that exogenously applied serotonin has profound impact on SA1 impulses evoked by whisker hair deflection. The effect of exogenous serotonin shown here supports the serotonergic transmission at Merkel discs demonstrated in our recent study (Chang et al., 2016). On the other hand, the lack of effects on SA1 impulses by the agonists and/or antagonists to P2X receptors, glutamate receptors, GABA receptors, glycine receptors and histamine receptors does not favor the co-transmitter hypothesis of the aforementioned chemical messengers for Merkel discs. The present study also confirms our recent study (Chang et al., 2016) about the excitatory roles of both ionotropic and metabotropic 5-HT receptors in whisker afferent neurons. Moreover, we show that the activation of metabotropic 5-HT receptors in whisker afferent neurons potentiates the hyperpolarization-activated inward current (Ih).
In our study serotonin at low concentration potentiates SA1 responses and at high concentration almost completely abolished SA1 responses. The potentiation effect of serotonin at low concentration is most likely due to an additive effect of exogenous and endogenous serotonin on whisker afferent terminals. In contrast, prolonged application of a high concentration of exogenous serotonin could result in 5-HT receptor desensitization, which would lead to the failure of 5-HT receptor activation by endogenously released serotonin during tactile stimulation. Strong desensitization of 5-HT3 receptors indeed occurred in our study following its repeated activation. In addition to 5-HT3 receptors, previous studies have shown that metabotropic 5-HT receptors including 5-HT2A and 5-HT2B receptors are susceptible to agonist-induced desensitization (Porter et al., 2001, Hanley and Hensler, 2002). Consistent with the desensitization of both ionotropic and metabotropic 5-HT receptors, our results show that high concentration of serotonin suppressed both early and late phases of SA1 responses. This occlusion effect by a high concentration of a chemical messenger (serotonin in the present case) is a pharmacological approach to validate a transmitter. In our experiments serotonin at 10 μM (low concentration) and 2 mM (high concentration) were used to show potentiation and suppression of SA1 responses, respectively. It should be noted that the actual concentrations of serotonin may be much lower at Merkel disc sites because they are deep inside tissues in our whisker hair follicle preparations, and diffusion barriers as well as serotonin uptake by tissues may greatly reduce serotonin concentrations around Merkel discs.
The present study does not favor ATP, glutamate, GABA, glycine, or histamine for being a co-transmitter at Merkel discs of whisker hair follicles. Among them, ATP and glutamate were previously thought to be strong candidates of transmitters mediating tactile transmission at Merkel discs. Studies have shown the presence of vesicular glutamate transporters in Merkel cells (Maksimovic et al., 2013) and expression of P2X receptors in whisker afferent neurons (Ikeda and Gu, 2016). Furthermore, we have recently shown that 5-HT receptors and P2X receptors are co-expressed in the majority of large-sized whisker afferent neurons of the rats (Ikeda and Gu, 2016). The lack of effects on SA1 responses by glutamate, ATP and other tested candidates may suggest that their receptors are not present at the postsynaptic sites in Aβ-afferent terminals of Merkel discs. Alternatively, some of these chemical messengers may modulate Merkel disc functions in a slow time scale beyond our present experimental paradigm.
In the present study a novel finding about the whisker afferent neuron is that all whisker afferent neurons tested show large Ih currents. Ih currents are mediated by hyperpolarization-activated and cyclic nucleotide–gated (HCN) channels and involved in neuronal excitability. We have shown that the Ih currents in whisker afferent neurons can be significantly potentiated by serotonin. Previous studies in thalamic and cerebellar neurons have also found that Ih currents could be modulated by serotonin, which results in the changes of membrane potentials and action potential firing patterns (McCormick and Pape, 1990, Williams et al., 2002). Our finding that Ih currents became significantly enhanced in whisker afferent neurons of 5-HT3A−/− mice is likely to be a molecular compensation for the loss of 5-HT3 receptors. Compensatory changes in Ih currents have been shown in GABAα5−/− mice (Bonin et al., 2013). The involvement of HCN channels in serotonergic functions at Merkel discs is demonstrated by our finding that Ih currents in whisker afferent neurons were significantly potentiated by serotonin via metabotropic 5-HT receptors. The potentiation of Ih currents via metabotropic 5-HT receptor activation has been shown to be an underlying mechanism of serotonin-evoked slow currents in the neurons of the dorsal cochlear nucleus, which is also found to be the underlying mechanism of the prolonged neuronal excitation induced by serotonin (Tang and Trussell, 2015). Metabotropic 5-HT receptors in whisker afferent neurons were shown to be 5-HT2A and 5-HT2B receptors based on the pharmacological results in our previous work and also shown in the present study (Chang et al., 2016). These receptors are known to couple to Gq-proteins to activate phospholipase C, resulting in the generation of inositol-1,4,5-trisphophate and diacylglycerol (Hoyer et al., 2002). Inositol-1,4,5-trisphophate can release Ca2+ from intracellular Ca2+ stores and a previous study has shown that an increase of Ca2+ could enhance Ih currents (Luthi and McCormick, 1999). HCN channel gating is dependent on cyclic nucleotides, including cAMP. However, activation of 5-HT2A and 5-HT2B receptors will not increase cAMP production since adenylyl cyclase is not linked with Gq-proteins (Garnovskaya et al., 1995). Therefore, the potentiation of Ih currents in our study probably is not mediated by cAMP signaling pathway. In addition to the above signaling pathways, Src tyrosine kinase may be involved in the potentiation of Ih currents (Tang and Trussell, 2015).
