Abstract
We present the R package bigleaf (version 0.6.5), an open source toolset for the derivation of meteorological, aerodynamic, and physiological ecosystem properties from eddy covariance (EC) flux observations and concurrent meteorological measurements. A ‘big-leaf’ framework, in which vegetation is represented as a single, uniform layer, is employed to infer bulk ecosystem characteristics top-down from the measured fluxes. Central to the package is the calculation of a bulk surface/canopy conductance (Gs/Gc) and a bulk aerodynamic conductance (Ga), with the latter including formulations for the turbulent and canopy boundary layer components. The derivation of physical land surface characteristics such as surface roughness parameters, wind profile, aerodynamic and radiometric surface temperature, surface vapor pressure deficit (VPD), potential evapotranspiration (ET), imposed and equilibrium ET, as well as vegetation-atmosphere decoupling coefficients, is described. The package further provides calculation routines for physiological ecosytem properties (stomatal slope parameters, stomatal sensitivity to VPD, bulk intercellular CO2 concentration, canopy photosynthetic capacity), energy balance characteristics (closure, biochemical energy), ancillary meteorological variables (psychrometric constant, saturation vapor pressure, air density, etc.), customary unit interconversions and data filtering. The target variables can be calculated with a different degree of complexity, depending on the amount of available site-specific information. The utilities of the package are demonstrated for three single-level (above-canopy) eddy covariance sites representing a temperate grassland, a temperate needle-leaf forest, and a Mediterranean evergreen broadleaf forest. The routines are further tested for a two-level EC site (tree and grass layer) located in a Mediterranean oak savanna. The limitations and the ecophysiological interpretation of the derived ecosystem properties are discussed and practical guidelines are given. The package provides the basis for a consistent, physically sound, and reproducible characterization of biometeorological conditions and ecosystem physiology, and is applicable to EC sites across vegetation types and climatic conditions with minimal ancillary data requirements.
Introduction
The eddy covariance (EC) technique provides direct and continuous measurements of the exchange of heat, water vapor, carbon dioxide, and other trace gases between the surface and the lower atmosphere [1, 2]. The method has significantly contributed to our understanding of how this mass and energy exchange is controlled by environmental drivers such as radiation [3, 4], temperature, vapor pressure deficit (VPD) [5, 6], or soil water stress [7], and how it is modulated by meteorological extreme events such as heatwaves [8, 9]. EC data have proven useful to characterize climate and vegetation controls on the partitioning of available energy at the land surface [10] and the resulting surface hydrology [11]. EC data have further allowed a more detailed insight into the coupling of biogeochemical cycles, in particular carbon and water, and its modification by climate and surface conditions [12, 13].
These findings have been achieved by a large scientific community [14, 15], which maintains several hundred EC measurement sites around the globe. The increasing length of available EC data in combination with freely available data processing tools [16, 17], which are partly available in R [18, 19], underline the important role of EC data in present and future ecological and climate change research.
The analysis of EC data does not have to be restricted to direct or partitioned energy and mass flux measurements, but additional ecosystem properties can be derived from a joint analysis of fluxes and meteorological variables. Such additional information can help in obtaining a more comprehensive understanding of the biological and physical processes underlying the measured fluxes (Fig 1). For instance, the aerodynamic conductance (Ga) between the land surface and the instrument height is a key variable describing how effective the ecosystem can transfer mass and energy to the atmosphere. Knowledge of both Ga and the measured energy or mass fluxes allows to infer average conditions at the surface (e.g. temperature, atmospheric humidity, CO2 concentration). This is of interest as conditions at the canopy surface are in general more relevant for ecophysiological processes than those measured at instrument height some distance above the canopy [20].
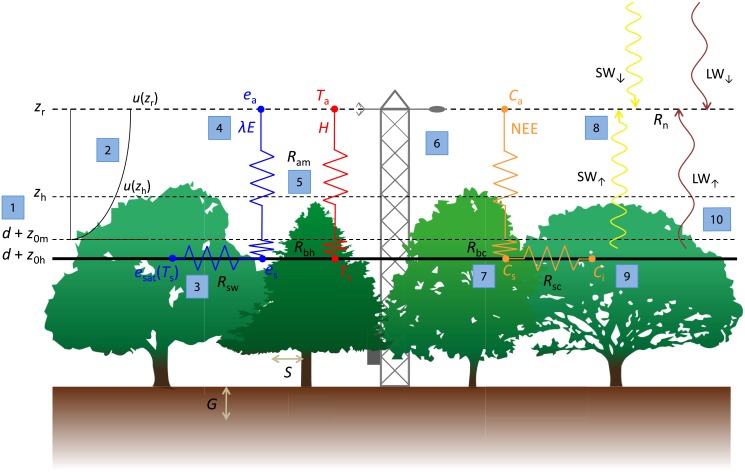
Fig 1. Illustration of the ‘big-leaf’ concept and main functions included in the bigleaf R package.
d is the displacement height, z0h is the roughness length for heat, z0m is the roughness length for momentum, zh is the average vegetation height, zr is the reference (=measurement) height, u is the horizontal wind speed, Rsw is the surface resistance to water vapor, Rsc is the surface resistance to CO2, Rbh is the canopy boundary layer resistance to heat transfer, Rbc is the canopy boundary layer resistance to CO2 transfer, Ram is the aerodynamic resistance to momentum transfer, esat is the saturation vapor pressure at the ‘big-leaf’ surface, es is the vapor pressure at the ‘big-leaf’ surface, ea is the vapor pressure at reference height, λE is the latent heat flux, Ts is the aerodynamic surface temperature, Ta is the air temperature, H is the sensible heat flux, Ci is the bulk intercellular CO2 concentration, Cs is the CO2 concentration at the ‘big-leaf’ surface, NEE is the net ecosystem exchange of CO2, SW↓ and SW↑ are the incoming and outgoing shortwave radiation, respectively, LW↓ and LW↑ are the incoming and outgoing longwave radiation, respectively, and Rn is the net radiation. Numbers denote the following functions: 1) roughness.parameters(); 2) stability.parameter(), stability.correction(), wind.profile(); 3) surface.conductance(), stomatal.sensitivity(), stomatal.slope(); 4) potential.ET(), equilibrium.imposed.ET(), WUE.metrics(); 5) aerodynamic.conductance(), decoupling(); 6) energy.closure(); 7) surface.conditions(); 8) light.response(), light.use.efficiency(); 9) intercellular.CO2(), photosynthetic.capacity(), biochemical.energy(), energy.use.efficiency(); 10) radiometric.surface.temp(). For details on the functions, see section ‘Package content’ or the respective R package help pages.
An important ecophysiological ecosystem property is the surface conductance (Gs). Its vegetation component (canopy conductance (Gc)) is an integrated measure of stomatal conductance and constitutes the main biological control on the exchange of water and carbon dioxide at the land surface. These two central bulk conductances (Ga and Gs) can be combined to assess the aerodynamic coupling between the vegetation and the atmosphere [21], which again indicates the relative importance of key meterological drivers and the degree of physiological control on evapotranspiration (ET) [21, 22]. Ecosystems well coupled to the atmosphere, such as aerodynamically rough forests, are more likely to exhibit stronger stomatal control on transpiration than low-statured ecosystems such as grasslands [21]. At the same time, ET is under stronger control of VPD in well-coupled ecosystems, whereas available energy has been identified as the decisive factor in poorly coupled ecosystems [22].
The derived Gs can be used to infer additional ecophysiological variables at ecosystem level such as intrinsic water-use efficiency metrics [23], intercellular CO2 concentration (Ci) [24], stomatal sensitivity to VPD [6, 25], or photosynthetic capacity [24, 26]. Many of these quantities can be seen as ecosystem scale analogues of parameters derived from leaf level measurements, and in theory constitute time-invariant quantities that characterize ecosystem functioning in a more comparable manner than flux measurements alone [27].
Since the EC method in its traditional application (i.e. single-level and time-averaged measurements) cannot resolve the vertical and horizontal distribution of ecosystem flux sources and sinks, the above described quantities inevitably lack information on the vertical and horizontal structure of the ecosystem as well as on its components (e.g. soil and vegetation) when they are inferred directly from the measured fluxes. Approaches directed to circumvent this limitation are two-level sensor systems [28, 29], techniques resolving the spatio-temporal variability of the fluxes [30], or the inversion of more detailed models which separate e.g. sunlit from shaded canopy fractions [31, 32], soil from canopy components [33], or which represent the canopy as a multi-layered system [34]. These alternative modeling approaches are able to give more detailed and more realistic insights into the underlying physical and physiological mechanisms. However, the additional complexity comes at the cost of higher computational demands as well as higher requirements on ancillary data for model parameterization. A much simpler and more direct way to infer ecosystem properties from EC data is to invert a ‘big-leaf’ model, in which measured fluxes are assumed to origin from a single, homogenous plane. This approach requires little site-specific ancillary information, is widely applicable across sites, and has been shown to give meaningful results within its limits of applicability and validity [35, 36]. Bulk ecosystem properties derived with a top-down ‘big-leaf’ approach are thus commonly presented in EC studies and have proven useful in characterizing vegetation behavior in various ecosystems and under contrasting conditions [10, 29, 37–41].
Despite their relevance for global change research and their widespread appearance, little effort has been put into the development of harmonized calculation protocols for these quantities, and as a consequence, calculated metrics are often not easily comparable, especially with respect to the wide variety of existing methodologies and formulations (e.g. [42]). In this paper, we describe the R package bigleaf, which provides functions to infer Ga, Gs and further physical as well as physiological bulk ecosystem properties from EC data and concurrent meteorological measurements in a consistent and standardized manner. In the following, the main equations are presented and their use is demonstrated for four contrasting EC sites. The limitations of the calculations, arising from methodological constraints and inherent limitations of the ‘big-leaf’ approach, as well as the consequences for the interpretation of the resulting variables, are discussed. The paper ends with practical guidelines on how to use the bigleaf package.
The bigleaf R package
Package design and availability
The bigleaf package is entirely written in the open source software R [43]. The package is available as a stable version from CRAN (https://cran.r-project.org/web/packages/bigleaf) or as a development version (continously updated with git version control) from http://www.bitbucket.org/juergenknauer/bigleaf. This paper describes package version 0.6.5 (git commit: fcada22). An overview of the main functions is illustrated in Fig 1. In the following, the theory underlying the package’s key functions is shortly presented. For technical details on the functions, the reader is directed to the functions’ help pages and examples therein.
The ‘big-leaf’ framework
All functions provided in this package are based on the ‘big-leaf’ framework (Fig 1) [44], which assumes that a single plane located at height d + z0h (d = displacement height, z0h = roughness length for heat) is the single source and sink of all mass and energy fluxes, and that wind speed is zero at height d + z0m (z0m = roughness length for momentum) and increases exponentially with height. This approach does not distinguish fluxes from different compartments of the ecosystem (e.g. soil and vegetation), nor does it account for vertical variations within the canopy or horizontal heterogeneity due to e.g. different species. The derived quantities at the ‘big-leaf’ surface must thus be regarded as average (but representative) conditions of the tower footprint. The main principle of the bigleaf package is to derive ecosystem surface properties from the observations using a top-down (inversion) approach.
Package content
Data filtering
The bigleaf package does not provide functionalities to pre-process raw EC data or to assess the quality of individual datapoints. Instead, the package relies on correctly pre-processed, aggregated, quality-flagged, and friction velocity (u*) filtered fluxes and meteorological measurements (e.g. [19, 45]). Further, some analyses presented in this paper are only meaningful if certain meteorological conditions are met (e.g. daytime or rainfree periods, see below).
