Abstract
Opioid-induced respiratory depression (OIRD) involves decreased sensitivity of ventilatory control systems to decreased blood levels of oxygen (hypoxia) and elevated levels of carbon dioxide (hypercapnia). Understanding the sites and mechanisms by which opioids elicit respiratory depression is pivotal for finding novel therapeutics to prevent and/or reverse OIRD. To examine the contribution of carotid body chemoreceptors OIRD, we used whole-body plethysmography to evaluate hypoxic (HVR) and hypercapnic (HCVR) ventilatory responses including changes in frequency of breathing, tidal volume, minute ventilation and inspiratory drive, after intravenous injection of morphine (10 mg/kg) in sham-operated (SHAM) and in bilateral carotid sinus nerve transected (CSNX) Sprague-Dawley rats. In SHAM rats, morphine produced sustained respiratory depression (e.g., decreases in tidal volume, minute ventilation and inspiratory drive) and reduced the HVR and HCVR responses. Unexpectedly, morphine-induced suppression of HVR and HCVR were substantially greater in CSNX rats than in SHAM rats. This suggests that morphine did not compromise the function of the carotid body-chemoafferent complex and indeed, that the carotid body acts to defend against morphine-induced respiratory depression. These data are the first in vivo evidence that carotid body chemoreceptor afferents defend against rather than participate in OIRD in conscious rats. As such, drugs that stimulate ventilation by targeting primary glomus cells and/or chemoafferent terminals in the carotid bodies may help to alleviate OIRD.
Keywords: morphine, respiratory depression, carotid body, chemoafferents, conscious rats
1. Introduction
The use of opioids to manage acute and chronic pain is limited by opioid-induced respiratory depression (OIRD) in operative and perioperative settings (Dahan et al., 2010). Morphine binds with relatively high affinity to μ-opioid receptors (μ-ORs) and with lesser affinity to δ-ORs and k-ORs (Chen et al., 1991; Christiansen and Reiff, 1991; Frances et al., 1990, 1992). Analgesic doses of morphine depress ventilation in humans by central and peripheral actions (Cepeda et al., 2003; Cashman and Dolin, 2004; Taylor et al., 2005) via activation of μ-ORs although co-activation of δ- or κ-ORs modulates μ-OR responses (Dahan et al., 2010; Trescott et al., 2008). In animals, opioids depress ventilation via central and peripheral mechanisms including (a) centrally-mediated depression of ventilatory drive, (b) chest-wall muscle rigidity, (c) increases in airways resistance, and (d) an increase pulmonary vascular resistance, which decreases gas-exchange (see Henderson et al., 2013, 2014; Shook et al., 1990).
Systemic morphine blunts hypoxic (HVR), hypercapnic (HCVR) and hypoxic-hypercapnic ventilatory responses in conscious rats (Emry et al., 2016; May et al., 2013a,b; Murphy et al., 1995) probably by actions in the brain since μ-ORs are expressed in numerous nuclei involved in respiratory control such as the nucleus tractus solitarius (NTS) (see Zhang et al., 2007). Moreover, microinjections of the μ-OR agonist, DAMGO, into medullary raphe regions of anesthetized rats blunt HVR whereas microinjections of DAMGO into the commissural NTS suppress HVR and HCVR (Zhang et al., 2007, 2009, 2011). The ability of morphine to depress HVR (Berkenbosch et al., 1997; Dahan et al., 1988; Sarton et al., 1999) and HCVR (Sarton et al., 1999) in humans, suggests that opioids and/or metabolites of opioids (Peat et al., 1991) depress carotid body (CB) function and central mechanisms responsive to these challenges (Dahan et al., 1998; Sarton et al., 1999). The possibility that opioids suppress HVR and HCVR via actions in CBs has been examined by intracarotid administration of the δ-OR agonist methionine-enkephalin (ME), and morphine on chemoreceptor activity in the carotid sinus nerve (CSN) of anaesthetized cats (McQueen and Ribeiro, 1980). ME profoundly inhibited chemoreceptor discharge whereas morphine was minimally active with lower doses increasing discharge (McQueen and Ribeiro, 1980). Chemoexcitation evoked by intracarotid injections of C02-saturated solutions were reduced by ME but were augmented by morphine. Kirby and McQueen (1986) confirmed that the rank order of opioid-induced depression of chemosensory discharge in cats was compatible with involvement of δ-ORs rather than μ-ORs. In contrast, Zimpfer et al (1983) found that morphine blunted hemodynamic responses elicited by chemoafferent stimulation in conscious dogs although whether this involved CBs or brain were not determined. The effects of opioids on resting CB and/or chemoafferent discharge or responses to hypoxic and/or hypercapnic challenges have not been examined in rats.
Despite the recent progress in understanding the mechanisms of OIRD (see Dahan et al., 2010; Boom et al., 2013), the role of CB chemoreceptors in these deleterious effects of morphine in vivo are unknown. To address this issue, we examined the effects of morphine (10 mg/kg, i.v.) on ventilatory responses elicited by a hypoxic (HX) or hypercapnic (HC) gas challenges in conscious Sprague-Dawley rats with prior sham-operation (SHAM) or bilateral CSN transection (CSNX) to disrupt chemoafferent input to the NTS. Our novel findings that morphine-induced suppression of ventilatory responses to HX and HC is exacerbated in CSNX rats provide the first in vivo evidence that CB chemoreceptors defend against, rather than participate in, the ventilatory depressant effects of morphine.
2. Materials and methods
2.1. Animals and surgeries
All studies were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 80–23) revised in 1996. The protocols were approved by the Institutional Animal Care and Use Committee at Galleon Pharmaceuticals, Inc (Philadelphia, PA). Thirty adult male Sprague Dawley rats (275–300 g) obtained from Harlan Laboratories (Indianapolis, IN) were used for this study. All rats were anesthetized with isoflurane (2.5%) and placed on a surgical station allowing body temperature to be maintained at 37°C via a thermal blanket (Harvard Apparatus, Holliston, MA). The adequacy of the anesthesia was regularly checked by nociceptive stimulus (toe pinch). The left jugular vein of all animals was catheterized (PE-50; Instech Solomon, Plymouth Meeting, PA) for delivery of morphine and exteriorized to the back of the neck. Bilateral CSNX was then performed in 15 rats while another 15 rats were sham-operated (SHAM). In CSNX rats, both CSNs were denervated at the point where they entered the glossopharyngeal nerve (Palmer et al., 2013; Gaston et al., 2014). In SHAM rats, the nerves were identified but not cut. The rats were allowed 7 days to recover from surgery. On the day of study, the catheters were flushed with normal saline (Hospira, Inc., Lake Forest, Illinois) at least 4h before starting the study. All studies were done in a quiet laboratory with relative humidity of 50 ± 2% and room temperature of 21.2 ± 0.2 °C.
2.2. Ventilatory parameters
Ventilatory parameters were continuously recorded in unrestrained freely-moving rats via a whole-body 12-chamber plethysmography system (PLY 3223; BUXCO Inc., Wilmington, NC, USA), as detailed previously (Henderson et al., 2013, 2014; May et al., 2013a,b) and as diagrammed in Fig. 1. Parameters were frequency of breathing (fR), tidal volume (Vt), minute ventilation (Ve), inspiratory time (Ti), and Vt/Ti, an index of inspiratory drive (Young et al., 2013; Lafierriere et al., 2005). The software constantly corrected digitized values for changes in chamber temperature and humidity and a rejection algorithm excluded motion-induced artifacts (Getsy et al., 2014).
Fig. 1.