In conclusion, the present study provide further evidence to consolidate the idea that Merkel discs are serotonergic synapses for tactile transmission in the whisker hair follicles. The study also identifies the excitatory role of HCN channels in mediating serotonin actions on whisker afferent neurons, which reveals the complexity of the epidermal serotonergic synapses in Merkel discs.
ACKNOWLWDGWMWNTS:
We thank Drs. S Kinnamon and E Larson for transferring 5-HT3A−/− to us from the Rocky Mountain Taste and Smell Center, an NIDCD-funded P30 core facility (P30DC004657 to Diego Restrepo) at the University of Colorado. This work was supported by NIH grants DE018661 and DE023090 to J.G.G.
Abbreviations:
- SA1 response
slowly adapting type 1 response
- 5-HT
5-hydroxytryptamine
- ATP
Adenosine triphosphate
- GABA
γ-Aminobutyric acid
- TCB-2
4-Bromo-3,6-dimethoxybenzocyclobuten-1-yl)methylamine hydrobromide
- BW 723C86
α-methyl-5-(2-thienylmethoxy)-1H-indole-3-ethanamine hydrochloride
- SR57227
1-(6-Chloro-2-pyridinyl)-4-piperidinamine hydrochloride
- DiI
(2Z)-2-[(E)-3-(3,3-dimethyl-1-octadecylindol-1-ium-2-yl)prop-2-enylidene]-3,3-dimethyl-1-octadecylindole
- PPADS
pyridoxalphosphate-6-azophenyl-2’,4’-disulfonic acid
- BBG
brilliant blue G
- (TNP-ATP
2’,3’-O-(2,4,6-Trinitrophenyl) adenosine-5’-triphosphate
- CNQX
antagonists 6-cyano-7-nitroquinoxaline-2,3-dione
- APV
D-(−)-2-Amino-5-phosphonopentanoic acid
- BIC
bicuculline
- STRY
strychnine
Footnotes
Author Contributions: W.C. performed electrophysiological recordings of tactile responses. H.K. performed patch-clamp recordings from DiI-labeled whisker afferent neurons. R.I. and J.L. were involved in preliminary experiments. J.G. designed the study and wrote the manuscript. All authors were involved in data collection and analysis, result discussion, and commented on the manuscript.
COMPETINGNG FINANCNCIAL INTERESTS: The authors declare no competing financial interests.