The package offers a basic data filtering routine (function filter.data()), which filters EC data based on the aforementioned criteria. The function consists of two parts: 1) Quality control: data points of bad quality (e.g. gap-filled with poor confidence) are discarded, and 2) Meteorological filtering: variables falling out of the (purpose-specific) accepted range (e.g. nighttime values, precipitation events) are filtered out. The filter.data() function returns the input data frame in wich time periods that do not fulfill the filter criteria are set to NA.
Constants, unit interconversions, and sign convention
The package combines all required constants into one list that can be evoked by calling bigleaf.constants(). This list is passed as a default argument to all functions that use one or more constants. Thus, individual constants do not have to be provided for any function call, but can be changed by calling the argument explicitly. As a basis for many calculation steps, common unit interconversions are provided:
Conductances between mass and molar units (m s−1 and mol m−2 s−1)
Water fluxes between mass and energy units (kg m−2 s−1 and W m−2)
Carbon fluxes between mass and molar units (g C m−2 d−1 and μmol CO2 m−2 s−1)
Atmospheric humidity between vapor pressure deficit (kPa), vapor pressure (kPa), specific humidity (kg kg−1), and relative humidity
Radiation between energy and molar units (W m−2 and μmol m−2 s−1)
The sign convention is that fluxes directed away from the surface are positive and those directed toward the surface are negative. Thus, negative net CO2 ecosystem exchange (NEE) values indicate a net uptake of CO2 by the ecosystem.
Meteorological variables
Most of the central functions in the bigleaf package require meteorological variables that are not commonly provided by the processed EC products, but which can be readily calculated from standard meteorological variables like air temperature, humidity, and atmospheric pressure. For reasons of space, the individual formulations are not presented here, instead the user is directed to the help page of the respective function and the references therein. All functions apply textbook calculations and include:
latent heat of vaporization: latent.heat.vaporization(Ta)
psychrometric constant: psychrometric.constant(Ta, p)
saturation vapor pressure and slope of the saturation vapor pressure curve: Esat.slope(Ta)
air density: air.density(Ta, p)
virtual temperature: virtual.temp(Ta, q)
wet-bulb temperature: wetbulb.temp(Ta, p, Da)
dew point: dew.point(Ta, Da)
where Ta is the air temperature (°C), p is the atmospheric pressure (kPa), q is the specific humidity (kg kg−1), and Da is the vapor pressure deficit (kPa). If p is not available, it can be approximated by the hypsometric equation as a function of site elevation (pressure.from.elevation()).
Aerodynamic conductance
Aerodynamic conductance to heat transfer (Gah) is central to the ‘big-leaf’ concept and multiple formulations have been proposed. Gah can be written as
| (1) |
where Ram is the aerodynamic resistance to momentum transfer with turbulence as the principal transport mechanism, and Rbh is the canopy (quasi-laminar) boundary layer resistance (“excess resistance”) to heat transfer, which is characterized by molecular diffusion as the dominant transport mechanism [46, 47]).
At EC sites, Gam can be calculated directly as (e.g. [46, 48](aerodynamic.conductance()):
| (2) |
where u* is friction velocity (m s−1) and u(zr) is wind speed (m s−1) at reference (=measurement height)(m).
Eq 2 implicitly accounts for the effects of atmospheric stability on Gam. Nevertheless, an alternative and frequently used formulation is provided, which explicitly accounts for the effects of atmospheric stability ([46]):
| (3) |
where k is the von Kármán constant (0.41), d is the zero plane displacement height (m), z0m is the roughness length for momentum (m), and ψh is the integrated form of the stability correction function for heat and water vapor. ψh is a function of the atmospheric stability parameter ζ = (zr − d)/L, where L is the Monin-Obukhov length. The function stability.correction() can be used to calculate ψh based on formulations suggested by [49] or [50]. The two roughness parameters d and z0m have to be determined a priori. The function roughness.parameters() provides three options: 1) an empirical approach assuming d and z0m as constant fractions of canopy height zh (by default d = 0.7zh and z0m = 0.1zh), 2) a semi-empirical approach estimating both z0m and d based on zh and leaf area index (LAI) according to [51] for data presented in [52], and 3) an approach that calculates z0m from the logarithmic wind profile equation with a prescribed d. Note that d and z0m, as well as all other ancillary variables (e.g. LAI), can be provided as time-varying vectors with the same length as the input data frame.
Multiple formulations have been suggested for the calculation of the canopy (quasi-laminar) boundary layer conductance to heat transfer (Gbh), which range from empirical to physically-based (see [53, 54] for an overview). [55] suggested a simple empirical relationship between Gbh and u* (Gb.Thom()):
| (4) |
Several further (semi-) empirical formulations have been suggested, but we restricted the functions to those best applicable to EC sites. In that respect, relationships based on the Reynolds number, which have been found to show a biphasic behavior [56], are currently not implemented. More mechanistic, but also parameter-rich approaches commonly require LAI and aerodynamically-relevant foliage characteristics (leaf width or leaf characteristic dimension). The formulation suggested by [51] is given by (Gb.Choudhury()):
| (5) |
where α is an attenuation coefficient modeled in dependence on LAI according to data presented in [57], u(zh) is wind speed (m s−1) at canopy height zh, and w is leaf width (m). Wind speed at height zh (or any other height z > d + z0m can be estimated from the logarithmic wind profile equation (wind.profile()):
| (6) |
where ψm is the integrated form of the stability correction function for momentum (as calculated in stability.correction()). A third model currently implemented in the bigleaf package was developed by [47] and simplified by [58](Gb.Su()):
| (7) |
where Cd is a foliage drag coefficient (assumed constant with a value of 0.2 [47]), fc is fractional vegetation cover, Ct is a heat transfer coefficient, and is the inverse Stanton number for bare soil surface [58]. Ct mainly depends on the leaf characteristic dimension and the number of leaf sides participating in heat transfer, see [47] and [58] for details. The denominator of Eq 7 is often referred to as the parameter (e.g. [54]), which is defined as:
| (8) |
From Eq 8 the roughness length for heat (z0h) can be determined.
Note that Gam is identical for different scalars in the atmosphere (heat, water vapor, CO2, and other trace gases), whereas Gb differs with respect to the quantity of interest. Gb of quantity x can be calculated based on Gbh [59]:
| (9) |
where Pr is the Prandtl number (0.71), and Scx is the Schmidt number for quantity x. For simplicity, the assumption is made that Gb is identical for heat and water vapor transfer (i.e. Gbh = Gbw). The more realistic difference of a few percent [59] is considered small compared to other uncertainties (see also [60]).
Since the calculations of Gam and Gbh are independent, the bulk aerodynamic conductance to heat transfer (Gah) can be calculated as the sum of the inverse versions of Eqs 2, 3 and 4–7. The main function aerodynamic.conductance() returns Gam, Gah, Gbh, Gac (aerodynamic conductance to CO2 transfer), Gbc, the corresponding resistances, and , ζ, as well as ψh. If one or more additional Schmidt numbers are provided, Ga and Gb are calculated for the respective quantities as well. Due to the modular structure of the functions, each of these components can also be calculated individually.
Surface conditions
EC measurements are accompanied by meteorological measurements taken at approximately the same height as the flux measurements, usually several meters above the canopy. If Ga is determined, the bulk transfer relations can be inverted and solved for the surface variable [23, 29]((surface.conditions())):
| (10) |
| (11) |
| (12) |
| (13) |
where H is the sensible heat flux (W m−2), ρ is the air density (kg m−3), cp is the heat capacity of dry air (J K−1 kg−1), e is vapor pressure (kPa), λE is the latent heat flux (W m−2), γ is the psychrometric constant (kPa K−1), esat is the saturation vapor pressure, D is the vapor pressure deficit (kPa), and C is the CO2 concentration. Subscripts a and s denote air and surface, respectively. Note that in Eqs 10–13 “surface conditions” refer to the notional canopy surface. It is also possible to infer conditions in the intercanopy airspace by replacing Gah in Eqs 10 and 11 or Gac in Eq 13 with Gam. The function surface.conditions() returns Ts, esat(Ts), es, Ds, qs, rHs, and Cs. This method can be applied to other atmospheric constituents measured at EC sites (e.g. methane, nitrogen oxides, ozone), provided that the corresponding Ga is known (see above).
An alternative estimate of surface temperature is based on the physical principle that any object emits longwave radiation in dependence of its temperature as described by the Stephan-Boltzmann relation. This radiometric surface temperature (Tr, in Kelvin) is given by (e.g. [61], radiometric.surface.temp()):
| (14) |
where LW↑ and LW↓ are longwave upward and longwave downward radiation (W m−2), respectively, σ is the Stefan-Boltzmann constant (W m−2 K−4), and ϵ is the emissivity of the surface.
Surface conductance
Surface conductance to water vapor (Gsw in m s−1), describes the conductance of the entire surface, i.e. including soil and plant canopy components. It is commonly calculated by inverting the Penman-Monteith (PM) equation (surface.conductance()):
| (15) |
where s is the slope of the saturation vapor pressure curve (kPa K−1), Rn is the net radiation (W m−2), G is the ground heat flux (W m−2), and S is the sum of all energy storage fluxes (W m−2).
Eq 15 implicitly assumes that Ga for water vapor equals Ga for heat, i.e. Gah = Gaw which corresponds to an amphistomatous vegetation where the transfer of both heat and water vapor occurs at both leaf sides. The hypostomatous case (water vapor transfer from one side only) is conceptually not straightforward at the canopy level [22, 42], and is thus currently not implemented in this package. Eq 15 further assumes that the energy balance is closed (i.e. Rn − G − S = λE + H). The derived Gsw and all subsequent derivations are sensitive to violations of this assumption [23, 62]. The function surface.conductance() offers the calculation of Gsw according to Eq 15, and a simplified (but also less realistic) formulation based on a simple flux-gradient approach, which assumes infinite Gah: Gsw = λE/λ/(Da/p). This formulation is equivalent to the one proposed by [63].
Vegetation-atmosphere decoupling
With both Gah and Gsw available, the degree of aerodynamic decoupling between the land surface and the atmosphere can be assessed with the decoupling coefficient Ω, which takes values between 0 and 1. Low values indicate well-coupled conditions and a high degree of physiological control on ET. Values close to 1 indicate the opposite, i.e. poorly coupled conditions and a low sensitivity of ET to Gsw [21, 22]. In its simplest and most commonly used form, Ω is given by [21] (decoupling()):
| (16) |
Eq 16 was modified by [64], who included the effects of radiative coupling between the vegetation and the atmosphere:
| (17) |
where Gr is the longwave radiative transfer conductance of the canopy (m s−1), calculated as (longwave.conductance()). Note that, as in the PM equation (Eq 15), Eqs 16 and 17 assume that the vegetation is amphistomatous [21].
Imposed and equilibrium evapotranspiration
The concept of decoupling is often used to characterize physiological and energy controls on transpiration. In addition it can help to quantify radiation and VPD controls on λE (e.g. [65]). λE can be written in an alternative way [21](equilibrium.imposed.ET()):
| (18) |
where
| (19) |
and
| (20) |
Eqs 19 and 20 are derived directly from the PM equation by letting Gah approach 0 or ∞, respectively. Thus, λEeq is the λE rate that would occur if the surface was completely decoupled from the atmosphere. In this case, λE is strongly controlled by Rn. Likewise, λEimp can be interpreted as the λE rate that would occur under fully coupled conditions, in which case λE is mainly dependent on Gsw and Da.
