Abstract
Background
Heart failure (HF) with preserved ejection fraction (HFpEF) is increasingly common clinically, now rivaling or exceeding HF with reduced EF. Sudden death is the leading mode of exodus in patients with HFpEF, but the underlying causes are largely unknown. Using ambulatory recordings in a rat model, we test the hypothesis that ventricular arrhythmias (VA) underlie sudden death in HFpEF.
Methods
Dahl salt-sensitive rats (7 weeks of age) were fed a high-salt diet to induce HFpEF (n=13) or a normal-salt diet (controls, n=9). Transthoracic echocardiography was performed to check systolic and diastolic function at 14-18 weeks of age. Telemetric electrocardiographic recordings were analyzed for QT interval duration, burden of premature ventricular contractions, spontaneous VA and heart rate variability (HRV). Survival was monitored twice daily.
Results
High-salt fed rats with clear diastolic dysfunction, preserved ejection fraction and HF signs were diagnosed with HFpEF at 14-15 weeks of age. QT and QTc intervals were prolonged in HFpEF rats compared to controls. HRV was reduced in HFpEF rats compared to controls. Spontaneous VA were more prevalent in HFpEF rats (6/13=46.1% vs. 0/9=0% in controls, p<0.05) and sudden death was observed in 4/13 HFpEF rats. Three of the 4 sudden deaths were associated with VA as the terminal rhythm.
Conclusions
In this rat model with phenotypically-verified HFpEF, sudden death was common, and generally associated with VA. Further clinical studies are warranted to determine whether these insights translate to sudden death in HFpEF patients.
Keywords: heart failure, ventricular arrhythmia, sudden death, heart rate variability, repolarization
Journal Subject Terms: Arrhythmias, Sudden Cardiac Death, Animal Models of Human Disease, Basic Science Research
Approximately 50% of patients with heart failure (HF) have preserved ejection fraction (EF; HFpEF), a proportion which continues to increase.1 Patients with HFpEF have a poor prognosis with a median survival of ~2 years and 5-year mortality of ~75%.2 HFpEF is often described as “single largest unmet need in cardiovascular medicine” largely due to the complex phenotypes and the enigmatic nature of disease pathogenesis.3 Multiple associated comorbidities (hypertension, obesity, diabetes mellitus, old age and renal dysfunction) and clinical phenotypes (diastolic dysfunction, pulmonary hypertension, atrial fibrillation and chronotropic incompetence) have complicated the understanding of HFpEF.4 Unlike HF with reduced ejection fraction (HFrEF), where multiple therapeutic agents have been shown to improve survival, all drugs or devices tested rigorously to date have failed in patients with HFpEF.5, 6 Cardiovascular death accounts for ~60% of all deaths, and sudden death is the leading mode of exodus, comprising 25% of all mortality in HFpEF patients.6–8 Although cardiac arrhythmias often underlie sudden death mechanistically, there is no known link between arrhythmias and sudden death in HFpEF.9 Relevant clinical data are lacking, perhaps because patients with HFpEF rarely have implanted devices which record heart rate. We thus turned to the Dahl salt-sensitive (DSS) rat model of HFpEF to investigate sudden death. This model develops delayed repolarization, easily-inducible ventricular arrhythmias (VA), and premature death.10
METHODS
The data, analytic methods, and study materials will be/have been made available to other researchers for purposes of reproducing the results or replicating the procedure.
Rat Model of HFpEF
All animal experiments were approved by the Cedars-Sinai Institutional Animal Care and Use Committee. Fig. 1A depicts the implementation of the DSS rat model of HFpEF.11–13 In brief, 7-week-old male DSS rats (Charles River Laboratories, MA) were randomly assigned to a high-salt (HS) diet (AIN-76A + 8% NaCl with irradiation, Research Diets, NJ) to induce HFpEF (n=13), or to a normal-salt (NS) diet (AIN-76A [0.3 % NaCl] with irradiation) to serve as controls (n=9). Transthoracic echocardiography was performed to measure systolic and diastolic function between 14 and 18 weeks of age. HS-fed DSS rats showed signs of HF from 14-15 weeks of age, which were scored as follows: appearance (0: normal, 1: mild distress, 2: severe distress), breathing (0: normal, 1: mild tachypnea, 2: labored breathing), mobility (0: normal, 1: decreased activity, 2: severely diminished), edema (0: normal, 1: mild edema, 2: severe edema), and weight (0: > 360g, 1: 300g < < 360g, 2: < 300g) (Fig. 1E). HS-fed rats with echo-verified diastolic dysfunction, normal EF and signs of HF were diagnosed with HFpEF and used for experiments. HS rats which failed to meet these criteria (~10 % due to absence of diastolic dysfunction or reduced EF) were excluded and omitted from analysis.