REFERENCES
- Bardoni R, Goldstein PA, Lee CJ, Gu JG, MacDermott AB (1997) ATP P2X receptors mediate fast synaptic transmission in the dorsal horn of the rat spinal cord. J Neurosci 17:5297–5304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basbaum AI, Bautista DM, Scherrer G, Julius D (2009) Cellular and molecular mechanisms of pain. Cell 139:267–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonin RP, Zurek AA, Yu J, Bayliss DA, Orser BA (2013) Hyperpolarization-activated current (In) is reduced in hippocampal neurons from Gabra5−/− mice. PLoS One 8:e58679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang W, Kanda H, Ikeda R, Ling J, DeBerry JJ, Gu JG (2016) Merkel disc is a serotonergic synapse in the epidermis for transmitting tactile signals in mammals. Proc Natl Acad Sci U S A 113:E5491–5500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnovskaya MN, Nebigil CG, Arthur JM, Spurney RF, Raymond JR (1995) 5-Hydroxytryptamine2A receptors expressed in rat renal mesangial cells inhibit cyclic AMP accumulation. Mol Pharmacol 48:230–237. [PubMed] [Google Scholar]
- Gu JG, MacDermott AB (1997) Activation of ATP P2X receptors elicits glutamate release from sensory neuron synapses. Nature 389:749–753. [DOI] [PubMed] [Google Scholar]
- Haeberle H, Fujiwara M, Chuang J, Medina MM, Panditrao MV, Bechstedt S, Howard J, Lumpkin EA (2004) Molecular profiling reveals synaptic release machinery in Merkel cells. Proc Natl Acad Sci U S A 101:14503–14508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halata Z, Grim M, Bauman KI (2003) Friedrich Sigmund Merkel and his “Merkel cell”, morphology, development, and physiology: review and new results. Anat Rec A Discov Mol Cell Evol Biol 271:225–239. [DOI] [PubMed] [Google Scholar]
- Hanley NR, Hensler JG (2002) Mechanisms of ligand-induced desensitization of the 5-hydroxytryptamine(2A) receptor. J Pharmacol Exp Ther 300:468–477. [DOI] [PubMed] [Google Scholar]
- Hoyer D, Hannon JP, Martin GR (2002) Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol Biochem Behav 71:533–554. [DOI] [PubMed] [Google Scholar]
- Iggo A, Muir AR (1969) The structure and function of a slowly adapting touch corpuscle in hairy skin. J Physiol 200:763–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda R, Cha M, Ling J, Jia Z, Coyle D, Gu JG (2014) Merkel cells transduce and encode tactile stimuli to drive Abeta-afferent impulses. Cell 157:664–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda R, Gu JG (2016) Electrophysiological property and chemical sensitivity of primary afferent neurons that innervate rat whisker hair follicles Molecular Pain (In Press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KO (2001) The roles and functions of cutaneous mechanoreceptors. Curr Opin Neurobiol 11:455–461. [DOI] [PubMed] [Google Scholar]
- Luthi A, McCormick DA (1999) Modulation of a pacemaker current through Ca(2+)-induced stimulation of cAMP production. Nat Neurosci 2:634–641. [DOI] [PubMed] [Google Scholar]
- Maksimovic S, Baba Y, Lumpkin EA (2013) Neurotransmitters and synaptic components in the Merkel cell-neurite complex, a gentle-touch receptor. Ann N Y Acad Sci 1279:13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maksimovic S, Nakatani M, Baba Y, Nelson AM, Marshall KL, Wellnitz SA, Firozi P, Woo SH, Ranade S, Patapoutian A, Lumpkin EA (2014) Epidermal Merkel cells are mechanosensory cells that tune mammalian touch receptors. Nature 509:617–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA, Pape HC (1990) Noradrenergic and serotonergic modulation of a hyperpolarization-activated cation current in thalamic relay neurones. J Physiol 431:319–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter RH, Malcolm CS, Allen NH, Lamb H, Revell DF, Sheardown MJ (2001) Agonist-induced functional desensitization of recombinant human 5-HT2 receptors expressed in CHO-K1 cells. Biochem Pharmacol 62:431–438. [DOI] [PubMed] [Google Scholar]
- Tachibana T, Nawa T (2002) Recent progress in studies on Merkel cell biology. Anat Sci Int 77:26–33. [DOI] [PubMed] [Google Scholar]
- Tang ZQ, Trussell LO (2015) Serotonergic regulation of excitability of principal cells of the dorsal cochlear nucleus. J Neurosci 35:4540–4551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SR, Christensen SR, Stuart GJ, Hausser M (2002) Membrane potential bistability is controlled by the hyperpolarization-activated current I(H) in rat cerebellar Purkinje neurons in vitro. J Physiol 539:469–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo SH, Lumpkin EA, Patapoutian A (2015) Merkel cells and neurons keep in touch. Trends Cell Biol 25:74–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo SH, Ranade S, Weyer AD, Dubin AE, Baba Y, Qiu Z, Petrus M, Miyamoto T, Reddy K, Lumpkin EA, Stucky CL, Patapoutian A (2014) Piezo2 is required for Merkel-cell mechanotransduction. Nature 509:622–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura M, Jessell T (1990) Amino acid-mediated EPSPs at primary afferent synapses with substantia gelatinosa neurones in the rat spinal cord. J Physiol 430:315–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman A, Bai L, Ginty DD (2014) The gentle touch receptors of mammalian skin. Science 346:950–954. [DOI] [PMC free article] [PubMed] [Google Scholar]