Abstract
Osteoarthritis (OA), osteochondrosis (OC), and synovial sepsis in horses cause loss of function and pain. Reliable biomarkers are required to achieve accurate and rapid diagnosis, with synovial fluid (SF) holding a unique source of biochemical information. Nuclear magnetic resonance (NMR) spectroscopy allows global metabolite analysis of a small volume of SF, with minimal sample preprocessing using a noninvasive and nondestructive method. Equine SF metabolic profiles from both nonseptic joints (OA and OC) and septic joints were analyzed using 1D 1H NMR spectroscopy. Univariate and multivariate statistical analyses were used to identify differential metabolite abundance between groups. Metabolites were annotated via 1H NMR using 1D NMR identification software Chenomx, with identities confirmed using 1D 1H and 2D 1H 13C NMR. Multivariate analysis identified separation between septic and nonseptic groups. Acetate, alanine, citrate, creatine phosphate, creatinine, glucose, glutamate, glutamine, glycine, phenylalanine, pyruvate, and valine were higher in the nonseptic group, while glycylproline was higher in sepsis. Multivariate separation was primarily driven by glucose; however, partial-least-squares discriminant analysis plots with glucose excluded demonstrated the remaining metabolites were still able to discriminate the groups. This study demonstrates that a panel of synovial metabolites can distinguish between septic and nonseptic equine SF, with glucose the principal discriminator.
Keywords: metabolomics, equine, synovial fluid, osteoarthritis, osteochondrosis, sepsis, nuclear magnetic resonance
Introduction
Conditions affecting the articular joints are common in horses resulting in loss of function, chronic pain, or subsequent inability to work, all of which represent economic and welfare concerns. These pathologies include osteoarthritis (OA), osteochondrosis (OC), and synovial sepsis, which can be life-threatening.1 Despite these conditions having a high prevalence and clinical relevance, diagnosis, staging, monitoring, and determination of an accurate prognosis remain challenging for practising veterinarians. Therefore, to differentiate equine articular joint pathologies, there is a need to identify reliable biomarkers of disease. Synovial fluid (SF) is located within the articular joint cavity, providing a pool of nutrients for surrounding tissues but primarily serving as a biological lubricant, containing molecules with low-friction and low-wear properties to articular surfaces.2 As SF is in close proximity to articular tissues primarily altered during joint pathology, this biofluid is an important source of biomarker discovery.3,4
To date, no validated markers that are both sensitive and specific to synovial sepsis have been identified within veterinary medicine, making early diagnosis a challenge. Together with clinical examination, diagnosis is based on SF culture and intracellular bacteria identification (both low sensitivity) as well as total protein, total nucleated cell count, and percentage of neutrophils whose results are open to interpretation and are affected by standard treatment protocols.5−8 Reduced levels of glucose in human and equine SF, due to an increase in synovial and neutrophil cell glycolytic activity in severe inflammation or infection, have previously been identified, with a serum-synovial glucose difference of >2.2 mmol/L considered supportive of a diagnosis of synovial sepsis.9,10 However, this parameter is nonspecific and can be influenced by multiple variables including synovial necrosis, diet, pain and white blood cell count. Elevations in lactate during the acute infection phase have previously been identified in SF of septic human, canine, and equine joints due to an increase in the consumption of glucose and subsequent production of lactate within an anaerobic environment.11−13 However, other studies have not been able to differentiate septic and nonseptic arthropathies based on lactate levels alone.14,15 Synovial d-lactate (produced via bacterial fermentation and a stereoisomer of mammalian l-lactate) was recently found to be unable to aid diagnosis of equine synovial sepsis.8
At present, no specific biomarkers have been identified for equine OA with none currently used as a diagnostic aid in clinical practice. Potential OA markers of interest include cartilage oligomeric matrix protein (COMP) and matrix metalloproteinases (MMPs), which have both been identified as elevated in human OA SF.16 Although detection of active MMPs in horses has demonstrated important potential in diagnosis, conversely, decreased levels of COMP have been identified in equine OA and found unable to stage the disease.17,18 In equine OC, elevated levels of a C-propeptide of cartilage type II procollagen and osteocalcin have shown potential as a diagnostic aid, although these markers have not translated to clinical practice.19−21 However, these protein markers identify pathology following significant cartilage degradation and bone remodelling opposed to an early disease state where potential intervention would prove most beneficial.