Figure 1. Experimental protocol and development of HFpEF.
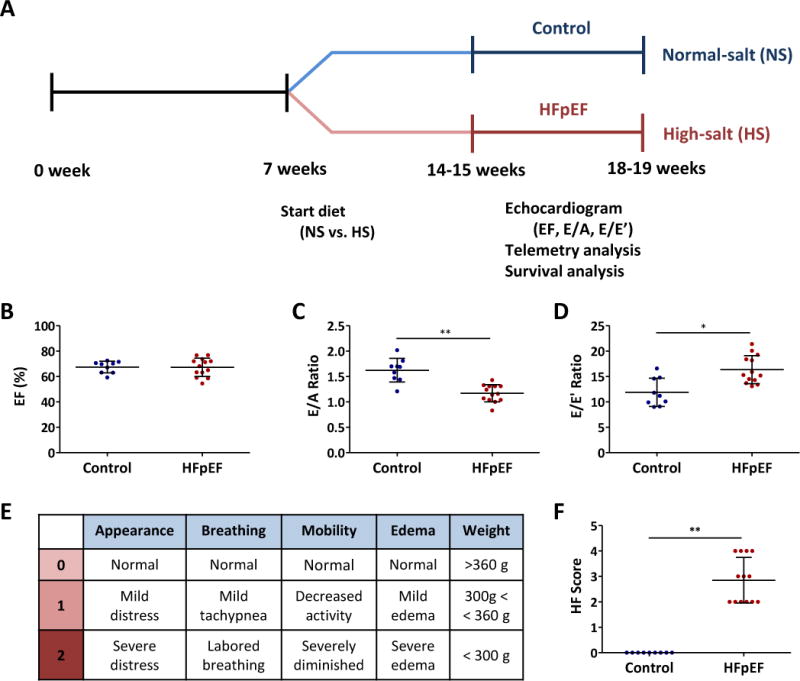
A. DSS rats were fed a HS or NS diet from 7 weeks of age. Echocardiography was performed to assess EF, E/A ratio and E/E’ ratio by 14-15 weeks of age. Telemetry devices were implanted to echo-proven HFpEF and control rats. Survival was monitored to identify sudden death. B. EF was preserved in HFpEF rats as well as in controls. C. E/A ratio was reduced in HFpEF rats compared to controls. D. E/E’ ratio was increased in HFpEF rats compared to controls. E. HF score was calculated from the following parameters; appearance, breathing, mobility, edema and weight. F. The HF score was increased in HFpEF rats compared to controls.
Transthoracic Echocardiogram
Rats were anesthetized with 2% isoflurane for transthoracic echocardiography (Vevo 770, VisualSonics, Toronto, Canada). Systolic function was measured with EF from images obtained in the parasternal short axis view as described.14 Diastolic function was evaluated from the apical four chamber views by measuring E/A and E/E’ ratios.11–14 E and A waves were measured with pulse wave Doppler mode between the tips of the mitral valve.11–14 E’ and A’ waves were measured with tissue Doppler mode at the septal corner of the mitral annulus.14 Three separate measurements from each animal were averaged.
Telemetry Device Implantation
Control and HFpEF rats were induced with 5% and maintained with 2% isoflurane for intraperitoneal telemetry device (CTA-F40, Data Sciences International, St. Paul, MN) implantation via an abdominal incision. The two device electrodes were sutured subcutaneously in a lead II configuration (negative electrode: right upper chest, positive electrode: left lower abdomen). Telemetry data were analyzed using Ponemah software (Data Sciences International). Premature ventricular contractions (PVC) were checked every 10 minutes and averaged for hourly PVC burden and 24-hour PVC burden. PVC were graded per Lown’s grading system (0: no PVC, 1: occasional PVC (<30 per hour), 2: frequent PVC (> 30 per hour), 3: multiform PVC, 4: repetitive PVC, 5: R-on-T PVC). ECG parameters (QT interval, QTc interval, RR interval, P wave, PR interval and QRS width) were measured three times and averaged. Heart rate was measured every 10 minutes over 24 hours to calculate standard deviation of average NN interval (SDANN). Continuous recording of NN interval for 5 minutes was used to check time and frequency domains of heart rate variability (HRV), such as standard deviation of NN interval (SDNN), SD1/SD2 from Poincare plots and fast Fourier transform analysis of low frequency (LF, 0.2 to 0.8 Hz) and high frequency (HF, 0.8 to 2.5 Hz). VA were defined as either ventricular tachycardia (VT, more than three consecutive ventricular beats, > 600 bpm) or ventricular fibrillation. Sustained VA were defined as lasting more than 30 seconds while non-sustained less than 30 seconds.