Potential evapotranspiration
Potential evapotranspiration (λEpot) is frequently used to characterize atmospheric demand and the degree of climatic aridity (e.g. [11]). Here, λEpot is by default calculated from the Priestley-Taylor equation [66] (potential.ET()):
| (21) |
where α is the Priestley-Taylor coefficient, which accounts for large-scale advection effects. Its value is usually set to 1.26, but it likely varies with surface conditions [67]). λEpot can further be calculated from the PM equation with a prescribed Gsw [6], which may correspond to typical maximum values (e.g. 95% quantile) found in the ecosystem:
| (22) |
Energy balance
The package contains basic functionalities to characterize energy balance closure at EC sites. The function energy.closure() quantifies the energy balance closure (Rn − G − S = λE + H) with both the slope method and the energy balance ratio (EBR) as described in [68]. The package further enables the calculation of biochemical energy (Sp), a small and therefore often neglected component of the energy balance: Sp = αNEE, where α = 0.422 J mol−1 denotes the biochemical energy taken up/released by photosynthesis/respiration per mole of CO2 fixed/respired [69]. The function energy.use.efficiency() provides a simple estimate of the energy use efficiency (EUE) of the ecosystem: EUE = Sp/Rn.
Physiological ecosystem quantities
For ecosystems that have a largely closed vegetation cover, and under conditions when canopy and soil surfaces are not wet, the derived Gs can be interpreted as a proxy for the canopy-integrated stomatal conductance (i.e. canopy conductance Gc) [36]. Gs may then be used to calculate additional physiological quantities. The function stomatal.slope() returns an estimate of the stomatal slope parameter G1 at ecosystem level, analogous to g1 at leaf level [41] (Note that in this paper, uppercase and lowercase letters denote physiological quantities at ecosystem and leaf-level, respectively). G1 is estimated using non-linear regression from the unified stomatal model (USO) [70]:
| (23) |
where G0 is the minimum canopy conductance (mol m−2 s−1), and GPP is gross primary productivity (μmol CO2 m−2 s−1). Ds and Cs represent conditions at the notional ‘big-leaf’ surface in this case (Eqs 12 and 13, respectively), but they are often replaced by the measured values at instrument height (i.e. Da and Ca [41]. G0 can either be estimated along with G1, or fixed to a user-defined value (e.g. set to 0). In addition to Eq 23, G1 can be calculated from the stomatal model proposed by [71], or from its modified version suggested by [72]. Note that absolute values and units of G1 differ across models. GPP is not directly measured at EC sites but inferred from NEE-partitioning algorithms (e.g. [73, 74]). GPP is further not directly analogous to leaf-level net photosynthesis (An), and ecosystem leaf day respiration, if available, may be subtracted from GPP to better represent canopy-level An [24, 75].
The package further includes several alternative water-use efficiency (WUE) metrics (WUE.metrics()) which can be calculated more readily from the measured fluxes, but which contain less physiological information [23]. Examples are WUE (= GPP/ET), inherent WUE (IWUE = (GPP Da)/ET) [12], or underlying WUE ()/ET) [13].
Stomatal sensitivity to VPD, a relevant indicator of vegetation water-use strategy, can be characterized with the following function [76] (stomatal.sensitivity()):
| (24) |
where the two parameters m (mol m−2 s−1 ln(kPa)−1) and b (mol m−2 s−1) represent the sensitivity of Gsw to Ds (Da can be used alternatively) and the reference Gsw at Ds of 1 kPa, respectively [6, 76].
Bulk canopy intercellular CO2 concentration (Ci in μmol mol−1) can be inferred from Fick’s first law analogously to the calculation of ci at leaf level (see e.g [24, 77], intercellular.CO2()):
| (25) |
where Cs is the CO2 concentration at the ‘big-leaf’ surface (μmol mol−1; Eq 13), which can also be approximated by Ca. Gsc denotes the surface conductance to CO2 (mol CO2 m−2 s−1) and is calculated as Gsc = Gsw/1.6.
With Ci available, the ‘big-leaf’ concept may be further expanded to calculate an estimate of basic photosynthetic parameters such as the maximum carboxylation rate (Vcmax) and maximum electron transport rate (Jmax) at canopy level (e.g. [24, 26, 78], photosynthetic.capacity()). The calculation is once more analogous to that at leaf level, where commonly the model developed by [79] is employed. Note however, that especially for Vcmax and Jmax the interpretation differs from that at leaf level (see Discussion). From the Rubisco-limited photosynthesis rate (when carboxylation is the rate limiting process i.e. GPP = GPPc, usually under high radiation), Vcmax (μmol m−2 s−1) can be calculated as:
| (26) |
where Kc (μmol mol−1) and Ko (mmol mol−1) are the Michaelis-Menten constants for CO2 and O2, respectively, Oi (mol mol−1) is the O2 concentration, and Γ* (μmol mol−1) is the photorespiratory CO2 compensation point (μmol mol−1). All photosynthetic parameters and their temperature responses (activation energies) are taken from [80] and assume infinite mesophyll conductance to CO2 transfer. Under conditions when Ribulose 1,5-bisphosphate (RuBP)-regeneration is limiting photosynthesis (i.e. GPP = GPPj), the electron transport rate J (μmol m−2 s−1) is given by:
| (27) |
Jmax is then calculated from the following relation:
| (28) |
where APPFDPSII is absorbed photosynthetic photon flux density (PPFD) by photosystem II (μmol m−2 s−1), and Θ is a curvature parameter. APPFDPSII is currently assumed to be a constant fraction of PPFD (by default APPFDPSII = 0.8PPFD), but a more realistic estimate of APPFD, depending on solar elevation angle and LAI, will be implemented in the future. Bulk canopy photosynthesis is assumed to be limited by either Rubisco activity (GPP = GPPc) or RuBP-regeneration (GPP = GPPj) at high and low radiation, respectively, and simple radiation thresholds are applied to separate the two limitation states. Vcmax and Jmax are temperature-dependent and are normalized to the reference temperature of 25°C (i.e. Vcmax,25 and Jmax,25) using a modified Arrhenius equation as described in e.g. [81] with default parameter values from [80] and [82].
Ecosystem light response curves (LRCs) are useful to characterize both the CO2 uptake rate at light saturation as well as the light utilization efficiency (i.e. the initial slope). The most frequently used model is the rectangular hyperbolic LRC, which can be written in a general form as [83] (light.response()):
| (29) |
where α is the initial slope of the light-response curve (μmol CO2 m−2 s−1 (μmol quanta m−2 s−1)−1), Reco is ecosystem respiration (μmol CO2 m−2 s−1), and PPFDref is the PPFD value at which GPPref (μmol CO2 m−2 s−1) is calculated (usually at saturating light, e.g. at 2000 μmol m−2 s−1). Additionally, a simple light-use efficiency (LUE) metric, defined as the ratio of cumulative GPP to cumulative PPFD, is available in the package (light.use.efficiency()).
Case studies
Single-level EC sites
Three sites with EC measurements at a single level above the canopy were chosen for the demonstration of the formulations described above: AT-Neu (Neustift), a managed grassland in Austria [84], DE-Tha (Tharandt), a high-statured (mean canopy height = 26.5m) spruce forest in Eastern Germany [85], and FR-Pue (Puechabon), a Mediterranean evergreen oak forest in southern France, which is subject to seasonal water stress [86]. The location as well as basic ecosystem properties for these sites are listed in Table 1. Data are freely available from the FLUXNET2015 dataset (http://fluxnet.fluxdata.org/data/fluxnet2015-dataset/; accessed on 2016-11-09). Subsetted dataframes are included in the package and are automatically loaded when the package is attached. Data underwent standard postprocessing (e.g u* filtering, gap-filling, NEE-partitioning) as detailed on the FLUXNET2015 webpage (http://fluxnet.fluxdata.org/data/fluxnet2015-dataset/data-processing/; accessed on 2018-04-19).
Table 1. Characteristics of the three single-level case study sites.
| site | lon (°) |
lat (°) |
elevation (m) |
MAP (mm) |
MAT (°C) |
vegetation type |
zh (m) |
max. LAI |
|---|---|---|---|---|---|---|---|---|
| AT-Neu | 11.32 | 47.12 | 970 | 852 | 6.30 | grassland | 0.5a | 6a |
| DE-Tha | 13.57 | 50.96 | 385 | 843 | 8.20 | spruce forest | 26.5 | 7.6 |
| FR-Pue | 3.60 | 43.74 | 48 | 883 | 13.50 | holm oak forest | 5.5 | 3.3 |
a highly variable throughout the growing season [84]. LAI = 5 in subsequent calculations
Seasonal courses of Gs, Ga and vegetation-atmosphere decoupling
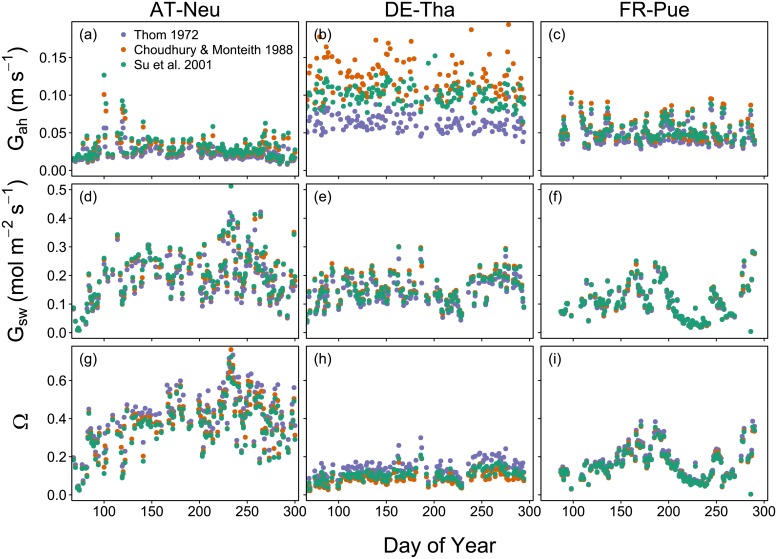
We calculated seasonal dynamics of aerodynamic and surface conductance to water vapor, as well as the decoupling coefficient Ω (Fig 2). The results reveal that Gah is relatively constant over the course of the year, but differs in magnitude across sites. As expected, highest values can be found in the aerodynamically rough spruce forest DE-Tha, and lowest values in the meadow AT-Neu. FR-Pue shows intermediate values. Differences between the different Gah versions result from different models of the bulk boundary layer conductance (Gbh; Eqs 4–7). The different Gbh formulations agree well for AT-Neu and FR-Pue, but lead to clear differences in estimated Gah for DE-Tha. This is likely because the Choudhury (Eq 5) and Su (Eq 7) models consider additional aerodynamic properties (e.g. leaf size, LAI) that are neglected in the Thom model (Eq 4). Thus, accounting for the low leaf characteristic dimension / leaf width and high LAI in DE-Tha leads to a higher Gah in the Su and especially in the Choudhury formulation compared to the Thom model. The differences in Gah among the formulations do not have strong effects on the derived Gsw and Ω. Gsw shows pronounced seasonal dynamics at all three sites. Lowest values correspond to inactive vegetation, as e.g. caused by soil water stress (DOY 190-240 in FR-Pue). The dynamics in Gsw are clearly reflected in Ω, the magnitude of which differs considerably across sites. AT-Neu (grassland) is relatively poorly coupled, whereas DE-Tha (forest) shows a high degree of coupling. All three sites show typical values for the respective vegetation type [87].