Metabolomics encompasses the comprehensive profiling of metabolic changes, including the study of metabolic pathways and quantification of unique biochemical molecules, within living systems.22 These small molecule metabolites include metabolic intermediates, secondary metabolites, hormones, and other signaling molecules.23 A major advantage of nuclear magnetic resonance (NMR) spectroscopy over other techniques, that is, mass spectrometry, is the analysis of native samples with a minimal level of sample preparation using a noninvasive and nondestructive method, subsequently producing results which are more reproducible and robust.24 Thus, this methodology holds huge potential in the analysis of biofluids to characterize the global metabolic profiles of various pathologies.25 Hugle et al. previously carried out NMR spectroscopy using human SF, identifying that septic arthritis SF could be distinguished from nonseptic arthritis SF via principal component analysis (PCA), although they were unable to identify the specific metabolites responsible for this discrimination.26 To date, only one peer-reviewed publication has used NMR to investigate the whole metabolic profile of equine SF by comparing normal and osteoarthritic SF.27 Lacitignola et al. identified ten differentially abundant metabolites, which included elevated levels of glucose and lactate in OA. No studies have analyzed equine SF using NMR to investigate synovial sepsis or OC, concurrently analyzed the metabolic profiles of multiple equine articular pathologies, or used multivariate analysis to investigate the metabolic profile as a whole.
In this study, we have used global metabolite identification using 1H NMR to identify potential metabolite biomarkers that allow differentiation between equine articular joint pathologies, potentially aiding accurate diagnosis.
Methods
Patient Groups and SF Collection
Following ethical approval and owner consent, excess aspirated SF (collected during clinical diagnostic investigations) was analyzed from joints of horses presenting to The Philip Leverhulme Equine Hospital, University of Liverpool between 2014 and 2016. SF was aspirated for diagnostic purposes from the affected joints at the start of surgical arthroscopy under general anesthesia with 500 μL of excess SF submitted for NMR metabolomic analysis. Pathological joints included the femorotibial, glenohumeral, metacarpophalangeal, metatarsophalangeal, and tarsocrural joints. Horses were divided into two main groups of joint pathology, septic, and nonseptic. The septic group was subdivided into six horses with synovial sepsis following a local penetrating wound and one foal diagnosed with synovial sepsis secondary to hematogenous spread. The nonseptic group consisted of four horses diagnosed with OA, two with meniscal tears and concurrent OA (MT), and six with OC. Diagnoses were determined via a combination of radiography, ultrasonography, arthroscopy and SF total protein, cytology, or bacterial culture as previously described.8 SF was immediately placed into uncoated 1.5 mL collection tubes and processed within an hour of collection. Particulate and cells were removed from the SF by centrifugation (4 °C, 2540g for 5 min) and then the cell-free supernatant transferred to a clean uncoated 1.5 mL collection tube, snap-frozen using liquid nitrogen, and stored at −80 °C. Samples were spun to ensure removal of cellular debris, which was critical to eliminate variance due to bacterial contamination in septic joints. To ascertain the most reproducible and robust collection protocol, biologically identical SF underwent ± centrifugation and supernatant removal followed by freezing using various methods at different temperatures. Multivariate statistical analysis of these samples indicated that the above method was most robust at reducing variance due to sample handling/processing (data not shown).
Sample Preparation
NMR samples were prepared within 48 h of acquisition. A 300 μL sample of thawed SF was diluted to a final volume containing 50% (v/v) SF, 40% (v/v) dd 1H2O (18.2 MΩ), 10% (v/v) 1 M PO43– pH 7.4 buffer (Na2HPO4, VWR International Ltd., Radnor, Pennsylvania, USA and NaH2PO4, Sigma-Aldrich, Gillingham, UK) in deuterium oxide (2H2O, Sigma-Aldrich), and 0.0025% (v/v) sodium azide (NaN3, Sigma-Aldrich). Samples were vortexed for 1 min, centrifuged at 13 000g and 4 °C for 2 min, and 590 μL transferred (taking care not to disturb any pelleted material) into 5 mm outer diameter NMR tubes using a glass pipet.