Survival Analysis
Survival was monitored from device implantation through study end-point (up to 26 weeks). Comparative medicine staff monitored rats twice a day and either recorded unexpected deaths or flagged very sick animals for prompt euthanasia. Common reasons were weakness (inability to reach water and chow), corporal edema and seizure. Sudden death was defined as death occurring within 24 hours of last being reported as well in unwitnessed cases.
Statistical Analysis
Continuous variables are shown as mean ± standard deviation (SD) and categorical variables are presented as numbers (percentages). Normal distribution was assessed using Kolmogorov-Smirnov test and homogeneity of variance was tested by Levene’s test. Student’s t-test was used for the comparison of variables with normal distribution, while Mann-Whitney test was used for the comparisons of non-normally distributed variables. Fisher’s exact test was used to compare categorical variables. Kaplan-Meier analysis was used to check survival with Log-rank test. Two-tailed p value was used to assess statistical significance (* denotes p value <0.05 and ** denotes p value < 0.001). SPSS v24. software was used for statistical analysis.
RESULTS
Development of HFpEF
HS-fed DSS rats showed diastolic dysfunction as evidenced by decreased E/A ratio (1.17±0.17 vs. 1.62±0.23 in controls, p<0.001, Fig. 1C, Supplemental Fig. 1B) and increased E/E’ ratio (16.4±2.8 vs. 11.9±2.8 in controls, p<0.05, Fig. 1D, Supplemental Fig. 1C) from echocardiography at 14-15 weeks of age. EF was preserved in both experimental groups (67±7 vs. 67±5 % in controls, p=0.94, Fig. 1B, Supplemental Fig. 1A). To assess severity of HF, we implemented a HF score (0~10) that was calculated from the following parameters: appearance, breathing, mobility, edema and weight (Fig. 1E). The HF score was increased in HS-fed rats (2.8 vs. 0 in controls, p<0.001, Fig. 1F). Echo-proven HS-fed rats with at least one sign of HF were diagnosed with HFpEF.
Increased PVC Burden
Rats with HFpEF appeared to have more frequent PVCs, and PVCs which were more complex, than did control rats, but the differences were not statistically significant (Supplemental Fig. 1D-K).
Delayed Repolarization
Delayed repolarization was evident in HFpEF rats (Fig. 2A): the QT interval was prolonged to 105±3 ms from 83±2 ms in controls (p<0.001, Fig. 2B). QTc interval (calculated as QT interval divided by square root of RR interval) was also prolonged (248±7 vs. 199±5 ms in controls, p<0.001, Fig 2C). RR interval was not different between the two groups nor were P wave, PR interval or QRS width (Fig. 2D-G).
Figure 2. Delayed repolarization in HFpEF rats.
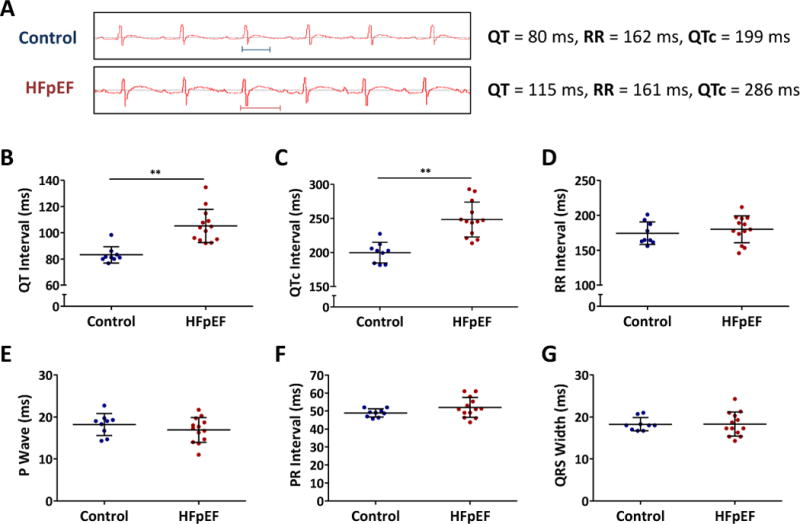
A. Representative ECG showing QT intervals in control and HFpEF rats. B. QT interval was prolonged in HFpEF rats compared to controls. C. QTc interval showed similar trend. D. RR interval was similar in both groups. E, F, G. P wave, PR interval and QRS width were unchanged in both control and HFpEF rats.
