Abstract
Ultrasonic-assisted extraction (UAE), using aqueous ethanol as the solvent, was firstly applied to extract phenolic compounds from Terminalia chebula Retz. fruits (T. chebula fruits). In this study, ethanol concentration (%), ultrasonic intensity (W/cm2), particle diameter (mm), extraction temperature (°C), ultrasonic time (min), liquid-solid ratio (mL/g) and extraction cycle were investigated by single-factor experiment and then optimized using a Box-Behnken design. The optimized result for UAE was 68% ethanol concentration, ultrasonic intensity of 3.6 W/cm2, solid-liquid ratio of 23 mg/mL, particle size of 0.18 mm and ultrasonic time of 20 min for 2 times at 70 °C. The yield of total phenolic was 448.7 ± 2.15 mg GAE/g DW under the above optimum conditions, which agreed with the predicted value (447.8 mg GAE/g DW). Compared to conventional solvent extraction (CSE), UAE extracts showed excellent DPPH radical, DPPH, ABTS scavenging activities and reducing power in a dose-dependent manner, and better than that of CSE extracts. Additionally, the extract of the T. chebula fruits was analyzed by HPLC-ESI/MS. In summary, UAE could effectively extract phenolic compounds from T. chebula fruits. In addition, the extract could be used as a potential source of natural antioxidants.
Introduction
Terminalia chebula Retz. (T. chebula) found in the tropical areas of the world and commonly called Chebulae Fructus (Hezi) in China. The plant belongs to the family Combretaceae [1]. It have traditionally been utilized as medicines for relieving bleeding piles, diarrhea, hiccoughing, sore throat and bladder diseases [2]. It has been reported that T. chebula fruits rich in phenolic compounds such as gallic acid (GA), ellagic acid (EA) and corilagin (CG). These compounds are powerful antioxidant, anti-inflammatory, cardiotonic, antibacterial and anticarcinogenic [3]. Based on the pharmacological activities of T. chebula fruits, it can be used as an important source to extract natural phenolic compounds. Hence, it is essential to optimize and develop a reliable extraction method to obtain high yield of phenolic compounds from T. chebula fruits.
Currently, natural antioxidants from herbs are attracting increasing attention for their potential utility. The active phenols and antioxidants from T. chebula fruits may be obtained by an extraction process for potential use in functional foods or nutraceuticals. Several extraction techniques have been employed for the extraction of phenolic compounds from T. Chebula fruits, such as reflux system in combination with water-ethanol and water-propylene glycol [4], and subcritical water extraction [5]. However, some antioxidant activities are usually decreased using traditionally extraction methods due to high temperature and long treatment time. Chemat et at. [6] have pointed the benefits of using ultrasonic treatment in food processing to decrease process energy, save time and increase shelf life. Ultrasonic-assisted extraction (UAE) has been reported to enhance dissolution of effective components by disrupting cell tissue, such as extracting Lutein and β-Carotene from Spinach [7] and total phenol from Spinach [8]. It has been reported that thermal function of ultrasound has effects on plant cells and tissues due to the fact that ultrasonic waves could produce heat and absorbed by herbs tissues [9, 10]. Additionally, ultrasound has mechanical effect via acoustic assisted cavitation along with efficient mass transfer of the cell content to the solvent due to collapse of bubbles [7, 11].
UAE has not been reported for extraction of total phenolics (TP) from T. chebula fruits, but may enhance yield or antioxidant activities over traditional solvent extraction methods. The objectives of this study were to (1) optimize the UAE process of phenolic compounds from T. chebula fruits by response surface methodology; (2) compare extraction yields and antioxidant activities from UAE and a traditional solvent extraction method; and (3) do phytochemical analyses of extract of T. chebula fruits using HPLC-ESI/MS.
Materials and methods
Materials
The fruits of T. chebula were bought from Shiyitang herbal Pieces limited liability Company (Harbin, China) in 2017. It was identified and authenticated by Associate Professor Junkai Wu at Heilongjiang University of Chinese Medicine, Harbin, Heilongjiang Province, China. The fruits which had reached physical maturity (yellowish green) were selected. The dried fruits were ground and sieved by the prescription sieves which was standardized by Pharmacopoeia of People’s Republic of China (2015). The lisenced specimens (Accession no. 1009011ch) have been well placed in the Herbarium of College of Veterinary Medicine (Northeast Agricultural University).
Reference substances (Gallic acid and Ascorbic acid; purity > 98% (w/w)) were both acquired from the Chinese Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). Folin-Phenol, 2,4,6-Tris (2-pyridyl)-s-triazine (TPTZ), 1,1-Diphenyl-2-picrylhydrazyl (DPPH), Trichloro acetic acid (TCA), [2,2'-azino-bis-(3-ethylbenzthiazoline-6-sulfonic acid)] ABTS, Ferric chloride, Riboflavin, 6-Hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic Acid (Trolox), Nitro blue tetrazolium (NBT), and Methionine were obtained from Sigma Chemical Co. (St. Louis, USA). The experiments were performed with purified distilled water obtained from Milli-Q academic water purification system (Millipore, Bedford, MA, USA). All other reagents were of analytical grade.
Quantification of total phenolic content
The quantification of total phenols in T. chebula fruit extracts were measured by Folin-Ciocalteu method and gallic acid was used as a reference substance [12]. Briefly, 0.1 mL of the gallic acid standard solution (0.05, 0.08, 0.10, 0.14, 0.17 and 0.2 mg/mL) or sample solution intermingled with distilled water (50 mL), Folin–Ciocalteu reagent (5 mL) and 1.5 mL of Na2CO3, and mixed in a brown volumetric flask. The reaction mixture was measured at 765 nm after incubation in the dark for 3 h. Linear regression method was applied for quantification through a six-point calibration curve. The TP content was then expressed as mg of Gallic acid equivalents per g of dry weight (DW).
Ultrasound-assisted extraction (UAE) optimization
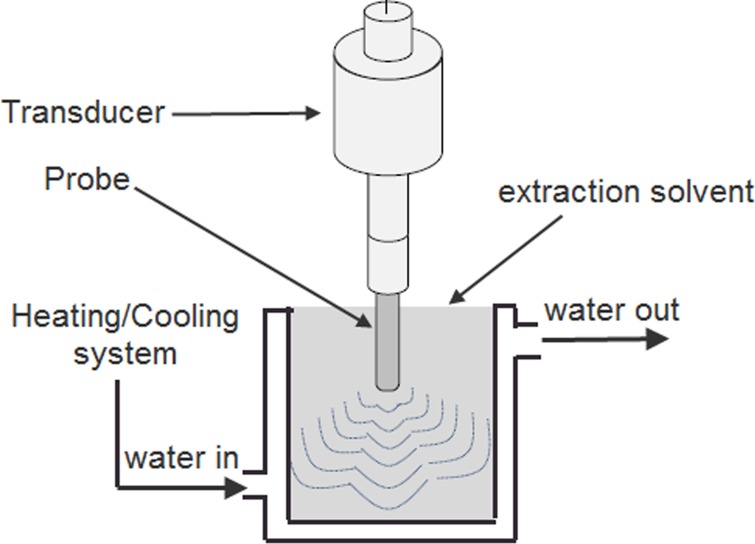
BILON-S650CT high-power ultrasonic processor (Shanghai Binlon Instrument CO., LTD, China) employed for ultrasonic extraction in a toughened glass tank in combination with an ultrasonic transducer (30 kHz, 650 W) and a power meter for changing the parameters during ultrasonic operation. The extraction temperature was maintained using a double layered extraction cells by cooling/heating systems (Fig 1).
Fig 1. Diagram of the UAE system.
A 12 mm acoustic horn (frequency range: 25–30 kHz; power range: 60–650 W; crushing capacity: 50–150 mL) was used to treat the solution with ultrasound placed 1 cm from the top of the extraction cell. The on/off time for the ultrasonic device was noted to be 1 s and 2 s, respectively. Taking into account the dissipated heat, ultrasonic power was calculated using calorimetric measurement and was expressed as ultrasonic intensity (UI) by using Eq (1) [13, 14]:
| (1) |
Where UI represents ultrasonic intensity (W/cm2), D represents ultrasonic reactor internal diameter (cm), and P is the value of power which is calculated according to the Eq (2) [13, 14].
| (2) |
Where P is the input power, m represents mass of the solvent (g), Cp (heat capacity) of the solvent in kJ/kg °C, and dT/dt is the temperature variation according to time (°C/s).
The value of input powers were adjusted to 100, 200, 300, 400, 500, and 600 W in single-factor experiments which were equivalent to 1.6, 3.3, 5.0, 6.7, 8.5 and 10.0 W/cm2, respectively. Single-factor experiments were performed at different conditions: ethanol concentration (0, 20, 40, 60, 80 and 95% (v/v)), ultrasonic intensity (0, 1.6, 3.3, 5.0, 6.7, 8.5 and 10.0 W/cm2), the range of particle diameters (0.15–2.00 mm) were determined by the prescription sieves (No. 1–9) which were standardized by Pharmacopoeia of People’s Republic of China (2015), 30, 40, 50, 60, 70 and 80 °C were taken as extraction temperatures, ultrasonic time (10, 15, 20, 25 and 35 min), liquid-solid ratio (10, 15, 20, 25, 30 and 35 mL/g) and the number of extraction chosen from 1 to 6. These experiments were repeated thrice. Finally, dried extracts were obtained following vacuum drying.