Fig 2. Seasonal courses of mean daily values of aerodynamic conductance to heat transfer (Gah), surface conductance to water vapor (Gsw), and decoupling coefficient (Ω) for the year 2012.
Data were filtered for rainfree periods (24h after rainfall excluded), daylight (PPFD > 200 μmol m−2 s−1), and positive λE. Gsw was calculated according to Eq 15, and Ω according to Eq 16. Three different Gah formulations (Eqs 2 and 4–7), denoted by different colors, were used as input variables for the respective functions.
Surface conditions
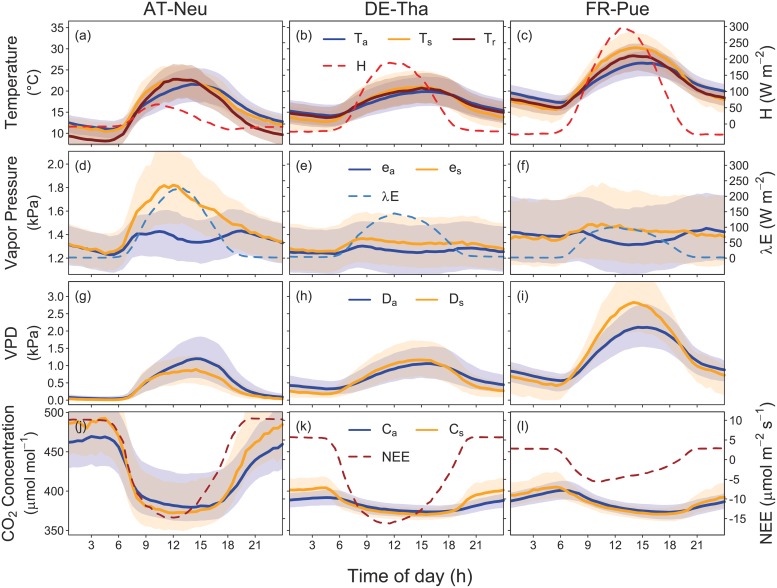
Fig 3 depicts mean diurnal courses of air temperature, vapor pressure, VPD, and CO2 concentration and the respective surface variables as calculated from Eqs 10–13 for the summer months June, July, and August (JJA) of all available site years. At all three sites, aerodynamic surface temperature Ts (Eq 10) exceeds air temperature at daytime and is lower at nighttime. Ts—Ta is largely parallel to the course of H throughout the day. The inferred temperature difference depends not only on the magnitude of H, but also on Gah. It follows that the grassland AT-Neu has a more pronounced temperature difference for the same H than the forest DE-Tha owing to its lower efficiency to transfer heat to the atmosphere (i.e. lower Gah). Temperature gradients are most pronounced at FR-Pue (approx. 4°C at midday) where a large fraction of the available energy goes into H. Radiometric surface temperature (Tr; Eq 14) generally agrees well with Ts, but shows biases at some timeperiods (e.g. AT-Neu at night). Differences between Ts and Tr can be caused by inappropriate emissivity values, biases in the estimated Gah, or differences in the spatial representativeness of radiation (LW↑) and flux (H) measurements.
Fig 3. Median diurnal courses of measured air and respective derived ‘big-leaf’ surface variables for the summer months of all available site years (JJA).
Lines depict median diurnal courses of all available site years and shaded areas the interquartile range. Surface conditions were calculated with Ga calculated from Eqs 2 and 7 (with Dl taken as 0.02, 0.008, and 0.035 m for AT-Neu, DE-Tha, and FR-Pue, respectively). Radiometric surface temperature in panels a-c was calculated according to Eq 14 assuming a constant longwave emissivity of 0.98.
The derived vapor pressure at the ‘big-leaf’ surface (es) exceeds the measured values at instrument height (ea) at all three sites during daytime. The water vapor gradient at AT-Neu is significantly higher than at the other two sites, which is caused by the relatively high λE and low Gah. The high es at AT-Neu leads to a decrease of surface VPD (Ds) compared to air VPD (Da). In contrast, the temperature effect on VPD is stronger than the moisture effect in DE-Tha and FR-Pue, with the consequence that Ds exceeds Da at daytime at these two sites. Future analyses should be directed to the question whether these patterns hold across sites and vegetation types.
The difference of CO2 concentration at the ‘big-leaf’ surface (Cs) to the concentration in the atmosphere (Ca) follows the diurnal pattern of NEE (Fig 3j–3l). Daytime photosynthetic CO2 uptake and nocturnal ecosystem respiration lead to lower or higher CO2 concentrations, respectively, at the surface compared to the air. The absolute differences are generally low (< 10 μmol mol−1), but may exceed 20 μmol mol−1 under conditions of high biological activity and low turbulent mixing.
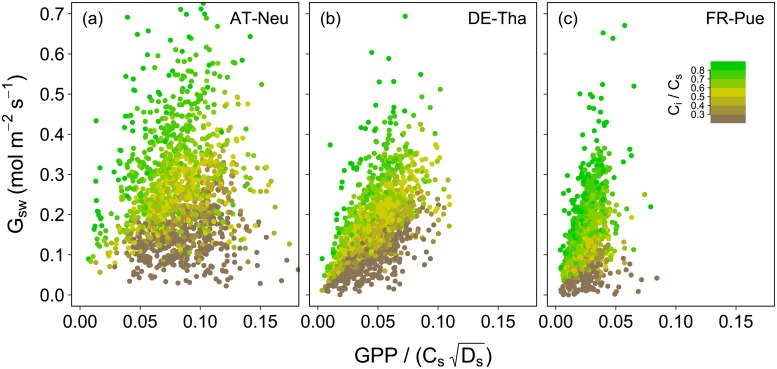
Relationship between Gs and GPP
Fig 4 illustrates the relationship between Gsw and the “stomatal index”, i.e. GPP adjusted for VPD and CO2 concentration [41] for the year 2012. The relationship between these two quantities characterizes intrinsic WUE (iWUE) at ecosystem level and provides essential information on the physiological basis of ecosystem WUE. The slope of the depicted relationship approximates the G1,USO parameter (“stomatal slope”) with higher slopes corresponding to a lower iWUE. Points in Fig 4 are colored according to the Ci/Cs ratio, which is again closely related to iWUE. High Ci/Cs correspond to high stomatal slopes and lower WUE, and the opposite is the case for low Ci/Cs. The relationship between Gsw and the “stomatal index” shows large scatter, especially at AT-Neu, which indicates variations of iWUE throughout the growing season. Such variations within one year may be caused by changes in phenology, LAI (as e.g. caused by mowing) or the onset of water stress.
Fig 4. Surface conductance (Gsw) plotted against .
The slope of the relationship corresponds approximately to the G1,USO parameter (Eq 23). Different colors denote the ratio of bulk intercellular CO2 concentration (Ci; Eq 25) to ‘big-leaf’ surface CO2 concentration (Cs; Eq 13). Shown are data for rainfree periods in the growing season of 2012 (see text for details on data filtering).
Two-level EC site
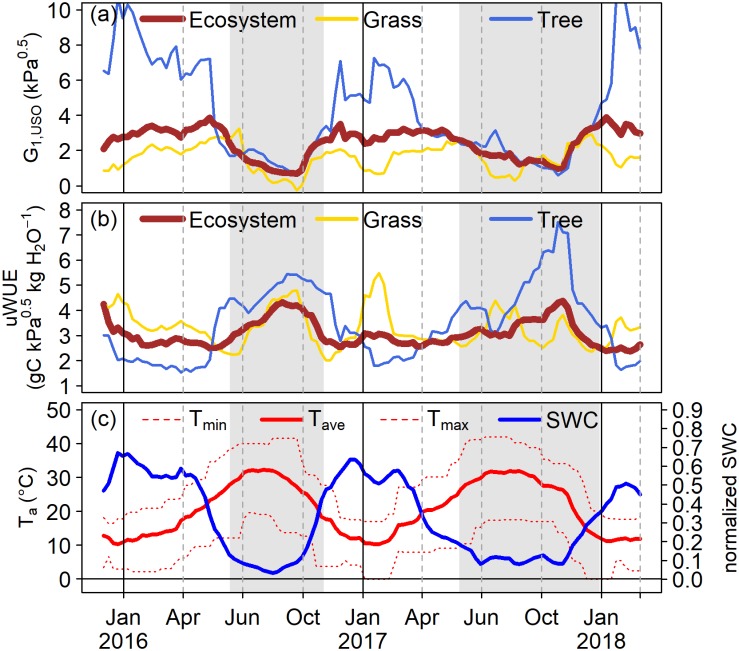
The package was further applied to data from the site ES-LMa (Majadas de Tietar), where fluxes and meteorology were measured at two different heights. The site (39°56’N; 5°46’W, 260 m a.s.l.) is an open woodland with a tree canopy cover (mainly Quercus ilex) of about 20% [88]. Ecosystem fluxes were measured at 15.5 m above ground (7 m above tree canopy height) and grass layer fluxes were measured with a second tower at 1.65 m height. Tree fluxes were derived as the differences of the ecosystem fluxes and the grass layer fluxes similar to [28, 29].
G1,USO and uWUE were calculated for a moving window of +/- 3 weeks which was shifted by one week for each calculation. This procedure was done for the ecosystem, grass layer and trees. Minimum, maximum and mean of mean daily air temperature and soil water content were calculated for the same period.
Differences in G1,USO follow clear seasonal patterns (Fig 5) depending on water availability, VPD (which follows air temperature), and the associated growth and senescence of the grass layer. Ecosystem G1,USO is relatively constant during the growing periods of 2016 and 2017 (winter and spring). G1,USO of the grass layer is more variable as compared to the ecosystem. This mirrors the seasonal dynamics and fast responses of the grass layer to environmental conditions. For G1,USO of the grass layer a pronounced increase is visible before G1,USO drops during the summer drought. The increase is due to the rapid drop in GPP as the grasses start wilting due to drying of the top soil, while λE reduces much slower due to soil evaporation from deeper layers. The subsequent drop in G1,USO is then caused by the continuous reduction in λE during the dry period as the deeper soil layers are also drying out. Q. ilex trees are rather isohydric and react to increasing VPD by closing their stomata to reduce water losses, which results in a decreasing G1,USO. In 2017, G1,USO of the trees decreases more slowly compared to 2016, which is most likely caused by several rain pulses that increased the water availability and reduced VPD as compared to the long lasting dry period in 2016. G1,USO (Fig 5a) and the uWUE (Fig 5b) show strongly anti-correlated patterns. As G1,USO increases the uWUE reduces and vice versa. The trees are able to strongly increase their uWUE as atmospheric humidity and soil water availability are reduced.
Fig 5.
(a) Time series of the stomatal slope parameter G1,USO and (b) underlying water-use efficiency (uWUE) calculated for the whole ecosystem (brown), the grass layer (yellow) and the trees (blue) between December 2015 and March 2018. (c) Time series of minimum, maximum and mean daily air temperature and normalized soil water content for the same period. Grey shaded areas denote dry periods associated with a wilted grass layer.