Decreased Heart Rate Variability
Averaged heart rate was measured in control and HFpEF rats over 24-hour period (Supplemental Fig. 2A). SDNN was reduced in HFpEF rats (5.4±1.9 vs. 9.0±1.9 in controls, p<0.001, Supplemental Fig. 2B). SDANN was also decreased in HFpEF rats (16.7±1.1 vs. 22.4±1.1 in controls, p<0.05, Supplemental Fig. 2C). SD1 and SD2 from Poincare plots (Supplemental Fig. 2D) were reduced in HFpEF rats (SD1 2.3±0.7 vs. 3.6±1.4 ms in controls, p <0.05, Supplemental Fig. 2E and SD2 7.3±2.7 vs. 12.2±12.8 ms in controls, p<0.001, Supplemental Fig. 2F). Fast Fourier transform of frequency domain (Supplemental Fig. 2G) also showed decreased HRV, evidenced by decreased LF and HF powers (LF 67.5±11.7 vs. 112.9±12.4 ms2 in controls, p<0.05, Supplemental Fig. 2H and HF 73.0±11.1 vs. 132.2±6.7 ms2 in controls, p<0.05, Supplemental Fig. 2I).
Spontaneous Ventricular Arrhythmias
Spontaneous VA were noted during telemetry in HFpEF rats but not in controls (Fig. 3A). In total, 6 HFpEF rats developed spontaneous VA (6/13=46.1 vs. 0/9=0% in controls, p<0.05, Fig. 3B). The six HFpEF rats were between 17-19 weeks of age. Electrolytes (Na, K and Ca), pH from arterial blood and creatinine (Cr) were all within normal limits in HFpEF rats (Supplemental Fig. 2J-N). Half of them (3/6=50%) were non-sustained VA (< 30 seconds) while the rest lasted >30 seconds (Fig. 3C). Three of 6 rats developed monomorphic VT (all non-sustained) while the other 3 showed polymorphic VT (all sustained) that degenerated into ventricular fibrillation.
Figure 3. Spontaneous development of VA in HFpEF rats.
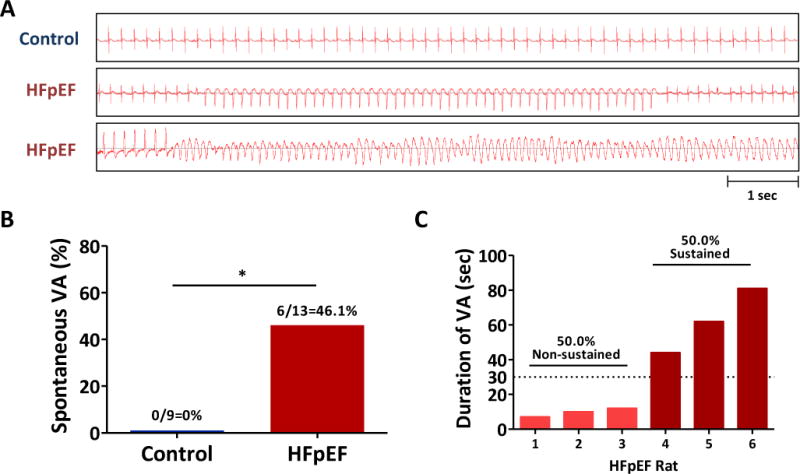
A. Representative tracings of telemetry devices in control and HFpEF rats. B. Increased incidence of spontaneous VA in HFpEF rats compared to controls. C. Proportion of non-sustained and sustained VA in HFpEF rats.
Sudden Death
Control and HFpEF rats were monitored until 26 weeks of age. While there was not a single death in controls, all HFpEF rats died by 26 weeks of age (Log-Rank <0.001, Fig. 4A). Reasons of mortality were either sudden death or mandated euthanasia due to weakness (inability to reach water and chow), severe corporal edema or seizure activity (Fig. 4B). Sudden death occurred in 4 of 13 HFpEF rats. Telemetry at the time of death showed VA as the cause in 3/4 cases (Fig. 4C, Supplemental Fig. 3A-B). The duration of VA preceding sudden death were 44, 62 and 81 seconds. One sudden death case showed prolonged ST depressions followed by bradycardic death (Fig. 4D); although this pattern looks ischemic, coronary disease is not a known feature of the DSS model. Further comparison between 9 non-sudden death rats and 4 sudden death rats did not reveal any significant differences in terms of HF score, QTc prolongation and parameters of HRV. Such an analysis would require a large number of animals (Supplemental Fig. 4A-F). Terminal HFpEF rats at the time of euthanasia maintained preserved EF along with normal electrolytes, oxygenation and kidney function (Supplemental Fig. 4G-L).