According to single-factor test, the parameters which affected the extraction process of TP from T. chebula fruits were optimized using a 17-run Box-Behnken Design (BBD). In addition, 12 factorial points and 5 axial points were used to determine the optimal extracting conditions. Three variables (X1, concentration of ethanol; X2, Ultrasonic intensity; X3, solid-liquid ratio) and three levels were given the values as 1 (high), 0 (intermediate) and −1 (low), are shown in Table 1. The formula of second-order polynomial mode is given below:
| (3) |
whereas Y represents the response; β0 was a constant; βi (linear), βii (quadratic) and βij (interactive) were the coefficients; Xi denotes independent variables. The terms XiXj and Xi2 represented the quadratic and interaction terms, respectively.
Table 1. Variables and experimental design levels for RSM.
| Independent variables | Coded symbols | Levels | ||
|---|---|---|---|---|
| -1 | 0 | 1 | ||
| Ethanol concentration (%) | X1 | 40 | 60 | 80 |
| Ultrasonic intensity (W/cm2) | X2 | 1.6 | 3.3 | 5.0 |
| Solid-liquid ratio (mg/mL) | X3 | 20 | 25 | 30 |
Conventional reflux extraction (CRE)
The reflux extraction of TP from the dried ripe T. chebula fruits was assessed by a previously described method [4]. The extraction parameters were temperature of 76 °C, ethanol concentration of 76.4%, liquid to solid ratio of 150 (mL/g), and time duration of 82 min. Additionally, extraction cycle 2 was used to exhaust the TP from the material. The extract was filtered and dried under vacuum.
In vitro antioxidant activity
Reducing power assay
The reducing power was assessed by a previously described method with some modifications [15]. The reaction containing 1.0 mL of UAE extract solution or CRE extract solution (0.03–0.08 mg/mL), 2.5 ml of K3Fe(CN)6 (1%, w/v) and 2.5 mL of 0.2 M Na2HPO4 (sodium phosphate buffer, pH 6.6) at 50 °C for 20 min. Then, Trichloroacetic acid (2.5 mL, 10%, w/v) was incorporated into the mixture and centrifuged at 5000 rpm for 10 min. Finally, 0.5 mL of Ferric chloride (0.1%) and the upper layer (5 mL) were mixed together. The absorbance was measured at 700 nm against a blank control (reaction mixture in which the solvent is used instead of sample solution) after 10 min. While, ascorbic acid was taken as positive control.
Ferric reducing antioxidant power (FRAP)
The reaction mixture containing 500 μL of the UAE extract solution or CRE extract solution (0.01–0.025 mg/mL), and FRAP reagent (5 mL) was incubated at 37 °C for 30 min. After incubation period, absorbance was measured against a blank control at 700 nm. Ascorbic acid was used as the positive control. FRAP reagent was made up of 0.3 M pH 3.6 Sodium acetate buffer, 10 mM TPTZ solution, 20 mM Ferric chloride solution in a volume ratio of 10:1:1. FRAP solution was standardized against FeSO4·7H2O (0.10, 0.14, 0.18, 0.22, 0.26 and 0.30 mM) and expressed as mM FeSO4·7H2O per g material.
DPPH radical scavenging assay
A previous method was used to measure DPPH radical scavenging activity as mentioned in literature [16] with some modifications. The reaction mixture containing 2.5 mL UAE extract solution or CRE extract solution (0.001–0.011 mg/mL) and 3.5 mL of 0.1 mM DPPH, kept in the dark at room temperature for 1 h. The absorbance of the sample and positive control (ascorbic acid) was measured against a blank control at 517 nm. The following formula was used to calculate DPPH radical scavenging activity.
| (4) |
where As represents the absorbance of distilled water alone, Ai is the absorbance of sample of different concentrations. The IC50 value shows that the concentration of the extract inhibited 50% DPPH radical formation.
ABTS radical scavenging assay
ABTS radical scavenging ability of the sample at different concentrations was determined as described previously with minor modifications [17]. 7 mM ABTS and 2.45 mM potassium persulfate reacted together in the dark at room temperature for 16 h to produce ABTS radical cation (ABTS+). 80% ethanol was mixed with ABTS+ solution to dilute it in order to obtain an absorbance of 0.700 ± 0.005 at 734 nm. The reaction mixture including 0.5 mL UAE extract solution or CRE extract solution (0.07–0.17 mg/mL) and ABTS+ solution (2 mL) was incubated at 37 °C for 30 min and examined against a blank control at 734 nm along with a positive control (ascorbic acid). The average value was calculated from three experimental replicates. The formula used for ABTS radical scavenging activity is as follows:
| (5) |
where As represents the absorbance of distilled water alone, Ai is the absorbance of sample of different concentrations. The IC50 value shows 50% inhibition of ABTS radical formation.
Superoxide radical scavenging assay
A previous method was applied to measure superoxide radical scavenging activity assay of TCFE with minor modifications [18]. Briefly, the mixture including 50 mM Tris–HCl buffer (4.5 mL, pH 8.2), 2 mL distilled water, 2 ml of UAE extract solution or CRE extract solution (0.01–0.05 mg/mL) and 0.5 mL of 25 mM pyrogallol solution was placed in an incubator at 37 °C for 5 min. Finally, 1.0 mL HCl (10 mM) was added to terminate the reaction. The absorbance of the reaction mixture and ascorbic acid (positive control) was noted against a blank control at 560 nm. The scavenging percentage was determined based on the formula:
| (6) |
where A0 denotes the absorbance of the control (distilled water), A1 represents absorbance of the sample, and A2 is the absorbance of the sample only (Tris–HCl buffer instead of pyrogallol solution). The IC50 value shows 50% inhibition of superoxide radical scavenging formation.
Identification of phenolic compounds
The HPLC/DAD/ESI-MS analysis was performed on an Agilent 1100 HPLC equipped with a Diode Array Detection (DAD) and an HP 1100 MSD API-electrospray (Agilent Technologies) operating both in positive and negative ionization mode.
Gemini C18 column (250 mm × 4.6 mm, 5 μm, Phenomenex analytical instruments Co., Ltd., America) was used for T. chebula fruit extract analysis. Mobile phase consisted of 0.1% formic acid (A) and methanol (B) at constant flow of 0.8 mL/min. The solvent gradient elution schedule was as follows: 0–12 min, 10–90% B; 12–14 min, 90–90% B; 14–14.1 min, 90–10%; 14.1–16 min, 10–10%. The injection volume was 10 μL and the column temperature was kept at 30 °C. The detection wavelength was kept at 254 nm.
Mass spectrometer operating conditions were: drying gas temperature of 350 °C at a flow rate of 10 L/min; nebulizer pressure of 30 psi; sheath gas temperature of 250 °C at a flow rate of 7 L/min; fragmenter voltage of 100 V; capillary voltage of 2500 V; and mass range of 100–1500 D.
Statistical analysis
All the experiments were repeated three times and values were expressed as means ± SD (n = 3). Stat-Ease Design-Expert 7.0.0 (Trial version, Stat-Ease Inc., Minneanopolis, MN, USA) software was employed for the experimental design and statistical analysis of response surface methodology. Mean values were regarded as significantly different at p < 0.05. Statistical analysis of the experimental data of the antioxidant tests were carried out using ANOVA (one-way analysis of variance) followed by Tukey test. SPSS software (version 18.0, SPSS Inc., Chicago, IL, USA) was used for the analysis data.
Results and discussion
Single-factor experimental analyses
Influence of ethanol concentration on TP extraction
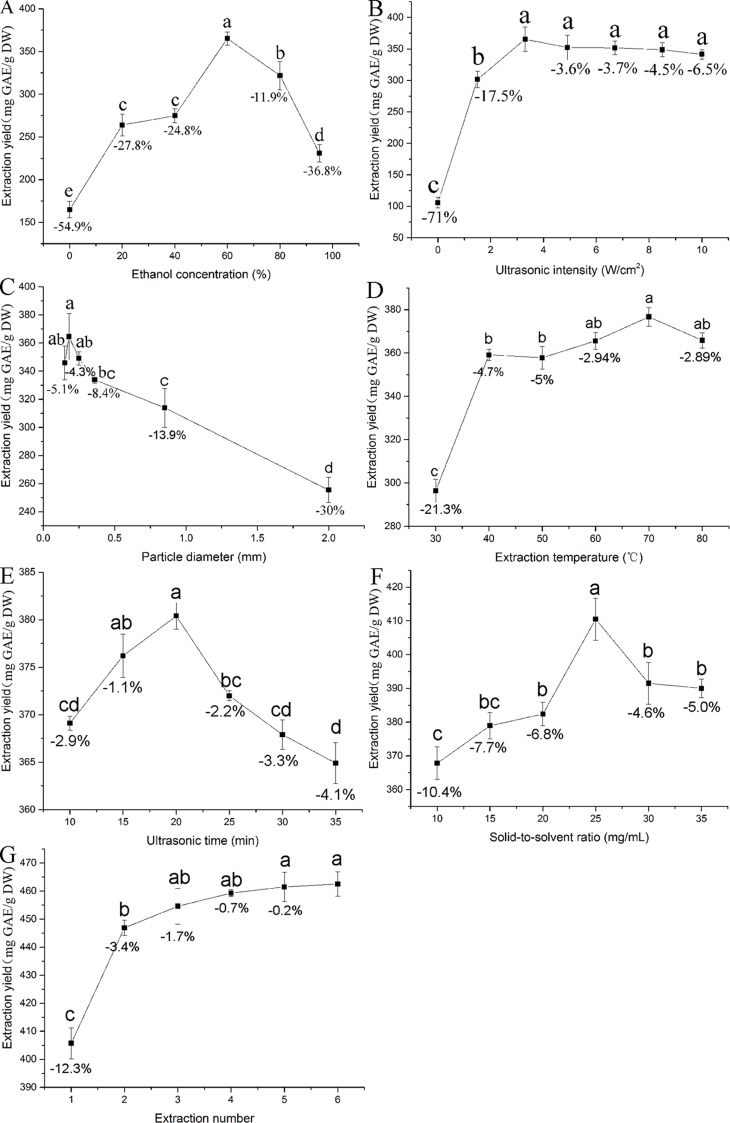
The concentration of solvent is an important factor in solid-liquid extraction system. Importantly, properties such as dielectric constant, surface tension, polarity, consistency, vapor pressure and solvent penetration and interaction with plant matrix have important effects on cavitation effect involved in UAE process [19]. Notably, ethanol concentration was set at 0, 20%, 40%, 60%, 80% and 100% to test the influence of different ethanol concentration on TP extraction. Other reaction conditions were as follows: ultrasonic intensity 5 W/cm2, particle diameters 0.25 mm, extraction temperature 60 °C, ultrasonic time 20 min, liquid-solid ratio 20 mL/g and extraction cycle 2.