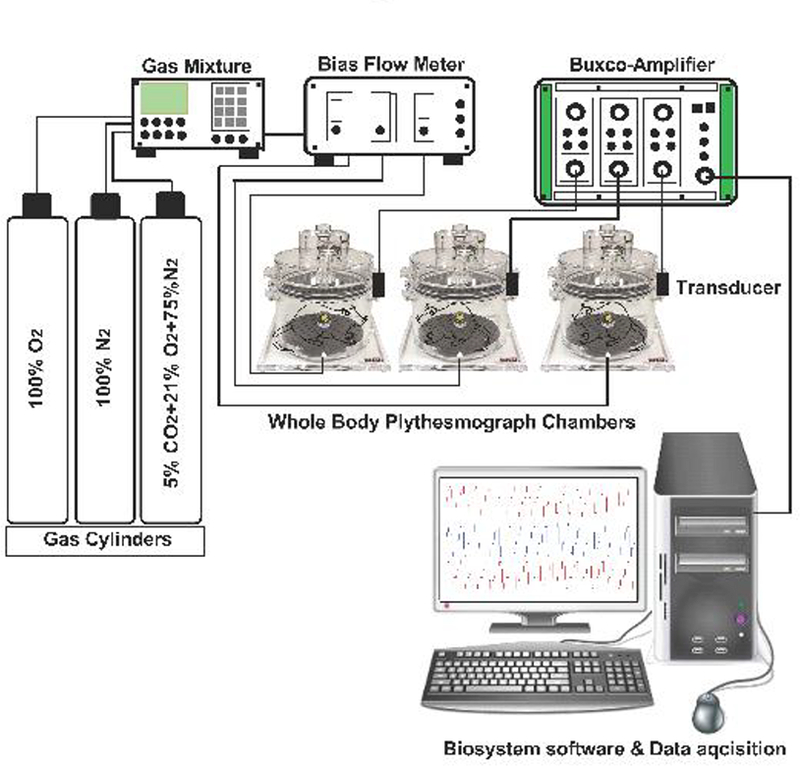
Schematic of Whole-body plethysmograph setup. Sham-operated (SHAM) and bilateral carotid sinus nerve transected (CNSX) rats were gradually acclimatized to the whole-body plethysmography chamber with a bias chamber air flow. A bias chamber air flow of at least 2 L/min was generated by connecting the chambers to a constant flow vacuum source. However, during normoxia, hypoxia and hypercapnia protocols, a bias flow of room air (PO2 of 21%), hypoxic (PO2 of 10%) and hypercapnic (PCO2 of 5%) gases were generated using a gas mixture. Gas mixture receives inflow from 100% N2, 100% O2 and 10% CO2 cylinders. Gas flow to each plethysmograph chamber (2 L/min) was controlled using flow meters. A respiratory waveform was generated from the expansion and contraction of the air that was exchanged between the rat and the chamber. The cyclic change in air volume during the respiratory cycle elicited oscillating airflow across a calibrated pneumotach in the wall of the plethysmograph chamber. Each pneumotach was calibrated (5.0 mL volume delivered in triplicate) on each study day prior to placing the rats in the chambers. At least 1 hour was permitted for rats to acclimate to the chamber before data collection began. Respiratory waveforms were amplified with Buxco-Amplifiers and captured continuously and stored using FinePointe software later data analysis. Barometric pressure, chamber temperature, chamber partial pressure of water, and body temperature were used to calculate a corrected tidal volume. The first three variables were assumed to be constant throughout the study at 740 mmHg, 25 °C, and 23.7 mmHg, respectively. Minute ventilation was calculated as the product of tidal volume and respiratory frequency.
2.3. Protocols
Rats were placed into the plethysmograph chambers and allowed to acclimatize for at least 60 min. Upon development of stable baseline recordings, the rats received a bolus injection of saline or morphine (10 mg/kg, i.v.). After 15 min, all rats were then subjected to a HX (10% O2, 90% N2) or to a HC (5% CO2, 21% O2, 74% N2) gas challenge of 20 min in duration. At this time, room-air was reintroduced to the chambers and the ventilatory parameters recorded for a further 15 min. The dose of morphine was chosen because it elicits a sustained decrease in Ve in conscious rats (May et al., 2013b; Young et al., 2013).
2.4. Data analysis
Data was continuously recorded and ventilatory parameters were averaged into 1 min time periods for statistical analyses and graphing. Morphine-induced respiratory depression in SHAM and CSNX rats were calculated as percentage change from pre-morphine baseline values. The post-morphine period was used to calculate percentage changes in ventilatory parameters elicited by the HX and HC challenges and upon return to room-air. All values are expressed as mean ± SEM. The data was analyzed by one-way or two-way repeated measures analyses of variance followed by Bonferroni corrections for multiple comparisons between means (May et al., 2013a,b).
3. Results
3.1. Ventilatory responses during the hypoxic and hypercapnic challenges - morphine studies
Prior to injection of morphine, all recorded ventilatory parameters in CSNX rats were similar to those of SHAM rats in both the hypoxia and hypercapnia studies (Table 1).
Table 1.
Changes in baseline parameters elicited by morphine
| Study | Parameter | Group | Pre | Post-morphine | %Max | %Total |
|---|---|---|---|---|---|---|
| Hypoxia | fR, breaths/min | SHAM | 127 ± 11 | 104 ± 6 | −19 ± 5a | −10 ± 3a |
| CSNX | 128 ± 20 | 98 ± 2 | −24 ± 7a | −8 ± 3a | ||
| Vt, ml | SHAM | 1.98 ± 0.09 | 1.58 ± 0.10 | −20 ± 3a | −22 ± 2a | |
| CSNX | 1.69 ± 0.10 | 1.25 ± 0.08 | −19 ± 5a | −22 ± 4a | ||
| Ve, ml/min | SHAM | 250 ± 26 | 163 ± 11 | −35 ± 4a | −30 ± 4a | |
| CSNX | 210 ± 27 | 123 ± 7 | −41 ± 5a | −29 ± 4a | ||
| Ti, sec | SHAM | 0.17 ± 0.01 | 0.29 ± 0.01 | +74 ± 6a | +53 ± 5a | |
| CSNX | 0.18 ± 0.02 | 0.31 ± 0.01 | +85 ±11a | +56 ± 10a | ||
| Te, sec | SHAM | 0.36 ± 0.03 | 0.31 ± 0.03 | −14 ± 4a | −14 ± 5a | |
| CSNX | 0.39 ± 0.05 | 0.31 ± 0.02 | −21 ± 5a | −14 ± 4a | ||
| Vt/Ti, ml/sec | SHAM | 12.0 ± 1.0 | 5.5 ± 0.4 | −54 ± 2a | −46 ± 3a | |
| CSNX | 10.0 ± 1.0 | 4.0 ± 0.2 | −59 ± 3a | −45 ± 3a | ||
| Hypercapnia | fR, breaths/min | SHAM | 100 ± 5 | 110 ± 3 | +11 ± 7 | +18 ± 4a |
| CSNX | 101 ± 3 | 111 ± 5 | +9 ± 2a | +16 ± 3a | ||
| VT, ml | SHAM | 2.35 ± 0.10 | 1.94 ± 0.03 | −17 ± 3a | −20 ± 2a | |
| CSNX | 2.16 ± 0.10 | 1.63 ± 0.05 | −19 ± 5a | −28 ± 4a | ||
| Ve, ml/min | SHAM | 233 ± 6 | 214 ± 8 | −8 ± 3a | −10 ± 3a | |
| CSNX | 218 ± 13 | 180 ± 8 | −17 ± 6a | −16 ± 4a | ||
| Ti, sec | SHAM | 0.20 ± 0.01 | 0.28 ± 0.01 | +40 ± 3a | +29 ± 3a | |
| CSNX | 0.21 ± 0.01 | 0.26 ± 0.02 | +26 ± 3a,b | +19 ± 3a,b | ||
| TE, sec | SHAM | 0.42 ± 0.03 | 0.27 ± 0.02 | −33 ± 6a | −31 ± 7a | |
| CSNX | 0.41 ± 0.03 | 0.29 ± 0.04 | −30 ± 2a | −28 ± 2a | ||
| Vt/Ti, ml/sec | SHAM | 11.8 ± 0.6 | 7.0 ± 0.2 | −41 ± 2a | −36 ± 2a | |
| CSNX | 10.6 ± 1.0 | 6.3 ± 0.6 | −39 ± 5a | −36 ± 4a |
The data are presented as mean ± S.E.M. SHAM, sham-operated rats. CSNX, carotid sinus nerve-transected rats. fR, frequency. Vt, tidal volume. Ve, minute ventilation. Ti, inspiratory time. Te, inspiratory time. There were 6 rats in each group.
P < 0.05, significant %change from post-morphine.
P < 0.05, post-morphine CSNX rats versus post-morphine SHAM rats.
3.1.1. Frequency of breathing
As shown in Fig. 2, the injection of morphine (10 mg/kg, i.v.) elicited an initial increase in fR in the HX (lower panel) and HC (middle panel) studies which was followed by a decrease in fR in the HX but not the HC study. As shown in Table 1, the maximum responses (column designated “%Max”) and total cumulative responses (column denoted %Total) elicited by morphine were similar in the SHAM and CSNX rats. As seen in the bottom left panel of Fig. 2, neither the HX nor HC challenge elicited significant increases in fR at any time-point in morphine-treated SHAM or CSNX rats. However, as seen in the bottom right panel of Fig. 2, there was a small cumulative (total) fall in fR during the HX challenge that was of equal magnitude in SHAM and CSNX rats. Moreover, there was a cumulative increase in fR during the HC challenge in morphine-treated SHAM rats that was absent in morphine-treated CSNX rats.
Fig. 2.