Figure 4. Sudden death of HFpEF rats associated with VA.
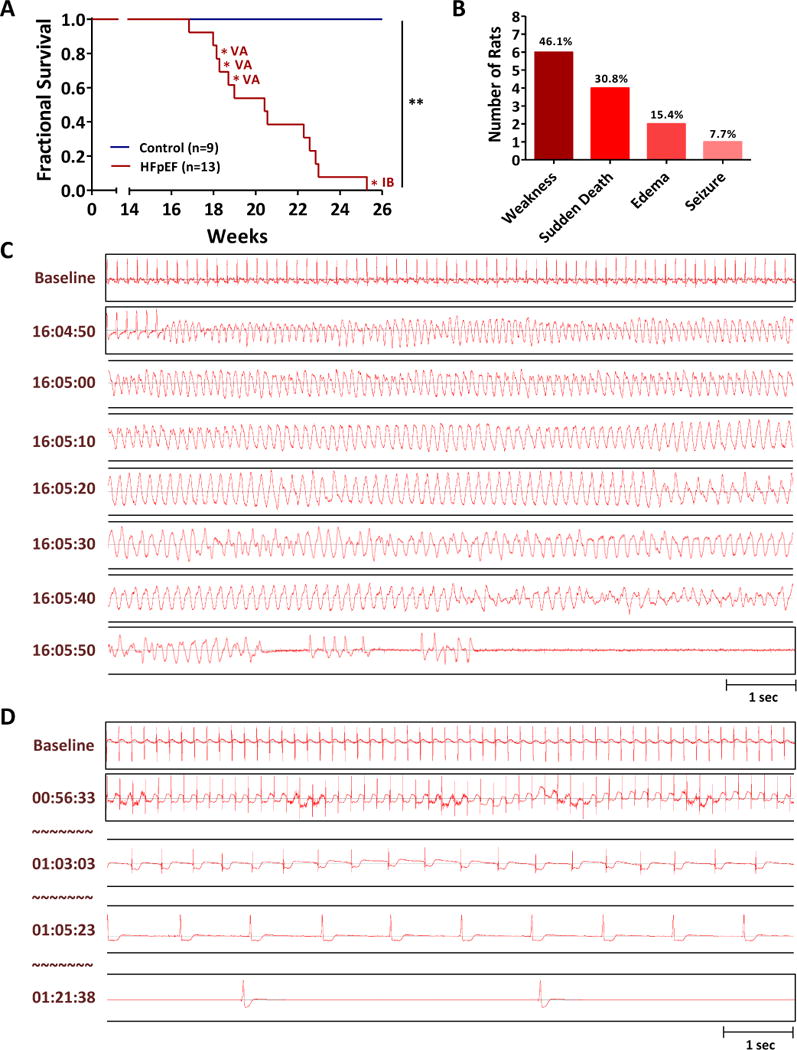
A. Survival analysis of control and HFpEF rats until 26 weeks of age. VA: ventricular arrhythmias, IB: ischemia followed by bradycardia B. Sudden death occurred in 4 of 13 HFpEF rats. C. Representative tracing of sudden death in a HFpEF rat showed VA as a cause of death. D. One sudden death case showed prolonged ischemia followed by bradycardic death.
DISCUSSION
Sudden death is the most common mode of exodus in HFpEF, however the etiology of sudden death in this patient population is not well-understood. Here we have tested the hypothesis that VA precipitates sudden death in HFpEF rats. Ambulatory recordings revealed delayed repolarization (QTc prolongation) and spontaneous VA in HFpEF rats but not in controls. Approximately 30% of HFpEF rats died suddenly, and 75% of ambulatory recordings during sudden death revealed underlying VA. HRV was reduced in HFpEF rats, which is a well-known predictor of arrhythmic death in heart failure.15 Our translational experiments support the hypothesis that VA precipitates sudden death in DSS rats with HFpEF.
