Abstract
Objective/Background
To review the effect of mechanical stretch on hypertrophic scars after burn injuries.
Methods
A systematic review of all controlled trials related to the effect of mechanical stretch on post burn hypertrophic scars was conducted. Studies of conservative scar managements that applied mechanical forces parallel to the scar surface, including stretching exercise, massage, and splinting, were appraised. Eligible studies published in English between 1995 and 2016 were extracted from The Cochrane Library, MEDLINE, CINAHL, Science direct, SPORTDiscus, and Physiotherapy Evidence Database Scale (PEDro). The journals were further screened with inclusion and exclusion criteria. PEDro was selected for further analysis and appraisal.
Results
There were 853 articles identified. After a standardized screening mechanism stipulated, only nine full-text articles were selected for critical appraisal using PEDro. There were five articles of high quality, two of fair quality, and two of poor quality. Detailed training regime and outcomes of nine studies were summarised, including two studies with stretching exercise, six studies with massage, and one study with splinting. The physical parameters of scar assessments and the range of motion on affected areas were compared.
Conclusion
From extensive literature search, there was no strong evidence indicating the positive effect of mechanical stretch using stretching exercise, massage, or splinting on hypertrophic scars. A firm conclusion cannot be drawn for the discrepancy of outcome measures and varied effectiveness. Most of the included studies lacked objective evaluation or control group for comparison. Further high quality studies with larger sample size and using standardized measurements are needed.
Keywords: burns, hypertrophic scars, mechanical stretch, massage, splinting, stretching exercise
Introduction
Hypertrophic scars are severe complications after burn injuries. The concomitant scar contractures will develop and expand to underlying connective tissue and muscles, resulting in limitation in joint range of motion (ROM) and participation of daily activities (Dewey, Richard, fit Parry, 2011). Despite dedicating investigations in preventing hypertrophic scars, scar contractures, and subsequent impairments, the complex pathogenesis and prolonged dynamic process make the treatment marginally effective (Blakeney, Rosenberg, Rosenberg, & Faber, 2008; Stubbs et al., 2011).
Conservative treatments were preferred in clinical settings to restrain the progression of scar and contracture for their noninvasive and easy-operation properties (Anthonissen, Daly, Janssens, & Van den Kerckhove, 2016). In recent years, the concept of “mechanotherapy” has inspired professionals to implement treatments from a mechanobiological basis (Huang, Holfeld, Schaden, Orgill, & Ogawa, 2013). In substantial basic research related to wound, hypertrophic scar, or keloid, skin tension was reported to have a strong relationship with inflammatory process, collagen orientation, and construction remolding in epidermis and dermis (Bouffard et al., 2008; Du et al., 2013; Junker, Kratz, Tollbäck, & Kratz, 2008). These laboratory tests showed that the influence of stretch on scar proliferation process was dosage-, stage-, and orientation-dependent, suggesting the necessity to explore the effective protocol of “stretch” comprised treatments in corresponding magnitude to prevent hypertrophic scar and contracture in clinical application (Akaishi, Akimoto, Ogawa, & Hyakusoku, 2008; Ogawa et al., 2012; Roques, 2002).
Although many guidelines stressed the importance of implementing mechanical stretch to improve scar texture, prevent or correct scar contracture, and increase ROM, consensus has seldom been reached regarding the detailed protocol and the magnitude of the stretching force. Therefore, this systematic review was conducted to evaluate the quality of published studies and summarise the effectiveness and regime for building up the practical guidelines.
Methods
Search strategy
Articles published from 1995 to 2016 were searched from the electronic database: Cochrane Central Register of Controlled Trials CENTRAL (The Cochrane Library), MEDLINE (1965 to most recent date available), CINAHL (1982 to most recent date available), Science direct, SPORTDiscus (1830+) and the Physiotherapy Evidence Database (PEDro). “Mechanical stretch” after burn injuries was defined as conservative scar managements that applied tensile force parallel to the scar, and stretching exercise, massage, and splinting were included in the analysis. Search syntax following professional standards were developed as: #1: MeSH descriptor: [Burns] explode all trees; #2: burn* or scald* or thermal injur*:ti, ab, kw; #3: MeSH descriptor: [Cicatrix, Hypertrophic] explode all trees; #4: scar* or cicatrix: ti, ab, kw; #5: #1 or #2 or #3 or #4; #6: MeSH descriptor: [splints] explode all trees; #7: MeSH descriptor: [massage] explode all trees; #8: stretch* or splint* or massage*: ti, ab, kw; #9: #6 or #7 or #8; #10: #5 and #9.