NMR Acquisition
One dimensional 1H NMR spectra with Carr–Purcell–Meiboom–Gill (CPMG) filter to attenuate signals from macromolecules such as albumins were acquired using a standard vendor pulse sequence (cpmgpr1d) on a 600 MHz NMR Bruker Avance III spectrometer with a TCI cryoprobe and chilled Sample-Jet autosampler. Spectra were acquired at 25 °C, with a 4 s interscan delay, 32 transients, and a 15 ppm spectral width. Software for acquisition and processing was carried out using Topsin 3.1 and IconNMR 4.6.7 with automated phasing and baseline correction and a standard vendor processing routine (exponential window function with 0.3 Hz line broadening).
Metabolite Annotation and Identification
All spectra were scrutinized to ensure spectra met community recommended quality control criteria prior to inclusion in statistical analysis.28 Quality control criteria included a flat baseline, water signal less than 0.4 ppm wide, and line-width half heights of representative beta anomeric glucose doublet all within one standard deviation. Spectra were then divided into spectral regions or “buckets” according to metabolite annotation from Chenomx NMR Suite 8.2 (330-mammalian metabolite library) with buckets attributed to multiple metabolites where peaks were found to overlap. Each spectrum was divided into 306 buckets with the intensity of each bucket divided by the bucket width to negate the intensity variance. Buckets were normalized to the median and Pareto scaled prior to statistical treatment. Metabolites annotated in Chenomx were also confirmed by a mixture of 1H 1D NMR and, where possible, to in-house 2D 1H 13C Heteronuclear Single Quantum Coherence NMR standards. Spectra and metabolite assignments including HMDB IDs and annotation level are all available in the Metabolights repository (www.ebi.ac.uk/metabolights/MTBLS543) and shown in Table 2.29 Among the metabolites identified was ethanol, likely through contamination during SF aspiration following surgical sterilization. Therefore, for multivariate analysis, the buckets attributed to ethanol were omitted.
Table 2. Metabolites Annotated in Synovial Fluid by Chenomx, Metabolites Subsequently Identified Using in-House Library Are Indicated by Metabolomics Standards Initiative (MSI) Level 1.29.
| database identifier | metabolite identification | reliability |
|---|---|---|
| HMDB00001 | 1-Methylhistidine | MS Level 2 |
| HMDB59655 | 2-Hydroxyglutarate | MS Level 2 |
| HMDB11743 | 2-Phenylpropionate | MS Level 2 |
| HMDB00357 | 3-Hydroxybutyrate | MS Level 2 |
| HMDB00355 | 3-Hydroxymethylglutarate | MS Level 2 |
| HMDB01149 | 5-Aminolevulinate | MS Level 2 |
| HMDB00042 | Acetate | MS Level 1 |
| HMDB00060 | Acetoacetate | MS Level 2 |
| HMDB01890 | Acetylcysteine | MS Level 2 |
| HMDB01432 | Agmatine | MS Level 2 |
| HMDB00729 | Alpha-Hydroxyisobutyrate | MS Level 2 |
| HMDB00186 | Alpha-Lactose | MS Level 2 |
| HMDB00043 | Betaine | MS Level 1 |
| HMDB00030 | Biotin | MS Level 1 |
| HMDB00094 | Citrate | MS Level 1 |
| HMDB00064 | Creatine | MS Level 1 |
| HMDB00562 | Creatinine | MS Level 1 |
| HMDB00143 | d-Galactose | MS Level 2 |
| HMDB00122 | d-Glucose | MS Level 1 |
| HMDB00108 | Ethanol | MS Level 1 |
| HMDB00142 | Formate | MS Level 2 |
| HMDB00663 | Glucarate | MS Level 2 |
| HMDB01401 | Glucose 6-phosphate | MS Level 2 |