Relative to HFrEF, HFpEF has historically received far less attention from either clinicians or basic scientists. Meanwhile, HFpEF has risen epidemically to comprise about half of all HF cases.1, 16. In HFrEF, ~45% of deaths are sudden and unexpected although this percentage is trending down due to advances in medical and device therapy.17 Cardiac arrhythmias are responsible for most sudden death cases in HFrEF,18, 19 but this conjecture is speculative in HFpEF due to the absence of relevant studies. Approximately 5% of HFpEF patients die annually, and sudden death is the most common mode of death.6, 7 In the I-Preserve trial, 60% of deaths were cardiovascular in HFpEF patients, with the following breakdown (as percent of all deaths): 26% sudden, heart failure (14%), cerebrovascular accident (9%) and myocardial infarction (5%).7 In the TOPCAT trial, 63.9% of all deaths in HFpEF patients were cardiovascular.6 Sudden death was the most common mode of death accounting for 24.3%, followed by heart failure (12.7%), myocardial infarction (6.3%) and stroke (4.8%).
While sudden deaths are mostly cardiac and often assumed to have been caused by lethal arrhythmias,19 the cause in any given case is unknown unless heart rhythm is fortuitously recorded. Given that lethal arrhythmias are responsible for most sudden deaths, ventricular tachycardia or fibrillation will be to blame in most cases, especially in the case of structural cardiac abnormality such as HF.9, 19 Although some studies suggest that pulseless electrical activity or asystole are more common than ventricular tachycardia or fibrillation in the case of cardiac arrest, as in the general population or in-hospital cardiac arrest, this may not be true in HF patients.20 Our study is the first to implicate VA as causal of sudden death in HFpEF.
A variety of hypotheses have been suggested to explain the increased incidence of VA and sudden death in HF. These include prolonged repolarization, abnormal calcium homeostasis, increased fibrosis, decreased conduction, autonomic nervous system imbalance and altered neurohumoral signaling.19, 21–23 Our recent pre-clinical study demonstrated delayed repolarization underlying high VA inducibility in the same rat model of HFpEF.10 Action potentials were prolonged and multiple re-entry circuits were responsible for an increased tendency to VA in HFpEF rats. Increased myocardial fibrosis further promoted multiple re-entry circuits. Although our previous study generated proposed mechanisms of VA in HFpEF, we did not record spontaneous VA, nor did we verify that VA actually causes sudden death. By implanting telemetry devices, we have shown here that HFpEF rats not only develop spontaneous VA but they also die suddenly due to spontaneous VA. Systematic rhythm monitoring in HFpEF populations will be required to determine if our findings have any clinical correlates.
HRV has long been investigated as an independent predictor of sudden death in heart failure.24 Our data are consistent with previous clinical reports that decreased HRV predicts sudden death in heart failure.15 Although HRV was reduced in HFpEF rats, it did not correlate with individual sudden death risk, perhaps due to the low number of animals studied.
Study Limitations
Our experimental study has several limitations. First, this model has been critiqued as being an admixture of HFpEF and HFrEF.25 Here we have phenotypically verified the diagnosis of HFpEF in all animals enrolled, removing the potential uncertainty. Additionally, we have shown evidence of preserved EF when these rats are euthanized due to weakness or seizures. Although we have not measured EF of sudden death rats (due to the sudden nature), in view of our previous observation that these HFpEF rats maintain preserved EF, our observations provide valuable translational insights into the mechanisms of sudden death in HFpEF. Second, even if HFpEF has been well-validated, this rat model might not truly capture the pathology of human HFpEF. Patients with HFpEF often have multiple comorbidities such as obesity, diabetes, coronary artery disease, chronic kidney disease, atrial arrhythmias and old age. While it will be very difficult or almost impossible to induce multiple comorbidities, we believe our model reproduces many important clinical features typical of human HFpEF: hypertension, hypertrophy, diastolic dysfunction, delayed repolarization, increased mortality and sudden death. Third, sudden death in rodents might not reflect the features of sudden death in humans. Currently there is no study investigating the causes of sudden death in human HFpEF. The hypothesis that VA commonly underlie sudden death can only be tested systematically in humans by using dedicated ambulatory recordings; the present findings have potential value in motivating such studies in HFpEF patients. Fourth, although HRV is a useful predictor of sudden death in HFrEF,15 it is not being clinically utilized to screen HFpEF patients. Further investigations are needed to test whether HRV might be useful in predicting sudden death in HFpEF patients.
Supplementary Material
What is Known?
Sudden death is the leading mode of death (~25%) in patients with heart failure with preserved ejection fraction (HFpEF).
The underlying mechanisms of sudden death in HFpEF are largely unknown.