Fig 2A showed that 60% ethanol gives the highest extraction yield, while water gives the lowest extraction yield in UAE process. Other reports have also found that 60% ethanol was suitable to extract TP or other phenolic compounds from T. chebula fruits using the reflux method [20]. Similar extraction yield of TP from T. chebula fruits was obtained using the same ethanol concentration by the reflux method and UAE, respectively. A remarkable extraction yield difference (p < 0.05) was found among the solvents with 60% ethanol concentration versus other concentrations, with the former giving 11.9–24.8% higher yield than 40% and 80% ethanol, respectively (Fig 2A). Therefore, an ethanol concentration of 60% was chosen for subsequent RSM experiments.
Fig 2. Effects of different extraction parameters (ethanol concentration, %; ultrasonic intensity, W/cm2; particle diameter, mm; extraction temperature, °C; ultrasonic time, min; solid-liquid ratio, mg/mL and extraction cycle) on yield of total phenolics from the T. chebula fruits.
Values were expressed as mean ± SD (n = 3), and evaluated by one-way AVONA followed by the Tukey test. Different letter and same letter were considered to be statistically significant (p < 0.05) and statistically insignificant (p > 0.05), respectively.
Influence of ultrasonic intensity on TP extraction
Ultrasonic power is defined as ultrasonic intensity, which involves the cavitation effect. We tested seven levels of ultrasonic intensity ranged from 0–10 W/cm2 to examine the effects on TP extraction yield. Other parameters are set as follows: ethanol concentration 60%, particle diameters 0.25 mm, extraction temperature 60 °C, ultrasonic time 20 min, liquid-solid ratio 20 mL/g and extraction cycle 2.
Fig 2B showed that increase in ultrasonic intensity from 0 to 3.3 W/cm2 enhances the yield of TP. The results revealed that increase in ultrasonic intensity enhances the cavitation effect which in turn facilitated the disruption of cell walls [21]. However, the extraction yield of TP declined when the ultrasonic intensity was over 3.3 W/cm2. The decrease in extraction yield with ultrasonic intensity may be due to the degradation of TP and cavitation effect. Intriguingly, TP were unstable and would be degraded under high ultrasonic intensity [22]. Significant (p < 0.05) differences in extraction yield were found among the ultrasonic intensities with the value of 3.3 W/cm2 versus the value of 1.6 W/cm2, the former giving 3.6–17.5% higher yield than the both experimental neighbors (Fig 2B). Hence, the ultrasonic intensity of 3.3 W/cm2 was selected for subsequent RSM experiments.
Effect of particle size on TP extraction
It is well known that particle size is another important parameter in solid-liquid extraction system. Generally, it has been previously reported that reduction in particle size leads to increase in the extraction yield due to increase in the surface area available for contact with the solvent [23]. In this study, T. chebula fruits sizes (varied from 0.15 to 2 mm) were used to investigate the influence of particle size on extraction yield. Other parameters are set as follows: ethanol concentration 60%, ultrasonic intensity 3.3 W/cm2, extraction temperature 60 °C, ultrasonic time 20 min, liquid-solid ratio 20 mL/g and extraction cycle 2.
Fig 2C showed that decreasing mean particle size causes an increase in the extraction yield of TP until the mean particle size reached 0.18 mm, which proved that a decrease in the particle size was beneficial to the migration of components from solid to the liquid. However, it was reported previously that smaller particle size leads to difficulty in extraction due to laborious diffusion [24]. Additionally, Fig 2C displayed that the yield of TP was slightly reduced when the particle size decreased from 0.18 to 0.15 mm. Similarly, it has been reported in other studies while extracting active compounds from herbs [25]. Compared to 0.18 mm particle size, the extraction yield of TP from T. chebula fruits decreased by 4.3 and 5.1% at the particle size of 0.15 and 0.25 mm respectively, but there was no marked statistical difference (p > 0.05) among the particle sizes with the value of 0.18 mm versus the value of both experimental neighbors. Thus, particle size of 0.18 mm was selected for the following experiments.
Influence of temperature on TP extraction
The extraction yield of TP was tested at extraction temperature from 30 to 80 °C. Other parameters are set as follows: ethanol concentration 60%, ultrasonic intensity 3.3 W/cm2, particle size 0.18 mm, ultrasonic time 20 min, liquid-solid ratio 20 mL/g and extraction cycle 2. It has been noted that increasing temperature causes an increase in the extraction yield of TP. The peak yield (376.3 mg GAE/g DW) was obtained at an extraction temperature of 70 °C. However, the extraction yield slightly decreased with further increase in extraction temperature (Fig 2D). It has been speculated that the combination of thermal and cavitation effects play a crucial role in UAE. The rise in temperature had a positive influence on extraction yield due to the reason that it accelerated the molecular movement and decreased the solvent viscidity [26, 27].
However, it should be kept in mind that high temperature could cause the degradation of the phenolic compounds [28]. Therefore, the increase in temperature could have both positive and negative effects. This finding was agreed with Altemimi who found that the thermal degradation of flavonoids and the decrease of number of acoustic cavitation bubbles were lead to decrease the amount of quercetin and rutin [29]. Importantly, no statistical significance (p > 0.05) was found among the extraction temperatures with the value of 70 °C versus the value of both experimental neighbors, thus a temperature of 70 °C was selected for the following experiments.
Effect of ultrasonic time on TP extraction
The extraction yield of TP was tested at ultrasonic time from 10 to 35 min. Other parameters are set as follows: ethanol concentration 60%, ultrasonic intensity 3.3 W/cm2, particle size 0.18 mm, extraction temperature 70 °C, liquid-solid ratio 20 mL/g and extraction cycle 2. Fig 2E represents the effect of ultrasonic time on the extraction yield of TP. The extraction yield increases from 10 to 20 min and reduced from 20 to 35 min. It was due to the fact that the release of bioactive compounds from the matrix cell walls could be accelerated. So the extraction yield of TP was increased by 20 min. However, extraction yield decreases and degradation of TP increases when the time interval exceeds 20 min. Surprisingly, the difference between the peak yield (380.1 mg GAE/g DW) and the adjacent yield (372.4 mg GAE/g DW) were statistically significant (P < 0.05), but minimum changes (2.2%) were observed in the yield of TP. Thus, an ultrasonic time of 20 min was chosen for subsequent tests.
Effect of solid-liquid ratio on TP extraction
The extraction yield of TP was tested at solid-liquid ratio from 10 to 35 mL/g. Other parameters are set as follows: ethanol concentration 60%, ultrasonic intensity 3.3 W/cm2, particle size 0.18 mm, extraction temperature 70 °C, liquid-solid ratio 20 mL/g and extraction cycle 2.
Fig 2F represents the yields of TP remained constant up to 25 mL/g, but decreases with increase in volume. Similarly, Sun et al. demonstrated that diffusion of the solvent into cells enhanced with the rise of solid-liquid ratio [24]. However, too high liquid-solid ratio can restrain the generation of aerosol and the cavitation effect. Similar results have been reported by Lu et al., who demonstrated that an increase in solvent volume did not boost yield [30]. Fig 2F also showed statistical significance (p < 0.05) among the liquid-solid ratio with the value of 25 mg/mL versus both experimental neighbors, the former giving 4.6–6.8% higher yield. Hence, 25 mg/mL was adopted as the center point for subsequent RSM experiments.
Effect of extraction cycle on TP extraction
Fig 2G showed that doubling the extraction cycle from 1 to 2 enhances significantly (p < 0.05) the yield of TP. The yield of TP extraction from extraction cycle 2 to 6 has slowly paced down. It has been found that there was no statistical difference (p > 0.05) between the extraction cycles with the value of 2 versus the value of 3, which indicated that TP has been completely extracted from the plant. In view of the extraction efficiency and energy cost, the extraction cycle 2 was chosen as the suitable extraction cycle.