Calculated ecosystem characteristics
Tables 2 and 3 present physical and physiological ecosystem properties, respectively, of the four study sites. All quantities represent median growing season values of multiple site years, i.e. have to be interpreted as multi-year averages. Site years used for the calculations were 2002-2012 for AT-Neu, 1996-2014 for DE-Tha, 2000-2014 for FR-Pue, and November 2015—November 2017 for ES-LMa. Growing season was delineated using filter.data() with tGPP = 0.5, ws = 15, min.int = 5 (relative GPP threshold, window size (days), minimum interval (days)). Data were filtered using site-specific, multi-year averaged u* thresholds, daytime conditions (PPFD > 200 μmol m−2 s−1), and rainfree periods (24h after rainfall excluded). Data were further filtered for Da > 0.01 kPa, λE > 0 W m−2 and Ta > 5C. Gah was calculated according to Eqs 2 and 7, unless stated otherwise. More information on the ancillary data used for the calculations can be found under http://www.bitbucket.org/juergenknauer/bigleaf/src/master/ancillary. Note that for this study, ancillary variables (e.g. LAI, zh, zr) were assumed to be constant throughout all site years. In many cases, however, they vary across the growing season or among years. Thus, for a more realistic representation of the calculated ecosystem properties, required ancillary variables, if available, should be provided at an adequate temporal resolution. In general, computations in the bigleaf package are fast, e.g. with a state-of-the-art PC it takes < 0.1 seconds to calculate Gs for 10 site years and 2-3 seconds to calculate all properties as shown in Tables 2 and 3.
Table 2. Median daytime physical ecosystem properties in the growing season calculated with the bigleaf package.
| AT-Neu | DE-Tha | FR-Pue | ES-LMa | ES-LMagrass | ES-LMatrees | |
|---|---|---|---|---|---|---|
| Ram (s m−1) | 31.6 | 7.1 | 10.9 | 13.0 | 28.7 | 11.6 |
| Rah,Thom (s m−1) | 47.3 | 15.6 | 20.4 | 23.9 | 46.2 | 21.8 |
| Rah,Choudhury (s m−1) | 38.0 | 8.1 | 18.1 | 21.3 | 74.4 | 21.2 |
| Rah,Su (s m−1) | 36.3 | 9.6 | 16.3 | 21.1 | 36.5 | 21.1 |
| Rac,Su (s m−1) | 37.8 | 10.3 | 18.0 | 23.6 | 39.2 | 24.0 |
| Rbh,Thom (s m−1) | 15.6 | 8.5 | 9.4 | 10.9 | 17.7 | 10.0 |
| Rbh,Choudhury (s m−1) | 6.3 | 1.0 | 7.0 | 8.0 | 41.9 | 9.3 |
| Rbh,Su (s m−1) | 4.8 | 2.5 | 5.3 | 8.0 | 8.3 | 9.4 |
| 1.6 | 2.2 | 2.1 | 1.9 | 1.5 | 2.0 | |
| 0.7 | 0.3 | 1.6 | 1.5 | 3.8 | 1.9 | |
| 0.5 | 0.7 | 1.2 | 1.5 | 0.7 | 1.9 | |
| ΩJarvis | 0.49 | 0.13 | 0.14 | 0.22 | 0.41 | 0.08 |
| ΩMartin | 0.38 | 0.10 | 0.12 | 0.19 | 0.35 | 0.07 |
| (m) | 0.05 | 2.65 | 0.55 | 0.80 | 0.02 | 0.80 |
| (m) | 0.04 | 1.42 | 0.48 | 0.78 | 0.02 | 0.86 |
| (m) | 0.05 | 1.74 | 0.43 | 0.38 | 0.05 | 0.48 |
| ζ | -0.021 | -0.085 | -0.034 | -0.052 | -0.030 | -0.017 |
| L (m) | -11.8 | -137.1 | -87.7 | -62.7 | -12.1 | -153.2 |
| u(zh)/u(zr) | 0.29 | 0.62 | 0.48 | 0.60 | 0.02 | 0.56 |
| Ts − Ta(°C) | 1.0 | 1.3 | 2.1 | 1.9 | 1.6 | 0.9 |
| Tr − Ta(°C) | 0.2 | 0.5 | 0.9 | 2.5 | 2.7 | 2.6 |
| es − ea (kPa) | 0.35 | 0.06 | 0.09 | 0.15 | 0.27 | 0.04 |
| Ds − Da (kPa) | -0.16 | 0.12 | 0.23 | 0.11 | -0.03 | 0.09 |
| Cs − Ca (μmol mol−1) | -13.8 | -3.3 | -2.8 | -3.1 | -4.7 | -1.8 |
| λEpot,PT (W m−2) | 247.5 | 310.7 | 353.0 | 333.9 | 187.3 | 152.9 |
| λEpot,PM (W m−2) a | 265.5 | 226.7 | 227.1 | 268.5 | 214.5 | 132.2 |
| λEeq (W m−2) | 196.4 | 246.6 | 280.1 | 265.0 | 148.6 | 121.3 |
| λEimp (W m−2) | 163.4 | 91.7 | 71.4 | 98.0 | 129.3 | 28.3 |
| EBR | 0.80 | 0.81 | 0.69 | 0.70 | 0.99 | 0.59 |
| EB slope | 0.72 | 0.76 | 0.64 | 0.67 | 0.98 | 0.43 |
| EB intercept (W m−2) | 21 | 18 | 19 | 10 | 3 | 27 |
| Sp (W m−2) | 6.2 | 5.5 | 2.8 | 2.3 | 2.0 | 1.3 |
| EUE | 0.038 | 0.022 | 0.012 | 0.012 | 0.021 | 0.008 |
a with Gsw,ref taken as the 95% quantile of Gsw
Table 3. Median daytime physiological ecosystem properties in the growing season calculated with the bigleaf package.
| AT-Neu | DE-Tha | FR-Pue | ES-LMa | ES-LMagrass | ES-LMatrees | |
|---|---|---|---|---|---|---|
| WUE (g C (kg H2O)−1) | 4.8 | 5.2 | 3.1 | 2.3 | 2.7 | 2.2 |
| IWUE (g C kPa (kg H2O)−1) | 5.3 | 5.4 | 4.2 | 3.1 | 3.4 | 3.1 |
| uWUE (g C kPa0.5 (kg H2O)−1) | 5.1 | 5.3 | 3.6 | 2.7 | 3.0 | 2.6 |
| Gsw (mol m−2 s−1) | 0.301 | 0.195 | 0.119 | 0.157 | 0.223 | 0.047 |
| m (mol m−2 s−1 ln(kPa)−1) | 0.080 | 0.091 | 0.089 | 0.099 | 0.067 | 0.060 |
| b (mol m−2 s−1) | 0.349 | 0.231 | 0.184 | 0.229 | 0.282 | 0.094 |
| G0,USO (mol m−2 s−1) | 0.090 | -0.007 | -0.015 | 0.014 | 0.040 | 0.034 |
| G1,USO (kPa0.5) a | 1.4 | 1.5 | 2.3 | 3.5 | 4.1 | 2.9 |
| G1,BB a | 6.6 | 7.7 | 10.4 | 13.7 | 14.8 | 11.9 |
| G1,LEUa, b | 5.5 | 6.0 | 10.7 | 9.7 | 9.0 | 26.0 |
| D0 (kPa) | 1.7 | 1.5 | 0.9 | 2.1 | 4.7 | 0.3 |
| Ci (μmol mol−1) | 231 | 213 | 233 | 297 | 316 | 310 |
| Ci/Cs | 0.61 | 0.57 | 0.62 | 0.74 | 0.79 | 0.77 |
| Vcmax,25 (μmol m−2 s−1) | 177.4 | 135.1 | 68.9 | 53.8 | 55.4 | 12.0 |
| Jmax,25 (μmol m−2 s−1) | 457.5 | 188.4 | 65.1 | 50.1 | 83.2 | 15.4 |
|
α (μmol CO2 m−2 s−1 (μmol quanta m−2 s−1)−1) |
0.106 | 0.079 | 0.037 | 0.037 | 0.044 | 0.098 |
| GPPref (μmol m−2 s−1) c | 34.8 | 24.0 | 12.9 | 12.5 | 13.8 | 8.6 |
| LUE (mol mol−1) | 0.027 | 0.020 | 0.010 | 0.008 | 0.009 | 0.002 |
a assuming g0 = 0;
b assuming D0 = 1.5 kPa;
c at 2000 μmol m−2 s−1
Discussion
Potential and limitations of the ‘big-leaf’ approach
All calculations implemented in the bigleaf package are based on the ‘big-leaf’ framework [35, 44], which reduces the ecosystem to a single, uniform plane (Fig 1). This approach thus assumes that vegetation as well as meteorological conditions are vertically and horizontally homogenous. One advantage of the ‘big-leaf’ approach is that calculations require no additional information on the EC site or commonly available variables only (e.g. LAI, vegetation height). Ecosystem properties are inferred directly from EC measurements, with no assumptions on the underlying ecosystem structure. The ‘big-leaf’ approach is further applicable to both single-level and two-level EC systems. In the latter case ecosystem properties can be derived for two ‘big leafs’, e.g. whole ecosystem and understory [28, 29] or whole ecosystem and grass layer (this study, Fig 5).
It is important to clarify that the bigleaf package exclusively applies a top-down approach, in which the ‘big-leaf’ framework is used to estimate ecosystem properties inversely from the measured fluxes. The package does not provide bottom-up model formulations, which apply a ‘big-leaf’ framework to up-scale simulated fluxes from leaf- to canopy-level. This up-scaling approach has been shown to be prone to integration errors [31, 89]. However, this type of error does not apply to the calculations in the bigleaf package because the ‘big-leaf’ framework is solely used for the derivation of bulk ecosystem properties and no up- or down-scaling is performed.
The fact that the top-down ‘big-leaf’ approach as applied in this package can only derive bulk ecosystem properties is also its most critical limitation. It is not possible to resolve the vertical distribution of the derived properties. For example, soil and vegetation components cannot be distinguished and the resulting properties will inevitably contain signals from both the soil and the vegetation. These drawbacks can only be circumvented by modeling approaches such as two-layer (soil/canopy) [33, 51] or dual-source (sun/shade) models [31], which attempt to resolve the flux contribution of different canopy fractions or ecosystem compartments. These alternative modeling frameworks are more complex and consequently require additional site-specific information (e.g. canopy clumping, canopy nitrogen profiles, etc.). They are thus mostly applied to a few sites where these additional model parameters are sufficiently well known (e.g. [90, 91]). The ‘big-leaf’ framework is thus most suitable for multi-site comparisons or for sites where little ancillary information is available, and where no detailed knowledge on the derived variable (e.g. canopy gradients) is required.
Interpretation of the derived physiological properties
The bigleaf package provides functions to calculate ecosystem-scale physiological variables such as Gs, G1, Ci, Vcmax, Jmax, and GPPref in the same manner as it is commonly done at leaf-level. Important in this context is that the interpretation of these bulk canopy variables is not as straightforward as that of their leaf-level analogues (see also [23]). This is due to 1) conceptual uncertainties (as discussed above), and 2) the presence of confounding physical factors. For instance, the intensity of the before-mentioned mixing of soil and vegetation signals increases with a decrease of vegetation density (i.e. LAI) of the ecosystem. [36] for instance showed that Gc is substantially overestimated in ecosystems with an LAI less than approx. 2. This does not mean that the calculation of Gs is meaningless in low-LAI ecosystems, but its physiological interpretation as Gc is increasingly compromised as vegetation cover decreases. For ecosystems with an LAI lower than 2-3, the inversion of a soil/canopy model [33] is likely more appropriate than the inversion of the ‘big-leaf’ model for the derivation of physiological variables.
In all ecosystems, confounding physical factors, which are non-existent or negligible at leaf-level, must be taken into account in order to extract a meaningful physiological signal. For example, evaporation (i.e. water fluxes not under plant control) occurring after rainfall will lead to an overestimation of the stomatal slope parameter G1, and thus to an underestimation of WUE, if such time-periods are not filtered out (see [23] for an overview of confounding factors and their associated uncertainties).