To avoid publication bias, additional studies were detected through online clinical trials registered websites (ClinicalTrials.gov, 2000; World Health Organization International Clinical Trials Registry Platform) and bibliographies of relevant publications.
Screening criteria
Studies were included according to the following criteria: 1) prospective controlled trials with full text available in English, including randomized controlled trial (RCT), non-RCT controlled clinical trials (CCT); 2) outcome measures were physical parameters related to scar and scar contracture; 3) interventions were stretching-, splinting-, and massage related. Subjects after burn injuries were not specified in terms of age, race, severity of injury, and stage of scars. Review articles and studies on the aetiology, laboratory tests, and assessments of scars were excluded. Two review authors independently assessed the title and abstract of articles and selected eligible trials. Then, the full texts were reviewed by the same reviewers to include studies using the prestipulated criteria. The disagreement was resolved by consultation with a third reviewer. The process was summarised through Preferred Reporting Items for Systematic Reviews (PRISMA).
Data extraction and quality assessment
The data was extracted independently by reviewers using a standard form, which contained characteristics of subjects, area and depth of injuries, mode and regime of therapies, and outcomes of scar and contracture from all groups. Study design and analytical methods were also recorded for quality appraisal using the Oxford Centre for Evidence-Based Medicine level of evidence (Oxford Centre for Evidence-Based Medicine, 2009) and PEDro Quality Scale (Maher, Sherrington, Herbert, Moseley, & Elkins, 2003). Studies were rated according to PEDro classification criteria: high quality = PEDro score 6–10; fair quality = PEDro score 4–5; poor quality = PEDro score ≤ 3.
Results
There were 853 articles identified from the electronic database followed by strategies and criteria stipulated in the method. After detailed screening of titles, abstracts, and contents, 12 full-text articles matched our selection criteria. Finally, nine studies with full text available were included in the quality assessment. The detailed PRISMA flow chart of the search process was summarised in the diagram (Figure 1).
Figure 1.
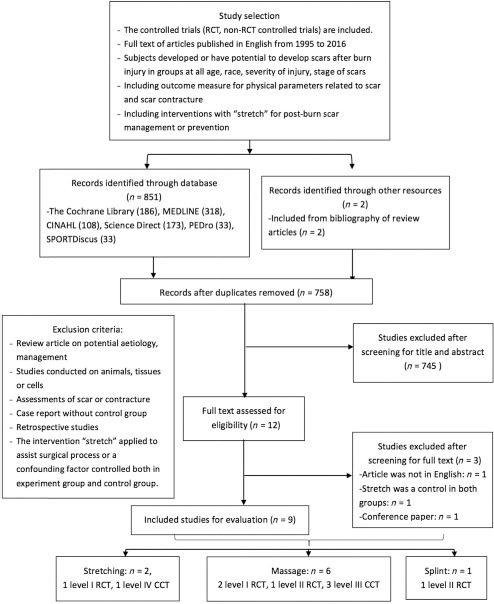
PRISMA flow chart of recruiting eligible studies.
Note. RCT = randomized controlled trial; CCT = case controlled trials.
Classification of selected studies
Among the nine evaluated studies, three were three level I RCTs (Cho et al., 2014; Okhovatian & Zoubine, 2007; Patiño, Novick, Merlo, & Benaim, 1999), two were level II RCTs for small sample size and short follow-up rate (Kolmus, Holland, Byrne, & Cleland, 2012; Silverberg, Johnson, & Moffat, 1996), three were level III CCTs (Morien, Garrison, & Smith, 2008; Roh, Cho, Oh, & Yoon, 2007; Roh, Seo, & Jang, 2010), and one was a level IV CCT (Godleski et al., 2013) for inadequate sample size. According to the PEDro criteria, five trials were of high quality, two were of fair quality, and two of poor quality. The details of PEDro scoring are listed in Table 1.
Table 1.