Optimization of extraction conditions using RSM
Fitting the model
Box-Behnken design (BBD) was used to optimize the three parameters for optimum extraction conditions. The response values and predicted values were obtained from 17 runs and factorial design, respectively (Table 2). The minimal difference (0–0.44%) between the predicted values and actual values proved the accuracy of the model. Multiple regressions applied for the analysis of the data and relativity between the test variable and response variable which was described by second-order polynomial equation:
| (7) |
where X1, X2 and X3 were represents ethanol concentration, ultrasonic intensity and solid-liquid ratio, respectively.
Table 2. The Box-Behnken experimental design with four independent variables.
| No. | X1 (%) | X2 (W/cm2) | X3 (mg/mL) | Actual value (mg GAE/g DW) | Predicted value (mg GAE/g DW) | Difference (%) |
|---|---|---|---|---|---|---|
| 1 | 40 | 1.6 | 25 | 388.8 | 390.52 | -0.44 |
| 2 | 80 | 1.6 | 25 | 401.1 | 400.58 | 0.13 |
| 3 | 40 | 5.0 | 25 | 398 | 398.53 | -0.13 |
| 4 | 80 | 5.0 | 25 | 427 | 425.27 | 0.41 |
| 5 | 40 | 3.3 | 20 | 408.9 | 408.38 | 0.13 |
| 6 | 80 | 3.3 | 20 | 434.2 | 435.93 | -0.40 |
| 7 | 40 | 3.3 | 30 | 406.7 | 404.98 | 0.42 |
| 8 | 80 | 3.3 | 30 | 413.7 | 414.23 | -0.13 |
| 9 | 60 | 1.6 | 20 | 402.9 | 401.70 | 0.30 |
| 10 | 60 | 5.0 | 20 | 421.6 | 421.60 | 0 |
| 11 | 60 | 1.6 | 30 | 392.7 | 392.70 | 0 |
| 12 | 60 | 5.0 | 30 | 404.3 | 405.50 | -0.30 |
| 13 | 60 | 3.3 | 25 | 444.6 | 444.26 | 0.08 |
| 14 | 60 | 3.3 | 25 | 445.3 | 444.26 | 0.23 |
| 15 | 60 | 3.3 | 25 | 442.4 | 444.26 | -0.42 |
| 16 | 60 | 3.3 | 25 | 443.8 | 444.26 | -0.10 |
| 17 | 60 | 3.3 | 25 | 445.2 | 444.26 | 0.21 |
ANOVA results (Table 3) showed that the Model p-value was remarkably significant (less than 0.0001). Significant model terms were X1, X2, X3, X1X2, X1X3, X12, X22 and X32 and there could be a chance of 0.01% that model “F-value” becomes large due to noise. The value of Lack of Fit is 3.68, not significant to the pure error. The chance of error in the "Lack of Fit F-value" was 12.01% due to noise signals. The value of "Adj R-Squared" was 0.9925 which is in agreement with "Pred R-Squared" value (0.9602). The noise ratio 39.85 (calculated by "Adeq Precision") showed an adequate signal which is a desirable value (greater than 4). So the model was appropriate and fit and could be employed to navigate the design space.
Table 3. ANOVA for response surface quadratic model analysis of variance table.
| Source | Sum of squares | Degree of freedom | Mean square | F-value | p-value |
|---|---|---|---|---|---|
| Model | 6597.89 | 9 | 733.10 | 237.17 | < 0.0001** |
| X1 | 677.12 | 1 | 677.12 | 219.06 | < 0.0001** |
| X2 | 534.65 | 1 | 534.65 | 172.97 | < 0.0001** |
| X3 | 315.01 | 1 | 315.01 | 101.91 | < 0.0001** |
| X1X2 | 69.72 | 1 | 69.72 | 22.56 | 0.0021** |
| X1X3 | 83.72 | 1 | 83.72 | 27.09 | 0.0012** |
| X2X3 | 12.60 | 1 | 12.60 | 4.08 | 0.0832 |
| X12 | 949.58 | 1 | 949.58 | 307.21 | < 0.0001** |
| X22 | 2741.65 | 1 | 2741.65 | 886.98 | < 0.0001** |
| X32 | 752.38 | 1 | 752.38 | 243.41 | < 0.0001** |
| Residual | 21.64 | 7 | 3.09 | ||
| Lack of Fit | 15.89 | 3 | 5.30 | 3.68 | 0.1201 |
| Pure Error | 5.75 | 4 | 1.44 | ||
| Cor Total | 6619.53 | 16 |
** Significance with p < 0.01
Response surface analysis
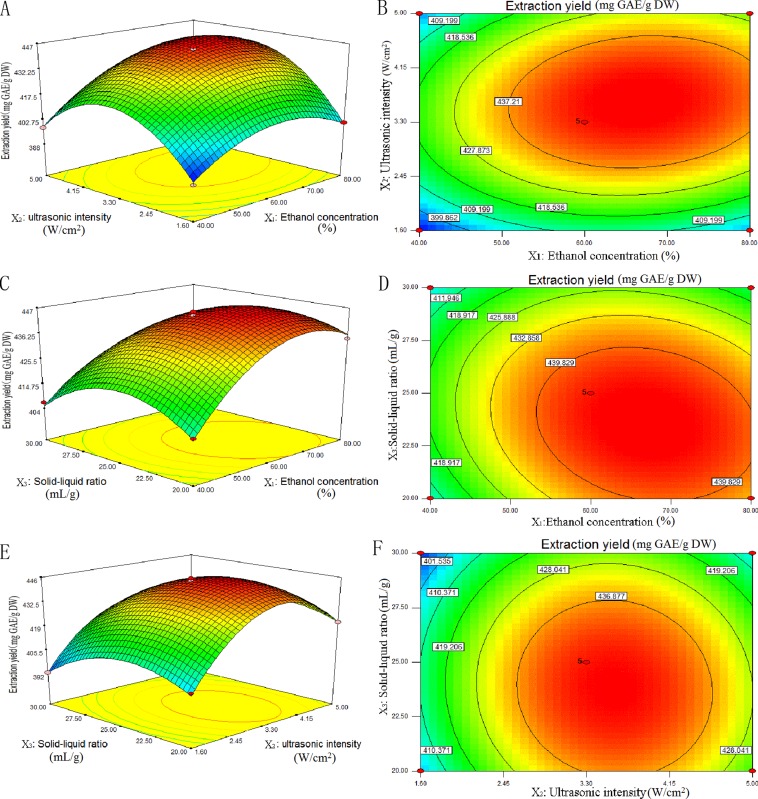
Two-dimensional contour plots and three-dimensional response surface displayed in Fig 3A–3F for independent variables (ethanol concentration, ultrasonic intensity and solid-liquid ratio). The results were obtained by maintaining the two variables unchanged which proved the variance in TP yield under UAE conditions.
Fig 3. Response surface (3D) and contour plots (2D) showing the effects of different extraction parameters (X1: ethanol concentration, %; X2: ultrasonic intensity, W/cm2; X3: solid-liquid ratio, mg/mL) added on the response Y.
The effects of ethanol concentration and ultrasonic intensity on the yield of TP were given in Fig 3A and 3B. The solid-liquid ratio was set at 25 mg/mL. On the whole, TP yield first increases and then decreases at different ethanol concentrations. On a smaller scale, yield of TP enhanced gently with rise in ethanol concentration at lower or higher ultrasonic intensity. However, the yield of TP markedly enhanced with rise in ethanol concentration near the central point. The change in TP yield with ultrasonic intensity also displayed a similar trend. The extraction yield of TP varied faster at higher ethanol concentration than at lower ethanol concentration. The two-dimensional contour plot (Fig 3B) was oval in shape, which indicated that the impact of interaction was significant between ethanol concentration and ultrasonic intensity on TP yield.
Fig 3C and 3D displays the effect of solid-liquid ratio and ethanol concentration on the yield of TP. The ultrasonic intensity was set at 3.3 W/cm2. The extraction yield of TP first increased with lower ethanol concentration, and then decreased gently with an increase in solid-liquid ratio. While, at higher ethanol concentration, the extraction yield of TP reduced quickly with a rise in solid-liquid ratio. In addition, near the central point of solid-liquid ratio, the yield of TP changed greatly with a rise in ethanol concentration. Fig 3D was also oval in shape, which proved that the interaction effect of ethanol concentration and ultrasonic intensity was significant on the yield of TP.
Fig 3E and 3F represents the influence of solid-liquid ratio and ultrasonic intensity on TP yield. The ethanol concentration was set at 60 °C. It showed that the yield of TP first enhances and then decreases at different ultrasonic intensities or solid-liquid ratios. Fig 3F was round in shape, which indicated that the effect of interaction of solid-liquid ratio and ultrasonic intensity on TP yield was insignificant.