What the Study Adds?
In a rodent model of HFpEF, sudden death was common and generally associated with ventricular arrhythmias.
HFpEF rats showed increased burden of premature ventricular contractions and reduced heart rate variability.
Acknowledgments
We thank Catherine Bresee, MS for the statistical analyses.
SOURCES OF FUNDING: This research was supported by NIH T32 HL116273, and Department of Defense grant PR150620. General laboratory support was provided by the Cedars-Sinai Board of Governors Heart Stem Cell Center, NIH R01 HL124074 and HL135866. EM is the Mark S. Siegel Family Distinguished Professor of the Cedars-Sinai Medical Center.
Footnotes
DISCLOSURES: None
References
- 1.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–9. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 2.Shah KS, Xu H, Matsouaka RA, Bhatt DL, Heidenreich PA, Hernandez AF, Devore AD, Yancy CW, Fonarow GC. Heart Failure With Preserved, Borderline, and Reduced Ejection Fraction: 5-Year Outcomes. J Am Coll Cardiol. 2017 doi: 10.1016/j.jacc.2017.08.074. [DOI] [PubMed] [Google Scholar]
- 3.Lewis GA, Schelbert EB, Williams SG, Cunnington C, Ahmed F, McDonagh TA, Miller CA. Biological Phenotypes of Heart Failure With Preserved Ejection Fraction. J Am Coll Cardiol. 2017;70:2186–2200. doi: 10.1016/j.jacc.2017.09.006. [DOI] [PubMed] [Google Scholar]
- 4.Shah SJ, Kitzman DW, Borlaug BA, van Heerebeek L, Zile MR, Kass DA, Paulus WJ. Phenotype-Specific Treatment of Heart Failure With Preserved Ejection Fraction: A Multiorgan Roadmap. Circulation. 2016;134:73–90. doi: 10.1161/CIRCULATIONAHA.116.021884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Massie BM, Carson PE, McMurray JJ, Komajda M, McKelvie R, Zile MR, Anderson S, Donovan M, Iverson E, Staiger C, Ptaszynska A, Investigators IP Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med. 2008;359:2456–67. doi: 10.1056/NEJMoa0805450. [DOI] [PubMed] [Google Scholar]
- 6.Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, Harty B, Heitner JF, Kenwood CT, Lewis EF, O’Meara E, Probstfield JL, Shaburishvili T, Shah SJ, Solomon SD, Sweitzer NK, Yang S, McKinlay SM, Investigators T Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;370:1383–92. doi: 10.1056/NEJMoa1313731. [DOI] [PubMed] [Google Scholar]
- 7.Zile MR, Gaasch WH, Anand IS, Haass M, Little WC, Miller AB, Lopez-Sendon J, Teerlink JR, White M, McMurray JJ, Komajda M, McKelvie R, Ptaszynska A, Hetzel SJ, Massie BM, Carson PE, Investigators IP Mode of death in patients with heart failure and a preserved ejection fraction: results from the Irbesartan in Heart Failure With Preserved Ejection Fraction Study (I-Preserve) trial. Circulation. 2010;121:1393–405. doi: 10.1161/CIRCULATIONAHA.109.909614. [DOI] [PubMed] [Google Scholar]
- 8.Vaduganathan M, Patel RB, Michel A, Shah SJ, Senni M, Gheorghiade M, Butler J. Mode of Death in Heart Failure With Preserved Ejection Fraction. J Am Coll Cardiol. 2017;69:556–569. doi: 10.1016/j.jacc.2016.10.078. [DOI] [PubMed] [Google Scholar]
- 9.Zipes DP, Wellens HJ. Sudden cardiac death. Circulation. 1998;98:2334–51. doi: 10.1161/01.cir.98.21.2334. [DOI] [PubMed] [Google Scholar]
- 10.Cho JH, Zhang R, Kilfoil PJ, Gallet R, de Couto G, Bresee C, Goldhaber JI, Marban E, Cingolani E. Delayed Repolarization Underlies Ventricular Arrhythmias in Rats with Heart Failure and Preserved Ejection Fraction. Circulation. 2017;136:2037–2050. doi: 10.1161/CIRCULATIONAHA.117.028202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gallet R, de Couto G, Simsolo E, Valle J, Sun B, Liu W, Tseliou E, Zile MR, Marban E. Cardiosphere-derived cells reverse heart failure with preserved ejection fraction (HFpEF) in rats by decreasing fibrosis and inflammation. JACC Basic Transl Sci. 2016;1:14–28. doi: 10.1016/j.jacbts.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doi R, Masuyama T, Yamamoto K, Doi Y, Mano T, Sakata Y, Ono K, Kuzuya T, Hirota S, Koyama T, Miwa T, Hori M. Development of different phenotypes of hypertensive heart failure: systolic versus diastolic failure in Dahl salt-sensitive rats. J Hypertens. 2000;18:111–20. doi: 10.1097/00004872-200018010-00016. [DOI] [PubMed] [Google Scholar]