PEDro Scoring.
| PEDro scale items | Cho et al., 2014 | Kolmus et al., 2012 | Roh et al., 2010 | Okhovatian and Zoubine, 2007 | Silverberg et al., 1996 | Patiño et al., 1999 | Roh et al., 2007 | Morien et al., 2008 | Godleski et al., 2013 |
|---|---|---|---|---|---|---|---|---|---|
| 1. Eligibility | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 2. Random allocation | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 |
| 3. Concealed allocation | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 4. Baseline comparability | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 5. Blind subjects | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 6. Blind therapists | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 7. Blind assessors | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 |
| 8. Key outcome measure and drop rate | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 9. Intention-to-treat analysis | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 |
| 10. Between-group statistical comparisons | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 |
| 11. Point measures & measures of variability | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 |
| Total score | 6/10 | 6/10 | 6/10 | 6/10 | 6/10 | 5/10 | 5/10 | 3/10 | 3/10 |
| RCT quality | High | High | High | High | High | Fair | Fair | Poor | Poor |
Note. PEDro = Physiotherapy Evidence Database Scale; RCT = randomized controlled trial.
Characteristics of subjects
The selected trials embodied 375 subjects in total, with sample size ranging from 8 to 160 and age ranging from 4 to 64 years old. Four trials specified the location of scars (Godleski et al., 2013; Kolmus et al., 2012; Roh et al., 2007; Roh et al., 2010; Silverberg et al., 1996), four trials underwent skin graft beforehand (Cho et al., 2014; Godleski et al., 2013; Kolmus et al., 2012; Morien et al., 2008), two trials were on patients aged < 18 years (Morien et al., 2008; Patiño et al., 1999) (Table 2). For ethnicity, one study incorporated a total of nine subjects, covering white, black, and Hispanic races (Silverberg et al., 1996).
Table 2.
Summary of Recruited Subjects and Outcome Measures.
| Specification | Age |
Skin graft |
Scar location |
Post injury days |
Outcome measure | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| < 18 y | axillary | Hand, wrist, or forearm | < 1 wk | 1 wk < 3 m | Within 1 y | 1 y | Scar | contracture | ||
| Stretch | ||||||||||
| Okhovatian and Zoubine, 2007 | ✓ | ✓* | ||||||||
| Godleski et al., 2013 | ✓ | ✓ | ✓* | |||||||
| Massage | ||||||||||
| Cho et al., 2014 | ✓ | ✓ | ✓* | |||||||
| Morien et al., 2008 | ✓ | ✓ | ✓ | ✓* | ||||||
| Roh et al., 2010 | ✓ | ✓ | ✓ | |||||||
| Roh et al., 2007 | ✓ | ✓ | ✓* | |||||||
| Patiño et al., 1999 | ✓ | ✓ | ||||||||
| Silverberg et al., 1996 | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| Splint | ||||||||||
| Kolmus et al., 2012 | ✓ | ✓ | ✓ | ✓ | ||||||
Note.
= significant improvement (p < .05).
Outcomes of Intervention
The duration of intervention ranged from 3 days to 6 months. For the post injury days for initiation treatments (within 48 hours–16 years after the injury), two trials specified the time within 1 week (Kolmus et al., 2012; Okhovatian & Zoubine, 2007), and scars in one trial had developed more than 1 year (Morien et al., 2008) (Table 2).
Regarding outcome measures, four studies assessed the subjective scar parameters using Vancouver Scar Scale (VSS), modified VSS or Patient and Observer Scar Assessment Scale (POSAS) (Patiño et al., 1999; Roh et al., 2007; Roh et al., 2010; Silverberg et al., 1996), and two studies involved objective scar measurement tools in the experiments, including high-resolution ultrasonic wave for measuring scar thickness, mexameter for scar melanin and erythema, tewameter for transepidermal water loss (TEWL), sebumeter for scar sebum, cutometer for scar elasticity, and laser Doppler for blood perfusion (Cho et al., 2014; Roh et al., 2010). Besides, four studies evaluated the ROM of scar-adjacent joints (Godleski et al., 2013; Morien et al., 2008; Okhovatian & Zoubine, 2007; Silverberg et al., 1996), and one of them assessed both the scar parameters and ROM (Silverberg et al., 1996). Overall, two out of six studies reported significantly improved scar property, and three out of four reported improved ROM of scar-adjacent joints.
Intervention strategy
Stretching (Table 3)
Table 3.