Experimental validation and optimization of extraction parameters
Optimum conditions obtained by using Design-Expert 7.0.0 software for ethanol, ultrasonic intensity and solid-liquid ratio were 67.63%, 3.64 W/cm2 and 23, respectively. Single-factor analysis was used for the determination of other parameters. While the value of TP obtained under the above conditions was 447.8 mg GAE/g DW. While the value of TP obtained under the above conditions was 447.8 mg GAE/g DW. The conditions modified for the ease of operation are: 68% ethanol concentration, 3.6 W/cm2 ultrasonic intensity and a value of 23 for solid-liquid ratio. Under the above modified conditions, the value of TP obtained was 448.7 ± 2.15 mg GAE/g DW (N = 3) which was higher than the non-optimized conditions and near to the predicted value, showed that the model was fit for the extraction of TP (Table 4).
Table 4. Predicted and experimental values of the responses at optimum conditions.
| Optimum condition | Extraction yield (mg GAE/g DW) | |||
|---|---|---|---|---|
| Ethanol concentration (%) | Ultrasonic intensity (W/cm2) | Solid-liquid ratio (mg/mL) | Experimental | Predicted |
| 68 (67.63) | 3.6 (3.64) | 23 (23.43) | 448.7 ± 2.15 | 447.8 |
Comparison with the soxhlet extraction method
Soxhlet extraction and UAE results of TP from dried ripe fruit of T. chebula approved that UAE gave a similar extraction yield (448.7 mg GAE/g DW) to the Soxhlet extraction value (443.5 mg GAE/g DW). However, the Soxhlet extraction approach required more solvent and consume more time than the UAE. Additionally, bioactivity of TP could be weakened by the longtime treatment under high temperature. Therefore, it could be concluded that UAE was better suited to extract TP from T. chebula fruits than Soxhlet extraction method.
Antioxidant activity in vitro
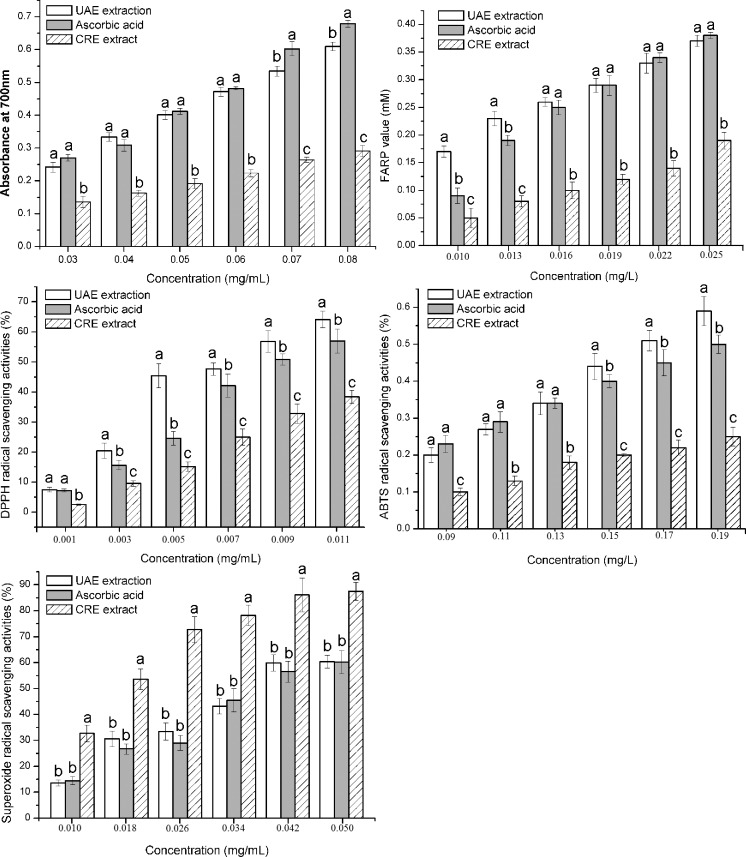
Generally, several approaches are needed to evaluate the antioxidant activities of a plant extract. During reducing power assay, antioxidants caused the reduction of Fe3+ to Fe2+ by donating an electron. So reducing power is widely used as a potential indicator for antioxidant activity [31]. The reducing potential of Ascorbic acid, UAE extract and CRE extract are shown in Fig 4A. The reducing powers exhibited a dose-dependent response. Coefficient of determination (R2) for UAE extract, Ascorbic acid and CRE extract were 0.9963, 0.9831 and 0.9964, respectively. The reducing power of UAE extract and positive control were almost at the same level at 0.03–0.06 mg/mL (p > 0.05), while the UAE extract was lower than the positive control at 0.07–0.08 mg/mL (p < 0.05). As expected, the scavenging activity of the CRE extract was much weaker than UAE extract and Ascorbic acid at 0.03–0.08 mg/mL (p < 0.05). The slopes of the trend lines for UAE extract, Ascorbic acid and CRE extract were 7.1829, 8.5543 and 3.1714 respectively, which also indicated that the reducing power of UAE extract increased slower than Ascorbic acid and faster then CRE extract. The sample solutions with high concentration were not further tested due to the fact that absorbance should be lower than 1 for preventing unacceptable relative errors.
Fig 4. Antioxidant activities of the UAE extract, the CRE extract and Ascorbic acid.
(A) Reducing power, (B) FRAP, (C) DPPH radical-scavenging activity, (D) ABTS radical-scavenging activity and (E) superoxide radical-scavenging activity. Values were expressed as mean ± SD (n = 3), and evaluated by one-way AVONA followed by the Tukey test. Different letter and same letter were considered to be statistically significant (p < 0.05) and statistically insignificant (p > 0.05), respectively.
In order to evaluate the total antioxidant activity of T. chebula fruit extract, an accurate and simple FRAP (ferric reducing antioxidant power) assay was used [32]. As shown in Fig 4B, the FRAP of UAE extract was greater than Ascorbic acid at 0.010–0.013 mg/mL (p < 0.05). Nevertheless, both of them were almost at the same level at 0.013–0.025 mg/mL (p > 0.05). Additionally, both of them were obviously higher than CRE extract (p < 0.05).
DPPH free radical is a stable free radical which has been widely employed to evaluate the in vitro antioxidant activities. Fig 4C showed the scavenging effects of Ascorbic acid and T. chebula fruit extract on DPPH free radicals. The DPPH free radical scavenging activity of UAE extract was far stronger than that of Ascorbic acid at 0.003–0.011 mg/mL (p < 0.05). Moreover, there is a high correlation (R2 = 0.9130) between the DPPH scavenging activity and contents. IC50 value of UAE extract and Ascorbic acid was 0.0066 and 0.0088 mg/mL, respectively. These findings indicated that UAE extract had stronger DPPH radical scavenging ability than Ascorbic acid. However, the DPPH radical scavenging ability of CRE extract was weaker than Ascorbic acid at 0.001–0.011 mg/mL (p < 0.05).
ABTS·+ could generate a blue-green radical cation on oxidation. Some antioxidants can react with ABTS radical, which leads to fading of the mixture solution. Therefore, ABTS method could be used to assess antioxidant activity [33]. As shown in Fig 4D, the tested samples and Ascorbic acid showed dose-dependent activities. ABTS scavenging activities of Ascorbic acid and UAE extract were almost found at the same level at concentration ranged from 0.09–0.13 mg/mL (p > 0.05). However, the scavenging activity of UAE extract was stronger than Ascorbic acid at 0.15–0.19 mg/mL (p < 0.05). The IC50 obtained for UAE extract and ascorbic acid was 0.17 and 0.19 mg/mL, respectively. Intriguingly, a previous study reported the same IC50 value (0.19 mg/mL) for ascorbic acid [34]. These findings revealed that the UAE extract showed similar ABTS scavenging activity as Ascorbic acid. Meanwhile, it has been noted that both the UAE extract and ascorbic acid had stronger ABTS scavenging activities than the CRE extract at 0.09–0.19 mg/mL (p < 0.05).
The superoxide anion could be produced in vivo by pyrogallic acid directly under alkaline conditions. Superoxide radical can further interact with other molecules to generate hydrogen peroxide and hydroxyl radical, which caused oxidative damage in DNA, lipids and proteins [35]. Antioxidants were able to scavenge superoxide anion radicals due to its ability to reduce the reaction (auto-oxidation) of pyrogallic acid. It has been noted that the superoxide radical scavenging activity of UAE extract was similar to that of ascorbic acid at 0.01–0.05 mg/mL (p > 0.05) (Fig 4E). However, the result could not be explained in this paper that the superoxide radical scavenging activity of the CRE extract was stronger than that of the UAE extract or Ascorbic acid (p < 0.05). The IC50 obtained for the UAE extract, ascorbic acid and the CRE extract on ABTS radical was 0.038, 0.038 and 0.016 mg/mL, respectively. Notably, our findings showed that T. chebula fruit extract was capable of relieving the oxidative injury by the superoxide anion.
It can be seen from the above results that the antioxidant activities of the UAE extracts were stronger than that of the CRE extract except the superoxide radical scavenging activity. It has been inferred that some active compounds were destroyed by the longtime extract (Comparison with the Soxhlet extraction method), although there are similar yield between the UAE extract and the CRE extract.