In general, uncertainties of physiological variables propagate with each calculation step. For example, Ci as calculated by Eq 25 is affected by uncertainties in both input variables Gs and GPP. Photosynthetic parameters are affected by the same uncertainties and in addition by assumptions made for their calculation. It follows that with increasing number of calculation steps following the derivation of Gs, uncertainties increase and the meaningfulness of the derived variables depends critically on the applied data filtering and the quality of the (original or partitioned) data.
As discussed above, all physiological variables are integrated over the entire canopy and represent bulk canopy properties (expressed in units per ground area instead of leaf area). They are thus not directly comparable to leaf-level measurements taken at a particular location in the canopy. The discrepancies between leaf and ecosystem values will be most pronounced for variables with a distinct profile within the canopy (e.g. Vcmax and Jmax [31]), and probably less relevant for G1.
General package usage guidelines
Data filtering
For most applications, it is recommended to apply a basic data filter that removes unreliable measurements or certain meteorological conditions. The optimal type of filter depends on the purpose of the study and the variable of interest. For example, it is advisable to exclude negative λE values from the calculation of Gs in order to minimize the occurrence of negative Gs estimates which are not readily interpretable. Furthermore, periods outside the growing season or following rainfall should be removed if Gs is interpreted in an ecophysiological context. Ga and surface conditions on the other hand can in principle be calculated for all conditions. In general, data that do not fulfill the assumptions of the EC method, or that were gap-filled with low confidence, should be discarded. Depending on the filter settings and the conditions at the site, this can lead to a considerable fraction of missing values in the dataset. This is generally not a problem for the subsequent analyses in this package (missing input data simply return NA again), but some (regression-based) functions may require a minimum number of available data in order to return robust results.
Treatment of uncertainties
The derived variables in the bigleaf package are affected by several sources of uncertainty, which may be classified as 1) random errors in the measured fluxes [92, 93], 2) systematic errors in the fluxes due to e.g. energy-balance non-closure, advection problems [94, 95] and 3) conceptual uncertainties. The complex nature of uncertainties in EC measurements and the associated computational challenges to adequately account for and propagate all sources of uncertainty in the derived variables are the main reasons why the bigleaf package does not offer uncertainty estimates for each output interval. To account for one or more of the outlined sources of uncertainties, the use of wrapper functions is the most meaningful approach. These functions (often in specialized R packages) apply e.g. Monte Carlo (parameter sensitivity on the derived variables) or bootstrapping (random data sampling with replacement) techniques without the need to modify the functions in bigleaf. Some simple examples on the use of such wrapper functions are given in the vignette of the bigleaf package (accessible in R with browseVignettes(“bigleaf”).
Use of the derived properties
The majority of the derived properties in the bigleaf package are intended to be primarily diagnostic, i.e. results serve to provide a more mechanistic understanding of the observed fluxes, which enables a more comprehensive analysis and interpretation of ecosystem surface-atmosphere gas exchange. These diagnostics provide additional insights on the underlying physical or physiological processes and are often directly comparable across sites and climatic conditions. Some variables may further be helpful for the parameterization, calibration, or evaluation of bottom-up models. For that purpose, two major prerequisites must be fulfilled: (1) the variable of interest derived with a top-down (inversion) approach must be at the same organizational scale as the one calculated in the bottom-up model, and (2) the framework and the assumptions made in the two approaches must be consistent. For example, both the dynamics and magnitude of the simulated degree of atmosphere-canopy decoupling (Ω) by land surface models can be directly compared with the Ω values derived from this package [87]. This also applies to other characteristics such as Ga, Gs, or WUE and LUE metrics that are simulated as (emergent) bulk surface properties in models. In contrast, physiological bulk canopy parameters such as Ci should not be compared to leaf-level ci values as simulated by multi-layer models. Likewise, bulk canopy or Vcmax,25 cannot be used to parameterize leaf-level vcmax,25 in multi-layer models. In any case, it is imperative that uncertainties specific to the EC-method (as summarized in the previous section) are taken into account when derived properties are used for bottom-up modeling purposes.
Conclusions
The presented R package bigleaf provides a framework for the derivation of physical and physiological ecosystem properties at EC sites in a consistent and reproducible manner and with minimal requirements regarding ancillary site data. The package thus has the potential to increase the comparability of the provided calculations as well as their applicability across sites. The functions will be useful in complementing the analysis of land-atmosphere mass and energy fluxes by providing a basic level of process understanding. The availability of additional ecosystem surface characteristics as provided by the bigleaf package will be key in interpreting ever-increasing records of EC data and the responses of land-atmosphere exchange to global environmental change. The open source and version control environment further enable the continuous development of the package and encourage community input.
Acknowledgments
This work used eddy covariance data acquired and shared by the FLUXNET community, including these networks: AmeriFlux, AfriFlux, AsiaFlux, CarboAfrica, CarboEuropeIP, CarboItaly, CarboMont, ChinaFlux, Fluxnet-Canada, GreenGrass, ICOS, KoFlux, LBA, NECC, OzFlux-TERN, TCOS-Siberia, and USCCC. The ERA-Interim reanalysis data are provided by ECMWF and processed by LSCE. The FLUXNET eddy covariance data processing and harmonization was carried out by the European Fluxes Database Cluster, AmeriFlux Management Project, and Fluxdata project of FLUXNET, with the support of CDIAC and ICOS Ecosystem Thematic Center, and the OzFlux, ChinaFlux and AsiaFlux offices. We thank Clemens G. Klein for technical support with Fig 1. We additionally thank Arnaud Carrara for providing flux and ancillary data for the site ES-LMa. SZ was supported by the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (QUINCY; grant no. 647204). MM received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Sklodowska-Curie grant agreement no. 721995 (TRuStEE). MM and TSEM thank the Alexander von Humboldt foundation for supporting the research activity in Majadas de Tietar through the Max Planck Research Prize to Markus Reichstein.
Data Availability
All data necessary for replication are publicly available. The presented software is available from an online repository (https://bitbucket.org/juergenknauer/bigleaf). Eddy covariance data for AT-Neu, DE-Tha, and FR-Pue are available online from the FLUXNET2015 webpage (http://fluxnet.fluxdata.org/data/fluxnet2015-dataset/), and eddy covariance data for ES-LMa are available from an online repository (https://zenodo.org/record/1314194#.W09H-3qPWi5).
Funding Statement
SZ was supported by the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (QUINCY; grant no. 647204). MM received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Sklodowska-Curie grant agreement no. 721995 (TRuStEE). MM and TSEM thank the Alexander von Humboldt foundation for supporting the research activity in Majadas de Tietar through the Max Planck Research Prize to Markus Reichstein. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Aubinet M, Grelle A, Ibrom A, Rannik Ü, Moncrieff J, Foken T, et al. Estimates of the annual net carbon and water exchange of forests: the EUROFLUX methodology. Advances in Ecological Research. 1999;30:113–175. 10.1016/S0065-2504(08)60018-5 [DOI] [Google Scholar]
- 2. Baldocchi DD, Falge E, Gu L, Olson R, Hollinger D, Running S, et al. FLUXNET: A new tool to study the temporal and spatial variability of ecosystem-scale carbon dioxide, water vapor, and energy flux densities. Bulletin of the American Meteorological Society. 2001;82(11):2415–2434. 10.1175/1520-0477(2001)082%3C2415:FANTTS%3E2.3.CO;2 [DOI] [Google Scholar]
- 3. Knohl A, Baldocchi DD. Effects of diffuse radiation on canopy gas exchange processes in a forest ecosystem. Journal of Geophysical Research: Biogeosciences. 2008;113(G2). 10.1029/2007JG000663 [DOI] [Google Scholar]
- 4. Mercado LM, Bellouin N, Sitch S, Boucher O, Huntingford C, Wild M, et al. Impact of changes in diffuse radiation on the global land carbon sink. Nature. 2009;458(7241):1014–1017. 10.1038/nature07949 [DOI] [PubMed] [Google Scholar]
- 5. Law BE, Falge E, Gu L, Baldocchi DD, Bakwin P, Berbigier P, et al. Environmental controls over carbon dioxide and water vapor exchange of terrestrial vegetation. Agricultural and Forest Meteorology. 2002;113(1):97–120. 10.1016/S0168-1923(02)00104-1 [DOI] [Google Scholar]
- 6. Novick KA, Ficklin DL, Stoy PC, Williams CA, Bohrer G, Oishi AC, et al. The increasing importance of atmospheric demand for ecosystem water and carbon fluxes. Nature Climate Change. 2016;6(11):1023–1027. 10.1038/nclimate3114 [DOI] [Google Scholar]
- 7. Keenan T, Sabate S, Gracia C. Soil water stress and coupled photosynthesis–conductance models: Bridging the gap between conflicting reports on the relative roles of stomatal, mesophyll conductance and biochemical limitations to photosynthesis. Agricultural and Forest Meteorology. 2010;150(3):443–453. 10.1016/j.agrformet.2010.01.008 [DOI] [Google Scholar]
- 8. Ciais P, Reichstein M, Viovy N, Granier A, Ogée J, Allard V, et al. Europe-wide reduction in primary productivity caused by the heat and drought in 2003. Nature. 2005;437(7058):529–533. 10.1038/nature03972 [DOI] [PubMed] [Google Scholar]
- 9. Teuling AJ, Seneviratne SI, Stöckli R, Reichstein M, Moors E, Ciais P, et al. Contrasting response of European forest and grassland energy exchange to heatwaves. Nature Geoscience. 2010;3(10):722–727. 10.1038/ngeo950 [DOI] [Google Scholar]