Stretching, Massage, Splinting Protocols, and Outcomes.
| Author | Population and Patients | Scar type | Experiment group | Control group | Time of treatment initiation | Regime | Intervention length | Follow-up length | Outcome measure (+/ - /0): + p < .05 |
|---|---|---|---|---|---|---|---|---|---|
| Stretching | |||||||||
| Okhovatian and Zoubine, 2007; Level I RCT | Iran; n = 30; age = CG 36 ± 10, EG 39 ± 9 | Group match burn injured patients | Burn-specific rehabilitation protocol: early initiation, stretching exercise, active exercise, daily activities, (n = 15) | CT: (n = 15) | EG: 1st d of admission and 3rd d after grafting CG: 2 weeks after admission, 10–15 d after grafting | CG: 15–20 min/session, 1 session/d EG: 30–45 min/session, 2–3 session/d | CG: 26 ± 15 EG: 22 ± 12 | Pre-post | No. of contracture (ROM < functional range): +, thrombosis: 0; length of stay 0, skin graft 0; |
| Godleski et al., 2013; Level IV CCT | America; n = 9; age = 39.4 ± 13.5; TBSA: 40.3 ± 21.8 | After skin graft After burn injuries | Intensive stretch for active area + CT | CT: Strengthening, mobility, self-care activities | 61.4 ± 25.5 d | > 1hr/d (30-min OT, 30-min PT) | 61.4 ± 25.5 | 0, 4 wk | Within group comparison: Goniometry + Finger flexion + Kapandji opposition scale + Largest gain in week 1 |
| Massage | |||||||||
| Cho et al., 2014; Level I RCT | Korean n = 146; EG: age = 46.06 ± 8.63 TBSA: 37.25 ± 18.60, CG: age = 47.21 ± 8.22 | Hypertrophic scars after acute management of burns, including skin graft | CT + massage (Effleurage, friction, petrissage massage after cream, oil, and lotion; (n = 80) | CT (ROM + silicone gel + pressure therapy + intralesional corticosteroid injection + cream/oil; (n = 80) | CG:156.47 ± 56.48 d EG: 148.77 ± 56.85 d | 3 sessions/ week, 30 min/ session for each area | CG: 35.85 ± 11.80 d EG: 34.69 ± 22.53 d | Pre-post | - pruritus (VAS): +; Itching scale +; scar thickness +, melanin and erythema +, TEWL +, scar sebum 0, scar elasticity 0 |
| Morien et al., 2008; Level III CCT | America; n = 8; age = 13.5 ± 2.6 (10–17 y) | Well-healed skin grafts > 2 y after third-degree burns | 5-min effleurage, 5-min stretching and rolling strokes, 2–5-min friction, 5-min lengthening and rolling | Contralateral scar site without massage | 2–16 y after burn injury | 1 session/d, 20 -25 min/ session | 5 children for 4 -5d, 3 for 3 d | Pre-post | - Subjective reported mood: 0, - ROM of scar adjacent joints: EG:+, CG: 0 |
| Roh et al., 2010; Level III, CCT | Korean; n = 26, age > 18 y, EG: age = 37.7 ± 13.67, TBSA: 29.54 ± 16.44 | Partial- or full-thickness burn on forearm or hand | skin rehabilitation nursing program: light palm stroking, acupressure and occlusive dressing (n = 13) | CT without massage (n = 13) | EG: 3.46 ± 2.40 mo; CG: 3.38 ± 2.26 mo | 30 min/session, 3 session/wk | 3 mo | Pre-post | - scar thickness (ultrasound) 0, - blood perfusion (Laser Doppler Imager) 0, - POSAS: 0, - depression (CESD): 0, - BSHS-B-K: 0 |
| Roh et al., 2007; Level III, CCT | Korean; n = 34, age > 18 y, EG: age = 33.3 ± 8.3, CG: age = 39.1 ± 8.2; | post burn scar at hand or forearm, partial- or full-thickness | massage, light stroking of palm, acupressure on unscarred areas on forearm and hand (n = 18) | conventional without massage (n = 17) | EG: 127 ± 171.1; CG: 95.3 ± 83.7 | Care giver massage 10 min/d, skin rehabilitation massage therapy 30 min/wk | 3 mo | 0, 3 mo, subjective skin status at 3 mo | total VSS and sub scores: +; skin status (subjective): +, depression (CES-D) +, itchiness (Itch Man Scale) + |
| Patiño et al., 1999; Level I RCT | Argentina, children, n = 30, EG: age = 59.4 ± 5.3 months; CG: age = 51.3 ± 4.1 months | Paediatric patients, HTS > 30% TBSA. Worst 10 cm2 area of HTS identified by VSS | pressure garment and friction massage with plain cream | pressure garments only | unknown | massage 10 min/d, daily | 3 mo | 0, 3 mo, subjective skin status at 3 mo | modified VSS 0 |
| Silverberg et al., 1996; Level II RCT | USA; n = 10, (white = 3, black = 4, hispanic = 3; mean age = 51 y, TBSA = 25.5%; | post burn scar at wrist; EG: 2 dorsal wrist burn, 3 volar wrist burn, CG:5 dorsal wrist burn | CT + soft tissue mobilization, (direct oscillation, friction massage) n = 5, mean age = 51 y, | CT (active assisted ROM) n = 5, | 1–11 mo after burn injury | 10–15min | 10–15 min | Pre-post | CG ROM: wrist extension: +; radial deviation: +. Total ROM: 0; VSS 0 EG ROM: wrist extension: +, ulnar deviation: +; Total ROM: 0; VSS 0 |