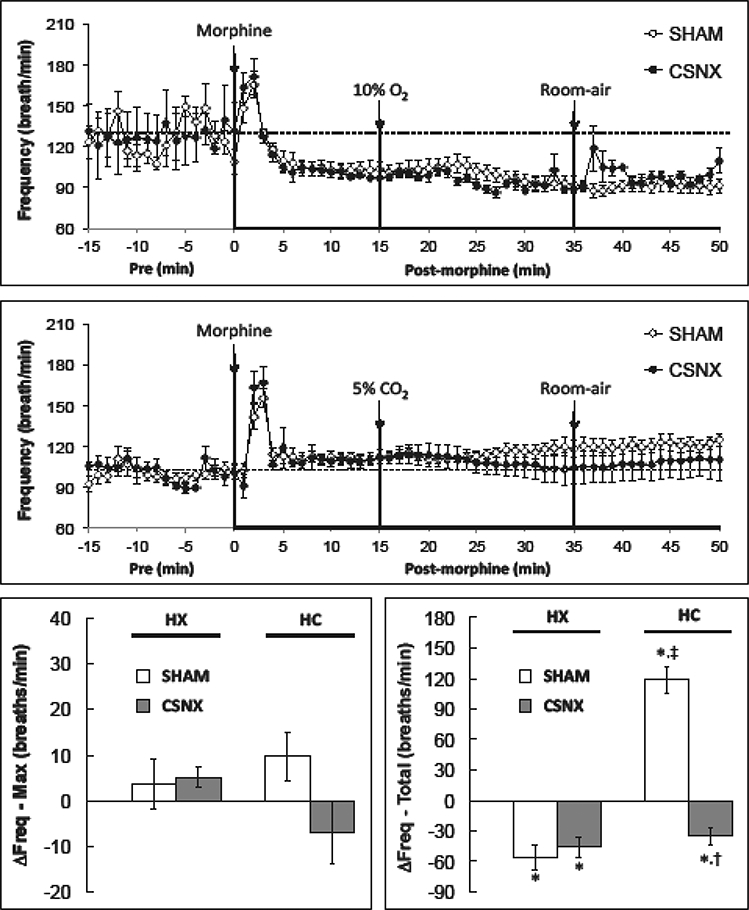
Changes in frequency of breathing in conscious sham-operated rats (SHAM) and in rats with bilateral carotid sinus nerve transection (CSNX) following bolus injection of morphine (10 mg/kg. i.v.), and then exposure to either hypoxic challenge (10% O2, 90% N2) for 20 min (top panel) or hypercapnic challenge (5% O2, 21% O2, 74% N2) for 20 min (middle panel) and then re-exposure to room-air. The maximal and total changes in Frequency of breathing (Freq) are shown in the bottom left and right panels, respectively. The data are presented as mean ± SEM. There were 6 rats in each group.
3.1.2. Inspiratory and expiratory times
Morphine produced an increase in TI that was similar in the SHAM and CSNX rats in the HX (top panel) and HC (middle panel) studies (Fig. 3). Neither the HX nor the HC challenges elicited significant changes in TI (bottom panels). As shown in Fig. 4, morphine produced a decrease in Te (expiratory duration was shorter) that was similar in the SHAM and CSNX rats in both the HX (top panel) and HC (middle panel) studies. Although neither HX nor HC elicited significant changes in Te at any time point, the HX challenge did elicit a cumulative increase in Te (lengthening of expiratory duration) of equal magnitude in the SHAM and CSNX rats. The HC challenge did not elicit a cumulative increase in Te in SHAM or CSNC rats.
Fig. 3.
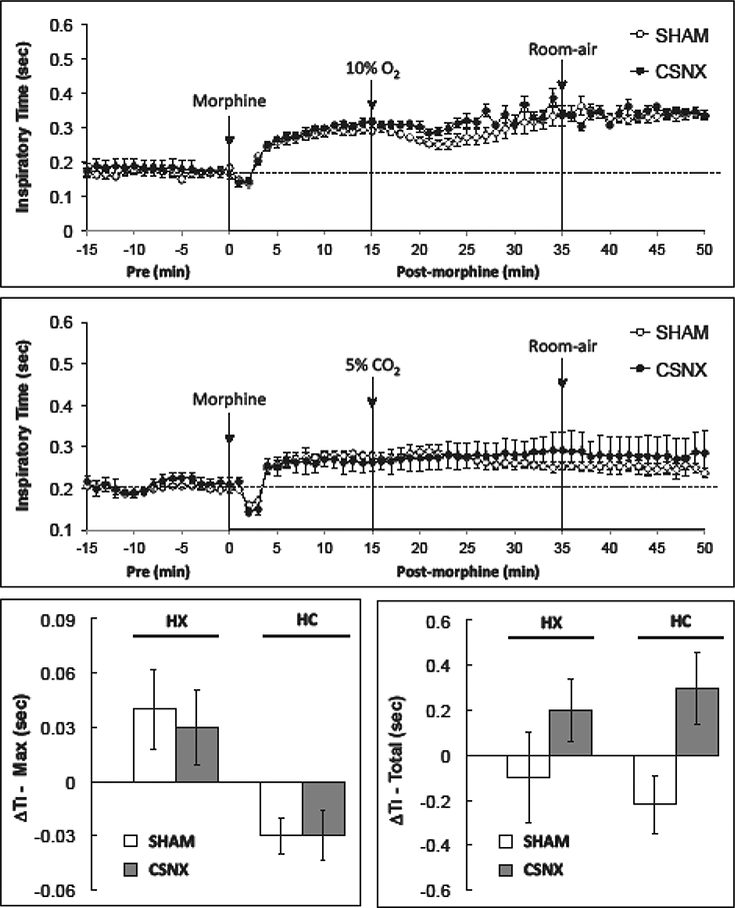
Changes in inspiratory time in conscious sham-operated rats (SHAM) and in rats with bilateral carotid sinus nerve transection (CSNX) following bolus injection of morphine (10 mg/kg. i.v.), and then exposure to hypoxic challenge (10% O2, 90% N2) for 20 min (top panel) or hypercapnic challenge (5% O2, 21% O2, 74% N2) for 20 min (middle panel) and then re-exposure to room-air. The maximal and total changes in inspiratory time (Ti) are shown in the bottom left and right panels, respectively. The data are presented as mean ± SEM. There were 6 rats in each group.
Fig. 4.
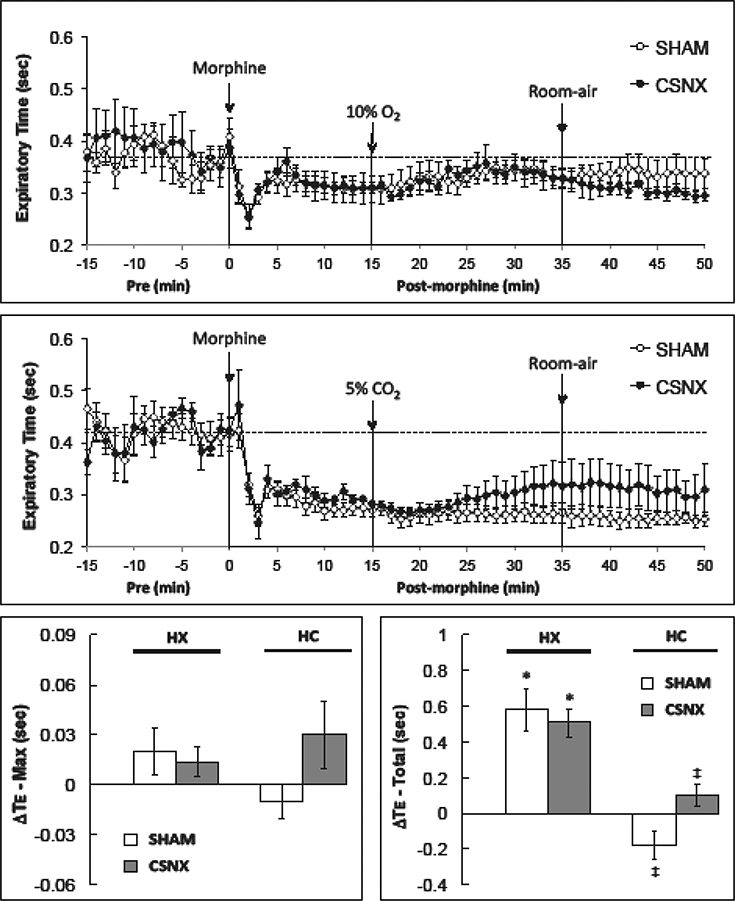
Changes in expiratory time in conscious sham-operated rats (SHAM) and in rats with bilateral carotid sinus nerve transection (CSNX) following bolus injection of morphine (10 mg/kg. i.v.), and then exposure to hypoxic challenge (10% O2, 90% N2) for 20 min (top panel) or hypercapnic challenge (5% O2, 21% O2, 74% N2) for 20 min (middle panel) and then re-exposure to room-air. The maximal and total changes in expiratory time (Te) are shown in the bottom left and right panels, respectively. The data are presented as mean ± SEM. There were 6 rats in each group.
3.1.3. Tidal volume
Morphine decreased Vt in the HX (top panel) and HC (middle panel) studies (Fig. 5). The maximum and total responses were similar in the SHAM and CSNX rats (Table 1). The HX and HC challenges increased Vt in morphine-treated SHAM rats (Fig. 5). The maximal and total increases in Vt elicited by HX and HC were smaller in CSNX rats than SHAM rats (bottom right panel of Fig. 5). Moreover, the maximal and total increases in Vt elicited by HC in morphine-treated SHAM and CSNX rats were substantially smaller than those elicited by HX in these rats.