| HMDB00131 | Glycerol | MS Level 1 |
| HMDB00123 | Glycine | MS Level 1 |
| HMDB00721 | Glycylproline | MS Level 2 |
| HMDB00128 | Guanidoacetate | MS Level 2 |
| HMDB00870 | Histamine | MS Level 2 |
| HMDB00764 | Hydrocinnamate | MS Level 2 |
| HMDB00678 | Isovalerylglycine | MS Level 2 |
| HMDB00190 | Lactate | MS Level 1 |
| HMDB00161 | l-Alanine | MS Level 1 |
| HMDB00646 | l-Arabinose | MS Level 2 |
| HMDB00062 | l-Carnitine | MS Level 2 |
| HMDB00174 | l-Fucose | MS Level 2 |
| HMDB00148 | l-Glutamate | MS Level 1 |
| HMDB00641 | l-Glutamine | MS Level 1 |
| HMDB00177 | l-Histidine | MS Level 1 |
| HMDB00687 | l-Leucine | MS Level 1 |
| HMDB00159 | l-Phenylalanine | MS Level 1 |
| HMDB00167 | l-Threonine | MS Level 2 |
| HMDB00158 | l-Tyrosine | MS Level 1 |
| HMDB00883 | l-Valine | MS Level 1 |
| HMDB01389 | Melatonin | MS Level 2 |
| HMDB01238 | N-Acetylserotonin | MS Level 2 |
| HMDB02055 | o-Cresol | MS Level 2 |
| HMDB00210 | Pantothenic acid | MS Level 2 |
| HMDB00821 | Phenylacetylglycine | MS Level 2 |
| HMDB01511 | Phosphocreatine | MS Level 2 |
| HMDB00239 | Pyridoxine | MS Level 2 |
| HMDB00243 | Pyruvate | MS Level 1 |
| HMDB00635 | Succinylacetone | MS Level 2 |
| HMDB00262 | Thymine | MS Level 1 |
| HMDB01878 | Thymol | MS Level 2 |
| HMDB00294 | Urea | MS Level 1 |
Statistical Analysis
SF spectra were divided into two groups: nonseptic and septic. Further separation of the groups was not possible due to limited sample number in this study. t tests and partial-least-squares discriminant analysis (PLS-DA) were carried out using MetaboAnalyst 3.5 (http://www.metaboanalyst.ca), which uses the R package of statistical computing software.30 For t tests, p < 0.05 was considered statistically significant following correction for multiple testing using the Benjamini-Hochberg false discovery rate method.31 Box plots were carried out using the software package SPSS 22. Quantile and PCA plots were produced using standard analytical routines in the software package R (https://cran.r-project.org/).
Results
Group Limitations
The patient groups were restricted to two main groups, septic or nonseptic (Table 1), due to a lack of sufficient cases of differing subdiagnoses. The joints from which each sample was collected were noted; however, without a larger study size, no separate grouping for differing joints was attempted.
Table 1. Further Clinical Characteristics of Group Patientsa.
| main groups |
subgroups |
||||||
|---|---|---|---|---|---|---|---|
| septic | nonseptic | septic |
nonseptic |
||||
| wound sepsis | haematogenous sepsis | OA | MT with OA | OC | |||
| number | 7 | 12 | 6 | 1 | 4 | 2 | 6 |
| mean age | 6 years 10 months | 7 years 7 months | 7 years 11 months | 0 years 2 months | 10 years 6 months | 12 years 0 months | 4 years 6 months |
| sex | 7M | 6F, 6M | 6M | 1M | 1F, 3M | 1F, 1M | 4F, 2M |
| joint | 1 × GH | 2 × GH | 1 × GH | ||||
| 1 × MCP | 3 × MCP | 1 × MCP | 2 × GH | 2 × FT | 3 × MCP | ||
| 1 × MTP | 4 × FT | 1 × MTP | 1 × FT | 1 × FT | |||
| 4 × TC | 1 × MTP | 4 × TC | 1 × MTP | 2 × TC | |||
| 2 × TC | |||||||
Abbreviations: Groups, OA = osteoarthritis, MT = meniscal tear and concurrent OA, OC = osteochrondrosis; sex, M = Male, F = Female; joints, GH = glenohumeral, MCP = metacarpophalangeal, FT = femorotibial, MTP = metatarsophalangeal, and TC = tarsocrural.