| Splinting | |||||||||
| Kolmus et al., 2012; Level II RCT | Melbourne; n = 52, age > 18 y EG: age = 43.5 ± 18.0 (3–50 y), TBSA:19.1 ± 14.2 CG: age = 49.4 ± 19.0, TBSA: 18.6 ± 10.6 (3–40 y) | Axillary burn requiring surgery; | Splint: shoulder splint (immobilisation abduction 90°) + CT (n = 27) | CT: stretching, strengthening and functional retraining (n = 25) | Usually 5 days after grafting | first 6 wk all day + 6 wk: overnight | 12 wk | 6, 12 wk | ROM (Plurimeter-V Inclinometer) - shoulder abduction: 0; - shoulder flexion: 0; - BSHS-B: 0; UEFI: 0; GST: 0 |
Note. BSHS-B = Burn Specific Health Scale-Brief questionnaire; BSHS-B-K = Korean Burn Specific Health Scale-Brief; CES-D = Korean Center for Epidemiologic Studies Depression Scale; CG = controlled group; CT = conventional treatment; EG = experimental group; GST = grocery shelving task; hr = hour; min = minute; mo = month; OT = occupational therapy; POSAS = patient and observer scar assessment scale; PT = physical therapy; ROM = range of motion; TBSA = total body surface area; TEWL = transepidermal water loss; UEFI = upper extremity functional index; VAS = visual analogue scale; VSS = Vancouver scar scale; y = year.
Among the two articles that examined the effect of stretching on scars and number of contracture, one RCT emphasised the early implementation of stretch exercise within 1 week after skin grafting (Okhovatian & Zoubine, 2007). In this trial, a burn rehabilitation protocol focused on early stretch as well as active exercise was prescribed from the first day of admission or the third day after grafting, with 60–135 minutes of daily intervention. Outcomes of the burn rehabilitation protocol were compared with a conventional treatment group which started 2 weeks later and followed by 15–20 minutes of exercise every day. Significant decrease in the number of contracture was reported compared with the conventional treatment group (Okhovatian & Zoubine, 2007).
The other CCT explored the effect of a 4-week intensive stretch on active scars in nine patients from 1–3 months after the injury, with more than 1 hour of stretch daily. Weekly changes were compared and largest gain of all measured ROM was found in the 1st week (Godleski et al., 2013).
Massage (Table 3)
There were four studies that examined the effect of massage on scars properties without measuring the limitation in ROM (Cho et al., 2014; Patiño et al., 1999; Roh et al., 2007; Roh et al., 2010). Among two other studies that investigated the limitation in joints, one took ROM as the only outcome measure for physical parameter of scars (Morien et al., 2008). One study included both scar parameter and ROM as outcome measures (Silverberg et al., 1996). Techniques of massage were described in detail in all six studies, including effleurage, friction, petrissage, stroke, and acupressure.
For scar parameters, two nonequivalent controlled trials (Roh et al., 2007; Roh et al., 2010) shared a similar post injury time, treatment regime, sample size (34 subjects in 2007 and 26 subjects in 2010) and predefined intervention duration (3 months). In the study conducted in 2007 whose weekly massage regime was for 30 minutes (Roh et al., 2007), total and sub scores of VSS showed overall significant improvement in experimental groups, whereas in studies conducted in 2010 with weekly 90 minutes of massage protocol, no significant difference was found either in objective scar measurement (thickness and blood perfusion) or subjective scar measurement (POSAS).
With an increased sample size to 146 and comparable study regime to Roh et al. (2010), Cho et al. (2014) recently displayed significant improvement after massage in the scar thickness, TEWL, melanin and erythema, through objective scar measurement tools of ultrasound, tewameter, and mexameter, whereas no significant difference was observed in scar sebum and elasticity. In this level I RCT, post injury days were 148.77 ± 56.85 days in the experiment group and 156.47 ± 56.48 days in the control group. The length of intervention was determined after the patient being discharged. The average treatment length was 1 month, incorporated with 90 minutes of weekly massage plus conventional treatments regime.