Identification of phenolic compounds
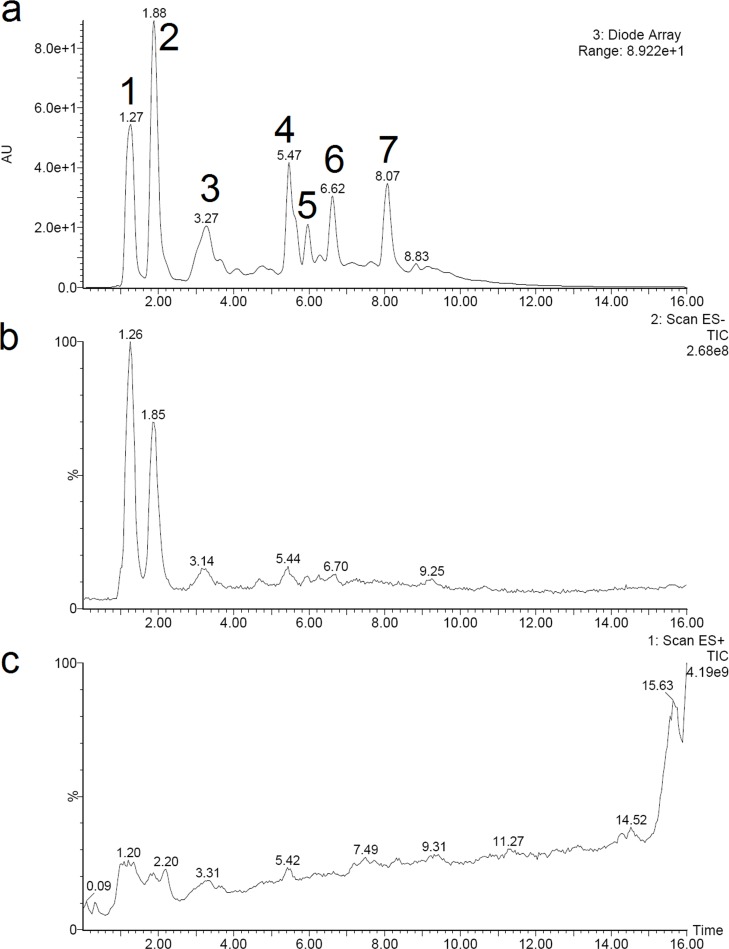
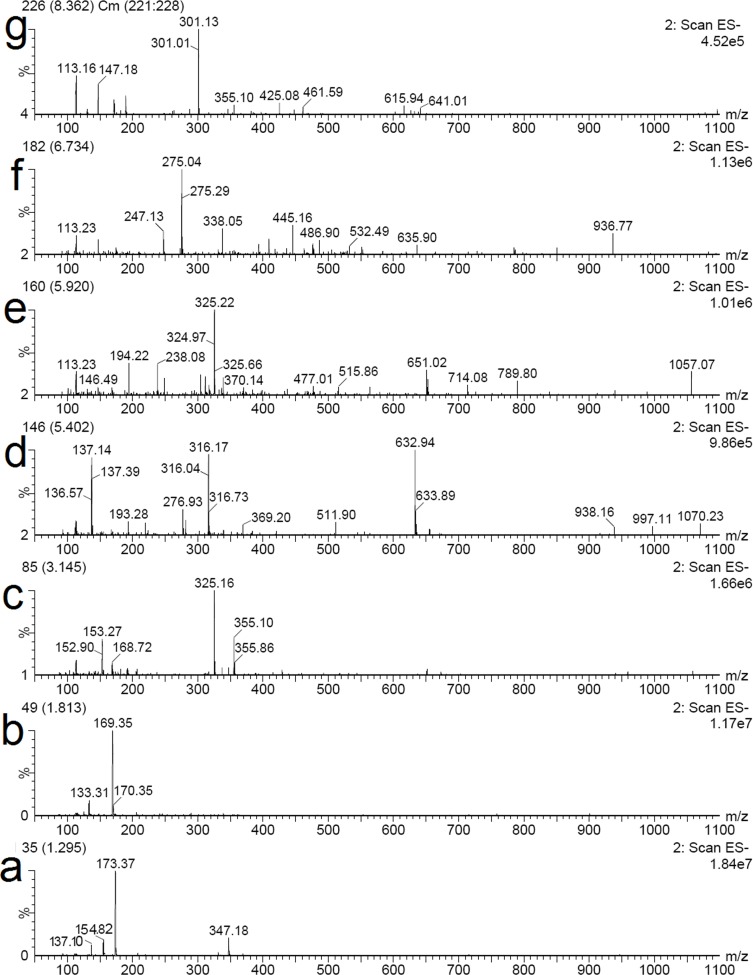
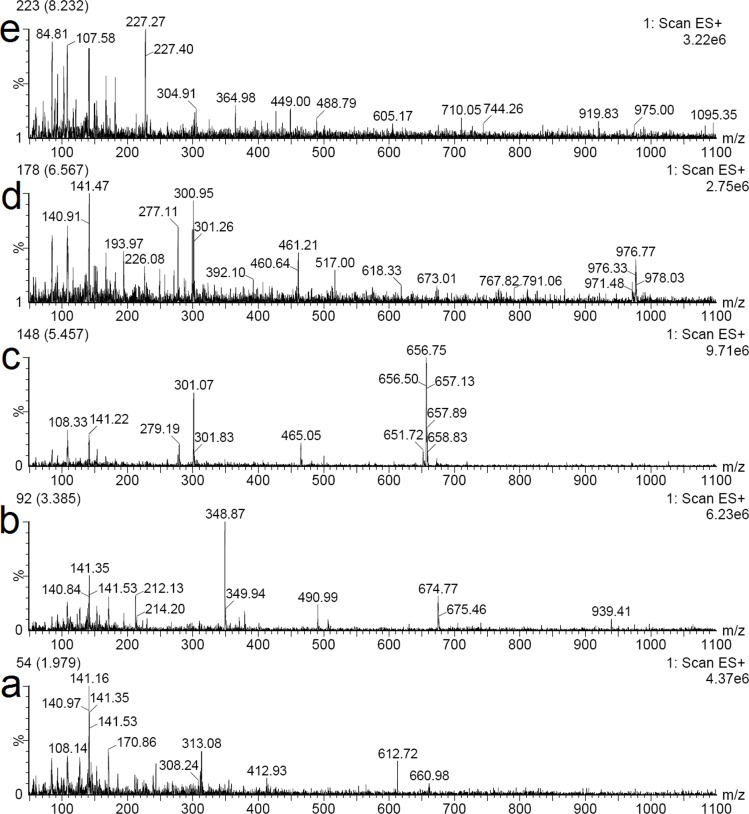
In order to identify the structures of main constituents in the T. chebula fruits extract, the sample was analyzed by HPLC–ESI-MS techniques in both negative and positive mode. Fig 5 showed that phenolic acids were detected sensitively in negative mode. The detected constituents exhibited their quasi-molecular ions [M–H]−. By careful studying on the mass spectra of these compounds (Figs 6 and 7) and comparing with reference data, 6 peaks in the T. chebula fruits extract were designated and identified (Table 5). They were shikimic acid (peak 1), gallic acid (peak 2), 5-O-galloylshikimic acid (peak 3), corilagin (peak 4), 3,4,8,9,10-Pentahydroxydibenzo [b, d] pyran-6-one (peak 6), ellagic acid (peak 7), respectively.
Fig 5.
The HPLC chromatogram (a), total ion chromatogram of mass spectrometer in negative ion mode (b) and positive ion mode (c) of the T. chebula fruits extract.
Fig 6.
The MS spectra in negative mode of seven representative compounds in the T. chebula fruits extract: shikimic acid (a), gallic acid (b), 5-O-galloylshikimic acid (c), corilagin (d), peak (e), 3,4,8,9,10-Pentahydroxydibenzo [b, d] pyran-6-one (f), ellagic acid (g).
Fig 7.
The MS spectra in postitive mode of seven representative compounds in the T. chebula fruits extract: gallic acid (a), 5-O-galloylshikimic acid (b), corilagin (c), 3,4,8,9,10-Pentahydroxydibenzo [b, d] pyran-6-one (d), ellagic acid (e).
Table 5. HPLC-ESI-MS fragmentation (negative and positive ion mode) of the compounds detected in T. chebula fruit extract.
| Rt (min) | Negative ion mode | Positive ion mode | Mol. weight | |
|---|---|---|---|---|
| Peak 1 | 1.27 | 173.37 [M-H]- | 174 | |
| 154.82 [M-H2O-H]- | ||||
| 137.10 [M-H2O-H2O-H]- | ||||
| Peak 2 | 1.88 | 169.35[M-H]- | 170.86 [M+H]+ | 170 |
| 781.02[M-H]- | 141.50 [M+H-CO]+ | |||
| Peak 3 | 3.27 | 325.16 [M-H]- | 348.87 [M+Na]+ | 326 |
| 674.77 [2M+ Na]+ | ||||
| Peak 4 | 5.47 | 632.94 [M-H]- | 656.75 [M+ Na]+ | 634 |
| 316.17 [M-2H]2- | ||||
| Peak 5 | 5.92 | 325.22 [M-H]- | 326 | |
| 651.2 [2M-H]- | ||||
| Peak 6 | 6.62 | 275.04 [M-H]- | 277.11 [M+H]+ | 276 |
| 247.13 [M-H-CO]- | ||||
| Peak 7 | 8.07 | 301.13[M-H]- | 605.17[2M+ H]+ | 302 |
Note: Peaks 1–7 represented shikimic acid, gallic acid, 5-O-galloylshikimic acid, corilagin, Peak 5 (unknown), 3,4,8,9,10-Pentahydroxydibenzo [b, d] pyran-6-one and ellagic acid, respectively.