Fig. 5.
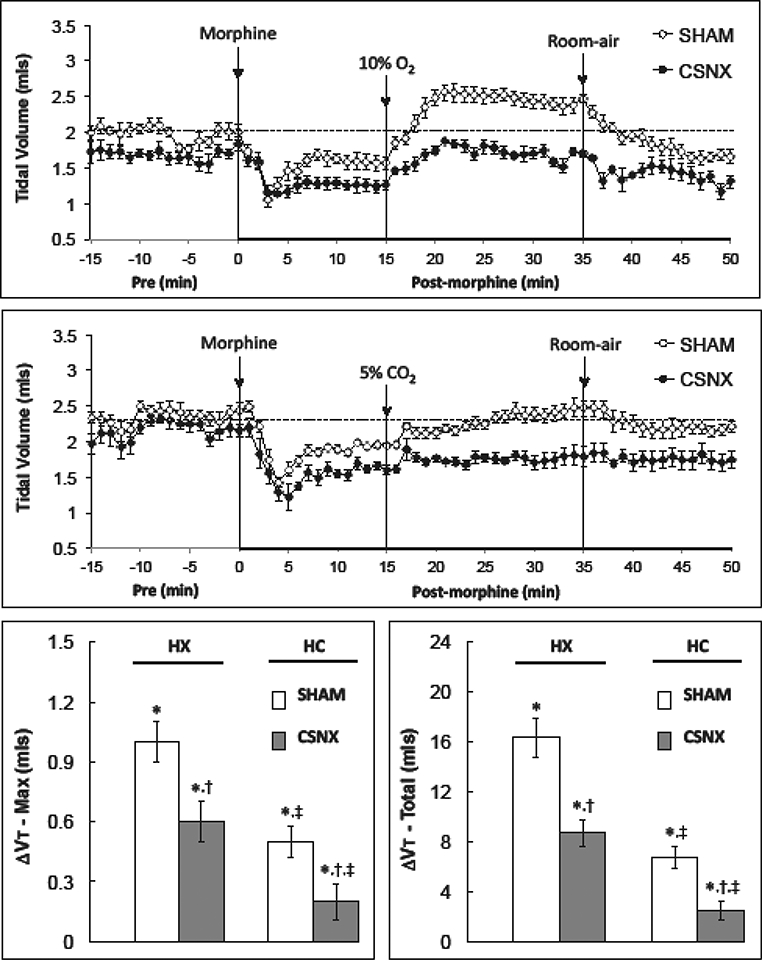
Changes in tidal volume in conscious sham-operated rats (SHAM) and in rats with bilateral carotid sinus nerve transection (CSNX) following bolus injection of morphine (10 mg/kg. i.v.), and then exposure to hypoxic challenge (10% O2, 90% N2) for 20 min (top panel) or hypercapnic challenge (5% O2, 21% O2, 74% N2) for 20 min (middle panel) and then re-exposure to room-air. The maximal and total changes in tidal volume (Vt) are shown in the bottom left and right panels, respectively. The data are presented as mean ± SEM. There were 6 rats in each group.
3.1.4. Minute ventilation
As summarized in Fig. 6, morphine elicited a decrease in Ve in both the HX and HC studies. The peak and total responses were similar in SHAM and CSNX rats (Table 1). The HX and HC challenge-induced increases in Ve were smaller in the morphine-treated CSNX rats than the morphine-treated SHAM rats (Fig. 6). The maximal and total increases in Ve elicited by HX and HC were smaller in CSNX rats than SHAM rats. Moreover, the maximum and total increases in Ve elicited by HC in morphine-treated SHAM and CSNX rats were substantially smaller than those elicited by HX.
Fig. 6.
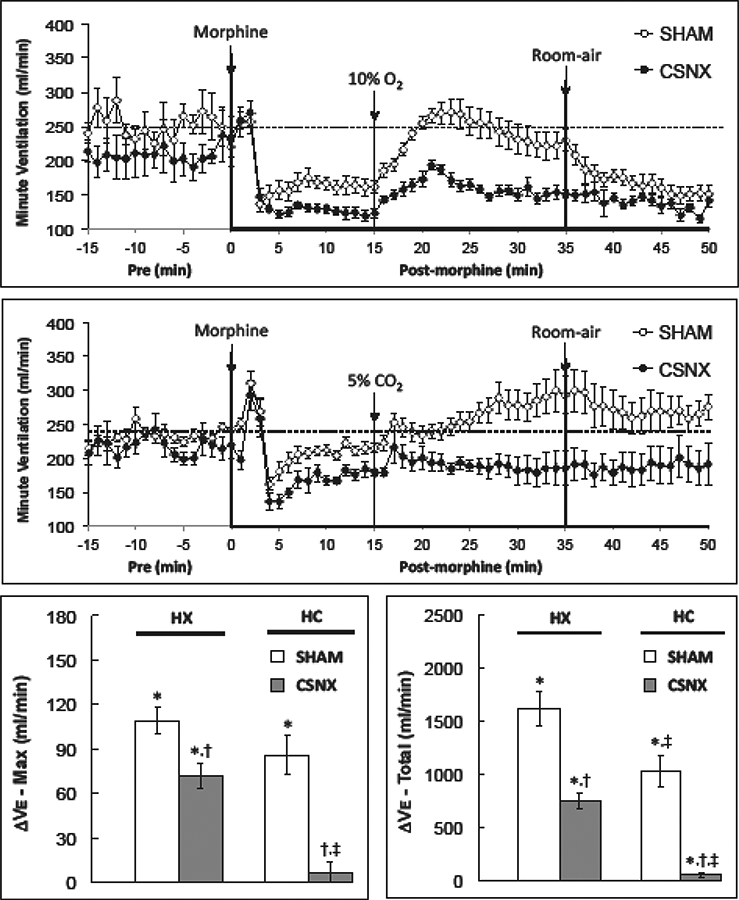
Changes in minute ventilation in conscious sham-operated rats (SHAM) and in rats with bilateral carotid sinus nerve transection (CSNX) following bolus injection of morphine (10 mg/kg. i.v.), and then exposure to hypoxic challenge (10% O2, 90% N2) for 20 min (top panel) or hypercapnic challenge (5% O2, 21% O2, 74% N2) for 20 min (bottom panel) and then re-exposure to room-air. Maximal and total changes in minute ventilation (Ve) are shown in the bottom left and right panels, respectively. The data are presented as mean ± SEM. There were 6 rats in each group.
3.1.5. Inspiratory Drive
Morphine elicited a decrease in Vt/Ti in both the HX and HC studies (Fig. 7). The peak and total responses were similar in SHAM and CSNX rats (Table 1). The HX- and HC-induced increases in Vt/Ti were smaller in morphine-treated CSNX rats than the morphine-treated SHAM rats (Fig. 7). Moreover, the maximum and total increases in Ve elicited by HC in morphine-treated SHAM and CSNX rats were smaller than those elicited by HX.
Fig. 7.
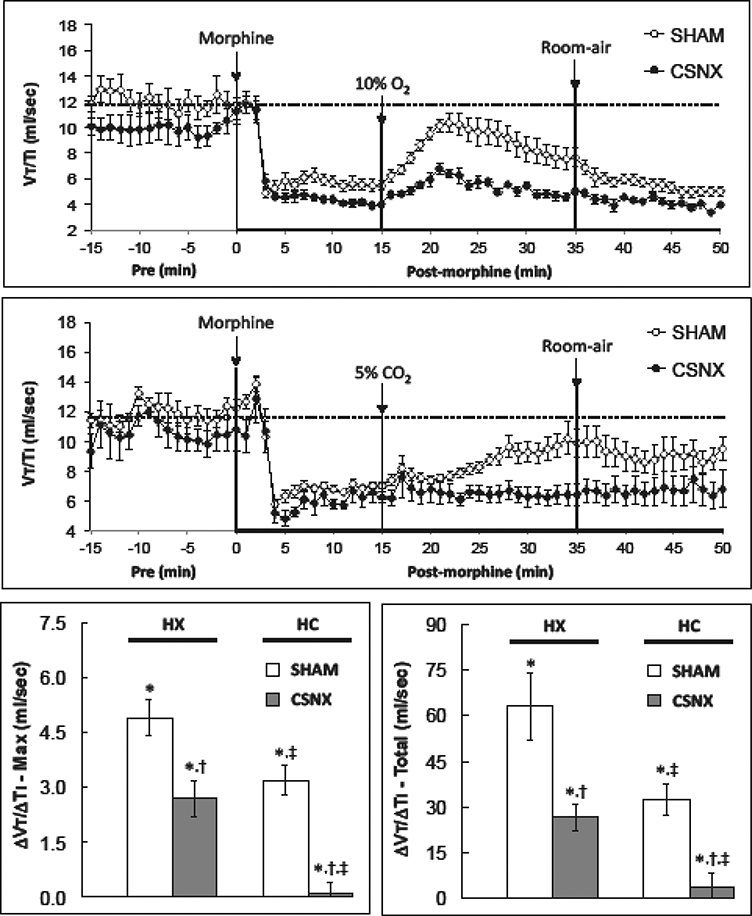
Changes in inspiratory drive (tidal volume/inspiratory time, Vt/Ti) in conscious sham-operated rats (SHAM) and in rats with bilateral carotid sinus nerve transection (CSNX) following bolus injection of morphine (10 mg/kg. i.v.), and then exposure to hypoxic challenge (10% O2, 90% N2) for 20 min (top panel) or hypercapnic challenge (5% O2, 21% O2, 74% N2) for 20 min (middle panel) and then re-exposure to room-air. Maximal and total changes in tidal volume/inspiratory time (Vt/Ti) are shown in the bottom left and right panels, respectively. The data are presented as mean ± SEM. There were 6 rats in each group.