Another two trials, one level I RCT (Patiño et al., 1999) conducted in 30 paediatric patients and the other level II RCT (Silverberg et al., 1996) performed in 10 multiracial Americans, showed no significant improvement in VSS scores under 10–15 minutes of daily massage (Patiño et al., 1999; Silverberg et al., 1996).
The only experiment that compared both ROM and scar parameter was performed by Silverberg et al. (1996) in 10 Americans, resulting in no significant improvement after 10–15 minutes of intervention. Another trial compared the massaged scars, which developed 2–16 years after injury, with contralateral scars without massage in children and a significant improvement was found after 5 days of 20–25 minutes of intervention (Morien et al., 2008).
Except for Silverberg et al. (1996), all the other five studies described the application of moisturiser during massage, such as cream, oil, lotion, cocoa butter (Cho et al., 2014; Morien et al., 2008; Patiño et al., 1999; Roh et al., 2007; Roh et al., 2010). Only Roh et al. (2007) applied tension on unscarred areas.
Splinting intervention (Table 3)
One level II RCT was identified using splints to stretch and immobilise the shoulder in 90° abduction after axillary burn in 52 adults. Unconscious patients in the Intensive Care Unit were also recruited. In total, 12 (23.1%) subjects dropped out from the study. Compared with conventional treatments composed of stretching using strengthening and functional training, shoulder ROM did not statistically differ after the supplement of static stretching splint after 6 weeks’ all-day and 6 weeks’ night wear (Kolmus et al., 2012).
Discussion
The quality of studies that explored the effect of “mechanical stretch” composed interventions on post burn scars and contractures were varied. Five out of nine studies were rated fair to poor quality for the lack of random allocation, assessor blind, intention to treat, or size of treatment effect measurement procedures. Generally, the subjects demonstrated significant improvement in scar parameters after stretching exercise with early initiation (within 1 week after surgery), and last more than 1 hour daily. The cooperation between a weekly 90-minute massage program and comprehensive conventional intervention would also ameliorate the scar outcomes, such as thickness and erythema. The improvement in ROM limited by scar contracture can be achieved by early initiated stretch exercise on active scar areas or massage comprised with multiple techniques, such as effleurage, stretching, rolling strokes, friction, lengthening, and rolling (Morien et al., 2008). Interventions that displayed non-significant results were considered to be caused by short daily treatment regime (around 10 minutes) or small sample size that failed to detect the significant difference.
It should be noted that two studies conducted by same author with similar intervention methods exhibited non-significant results after using objective scar measurement tools. The author inferred that this may due to the lack of large sample size and power, overestimate of the results in the trials, or using subjective scar measurements without assessor blinding (Roh et al., 2007; Roh et al., 2010). This highlights the importance of using standardised objective measurements in clinical trials, which contribute to the comparability and synthesis of outcomes from different studies. It also reflects a challenge of evaluating scar management strategy in clinical in which the response of patients’ scars to the treatments may be varied because of genetic factors, compliance to the conventional treatments, and participation of non-monitored daily activities. To determine the efficacy of a treatment strategy, an in vivo animal model could be another choice in terms of the availability of negative control group and comparability of genetic and environmental factors.
As for early implementation of stretch in post burn patients, it is worth noting that there were also emerging studies verifying the effect of tension reduction in preventing or reducing the severity of scar and related contracture formation (Atkinson, McKenna, Barnett, McGrath, & Rudd, 2005; Monstrey et al., 2014; Yagmur, Akaishi, Ogawa, & Guneren, 2010). Since wound healing and scar formation are closely connected dynamic processes, further trials could be conducted to further define the time of treatment initiation and the influence of intensity, frequency, and duration.
Although the effects of stretch are not only on scars but also on the underlying soft tissue, such as fascia, tendons, and muscles, it was generally accepted that the improvement in scar pliability and scar contracture would also increase the ROM of adjacent joints (Silverberg et al., 1996). There is a theoretical explanation that the stretch can disrupt fibrotic tissue mechanically and produce greater level of laminin and collagen, thereby increasing the pliability of scars as well as epidermal thickness (Shin & Bordeaux, 2012; Tokuyama, Nagai, Takahashi, Kimata, & Naruse, 2015). Therefore, considering the clinical importance and indivisibility of scar tissue and contracture, the authors also included both the parameters as outcome measures.