The mass spectrum of Fig 6A showed the negative quasi-molecular ion at m/z 173.37 [M-H]- and product ions at m/z 154.82 [M-H2O-H]-, 137.10 [M-H2O-H2O-H]-, which is similar to the characteristics of shikimic acid [36]. The mass spectrum of Fig 6B and Fig 7A showed the negative quasi-molecular ion at m/z 169.35 [M-H]- and the positive quasi-molecular ion at m/z 170.86 [M+H]+ and the signal at m/z 141.50 [M+H-CO]+ due to the loss of CO, which corresponds to the fragmentation regularity of gallic acid [37]. The mass spectrum of 5-O-galloylshikimic acid confirmed the presence of the following signals: the negative quasi-molecular ion [M-H]- at 325.16 m/z (Fig 6C), and the positive quasi-molecular ion [M+ Na]+ at 348.87 m/z and [2M+ Na]+ at 674.77 m/z (Fig 7B) [38]. The mass spectrum of corilagin confirmed the presence of the following signals: the negative quasi-molecular ion at [M-H]- at 632.94 m/z (Fig 6D) and [M-2H]2- at 316.17 and the positive quasi-molecular ion [M+ Na]+ at 656.75 m/z (Fig 7C). The ESI-MS spectrum of Fig 6E in addition to the quasi-molecular ion [M-H]- at m/z 325.22, shows another fraction ion at m/z 651.2 [2M-H]-, the molecular mass of the compound is 326. The ESI-MS spectrum of Fig 6F represented the quasi-molecular ion [M-H]- at m/z 275.04, and Fig 7D showed the quasi-molecular ion [M+ Na]+ at 277.11 m/z, which corresponds to the characteristics of 3,4,8,9,10-Pentahydroxydibenzo [b, d] pyran-6-one [39]. Finally, the mass spectrum of ellagic acid showed the negative quasi-molecular ion [M-H]- at 301.13 m/z (Fig 6G) and the positive quasi-molecular ion [2M+H]+ at 605.17 (Fig 7E) [40].
Manna, K., et al reported that shikimic acid reversed the H2O2 induced oxidative damage in hepatocytes by inhibition of NF-kappa B, activation of PI3K/Akt/Nrf2 pathway and reduction of apoptosis [41]. Moreover, it has been proved that gallic acid, 5-O-galloylshikimic acid, corilagin, 3,4,8,9,10-Pentahydroxydibenzo [b, d] pyran-6-one and ellagic acid had significant antioxidant activities [42–44]. Therefore, these compounds could be responsible for the antioxidant activities of T. chebula fruits extract.
Conclusions
RSM was successfully applied to optimize the extraction parameters for total phenolics (TP) yield from the T. chebula fruit. The optimal combination was determined to be an ethanol concentration of 68%, ultrasonic intensity of 3.6 W/cm2, solid-liquid ratio of 23 mg/mL, particle size of 0.18 mm and ultrasonic time of 20 min for 2 times at 70 °C. The yield of TP reached 448.7 ± 2.15 mg GAE/g DW under these conditions. Besides, the antioxidant activities of the UAE extract were stronger than that of the CRE.
Data Availability
All relevant data are within the paper.
Funding Statement
This research was supported by the National Natural Science Foundation of China (grant number 31572559); and Academic Backbone Project of Northeast Agricultural University (grant number 16XG16).
References
- 1.Prado P, Thibaut T. Differences between epiphytic assemblages on introduced Caulerpa taxifolia and coexisting eelgrass (Zostera capricorni) in Botany Bay (NSW, Australia). Sci Mar. 2008;72(4):645–54. 10.3989/scimar.2008.72n4645 PubMed PMID: WOS:000262997400003. [DOI] [Google Scholar]
- 2.Bag A, Bhattacharyya SK, Chattopadhyay RR. The development of Terminalia chebula Retz. (Combretaceae) in clinical research. Asian Pacific Journal of Tropical Biomedicine. 2013;3(3):244–52. 10.1016/S2221-1691(13)60059-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Avula B, Wang Y-H, Wang M, Shen Y-H, Khan IA. Simultaneous Determination and Characterization of Tannins and Triterpene Saponins from the Fruits of Various Species of Terminalia and Phyllantus emblica Using a UHPLC-UV-MS Method: Application to Triphala. Planta Med. 2013;79(2):181–8. 10.1055/s-0032-1328089 PubMed PMID: WOS:000313965100014. [DOI] [PubMed] [Google Scholar]
- 4.Tubtimdee C, Shotipruk A. Extraction of phenolics from Terminalia chebula Retz with water–ethanol and water–propylene glycol and sugaring-out concentration of extracts. Sep Purif Technol. 2011;77(3):339–46. 10.1016/j.seppur.2011.01.002 [DOI] [Google Scholar]
- 5.Rangsriwong P, Rangkadilok N, Satayavivad J, Goto M, Shotipruk A. Subcritical water extraction of phenolic compounds from Terminalia chebula Retz. fruits. Sep Purif Technol. 2009;66(1):51–6. 10.1016/j.seppur.2008.11.023 [DOI] [Google Scholar]
- 6.Chemat F, Zill e H, Khan MK. Applications of ultrasound in food technology: Processing, preservation and extraction. Ultrason Sonochem. 2011;18(4):813–35. 10.1016/j.ultsonch.2010.11.023 . [DOI] [PubMed] [Google Scholar]
- 7.Altemimi A, Lightfoot DA, Kinsel M, Watson DG. Employing Response Surface Methodology for the Optimization of Ultrasound Assisted Extraction of Lutein and beta-Carotene from Spinach. Molecules. 2015;20(4):6611–25. 10.3390/molecules20046611 PubMed PMID: WOS:000354480700085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Altemimi A, Choudhary R, Watson DG, Lightfoot DA. Effects of ultrasonic treatments on the polyphenol and antioxidant content of spinach extracts. Ultrason Sonochem. 2015;24:247–55. 10.1016/j.ultsonch.2014.10.023 . [DOI] [PubMed] [Google Scholar]
- 9.Wu MY, Wu J. In-vitro investigations on ultrasonic control of water chestnut. J Aquat Plant Manage. 2007;45:76–83. PubMed PMID: WOS:000250536800002. [Google Scholar]
- 10.Jambrak AR, Mason TJ, Paniwnyk L, Lelas V. Ultrasonic effect on pH, electric conductivity, and tissue surface of button mushrooms, brussels sprouts and cauliflower. Czech J Food Sci. 2007;25(2):90–100. 10.17221/757-CJFS PubMed PMID: WOS:000245151600005. [DOI] [Google Scholar]
- 11.Soria AC, Villamiel M. Effect of ultrasound on the technological properties and bioactivity of food: a review. Trends Food Sci Tech. 2010;21(7):323–31. 10.1016/j.tifs.2010.04.003 PubMed PMID: WOS:000280121300001. [DOI] [Google Scholar]
- 12.Singleton VL, Orthofer R, Lamuela-Raventós RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Method Enzymol 1999;299:152–78. [Google Scholar]
- 13.Roslan J, Kamal SMM, Yunos KFM, Abdullah N. Optimization of Enzymatic Hydrolysis of Tilapia Muscle (Oreochromis niloticus) using Response Surface Methodology (RSM). Sains Malays. 2014;43(11):1715–23. PubMed PMID: WOS:000345535300010. [Google Scholar]
- 14.Boukroufa M, Boutekedjiret C, Petigny L, Rakotomanomana N, Chemat F. Bio-refinery of orange peels waste: A new concept based on integrated green and solvent free extraction processes using ultrasound and microwave techniques to obtain essential oil, polyphenols and pectin. Ultrason Sonochem. 2015;24:72–9. 10.1016/j.ultsonch.2014.11.015 PubMed PMID: WOS:000349726500010. [DOI] [PubMed] [Google Scholar]
- 15.Konyalioglu S, Saglam H, Kivcak B. alpha-Tocopherol, flavonoid, and phenol contents and antioxidant activity of Ficus carica leaves. Pharm Biol. 2005;43(8):683–6. 10.1080/13880200500383538 PubMed PMID: ISI:000234398600007. [DOI] [Google Scholar]
- 16.Naik GH, Priyadarsini KI, Naik DB, Satav JG, Mohan H. Antioxidant, antiradical activity and phytochemical analysis of the aqueous extract of Terminalia chebula. Xi Biennial Meeting of the Society for Free Radical Research International. 2002:551–5. PubMed PMID: WOS:000182408200103. [Google Scholar]
- 17.Suzek H, Celik I, Dogan A, Yildirim S. Protective effect and antioxidant role of sweetgum (Liquidambar orientalis) oil against carbon tetrachloride-induced hepatotoxicity and oxidative stress in rats. Pharm Biol. 2016;54(3):451–7. 10.3109/13880209.2015.1045086 PubMed PMID: ISI:000368607100006. [DOI] [PubMed] [Google Scholar]