- 10. Wilson KB, Baldocchi DD, Aubinet M, Berbigier P, Bernhofer C, Dolman H, et al. Energy partitioning between latent and sensible heat flux during the warm season at FLUXNET sites. Water Resources Research. 2002;38(12). 10.1029/2001WR000989 [DOI] [Google Scholar]
- 11. Williams CA, Reichstein M, Buchmann N, Baldocchi D, Beer C, Schwalm C, et al. Climate and vegetation controls on the surface water balance: Synthesis of evapotranspiration measured across a global network of flux towers. Water Resources Research. 2012;48(6). 10.1029/2011WR011586 [DOI] [Google Scholar]
- 12. Beer C, Ciais P, Reichstein M, Baldocchi D, Law B, Papale D, et al. Temporal and among-site variability of inherent water use efficiency at the ecosystem level. Global Biogeochemical Cycles. 2009;23(2). 10.1029/2008GB003233 [DOI] [Google Scholar]
- 13. Zhou S, Yu B, Huang Y, Wang G. The effect of vapor pressure deficit on water use efficiency at the subdaily time scale. Geophysical Research Letters. 2014;41 10.1002/2014GL060741 [DOI] [Google Scholar]
- 14. Baldocchi D. Turner Review No. 15.’Breathing’ of the terrestrial biosphere: lessons learned from a global network of carbon dioxide flux measurement systems. Australian Journal of Botany. 2008;56(1):1–26. 10.1071/BT07151 [DOI] [Google Scholar]
- 15. Pastorello G, Papale D, Chu H, Trotta C, Agarwal D, Canfora E, et al. A new data set to keep a sharper eye on land-air exchanges. Eos, Transactions American Geophysical Union (Online). 2017;98(8). [Google Scholar]
- 16. Beringer J, McHugh I, Hutley LB, Isaac P, Kljun N. Dynamic INtegrated Gap-filling and partitioning for OzFlux (DINGO). Biogeosciences. 2017;14(6):1457 10.5194/bg-14-1457-2017 [DOI] [Google Scholar]
- 17. Kljun N, Calanca P, Rotach M, Schmid H. A simple two-dimensional parameterisation for Flux Footprint Prediction (FFP). Geoscientific Model Development. 2015;8(11):3695 10.5194/gmd-8-3695-2015 [DOI] [Google Scholar]
- 18. Metzger S, Durden D, Sturtevant C, Luo H, Pingintha-Durden N, Sachs T, et al. eddy4R 0.2. 0: a DevOps model for community-extensible processing and analysis of eddy-covariance data based on R, Git, Docker, and HDF5. Geoscientific Model Development. 2017;10(9):3189 10.5194/gmd-10-3189-2017 [DOI] [Google Scholar]
- 19. Wutzler T, Lucas-Moffat A, Migliavacca M, Knauer J, Sickel K, Šigut L, et al. Basic and extensible post-processing of eddy covariance flux data with REddyProc. Biogeosciences Discussions. 2018. 10.5194/bg-2018-56 [DOI] [Google Scholar]
- 20. Grantz D, Meinzer F. Stomatal response to humidity in a sugarcane field: simultaneous porometric and micrometeorological measurements. Plant, Cell & Environment. 1990;13(1):27–37. 10.1111/j.1365-3040.1990.tb01296.x [DOI] [Google Scholar]
- 21. Jarvis P. Coupling of carbon and water interactions in forest stands. Tree physiology. 1986;2(1-2-3):347–368. 10.1093/treephys/2.1-2-3.347 [DOI] [PubMed] [Google Scholar]
- 22. McNaughton K, Jarvis P. Effects of spatial scale on stomatal control of transpiration. Agricultural and Forest Meteorology. 1991;54(2):279–302. 10.1016/0168-1923(91)90010-N [DOI] [Google Scholar]
- 23. Knauer J, Zaehle S, Medlyn BE, Reichstein M, Williams CA, Migliavacca M, et al. Towards physiologically meaningful water-use efficiency estimates from eddy covariance data. Global Change Biology. 2018;24:694–710. 10.1111/gcb.13893 [DOI] [PubMed] [Google Scholar]
- 24. Kosugi Y, Takanashi S, Ueyama M, Ohkubo S, Tanaka H, Matsumoto K, et al. Determination of the gas exchange phenology in an evergreen coniferous forest from 7 years of eddy covariance flux data using an extended big-leaf analysis. Ecological Research. 2013;28(3):373–385. 10.1007/s11284-012-1019-4 [DOI] [Google Scholar]
- 25. Migliavacca M, Meroni M, Manca G, Matteucci G, Montagnani L, Grassi G, et al. Seasonal and interannual patterns of carbon and water fluxes of a poplar plantation under peculiar eco-climatic conditions. Agricultural and Forest Meteorology. 2009;149(9):1460–1476. 10.1016/j.agrformet.2009.04.003 [DOI] [Google Scholar]
- 26. Ueyama M, Tahara N, Iwata H, Euskirchen ES, Ikawa H, Kobayashi H, et al. Optimization of a biochemical model with eddy covariance measurements in black spruce forests of Alaska for estimating CO2 fertilization effects. Agricultural and Forest Meteorology. 2016;222:98–111. 10.1016/j.agrformet.2016.03.007 [DOI] [Google Scholar]
- 27. Reichstein M, Bahn M, Mahecha MD, Kattge J, Baldocchi DD. Linking plant and ecosystem functional biogeography. Proceedings of the National Academy of Sciences. 2014;111(38):13697–13702. 10.1073/pnas.1216065111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Baldocchi DD, Vogel CA, Hall B. Seasonal variation of energy and water vapor exchange rates above and below a boreal jack pine forest canopy. Journal of Geophysical Research: Atmospheres (1984–2012). 1997;102(D24):28939–28951. 10.1029/96JD03325 [DOI] [Google Scholar]
- 29. Blanken P, Black T. The canopy conductance of a boreal aspen forest, Prince Albert National Park, Canada. Hydrological Processes. 2004;18(9):1561–1578. 10.1002/hyp.1406 [DOI] [Google Scholar]
- 30. Xu K, Metzger S, Desai AR. Surface-atmosphere exchange in a box: Space-time resolved storage and net vertical fluxes from tower-based eddy covariance. Agricultural and Forest Meteorology. 2018;255:81–91. 10.1016/j.agrformet.2017.10.011 [DOI] [Google Scholar]
- 31. De Pury D, Farquhar G. Simple scaling of photosynthesis from leaves to canopies without the errors of big-leaf models. Plant, Cell & Environment. 1997;20(5):537–557. 10.1111/j.1365-3040.1997.00094.x [DOI] [Google Scholar]
- 32. Wang YP, Leuning R. A two-leaf model for canopy conductance, photosynthesis and partitioning of available energy I:: Model description and comparison with a multi-layered model. Agricultural and Forest Meteorology. 1998;91(1):89–111. 10.1016/S0168-1923(98)00061-6 [DOI] [Google Scholar]
- 33. Shuttleworth WJ, Wallace J. Evaporation from sparse crops-an energy combination theory. Quarterly Journal of the Royal Meteorological Society. 1985;111(469):839–855. 10.1002/qj.49711146910 [DOI] [Google Scholar]
- 34. Baldocchi D, Harley P. Scaling carbon dioxide and water vapour exchange from leaf to canopy in a deciduous forest. II. Model testing and application. Plant, Cell & Environment. 1995;18(10):1157–1173. 10.1111/j.1365-3040.1995.tb00626.x [DOI] [Google Scholar]
- 35. Raupach M, Finnigan J. ‘Single-layer models of evaporation from plant canopies are incorrect but useful, whereas multilayer models are correct but useless’: Discuss. Functional Plant Biology. 1988;15(6):705–716. [Google Scholar]
- 36. Kelliher F, Leuning R, Raupach M, Schulze ED. Maximum conductances for evaporation from global vegetation types. Agricultural and Forest Meteorology. 1995;73(1):1–16. 10.1016/0168-1923(94)02178-M [DOI] [Google Scholar]
- 37. Kumagai T, Saitoh TM, Sato Y, Morooka T, Manfroi OJ, Kuraji K, et al. Transpiration, canopy conductance and the decoupling coefficient of a lowland mixed dipterocarp forest in Sarawak, Borneo: dry spell effects. Journal of Hydrology. 2004;287(1):237–251. 10.1016/j.jhydrol.2003.10.002 [DOI] [Google Scholar]
- 38. Launiainen S. Seasonal and inter-annual variability of energy exchange above a boreal Scots pine forest. Biogeosciences. 2010;7(12):3921–3940. 10.5194/bg-7-3921-2010 [DOI] [Google Scholar]
- 39. Khatun R, Ohta T, Kotani A, Asanuma J, Gamo M, Han S, et al. Spatial variations in evapotranspiration over East Asian forest sites. I. Evapotranspiration and decoupling coefficient. Hydrological Research Letters. 2011;5:83–87. 10.3178/hrl.5.83 [DOI] [Google Scholar]
- 40. Baldocchi D, Ma S. How will land use affect air temperature in the surface boundary layer? Lessons learned from a comparative study on the energy balance of an oak savanna and annual grassland in California, USA. Tellus B: Chemical and Physical Meteorology. 2013;65(1):19994 10.3402/tellusb.v65i0.19994 [DOI] [Google Scholar]
- 41. Medlyn BE, De Kauwe MG, Lin YS, Knauer J, Duursma RA, Williams CA, et al. How do leaf and ecosystem measures of water-use efficiency compare? New Phytologist. 2017;216(3):758–770. 10.1111/nph.14626 [DOI] [PubMed] [Google Scholar]
- 42. Goldberg V, Bernhofer C. Testing different decoupling coefficients with measurements and models of contrasting canopies and soil water conditions. Annales Geophysicae. 2008;26(7):1977–1992. 10.5194/angeo-26-1977-2008 [DOI] [Google Scholar]
- 43.R Core Team. R: A Language and Environment for Statistical Computing; 2017. Available from: https://www.R-project.org/.
- 44.Monteith J. Evaporation and environment. In: Fogg GE, editor. Symp. Soc. Exp. Biol. vol. 19. Cambridge University Press; 1965. p. 205–234. [PubMed]
- 45. Papale D, Reichstein M, Aubinet M, Canfora E, Bernhofer C, Kutsch W, et al. Towards a standardized processing of Net Ecosystem Exchange measured with eddy covariance technique: algorithms and uncertainty estimation. Biogeosciences. 2006;3:571–583. 10.5194/bg-3-571-2006 [DOI] [Google Scholar]
- 46. Verma S. Aerodynamic resistances to transfers of heat, mass and momentum In: Black T, Spittlehouse D, Novak M, Price D, editors. Estimation of areal evapotranspiration. vol. 177 International Association of Hydrological Sciences; 1989. p. 13–20. [Google Scholar]
- 47. Massman W. A model study of for vegetated surfaces using ‘localized near-field’Lagrangian theory. Journal of Hydrology. 1999;223(1):27–43. 10.1016/S0022-1694(99)00104-3 [DOI] [Google Scholar]
- 48. Monteith J, Unsworth M. Principles of Environmental Physics. 3rd ed Academic Press; 2008. [Google Scholar]
- 49. Businger JA, Wyngaard JC, Izumi Y, Bradley EF. Flux-profile relationships in the atmospheric surface layer. Journal of the Atmospheric Sciences. 1971;28(2):181–189. 10.1175/1520-0469(1971)028%3C0181:FPRITA%3E2.0.CO;2 [DOI] [Google Scholar]
- 50. Dyer A, Hicks B. Flux-gradient relationships in the constant flux layer. Quarterly Journal of the Royal Meteorological Society. 1970;96(410):715–721. 10.1002/qj.49709641012 [DOI] [Google Scholar]
- 51. Choudhury B, Monteith J. A four-layer model for the heat budget of homogeneous land surfaces. Quarterly Journal of the Royal Meteorological Society. 1988;114(480):373–398. 10.1002/qj.49711448006 [DOI] [Google Scholar]
- 52. Shaw RH, Pereira A. Aerodynamic roughness of a plant canopy: a numerical experiment. Agricultural Meteorology. 1982;26(1):51–65. 10.1016/0002-1571(82)90057-7 [DOI] [Google Scholar]
- 53. Hong J, Kim J, Byun Y. Uncertainty in carbon exchange modelling in a forest canopy due to kB−1 parametrizations. Quarterly Journal of the Royal Meteorological Society. 2012;138(664):699–706. 10.1002/qj.944 [DOI] [Google Scholar]
- 54. Verhoef A, De Bruin H, Van Den Hurk B. Some practical notes on the parameter kB−1 for sparse vegetation. Journal of Applied Meteorology. 1997;36(5):560–572. 10.1175/1520-0450(1997)036%3C0560:SPNOTP%3E2.0.CO;2 [DOI] [Google Scholar]