3.2. Ventilatory responses upon return to room-air
As seen in Figs. 2–7, the changes in ventilatory parameters upon return to room-air after the HX and HC challenges were in general unremarkable and similar in nature in the SHAM and CSNX rats. The values stayed at levels seen at the end of the HX and HC levels, or gradually fell back toward the morphine levels (see upper panels of Figs. 5 and 6). The total changes in ventilatory parameters (total %changes from post-morphine levels) during post-HX (top panel) and post-HC (bottom panel) are summarized in Fig. 8. The post-HX decrease in fR and the increase in Te observed in the SHAM rats were not observed in CSNX rats. Moreover, the post-HC increases in fR, Vt, Ve and Vt/Ti and the decrease in Ti observed in SHAM rats were not observed in CSNX rats.
Fig. 8.
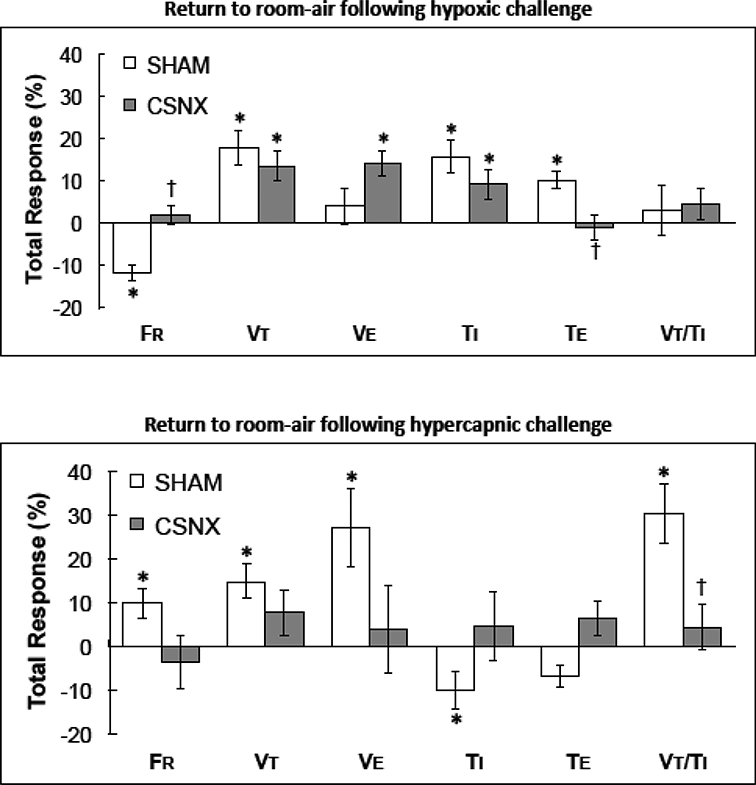
Total changes in frequency of breathing (fR), tidal volume (Vt), minute ventilation (Ve), inspiratory time (TI), expiratory time (Te) and tidal volume/inspiratory time (Vt/ Ti) upon return to room-air following exposure to either hypoxic (10% O2, 90% N2) challenge (top panel) or hypercapnic (5% O2, 21% O2, 74% N2) challenge (bottom panel) in morphine-treated sham-operated rats (SHAM) and in morphine-treated rats with bilateral carotid sinus nerve transection (CSNX) * P < 0.05, significant change from pre-hypoxia levels. †P< 0.05, CSNX versus SHAM.
3.3. Comparison between naive SHAM and CSNX rats
Comparisons of the changes in Ve elicited by HX or HC challenges in rats that received saline or morphine (10 mg/kg, i.v.) are shown in Table 2. The maximum and total increases in Ve elicited by HX challenge were about 50% less in CSNX rats than in SHAM rats. Morphine suppressed the increases in Ve elicited by HX challenge in SHAM rats but did not diminish the residual responses in CSNX rats. The increases in Ve elicited by HC were similar in SHAM and CSNX rats. The maximum and total responses elicited by HC were markedly diminished (about 80%) by morphine in SHAM rats. The HC-induced responses were virtually eliminated in CSNX rats (reduction of about 95%).
Table 2.
Arithmetic changes in ventilatory parameters elicited by hypoxic or hypercapnic challenges
| Study | Parameter | Group | Pre | Challenge | Room-air |
|---|---|---|---|---|---|
| Hypoxia | ΔfR, breaths/min | SHAM | 104 ± 6 | −56 ± 16a | −160 ± 21a |
| CSNX | 98 ± 2 | −46 ± 14a | +5 ± 18b | ||
| ΔVt, ml | SHAM | 1.58 ± 0.10 | +16.3 ±1.6 | +4.0 ± 0.8 | |
| CSNX | 1.25 ± 0.08 | 8.7 ± l.la | −19 ± 5b | ||
| ΔVe, ml/min | SHAM | 250 ± 26 | 163 ± 11 | −35 ± 4b | |
| CSNX | 210 ± 27 | 123 ± 7a | −41 ± 5b | ||
| ΔTI, sec | SHAM | 0.17 ±0.01 | 0.29 ± 0.01 | +74 ± 6b | |
| CSNX | 0.18 ±0.02 | 0.31 ± 0.01 | +85 ±llb | ||
| ΔVt/Ti, ml/sec | SHAM | 12.0 ±1.0 | 5.5 ± 0.4 | −54 ± 2b | |
| CSNX | 10.0 ±1.0 | 4.0 ± 0.2a | −59 ± 3b | ||
| Hypercapnia | ΔfR, breaths/min | SHAM | 100 ± 5 | 110 ± 3 | +11 ± 7 |
| CSNX | 101 ± 3 | 111 ±5 | +9± 2b | ||
| ΔVt, ml | SHAM | 2.35 ± 0.10 | 1.94 ± 0.03 | −17 ± 3b | |
| CSNX | 2.16 ± 0.10 | 1.63 ± 0.05a | −19 ± 5b | ||
| ΔVe, ml/min | SHAM | 233 ± 6 | 214 ±8 | −8±3b | |
| CSNX | 218 ± 13 | 180 ±8 | −17 ± 6b | ||
| ΔTi, sec | SHAM | 0.20 ±0.01 | 0.28 ±0.01 | +40 ± 3b | |
| CSNX | 0.21 ±0.01 | 0.26 ±0.02 | +26 ± 3b,c | ||
| ΔVt/Ti, ml/sec | SHAM | 11.8 ±0.6 | 7.0 ±0.2 | −41 ± 2b | |
| CSNX | 10.6 ± 1.0 | 6.3 ±0.6 | −39 ± 5b |
The data are presented as mean ± S.E.M. SHAM, sham-operated rats. CSNX, carotid sinus nerve-transected rats. fR, frequency. Vt, tidal volume. Ve, minute ventilation. TI, inspiratory time. Vt/Ti, tidal/volume/inspiratory time. There were 6 rats in each group.
P < 0.05, post-morphine CSNX rats versus post-morphine SHAM rats.
P < 0.05, significant %change from post-morphine.
P < 0.05, morphine response in CSNX rats versus morphine response in SHAM rats.
4. Discussion
To our knowledge, this is the first in vivo evidence that CB chemoreceptors defend against, rather than participate in the ventilatory depressant effects of morphine. Key findings were that morphine-induced respiratory depression per se and suppression of ventilatory responses to HX and HC challenges were more pronounced in CSNX than SHAM rats, and morphine virtually abolished ventilatory responses to HC in SHAM and CSNX rats whereas residual ventilatory responses elicited by HX were minimally affected by morphine. Whether enhanced activity of this (normally) subsidiary pathway plays a role in the substantial recovery of HVR after weeks in CSNX rats (Martin-Body et al., 1986; Roux et al., 2000) is unknown. Concomitant bilateral section of glossopharyngeal nerves and abdominal vagus nerves markedly diminishes recovery of HVR in CSNX rats (Martin-Body et al., 1986). It could be envisioned that residual responses to HX in CSNX rats is due to activity of the above nerves or other potential chemoreceptor structures and that time-dependent increase in efficacy of these systems develops to allow for a fuller HVR. An intriguing aspect of these findings is that it this subsidiary pathway appears insensitive to morphine in early days of CSNX. We are exploring whether this subsidiary system remains insensitive to morphine at later times after CSNX.