- 18.Huang B, Ke H, He J, Ban X, Zeng H, Wang Y. Extracts of Halenia elliptica exhibit antioxidant properties in vitro and in vivo. Food Chem Toxicol. 2011;49(1):185–90. 10.1016/j.fct.2010.10.015 [DOI] [PubMed] [Google Scholar]
- 19.Sun Y, Liu D, Chen J, Ye X, Yu D. Effects of different factors of ultrasound treatment on the extraction yield of the all-trans-β-carotene from citrus peels. Ultrason Sonochem. 2011;18(1):243–9. 10.1016/j.ultsonch.2010.05.014 [DOI] [PubMed] [Google Scholar]
- 20.Tubtimdee C, Shotipruk A. Extraction of phenolics from Terminalia chebula Retz with water-ethanol and water-propylene glycol and sugaring-out concentration of extracts. Sep Purif Technol. 2011;77(3):339–46. 10.1016/j.seppur.2011.01.002 PubMed PMID: WOS:000288738500008. [DOI] [Google Scholar]
- 21.Entezari MH, Nazary SH, Khodaparast MHH. The direct effect of ultrasound on the extraction of date syrup and its micro-organisms. Ultrason Sonochem. 2004;11(6):379–84. 10.1016/j.ultsonch.2003.10.005 PubMed PMID: WOS:000223531700005. [DOI] [PubMed] [Google Scholar]
- 22.Wang BC, Wang QH, Zhu LC, Yuan FW. Degrade naphthalene using cells immobilized combining with low-intensity ultrasonic technique. Colloid Surface B. 2007;57(1):17–21. 10.1016/j.colsurfb.2006.12.003 PubMed PMID: WOS:000246750200003. [DOI] [PubMed] [Google Scholar]
- 23.Skotti E, Anastasaki E, Kanellou G, Polissiou M, Tarantilis PA. Total phenolic content, antioxidant activity and toxicity of aqueous extracts from selected Greek medicinal and aromatic plants. Ind Crop Prod. 2014;53:46–54. 10.1016/j.indcrop.2013.12.013 [DOI] [Google Scholar]
- 24.Sun YS, Liu ZB, Wang JH, Yang SF, Li BQ, Xu N. Aqueous ionic liquid based ultrasonic assisted extraction of four acetophenones from the Chinese medicinal plant Cynanchum bungei Decne. Ultrason Sonochem. 2013;20(1):180–6. 10.1016/j.ultsonch.2012.07.002 PubMed PMID: WOS:000311130000026. [DOI] [PubMed] [Google Scholar]
- 25.Hassan M, Essam T, Yassin AS, Salama A. Optimization of rhamnolipid production by biodegrading bacterial isolates using Plackett-Burman design. Int J Biol Macromol. 2016;82:573–9. 10.1016/j.ijbiomac.2015.09.057 PubMed PMID: WOS:000367425100072. [DOI] [PubMed] [Google Scholar]
- 26.Zhang G, He L, Hu M. Optimized ultrasonic-assisted extraction of flavonoids from Prunella vulgaris L. and evaluation of antioxidant activities in vitro. Innov Food Sci Emerg. 2011;12(1):18–25. 10.1016/j.ifset.2010.12.003 [DOI] [Google Scholar]
- 27.Wang CX, Li YP, Yao LH, Wu GJ, Chang J, Shu CH, et al. Optimization of Ultrasonic-assisted Extraction of Flavonoid from Portulaca oleracea L. by Response Surface Methodology and Chemical Composition Analysis. J Korean Soc Appl Bi. 2014;57(5):647–53. 10.1007/s13765-014-4058-4 PubMed PMID: WOS:000345310300015. [DOI] [Google Scholar]
- 28.Al-Farsi MA, Lee CY. Optimization of phenolics and dietary fibre extraction from date seeds. Food Chem. 2008;108(3):977–85. 10.1016/j.foodchem.2007.12.009 [DOI] [PubMed] [Google Scholar]
- 29.Altemimi A, Watson DG, Kinsel M, Lightfoot DA. Simultaneous extraction, optimization, and analysis of flavonoids and polyphenols from peach and pumpkin extracts using a TLC-densitometric method. Chem Cent J. 2015;9. doi: ARTN 39 10.1186/s13065-015-0113-4 PubMed PMID: WOS:000356582500001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang L, Li D, Bao C, You J, Wang Z, Shi Y, et al. Ultrasonic extraction and separation of anthraquinones from Rheum palmatum L. Ultrason Sonochem. 2008;15(5):738–46. 10.1016/j.ultsonch.2007.12.008 [DOI] [PubMed] [Google Scholar]
- 31.Zheng Y, Li Y, Wang WD. Optimization of ultrasonic-assisted extraction and in vitro antioxidant activities of polysaccharides from Trametes orientalis. Carbohyd Polym. 2014;111:315–23. 10.1016/j.carbpol.2014.04.034 PubMed PMID: WOS:000340302100036. [DOI] [PubMed] [Google Scholar]
- 32.Wu T, McCallum JL, Wang S, Liu R, Zhu H, Tsao R. Evaluation of antioxidant activities and chemical characterisation of staghorn sumac fruit (Rhus hirta L.). Food Chem. 2013;138(2–3):1333–40. 10.1016/j.foodchem.2012.10.086 . [DOI] [PubMed] [Google Scholar]
- 33.Lv LZ, Wei L, Chen DY, Liu JB, Lin SY, Ye HQ, et al. Optimization of ultrasound-assisted extraction of polyphenols from maize filaments by response surface methodology and its identification. Journal of Applied Botany and Food Quality. 2015;88:152–63. 10.5073/Jabfq.2015.088.022 PubMed PMID: WOS:000361900900001. [DOI] [Google Scholar]
- 34.Ye C-L, Hu W-L, Dai D-H. Extraction of polysaccharides and the antioxidant activity from the seeds of Plantago asiatica L. Int J Biol Macromol. 2011;49(4):466–70. 10.1016/j.ijbiomac.2011.05.026 [DOI] [PubMed] [Google Scholar]
- 35.Liu J, Jia L, Kan J, Jin CH. In vitro and in vivo antioxidant activity of ethanolic extract of white button mushroom (Agaricus bisporus). Food Chem Toxicol. 2013;51:310–6. 10.1016/j.fct.2012.10.014 . [DOI] [PubMed] [Google Scholar]
- 36.Avula B, Wang Y-H, Smillie TJ, Khan IA. Determination of Shikimic Acid in Fruits of Illicium Species and Various Other Plant Samples by LC–UV and LC–ESI–MS. Chromatographia. 2008;69(3–4):307–14. 10.1365/s10337-008-0884-z [DOI] [Google Scholar]
- 37.Bedner M, Duewer DL. Dynamic Calibration Approach for Determining Catechins and Gallic Acid in Green Tea Using LC-ESI/MS. Anal Chem. 2011;83(16):6169–76. 10.1021/ac200372d PubMed PMID: WOS:000293758800009. [DOI] [PubMed] [Google Scholar]
- 38.Pawlowska AM, De Leo M, Braca A. Phenolics of Arbutus unedo L. (Ericaceae) fruits: Identification of anthocyanins and gallic acid derivatives. J Agr Food Chem. 2006;54(26):10234–8. 10.1021/jf062230o PubMed PMID: WOS:000242941700086. [DOI] [PubMed] [Google Scholar]
- 39.Pfundstein B, El Desouky SK, Hull WE, Haubner R, Erben G, Owen RW. Phenolic compounds in the fruits of Egyptian medicinal plants (Terminalia bellerica, Terminalia chebula and Terminalia horrida): Characterization, quantitation and determination of antioxidant capacities. Phytochemistry. 2010;71(10):1132–48. 10.1016/j.phytochem.2010.03.018 PubMed PMID: WOS:000279412900012. [DOI] [PubMed] [Google Scholar]
- 40.Lee JH, Johnson JV, Talcottt ST. Identification of ellagic acid conjugates and other phenolics in muscadine grapes by HPLC-ESI-MS. J Agr Food Chem. 2005;53(15):6003–10. 10.1021/jf050468r PubMed PMID: WOS:000230702400028. [DOI] [PubMed] [Google Scholar]
- 41.Manna K, Khan A, Das DK, Kesh SB, Das U, Ghosh S, et al. Protective effect of coconut water concentrate and its active component shikimic acid against hydroperoxide mediated oxidative stress through suppression of NF-kappa B and activation of Nrf2 pathway. J Ethnopharmacol. 2014;155(1):132–46. 10.1016/j.jep.2014.04.046 PubMed PMID: WOS:000340854000012. [DOI] [PubMed] [Google Scholar]
- 42.Zhang ZG, Liu GW, Li XB, Gao L, Guo CM, Wang HB, et al. Evaluation of the Change of Serum Copper and Zinc Concentrations of Dairy Cows with Subclinical Ketosis. Biol Trace Elem Res. 2010;138(1–3):8–12. 10.1007/s12011-009-8606-4 PubMed PMID: WOS:000284382500002. [DOI] [PubMed] [Google Scholar]
- 43.Tung Y-T, Wu J-H, Huang C-C, Peng H-C, Chen Y-L, Yang S-C, et al. Protective effect of Acacia confusa bark extract and its active compound gallic acid against carbon tetrachloride-induced chronic liver injury in rats. Food Chem Toxicol. 2009;47(6):1385–92. 10.1016/j.fct.2009.03.021 [DOI] [PubMed] [Google Scholar]
- 44.Zhang ZJ, Liao LP, Moore J, Wu T, Wang ZT. Antioxidant phenolic compounds from walnut kernels (Juglans regia L.). Food Chem. 2009;113(1):160–5. 10.1016/j.foodchem.2008.07.061 PubMed PMID: WOS:000260711100025. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.