The conclusion of effectiveness of stretch on scar property and contracture should be drawn carefully for all stretch-incorporated treatments were applied in combination with other treatments, such as active exercise, pressure garment, moisturisation, or functional retraining. And the outcome measures remain incomparable for the inconsistent content or tools. Under these circumstance, it remains a challenge to verify the effect of one intervention modality.
Limitations
The limitation of this systematic review is that only a small number of studies met the inclusion criteria. Most of the studies were not assessed by a blind assessor; thus, it may contribute to assessment bias. Among the nine selected trials, the treatment regime and the outcome measures were varied, thereby adding the difficulty to analyse and interpret the findings. Moreover, there was a lack of clear explanation on the theoretical framework behind mechanical stretching.
Conclusion
Stretch is one of the most commonly used therapeutic techniques adopted for scar management. However, there seems a lack of understanding regarding the exact mechanism of stretching in the improvement of scar conditions. The direction, magnitudes, duration, and frequency of stretching were not clearly defined in the therapy regime, thus arising problems in proving its efficacy. Further high quality clinical trials on scar management are needed to generate the evidence to show its effectiveness. Future research should focus more on comparison among detailed regime of intervention application using a larger sample size. Basic science study should also be conducted to identify the underlying mechanism of stretching on the fibroblasts of the scar tissues.
Acknowledgements
The authors thank all of the researchers who conducted trials involved in the study.
Funding/Support:
No financial or grant support was received for this study.
Conflicts of interest:
All authors declare that they have no conflicts of interest.
References
- Akaishi S., Akimoto M., Ogawa R., & Hyakusoku H.. (2008). The relationship between keloid growth pattern and stretching tension: Visual analysis using the finite element method. Annals of Plastic Surgery, 60, 445–451. [DOI] [PubMed] [Google Scholar]
- Anthonissen M., Daly D., Janssens T., & Van den Kerckhove E.. (2016). The effects of conservative treatments on burn scars: A systematic review. Burns, 42, 508–518. [DOI] [PubMed] [Google Scholar]
- Atkinson J. A. M., McKenna K. T., Barnett A. G., McGrath D. J., & Rudd M.. (2005). A randomized, controlled trial to determine the efficacy of paper tape in preventing hypertrophic scar formation in surgical incisions that traverse Langer's skin tension lines. Plastic and Reconstructive Surgery, 116, 1648–1656. [DOI] [PubMed] [Google Scholar]
- Blakeney P. E., Rosenberg L., Rosenberg M., & Faber A. W.. (2008). Psychosocial care of persons with severe burns. Burns, 34, 433–440. [DOI] [PubMed] [Google Scholar]
- Bouffard N. A., Cutroneo K. R., Badger G. J., White S. L., Buttolph T. R., Ehrlich H. P. et al. (2008). Tissue stretch decreases soluble TGF-beta1 and type-1 procollagen in mouse subcutaneous connective tissue: Evidence from ex vivo and in vivo models. Journal of Cellular Physiology, 214, 389–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho Y. S., Jeon J. H., Hong A., Yang H. T., Yim H., Cho Y. S. et al. (2014). The effect of burn rehabilitation massage therapy on hypertrophic scar after burn: A randomized controlled trial. Burns, 40, 1513–1520. [DOI] [PubMed] [Google Scholar]
- ClinicalTrial.gov,. (2000, February). Retrieved August 1, 2016, from: https://clinicaltrials.gov.