That morphine elicited an initial increase in fR is consistent with our previous evidence that this opioid initially enhances fR in conscious Sprague-Dawley rats by activation of peripheral μ-ORs (Henderson et al., 2013, 2014). Morphine lowered fR in the HX but not HC group. However, actual levels reached after injection of morphine were similar in both groups. As such, the fall in fR in the HX but not HC study is likely a reflection of higher resting baseline values in the HX study. Overall, maximal and total changes in ventilatory parameters elicited by morphine were similar in SHAM and CSNX rats. These findings imply that the initial effects of morphine on breathing (e.g., marked reduction in Vt rather than fR) may be due mainly to actions in the brain and that the presence or absence of the CB is inconsequential. It is equally plausible that morphine suppresses CB function such that the effects of morphine would be expected to be similar in SHAM and CSNX rats.
As was expected (Henderson et al., 2013, 2014; May et al., 2013a), the increases in fR (and concomitant decreases in TI and Te) and Vt, Ve and Vt/Ti (respiratory drive) elicited by the HC and HX challenges were substantially smaller in morphine-treated SHAM rats than in their saline-treated counterparts. However, it was evident that the ventilatory depressant effects of morphine were substantially greater against HC than HX. Specifically, HX elicited robust increases in Vt, Ve and Vt/Ti in morphine-treated rats whereas HC elicited minimal responses. This suggests that morphine is less able to suppress central pathways that process the ventilatory responses to HX (Gozal et al., 2000; Costa et al., 2014) than those that process responses to HC (Shimokawa et al., 2005; Guyenet et al., 2010). Opioids elicit complex effects on brainstem circuitry regulating breathing including slowing of respiratory-related neurotransmission via failure of rhythmic drive from pre-inspiratory neurons to the pre-Botzinger complex inspiratory networks that due to morphine, are depressed below threshold for spontaneous rhythmic activity (Mellen et al., 2003).
With respect to central HX pathways, it is known that microinjection of opioid μ-receptor agonists into the medullary raphe region (Zhang et al., 2009) and commissural NTS (Zhang et al., 2011) suppress HVR. Morphine has numerous potential sites/mechanisms to suppress ventilatory responses to HC. For example, activation of opioid μ-receptors in conscious rats (Emry et al., 2016) or selectively in caudal medullary raphe region in anesthetized rats (Zhang et al., 2007) inhibits HCVR, whereas systemic morphine (10 mg/ kg, iv) immediately reduces resting discharge rate and C02-sensitivity of retrotrapezoid nucleus neurons (Fortuna et al., 2009), which play a vital role in overall responses to HC (Guyenet et al., 2010). Increases in pC02 drive respiration in part via the carbonic anhydrase-catalyzed conversion of CO2 plus H2O to H2CO3, which spontaneously decomposes to HCO3- and H+ (Guyenet et al., 2010). Although direct evidence in the brain is lacking, morphine inhibits erythrocyte carbonic anhydrase (Coban et al., 2007) and inhibits acidsensing ion channels (ASICs) in rat dorsal root neurons (Cai et al., 2014). Furthermore, morphine increases secretion of melatonin (Esposti et al., 1988), which attenuates CB chemoreceptor response of rats to hypercapnic acidosis (Tjong et al., 2004). As such, morphine may directly inhibit activity of the CB and central neurons responsive to CO2, generation of H+ and mechanisms by which CO2/H+ ions activate these neurons. This combination of effects of morphine appears to suppress HCVR to a greater extent than those mediating HVR. Differential effects of morphine on HVR and HCVR are highlighted by contrasting temporal changes in ventilatory parameters in morphine-treated rats in response to HX and HC. Specifically, HX-induced increases in respiratory drive (Vt/Ti) occurred relatively rapidly whereas the increase in respiratory drive elicited by HC challenge developed much more slowly (see Fig. 7).
We have reported that intravenous administration of a 10 mg/kg dose of morphine to freely-moving male Sprague-Dawley rats elicits a relatively minor increase in core body temperature (0.5°C) that are accompanied by falls in blood pH, pO2 and sO2 with increases in blood pCO2 and Alveolar-arterial gradient. These responses, indicative of ventilatory depression and enhanced ventilation-perfusion mismatch, lasted for about 60 min and 30 min, respectively. As such it is evident the ventilatory depressant effects of morphine reported in the present study translate into tangible and important changes in arterial blood-gas chemistry. Therefore, as expected, HVR was markedly diminished in saline-treated CSNX rats whereas HCVR was not (Gaston et al., 2014; Martin-Body et al., 1986; Roux et al., 2000; Fatemian et al., 2003). This does not discount the role of the CB in HCVR but shows that central processes can cover for the loss of CB input (Fatemian et al., 2003). That morphine-induced suppression of HVR and HCVR was exacerbated in CSNX rats suggests that CBs are not compromised by morphine and indeed play a role in defending against its deleterious effects. Our data are consistent with evidence that (a) lower doses of morphine enhance CB chemoreceptor discharge in cats and that higher doses caused minimal suppression (McQueen and Ribeiro, 1980) and (b) activation of chemoafferents by intracarotid injections of CO2 rich solutions was augmented by morphine (McQueen and Ribeiro, 1980). The ability of the CB to defend against morphine may be underestimated in our studies since the loss of CB input blunts central sensitivity to CO2 in humans (Fatemian et al., 2003) and dogs (Blain et al., 2010). In addition, experimental approaches across range of species estimated that, up to one-third of the increase in HCVR is CB chemoreceptor-mediated (Forster and Smith, 2010). More importantly, the CB chemoreceptors drive the initial HCVR in rats immediately after a change in inspired CO2 (Cummings and Frappel, 2009). There is also evidence that CB chemoreceptor activity modulates the CO2 sensitivity of the central chemoreceptors, suggesting the interdependent theory of central and peripheral chemoreceptors (Forester and Smith, 2010). Therefore, CSNX could directly impact the HCVR, as could any changes to glomus cells CO2/pH sensitivity. The different effects of morphine on HX- and HC-induced increases in Vt/Ti in CSNX rats highlights the differential role of CBs. Specifically, the pattern of response during HX challenge in morphine-treated CSNX rats (i.e., rapid increase in Vt/Ti followed by waning) was similar to that in morphine-treated SHAM rats (see Fig. 7). The gradual increase in Vt/Ti that occurred during HC in morphine-treated SHAM rats was absent in morphine-treated CSNX rats.
A striking feature of this study was the different status of ventilatory variables in morphine-treated rats upon return to room-air following HX and HC challenges. The increases in Vt, Ve and Vt/Ti that occurred during HX challenge merely subsided back to morphine-induced levels before the start of the challenge. As such, it is evident that a 20 min HX challenge is insufficient to alter the continuing effects of morphine and/or it major metabolites (Christrup, 1997; De Gregor et al., 2012). In contrast, the persistent increases in Vt, Ve and Vt/Ti that occurred after HC challenge (responses reached levels equivalent to those before injection of morphine) in SHAM rats were prevented by CSN transection (i.e., after return to room-air). As such, a 20 min HC challenge was sufficient to elicit sustained reversal of the deleterious effects of morphine/metabolites on ventilation. Morphine-treated rats were still sedated during and after HC challenge and behaved similarly to those in the HX study. This raises questions whether C02 and/or H+ ions combat the actions of morphine on (e.g., displace it from) targets such as carbonic anhydrase and ASICs.
Although HC is a potent activator of the sympathetic nervous system (Gamble et al., 1990), morphine-treated rats showed no obvious behavioral changes during the HC challenge. This does not negate the possibility that “arousal” in central circuitry driving respiration occurred in some manner during HC challenge although Gamble and Milne (1990) provided evidence that moderate HC powerfully depresses flexor withdrawal responses to noxious stimuli by mechanism involving release of endogenous opioids but not circulating catecholamines. This raises the strong possibility that the state of arousal of the rat (with or without morphine) may have important influence on the ventilatory responses to HC gas challenge. Another question is why the effects of HC challenge are so long-lasting. Again, this is not due to blockade of opiate receptors per se or enhanced degradation/clearance of morphine and/or metabolites because the rats still displayed the sedative effects of morphine. Addressing the mechanisms by which HC challenge reverses morphine-induced respiratory depression may lead to novel therapeutic approaches to combat OIRD without affecting the sedative or analgesic actions of the compounds.