- 55. Thom A. Momentum, mass and heat exchange of vegetation. Quarterly Journal of the Royal Meteorological Society. 1972;98(415):124–134. 10.1002/qj.49709841510 [DOI] [Google Scholar]
- 56. Garratt J, Hicks B. Momentum, heat and water vapour transfer to and from natural and artificial surfaces. Quarterly Journal of the Royal Meteorological Society. 1973;99(422):680–687. 10.1002/qj.49709942209 [DOI] [Google Scholar]
- 57. McNaughton K, Van den Hurk B. A ‘Lagrangian’revision of the resistors in the two-layer model for calculating the energy budget of a plant canopy. Boundary-Layer Meteorology. 1995;74(3):261–288. 10.1007/BF00712121 [DOI] [Google Scholar]
- 58. Su Z, Schmugge T, Kustas W, Massman W. An evaluation of two models for estimation of the roughness height for heat transfer between the land surface and the atmosphere. Journal of Applied Meteorology. 2001;40(11):1933–1951. 10.1175/1520-0450(2001)040%3C1933:AEOTMF%3E2.0.CO;2 [DOI] [Google Scholar]
- 59. Hicks B, Baldocchi D, Meyers T, Hosker RP Jr., Matt D. A preliminary multiple resistance routine for deriving dry deposition velocities from measured quantities. Water, Air, and Soil Pollution. 1987;36(3-4):311–330. 10.1007/BF00229675 [DOI] [Google Scholar]
- 60. Jarvis PG, McNaughton K. Stomatal control of transpiration: scaling up from leaf to region. Advances in Ecological Research. 1986;15:1–49. 10.1016/S0065-2504(08)60119-1 [DOI] [Google Scholar]
- 61. Wang W, Liang S, Meyers T. Validating MODIS land surface temperature products using long-term nighttime ground measurements. Remote Sensing of Environment. 2008;112(3):623–635. 10.1016/j.rse.2007.05.024 [DOI] [Google Scholar]
- 62. Wohlfahrt G, Haslwanter A, Hörtnagl L, Jasoni RL, Fenstermaker LF, Arnone JA, et al. On the consequences of the energy imbalance for calculating surface conductance to water vapour. Agricultural and Forest Meteorology. 2009;149(9):1556–1559. 10.1016/j.agrformet.2009.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. McNaughton K, Black TA. A study of evapotranspiration from a Douglas fir forest using the energy balance approach. Water Resources Research. 1973;9(6):1579–1590. 10.1029/WR009i006p01579 [DOI] [Google Scholar]
- 64. Martin P. The significance of radiative coupling between vegetation and the atmosphere. Agricultural and Forest Meteorology. 1989;49(1):45–53. 10.1016/0168-1923(89)90061-0 [DOI] [Google Scholar]
- 65. Martin T, Brown K, Kučera J, Meinzer F, Sprugel D, Hinckley T. Control of transpiration in a 220-year-old Abies amabilis forest. Forest Ecology and Management. 2001;152(1):211–224. 10.1016/S0378-1127(00)00604-6 [DOI] [Google Scholar]
- 66. Priestley C, Taylor R. On the assessment of surface heat flux and evaporation using large-scale parameters. Monthly Weather Review. 1972;100(2):81–92. 10.1175/1520-0493(1972)100%3C0081:OTAOSH%3E2.3.CO;2 [DOI] [Google Scholar]
- 67. De Bruin H. A model for the Priestley-Taylor parameter α. Journal of Climate and Applied Meteorology. 1983;22(4):572–578. 10.1175/1520-0450(1983)022%3C0572:AMFTPT%3E2.0.CO;2 [DOI] [Google Scholar]
- 68. Wilson K, Goldstein A, Falge E, Aubinet M, Baldocchi D, Berbigier P, et al. Energy balance closure at FLUXNET sites. Agricultural and Forest Meteorology. 2002;113(1):223–243. 10.1016/S0168-1923(02)00109-0 [DOI] [Google Scholar]
- 69. Meyers TP, Hollinger SE. An assessment of storage terms in the surface energy balance of maize and soybean. Agricultural and Forest Meteorology. 2004;125(1):105–115. 10.1016/j.agrformet.2004.03.001 [DOI] [Google Scholar]
- 70. Medlyn BE, Duursma RA, Eamus D, Ellsworth DS, Prentice IC, Barton CV, et al. Reconciling the optimal and empirical approaches to modelling stomatal conductance. Global Change Biology. 2011;17(6):2134–2144. 10.1111/j.1365-2486.2010.02375.x [DOI] [Google Scholar]
- 71. Ball JT, Woodrow IE, Berry JA. A model predicting stomatal conductance and its contribution to the control of photosynthesis under different environmental conditions In: Biggins J, editor. Progress in photosynthesis research. Martinus Nijhoff Publishers, Dordrecht, Netherlands; 1987. p. 221–224. [Google Scholar]
- 72. Leuning R. A critical appraisal of a combined stomatal-photosynthesis model for C3 plants. Plant, Cell & Environment. 1995;18(4):339–355. 10.1111/j.1365-3040.1995.tb00370.x [DOI] [Google Scholar]
- 73. Reichstein M, Falge E, Baldocchi D, Papale D, Aubinet M, Berbigier P, et al. On the separation of net ecosystem exchange into assimilation and ecosystem respiration: review and improved algorithm. Global Change Biology. 2005;11(9):1424–1439. 10.1111/j.1365-2486.2005.001002.x [DOI] [Google Scholar]
- 74. Lasslop G, Reichstein M, Papale D, Richardson AD, Arneth A, Barr A, et al. Separation of net ecosystem exchange into assimilation and respiration using a light response curve approach: critical issues and global evaluation. Global Change Biology. 2010;16(1):187–208. 10.1111/j.1365-2486.2009.02041.x [DOI] [Google Scholar]
- 75. Wohlfahrt G, Gu L. The many meanings of gross photosynthesis and their implication for photosynthesis research from leaf to globe. Plant, Cell & Environment. 2015;38(12):2500–2507. 10.1111/pce.12569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Oren R, Sperry J, Katul G, Pataki D, Ewers B, Phillips N, et al. Survey and synthesis of intra-and interspecific variation in stomatal sensitivity to vapour pressure deficit. Plant, Cell & Environment. 1999;22(12):1515–1526. 10.1046/j.1365-3040.1999.00513.x [DOI] [Google Scholar]
- 77. Keenan T, Sabate S, Gracia C. The importance of mesophyll conductance in regulating forest ecosystem productivity during drought periods. Global Change Biology. 2010;16(3):1019–1034. 10.1111/j.1365-2486.2009.02017.x [DOI] [Google Scholar]
- 78. Rayment M, Loustau D, Jarvis P. Photosynthesis and respiration of black spruce at three organizational scales: shoot, branch and canopy. Tree Physiology. 2002;22(4):219–229. 10.1093/treephys/22.4.219 [DOI] [PubMed] [Google Scholar]
- 79. Farquhar G, von Caemmerer S, Berry J. A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta. 1980;149(1):78–90. 10.1007/BF00386231 [DOI] [PubMed] [Google Scholar]
- 80. Bernacchi C, Singsaas E, Pimentel C, Portis AR Jr., Long S. Improved temperature response functions for models of Rubisco-limited photosynthesis. Plant, Cell & Environment. 2001;24(2):253–259. 10.1111/j.1365-3040.2001.00668.x [DOI] [Google Scholar]
- 81. Medlyn BE, Dreyer E, Ellsworth D, Forstreuter M, Harley P, Kirschbaum M, et al. Temperature response of parameters of a biochemically based model of photosynthesis. II. A review of experimental data. Plant, Cell & Environment. 2002;25(9):1167–1179. 10.1046/j.1365-3040.2002.00891.x [DOI] [Google Scholar]
- 82. Bernacchi C, Pimentel C, Long S. In vivo temperature response functions of parameters required to model RuBP-limited photosynthesis. Plant, Cell & Environment. 2003;26(9):1419–1430. 10.1046/j.0016-8025.2003.01050.x [DOI] [Google Scholar]
- 83. Falge E, Baldocchi D, Olson R, Anthoni P, Aubinet M, Bernhofer C, et al. Gap filling strategies for defensible annual sums of net ecosystem exchange. Agricultural and Forest Meteorology. 2001;107(1):43–69. 10.1016/S0168-1923(00)00225-2 [DOI] [Google Scholar]
- 84. Wohlfahrt G, Hammerle A, Haslwanter A, Bahn M, Tappeiner U, Cernusca A. Seasonal and inter-annual variability of the net ecosystem CO2 exchange of a temperate mountain grassland: Effects of weather and management. Journal of Geophysical Research: Atmospheres. 2008;113(D8). 10.1029/2007jd009286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Grünwald T, Bernhofer C. A decade of carbon, water and energy flux measurements of an old spruce forest at the Anchor Station Tharandt. Tellus B. 2007;59(3):387–396. 10.1111/j.1600-0889.2007.00259.x [DOI] [Google Scholar]
- 86. Rambal S, Ourcival JM, Joffre R, Mouillot F, Nouvellon Y, Reichstein M, et al. Drought controls over conductance and assimilation of a Mediterranean evergreen ecosystem: scaling from leaf to canopy. Global Change Biology. 2003;9(12):1813–1824. 10.1111/j.1365-2486.2003.00687.x [DOI] [Google Scholar]
- 87. De Kauwe MG, Medlyn BE, Knauer J, Williams CA. Ideas and perspectives: how coupled is the vegetation to the boundary layer? Biogeosciences. 2017;14:4435–4453. [Google Scholar]
- 88. Casals P, Gimeno C, Carrara A, Lopez-Sangil L, Sanz M. Soil CO2 efflux and extractable organic carbon fractions under simulated precipitation events in a Mediterranean Dehesa. Soil Biology and Biochemistry. 2009;41(9):1915–1922. 10.1016/j.soilbio.2009.06.015 [DOI] [Google Scholar]
- 89. Luo X, Chen JM, Liu J, Black TA, Croft H, Staebler R, et al. Comparison of Big-Leaf, Two-Big-Leaf, and Two-Leaf Upscaling Schemes for Evapotranspiration Estimation Using Coupled Carbon-Water Modeling. Journal of Geophysical Research: Biogeosciences. 2018;123(1):207–225. [Google Scholar]
- 90. Reichstein M, Tenhunen J, Roupsard O, Ourcival JM, Rambal S, Miglietta F, et al. Inverse modeling of seasonal drought effects on canopy CO2/H2O exchange in three Mediterranean ecosystems. Journal of Geophysical Research: Atmospheres. 2003;108(D23). 10.1029/2003JD003430 [DOI] [Google Scholar]
- 91. Wolf A, Akshalov K, Saliendra N, Johnson DA, Laca EA. Inverse estimation of Vcmax, leaf area index, and the Ball-Berry parameter from carbon and energy fluxes. Journal of Geophysical Research: Atmospheres. 2006;111(D8). 10.1029/2005JD005927 [DOI] [Google Scholar]
- 92. Hollinger DY, Richardson AD. Uncertainty in eddy covariance measurements and its application to physiological models. Tree physiology. 2005;25(7):873–885. 10.1093/treephys/25.7.873 [DOI] [PubMed] [Google Scholar]
- 93. Richardson AD, Aubinet M, Barr AG, Hollinger DY, Ibrom A, Lasslop G, et al. Uncertainty quantification In: Eddy Covariance. Springer; 2012. p. 173–209. [Google Scholar]
- 94. Leuning R, Van Gorsel E, Massman WJ, Isaac PR. Reflections on the surface energy imbalance problem. Agricultural and Forest Meteorology. 2012;156:65–74. 10.1016/j.agrformet.2011.12.002 [DOI] [Google Scholar]
- 95. Finnigan J. An introduction to flux measurements in difficult conditions. Ecological Applications. 2008;18(6):1340–1350. 10.1890/07-2105.1 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data necessary for replication are publicly available. The presented software is available from an online repository (https://bitbucket.org/juergenknauer/bigleaf). Eddy covariance data for AT-Neu, DE-Tha, and FR-Pue are available online from the FLUXNET2015 webpage (http://fluxnet.fluxdata.org/data/fluxnet2015-dataset/), and eddy covariance data for ES-LMa are available from an online repository (https://zenodo.org/record/1314194#.W09H-3qPWi5).