- Dewey W. S., Richard R. L., & Parry I. S.. (2011). Positioning, splinting, and contracture management. Physical Medicine and Rehabilitation Clinics of North America, 22, 229–247. [DOI] [PubMed] [Google Scholar]
- Du Q. C., Zhang D. Z., Chen X. J., Lan-Sun G., Wu M., & Xiao W. L.. (2013). The effect of p38MAPK on cyclic stretch in human facial hypertrophic scar fibroblast differentiation. PLoS ONE, 8, e75635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godleski M., Oeffling A., Bruflat A. K., Craig E., Weitzenkamp D., & Lindberg G.. (2013). Treating burn-associated joint contracture: Results of an inpatient rehabilitation stretching protocol. Journal of Burn Care and Research, 34, 420–426. [DOI] [PubMed] [Google Scholar]
- Huang C., Holfeld J., Schaden W., Orgill D., & Ogawa R.. (2013). Mechanotherapy: Revisiting physical therapy and recruiting mechanobiology for a new era in medicine. Trends in Molecular Medicine, 19, 555–564. [DOI] [PubMed] [Google Scholar]
- Junker J. P. E., Kratz C., Tollbäck A., & Kratz G.. (2008). Mechanical tension stimulates the transdifferentiation of fibroblasts into myofibroblasts in human burn scars. Burns, 34, 942–946. [DOI] [PubMed] [Google Scholar]
- Kolmus A. M., Holland A. E., Byrne M. J., & Cleland H. J.. (2012). The effects of splinting on shoulder function in adult burns. Burns, 38, 638–644. [DOI] [PubMed] [Google Scholar]
- Maher C. G., Sherrington C., Herbert R. D., Moseley A. M., & Elkins M.. (2003). Reliability of the PEDro scale for rating quality of randomized controlled trials. Physical Therapy, 83, 713–721. [PubMed] [Google Scholar]
- Monstrey S., Middelkoop E., Vranckx J. J., Bassetto F., Ziegler U. E., Meaume S. et al. (2014). Updated scar management practical guidelines: Non-invasive and invasive measures. Journal of Plastic, Reconstructive and Aesthetic Surgery, 67, 1017–1025. [DOI] [PubMed] [Google Scholar]
- Morien A., Garrison D., & Smith N. K.. (2008). Range of motion improves after massage in children with burns: A pilot study. Journal of Bodywork and Movement Therapies, 12, 67–71. [DOI] [PubMed] [Google Scholar]
- Ogawa R., Okai K., Tokumura F., Mori K., Ohmori Y., Huang C. et al. (2012). The relationship between skin stretching/contraction and pathologic scarring: The important role of mechanical forces in keloid generation. Wound Repair and Regeneration, 20, 149–157. [DOI] [PubMed] [Google Scholar]
- Okhovatian F., & Zoubine N.. (2007). A comparison between two burn rehabilitation protocols. Burns, 33(4), 429–434. [DOI] [PubMed] [Google Scholar]
- Oxford Centre for Evidence-Based Medicine. (2009). Oxford Centre for Evidence-based Medicine: Levels of evidence (March 2009). Retrieved July 19, 2016, from: http://www.cebm.net/oxford-centre-evidence-based-medicine-levels-evidence/.
- Patiño O., Novick C., Merlo A., & Benaim F.. (1999). Massage in hypertrophic scars. Journal of Burn Care and Rehabilitation, 20, 268–271. [PubMed] [Google Scholar]
- Roh Y. S., Cho H., Oh J. O., & Yoon C. J.. (2007). Effects of skin rehabilitation massage therapy on pruritus, skin status, and depression in burn survivors. Journal of Korean Academy of Nursing, 37, 221–226. [DOI] [PubMed] [Google Scholar]
- Roh Y. S., Seo C. H., & Jang K. U.. (2010). Effects of a skin rehabilitation nursing program on skin status, depression, and burn-specific health in burn survivors. Rehabilitation Nursing, 35, 65–69. [DOI] [PubMed] [Google Scholar]
- Roques C.. (2002). Massage applied to scars. Wound Repair and Regeneration, 10(2), 126–128. [DOI] [PubMed] [Google Scholar]
- Shin T. M., & Bordeaux J. S.. (2012). The role of massage in scar management: A literature review. Dermatologic Surgery, 38(3), 414–423. [DOI] [PubMed] [Google Scholar]
- Silverberg R., Johnson J., & Moffat M.. (1996). The effects of soft tissue mobilization on the immature burn scar: Results of a pilot study. Journal of Burn Care and Research, 17, 252–259. [DOI] [PubMed] [Google Scholar]
- Stubbs T. K., James L. E., Daugherty M. B., Epperson K., Barajaz K. A., Blakeney P. et al. (2011). Psychosocial impact of childhood face burns: A multicenter, prospective, longitudinal study of 390 children and adolescents. Burns, 37, 387–394. [DOI] [PubMed] [Google Scholar]
- Tokuyama E., Nagai Y., Takahashi K., Kimata Y., & Naruse K.. (2015). Mechanical stretch on human skin equivalents increases the epidermal thickness and develops the basement membrane. PLoS ONE, 10, e0141989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization International Clinical Trials Registry Platform. Retrieved August 1, 2016, from: http://apps.who.int/trialsearch/. [Google Scholar]
- Yagmur C., Akaishi S., Ogawa R., & Guneren E.. (2010). Mechanical receptor-related mechanisms in scar management: a review and hypothesis. Plastic and Reconstructive Surgery, 126, 426–434. [DOI] [PubMed] [Google Scholar]