The second striking observations were that HC did not reverse the ventilatory depressant effects of morphine in CSNX rats and that the depressant effects of morphine were intact upon return to room-air. It is apparent that the ability of HC to elicit a full recovery of the deleterious effects of morphine reside in the CB or that an active CB-CSN input to the brain is necessary for central C02 to overcome the effects of morphine. These findings suggest that CB chemoreceptors play a vital role in allowing elevated levels of blood C02 that occur during morphine challenge (Dahan et al., 2010) to stimulate ventilation. As such, therapeutic strategies that directly activate the CB or enhance actions of C02/H+ ions on glomus cells may effectively combat OIRD.
In rats, the disposition of morphine and its major biologically-active metabolite, morphine-3-glucuronide, after peripheral administration have been investigated extensively (see Smith et al., 1990; Alnouti et al., 2007; South et al., 2009). For example, South et al (2009) reported that following intravenous injection of a 10 mg/kg dose of morphine to male Sprague-Dawley rats (same dose and route of administration, and rat and gender strain as used in the present study), the blood levels of morphine (all approximate values read from the manuscript figs) peaked at 7 μΜ at 2.5 minutes (time of initial maximal effects in our study) and were at 1 μΜ at 50 minutes (time of last measurement in our study when many effects of morphine were still sustained). In addition, blood concentrations of morphine-3-glucuronide were 7.5 μΜ at 2.5 minutes and 7 μΜ at 50 minutes. These findings are certainly supportive of a variety of evidence that the biological effects of morphine are due both to the parent molecule and the metabolite, morphine-3-glucuronide (Smith et al., 1990; Alnouti et al., 2007; South et al., 2009).
The issue of how the magnitude of effects of morphine on baseline parameters in sham and CSNX rats affects the subsequent ventilatory responses to the hypoxic and hypercapnic gas challenges is vital to understanding how morphine exerts its effects on the gas challenges. With respect to fR, TI and Te, it is evident that morphine elicited similar changes in these parameters in SHAM and CSNX rats and that neither HX or HC challenges elicited noticeable changes in these morphine-treated rats. However, it is evident that morphine elicited greater reductions in Vt and Ve in CSNX rats than in SHAM rats and that the subsequent increases in these parameters elicited by the HX and HC challenges were substantially smaller in CSNX rats. In contrast, although morphine elicited similar reductions in respiratory drive (Vt/Ve) in SHAM and CSNX rats, the increases in respiratory drive elicited by the HX and HC challenges were markedly smaller in the CSNX rats. Taken together, it would appear that the post-morphine baseline values do not necessarily predict the degree by which the ventilatory responses will be compromised in SHAM and CSNX rats.
In summary, our data suggest that the CB-chemoafferent complex plays a key role in defending against OIRD. The findings raise intriguing questions regarding the precise mechanisms by which e CB chemoreceptors defend against deleterious the effects of morphine on breathing and how HC and accompanying generation of H+ ions are able to reverse these deleterious actions of morphine. Our work is particularly relevant to recent findings that the HCVR remains depressed in chronic morphine-treated rats despite these rats having developed tolerance to the analgesic effects of the opioid (Emery et al., 2016).
Acknowledgements
The authors would like to thank Dr. James N. Bates for critiquing the manuscript.
Funding: This study was supported by a National Institutes of Health (NIH) Program Project Grant (1P-01-HL-101871 to SJL), and grants from Galleon Pharmaceuticals (SJL). Galleon Pharmaceuticals had involvement in study design and in the decision to submit the article for publication.
Footnotes
Competing interests: The authors have declared that no competing interests exist.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alnouti YM, Shelby MK, Chen C, Klaassen CD, 2007. Influence of phenobarbital on morphine metabolism and disposition: LC-MS/MS determination of morphine and morphine-3-glucruonide in Wistar-Kyoto rat serum, bile, and urine. Curr. Drug Metab 8, 79–89. [DOI] [PubMed] [Google Scholar]
- Berkenbosch A, Teppema LJ, Olievier CN, Dahan A, 1997. Influences of morphine on the ventilatory response to isocapnic hypoxia. Anesthesiology 86, 1342–1349. [DOI] [PubMed] [Google Scholar]
- Blain GM, Smith CA, Henderson KS, Dempsey JA, 2010. Peripheral chemoreceptors determine the respiratory sensitivity of central chemoreceptors to CO2. J. Physiol 588, 2455–2471. DOI: 10.1113/jphysiol.2010.187211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boom M, Olofsen E, Neukirchen M, Fussen R, Hay J, Groeneveld GJ, Aarts L, Sarton E, Dahan A, 2013. Fentanyl utility function: a risk-benefit composite of pain relief and breathing responses. Anesthesiology 119, 663–674. DOI: 10.1097/ALN.0b013e31829ce4cb [DOI] [PubMed] [Google Scholar]
- Cai Q, Qiu CY, Qiu F, Liu TT, Qu ZW, Liu YM, Hu WP, 2014. Morphine inhibits acidsensing ion channel currents in rat dorsal root ganglion neurons. Brain Res. 1554, 12–20. DOI: 10.1016/j.brainres.2014.01.042 [DOI] [PubMed] [Google Scholar]
- Cashman JN, Dolin SJ, 2004. Respiratory and haemodynamic effects of acute postoperative pain management: evidence from published data. Brit. J. Anaesthesia 93, 212–223. DOI: 10.1093/bja/aeh180 [DOI] [PubMed] [Google Scholar]
- Cepeda MS, Farrar JT, Baumgarten M, Boston R, Carr DB, Strom BL, 2003. Side effects of opioids during short-term administration: effect of age, gender, and race. Clin. Pharmacol. Ther 74, 102–112. DOI: 10.1016/S0009-9236(03)00152-8 [DOI] [PubMed] [Google Scholar]
- Chen ZR, Irvine RJ, Somogyi AA, Bochner F, 1991. Mu receptor binding of some commonly used opioids and their metabolites. Life Sci. 48, 2165–2171. [DOI] [PubMed] [Google Scholar]
- Christensen CB, Reiff L, 1991. Morphine-6-glucuronide: Receptor binding profile in bovine caudate nucleus. Pharmacol. Toxicol 68, 151–153. [DOI] [PubMed] [Google Scholar]
- Christrup LL, 1997. Morphine metabolites. Acta. Anaesth. Scand 41, 116–122. [DOI] [PubMed] [Google Scholar]
- Coban TA, Beydemir S, Gülçin I, Ekinci D, 2007. Morphine inhibits erythrocyte carbonic anhydrase in vitro and in vivo. Biol. Pharmaceutic. Bull 30, 2257–2261. [DOI] [PubMed] [Google Scholar]
- Costa KM, Accorsi-Mendonça D, Moraes DJ, Machado BH, 2014. Evolution and physiology of neural oxygen sensing. Front. Physiol 5, 302 DOI: 10.3389/fphys.2014.00302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahan A, Sarton E, Teppema L, Olievier C., 1998. Sex-related differences in the influence of morphine on ventilatory control in humans. Anesthesiology 88, 903–913. [DOI] [PubMed] [Google Scholar]
- Dahan A, Aarts L, Smith TW, 2010. Incidence, reversal, and prevention of opioid-induced respiratory depression. Anesthesiology 112, 226–238. DOI: 10.1097/ALN.0b013e3181c38c25 [DOI] [PubMed] [Google Scholar]
- De Gregori S, De Gregori M, Ranzani GN, Allegri M, Minella C, Regazzi M, 2012. Morphine metabolism, transport and brain disposition. Metab. Brain Dis 27, 1–5. DOI: 10.1007/s11011-011-9274-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery MJ, Groves CC, Kruse TN, Shi C, Terman GW, 2016. Ventilation and the response to hypercapnia after morphine in opioid-naive and opioid-tolerant rats. Anesthesiology 124: 945–957. DOI: 10.1097/ALN.0000000000000997 [DOI] [PubMed] [Google Scholar]
- Esposti D, Esposti G, Lissoni P, Parravicini L, Fraschini F, 1988. Action of morphine on melatonin release in the rat. J. Pineal Res 15, 35–39. [DOI] [PubMed] [Google Scholar]
- Fatemian M, Nieuwenhuijs DJ, Teppema LJ, Meinesz S, van der Mey AG, Dahan A, Robbins PA, 2003. The respiratory response to carbon dioxide in humans with unilateral and bilateral resections of the carotid bodies. J. Physiol 549, 965–973. DOI: 10.1113/jphysiol.2003.042259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortuna MG, Stornetta RL, West GH, Guyenet PG, 2009. Activation of the retrotrapezoid nucleus by posterior hypothalamic stimulation. J. Physiol 587, 5121–5138. DOI: 10.1113/jphysiol.2009.176875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frances B, Gout R, Campistron G, Panconi E, Cros J, 1990. Morphine-6-glucuronide is more μ-selective and potent in analgesic tests than morphine. Prog. Clin. Biol. Res 328, 477–480. [PubMed] [Google Scholar]