- 13.Klotz S, Hay I, Zhang G, Maurer M, Wang J, Burkhoff D. Development of heart failure in chronic hypertensive Dahl rats: focus on heart failure with preserved ejection fraction. Hypertension. 2006;47:901–11. doi: 10.1161/01.HYP.0000215579.81408.8e. [DOI] [PubMed] [Google Scholar]
- 14.Liu J, Rigel DF. Echocardiographic examination in rats and mice. Methods Mol Biol. 2009;573:139–55. doi: 10.1007/978-1-60761-247-6_8. [DOI] [PubMed] [Google Scholar]
- 15.La Rovere MT, Pinna GD, Maestri R, Mortara A, Capomolla S, Febo O, Ferrari R, Franchini M, Gnemmi M, Opasich C, Riccardi PG, Traversi E, Cobelli F. Short-term heart rate variability strongly predicts sudden cardiac death in chronic heart failure patients. Circulation. 2003;107:565–70. doi: 10.1161/01.cir.0000047275.25795.17. [DOI] [PubMed] [Google Scholar]
- 16.Dunlay SM, Roger VL, Redfield MM. Epidemiology of heart failure with preserved ejection fraction. Nat Rev Cardiol. 2017;14:591–602. doi: 10.1038/nrcardio.2017.65. [DOI] [PubMed] [Google Scholar]
- 17.Shen L, Jhund PS, Petrie MC, Claggett BL, Barlera S, Cleland JGF, Dargie HJ, Granger CB, Kjekshus J, Kober L, Latini R, Maggioni AP, Packer M, Pitt B, Solomon SD, Swedberg K, Tavazzi L, Wikstrand J, Zannad F, Zile MR, McMurray JJV. Declining Risk of Sudden Death in Heart Failure. N Engl J Med. 2017;377:41–51. doi: 10.1056/NEJMoa1609758. [DOI] [PubMed] [Google Scholar]
- 18.Tomaselli GF, Marban E. Electrophysiological remodeling in hypertrophy and heart failure. Cardiovasc Res. 1999;42:270–83. doi: 10.1016/s0008-6363(99)00017-6. [DOI] [PubMed] [Google Scholar]
- 19.Tomaselli GF, Zipes DP. What causes sudden death in heart failure? Circ Res. 2004;95:754–63. doi: 10.1161/01.RES.0000145047.14691.db. [DOI] [PubMed] [Google Scholar]
- 20.Girotra S, Nallamothu BK, Spertus JA, Li Y, Krumholz HM, Chan PS, American Heart Association Get with the Guidelines-Resuscitation I Trends in survival after in-hospital cardiac arrest. N Engl J Med. 2012;367:1912–20. doi: 10.1056/NEJMoa1109148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sharma K, Kass DA. Heart failure with preserved ejection fraction: mechanisms, clinical features, and therapies. Circ Res. 2014;115:79–96. doi: 10.1161/CIRCRESAHA.115.302922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kamei K, Maehara K, Kimura J, Ishibashi T, Maruyama Y. Comprehensive analyses of arrhythmogenic substrates and vulnerability to ventricular tachycardia in left ventricular hypertrophy in salt-sensitive hypertensive rats. Circ J. 2007;71:390–6. doi: 10.1253/circj.71.390. [DOI] [PubMed] [Google Scholar]
- 23.Annoni EM, Xie X, Lee SW, Libbus I, KenKnight BH, Osborn JW, Tolkacheva EG. Intermittent electrical stimulation of the right cervical vagus nerve in salt-sensitive hypertensive rats: effects on blood pressure, arrhythmias, and ventricular electrophysiology. Physiol Rep. 2015;3 doi: 10.14814/phy2.12476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation. 1996;93:1043–65. [PubMed] [Google Scholar]
- 25.Valero-Munoz M, Backman W, Sam F. Murine Models of Heart Failure with Preserved Ejection Fraction: a “Fishing Expedition”. JACC Basic Transl Sci. 2017;2:770–789. doi: 10.1016/j.jacbts.2017.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