- Frances B, Gout R, Monsarrat B, Cros J, Zajac JM, 1992. Further evidence that morphine-6 betaglucuronide is a more potent opioid agonist than morphine. J. Pharmacol. Exp. Ther 262, 25–31. [PubMed] [Google Scholar]
- Gamble GD, Milne RJ, 1990. Hypercapnia depresses nociception: endogenous opioids implicated. Brain Res. 514, 198–205. [DOI] [PubMed] [Google Scholar]
- Gaston B, May WJ, Sullivan S, Yemen S, Marozkina NV, Palmer LA, Bates JN, Lewis SJ, 2014. Essential role of hemoglobin β−93-cysteine in post-hypoxia facilitation of breathing in conscious mice. J. Appl. Physiol 116, 1290–1299. DOI: 10.1152/japplphysiol.01050.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getsy PM, Davis J, Coffee GA, May WJ, Palmer LA, Strohl KP, Lewis SJ, 2014. Enhanced non-eupneic breathing following hypoxic, hypercapnic or hypoxic-hypercapnic gas challenges in conscious mice. Resp. Physiol. Neurobiol 204, 147–159. DOI: 10.1016/j.resp.2014.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozal D, Gozal E, Simakajornboon N, 2000. Signaling pathways of the acute hypoxic ventilator response in the nucleus tractus solitarius. Resp. Physiol 121, 209–221. [DOI] [PubMed] [Google Scholar]
- Guyenet PG, Stornetta RL, Abbott SB, Depuy SD, Fortuna MG, Kanbar R, 2010. Central CO2-chemoreception and integrated neural mechanisms of cardiovascular and respiratory control. J. Appl. Physiol 108, 995–1002. DOI: 10.1152/japplphysiol.00712.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson F, May WJ, Gruber R, Young AP, Palmer LA, Gaston B, Lewis SJ, 2013. Low dose morphine elicits ventilatory excitant and depressant responses in conscious rats: Role of peripheral μ-opioid receptors. Open J. Mol. Integr. Physiol 3, 111–124. DOI: 10.4236/ojmip.2013.33017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson F, May WJ, Gruber RB., Discala JF, Puscovic V, Young AP, Baby SM, Lewis SJ, 2014. Role of central and peripheral opiate receptors in the effects of fentanyl on ventilation, arterial blood-gas chemistry and analgesia in conscious rats. Resp. Physiol. Neurobiol 191, 95–105. DOI: 10.1016/j.resp.2013.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby GC, McQueen DS, 1986. Characterization of opioid receptors in the cat carotid body involved in chemosensory depression in vivo. Brit. J. Pharmacol 1986; 88: 889–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laferriere A, Colin-Durand J, Moss IR, 2005. Ontogeny of respiratory sensitivity and tolerance to the mu-opioid agonist fentanyl in rat. Develop. Brain Res 156, 210–217. DOI: 10.1016/j.devbrainres.2005.03.002 [DOI] [PubMed] [Google Scholar]
- Martin-Body RL, Robson GJ, Sinclair JD, 1986. Restoration of hypoxic respiratory responses in the awake rat after carotid body denervation by sinus nerve section. J. Physiol 380, 61–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May WJ, Gruber RB, Discala JF, Puskovic V, Henderson F, Palmer LA, Lewis SJ, 2013a. Morphine has latent deleterious effects on the ventilatory responses to a hypoxic challenge. Open J. Mol. Integr. Physiol 3, 166–180. DOI: 10.4236/ojmip.2013.34022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- May WJ, Henderson F, Gruber RB, Discala JF, Young AP, Bates JN, Palmer LA, Lewis SJ, 2013b. Morphine has latent deleterious effects on the ventilatory responses to a hypoxic-hypercapnic challenge. Open. J. Mol. Integr. Physiol 3, 134–145. DOI: 10.4236/ojmip.2013.33019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQueen DS, Ribeiro JA, 1980. Inhibitory actions of methionine-enkephalin and morphine on the cat carotid chemoreceptors. Brit. J. Pharmacol 71, 297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellen NM, Janczewski WA, Bocchiaro CM, Feldman JL, 2003. Opioid-induced quantal slowing reveals dual networks for respiratory rhythm generation. Neuron 2003; 37, 821–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy DJ, Joran ME, Grando JC, 1995. A non-invasive method for distinguishing central from peripheral nervous system effect of respiratory depressant drugs in conscious rats. Gen. Pharmacol 26, 569–575. [DOI] [PubMed] [Google Scholar]
- Palmer LA, May WJ, deRonde K, Brown-Steinke K, Gaston B, Bates JN, Gaston B, Lewis SJ, 2013. Ventilatory responses during and following exposure to a hypoxic challenge in conscious mice deficient or null in S-nitrosoglutathione reductase. Resp. Physiol. Neurobiol 185, 571–581. DOI: 10.1016/j.resp.2012.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peat SJ, Hanna MH, Woodham M, Knibb AA, Ponte J, 1991. Morphine-6-glucuronide: effects on ventilation in normal volunteers. Pain 45, 101–104. [DOI] [PubMed] [Google Scholar]
- Roux JC, Peyronnet J, Pascual O, Dalmaz Y, Pequignot JM, 2000. Ventilatory and central neurochemical reorganisation of O2 chemoreflex after carotid sinus nerve transection in rat. J Physiol. 522, 493–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarton E, Teppema L, Dahan A, 1999. Sex differences in morphine-induced ventilatory depression reside within the peripheral chemoreflex loop. Anesthesiology 90, 1329–1338. [DOI] [PubMed] [Google Scholar]
- Shimokawa N, Dikic I, Sugama S, Koibuchi N, 2005. Molecular responses to acidosis of central chemosensitive neurons in brain. Cell Signal. 17, 799–808. DOI: 10.1016/j.cellsig.2005.01.004 [DOI] [PubMed] [Google Scholar]
- Shook JE, Watkins WD, Camporesi EM, 1990. Differential roles of opioid receptors in respiration, respiratory disease, and opiate-induced respiratory depression. Am. Rev. Resp. Dis 142, 895–909. DOI: 10.1164/ajrccm/142.4.895 [DOI] [PubMed] [Google Scholar]
- Smith MT, Watt JA, Cramond T, 1990. Morphine-3-glucuronide: A potent antagonist of morphine analgesia. Life Sci. 1990; 47, 579–585. [DOI] [PubMed] [Google Scholar]
- South SM, Edwards SR, Smith MT, 2009. Antinociception versus serum concentration relationships following acute administration of intravenous morphine in male and female Sprague-Dawley rats: differences between the tail flick and hot plate nociceptive tests. Clin. Exp. Pharmacol. Physiol 36, 20–28. . doi: 10.1111/j.1440-1681.2008.05019.x. [DOI] [PubMed] [Google Scholar]
- Taylor S, Kirton OC, Staff I, Kozol RA, 2005. Postoperative day one: a high risk period for respiratory events. Am. J. Surg 190, 752–756. DOI: 10.1016/j.amjsurg.2005.07.015 [DOI] [PubMed] [Google Scholar]
- Trescot AM, Datta S, Lee M, Hansen H, 2008. Opioid pharmacology. Pain Physician 11(2 Suppl), S133–S153. [PubMed] [Google Scholar]
- Tjong YW, Chen Y, Liong EC, Ip SF, Tipoe GL, Fung ML, 2004. Melatonin attenuates rat carotid chemoreceptor response to hypercapnic acidosis. J. Pineal Res 36, 49–57. [DOI] [PubMed] [Google Scholar]
- Young AP, Gruber RB, Discala JF, May WJ, Palmer LA, Lewis SJ, 2013. Co-activation of μ- and δ-opioid receptors elicits tolerance to morphine-induced ventilatory depression via generation of peroxynitrite. Resp. Physiol. Neurobiol 186, 255–264. DOI: 10.1016/j.resp.2013.02.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Xu F, Zhang C, Liang X, 2007Activation of opioid mu-receptors in caudal medullary raphe region inhibits the ventilatory response to hypercapnia in anesthetized rats. Anesthesiology 107, 288–297. DOI: 10.1097/01.anes.0000270760.46821.67 [DOI] [PubMed] [Google Scholar]
- Zhang Z, Xu F, Zhang C, Liang X, 2009. Opioid mu-receptors in medullary raphe region affect the hypoxic ventilation in anesthetized rats. Resp. Physiol. Neurobiol 168, 281–288. DOI: 10.1016/j.resp.2009.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Zhuang J, Zhang C, Xu F, 2011. Activation of opioid μ-receptors in the commissural subdivision of the nucleus tractus solitarius abolishes the ventilatory response to hypoxia in anesthetized rats. Anesthesiology 115,: 353–363. DOI: 10.1097/ALN.0b013e318224cc1f [DOI] [PubMed] [Google Scholar]
- Zimpfer M, Beck A, Mayer N, Raberger G, Steinbereithner K, 1983. Effects of morphine on the control of the cardiovascular system by the carotid-sinus-reflex and by the carotid chemoreflex. Anaesthesist 32, 60–66. [PubMed] [Google Scholar]