Metabolite Annotation and Identification
NMR spectra for all groups showed a consistent set of metabolite signals present with multiple metabolites identified from 1D multiplet pattern overlap (Figure 1). The metabolite abundances within the septic group were less variable than the nonseptic group, which is perhaps expected given the nonseptic group included samples from two separate diagnoses (OA and OC). Of the 306 spectral bins in each extract, 203 (66%) were annotated to 71 metabolites, with identification confirmed for 55 of these metabolites (Table 2).
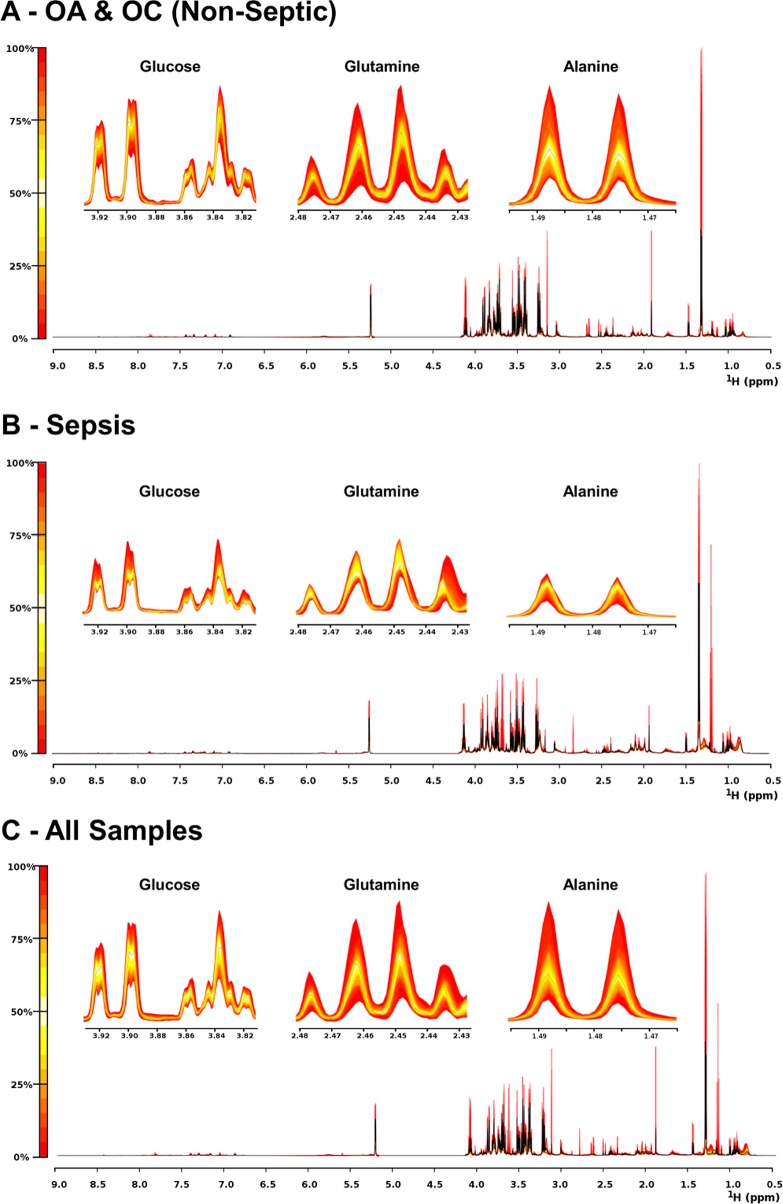
Figure 1.
Quantile plots of OA/OC (Non-Septic), sepsis, and all spectra depicting the median spectral plot (black line) and variation from the median within each group (yellow to red scale) for the full spectral range (9.0–0.5 ppm) and more detailed regions depicting selected peaks from three differentially abundant metabolites, glucose, glutamine, and alanine. Variation within the full spectra can most clearly be seen at 0.8 ppm.
Statistical Analysis and Differentially Abundant Metabolites
Of the 306 identified peaks, 180 were found to be differentially abundant between septic and nonseptic groups when analyzed using a univariate t test, p < 0.05, with 98 peaks assigned to 26 different metabolites (Table S1).
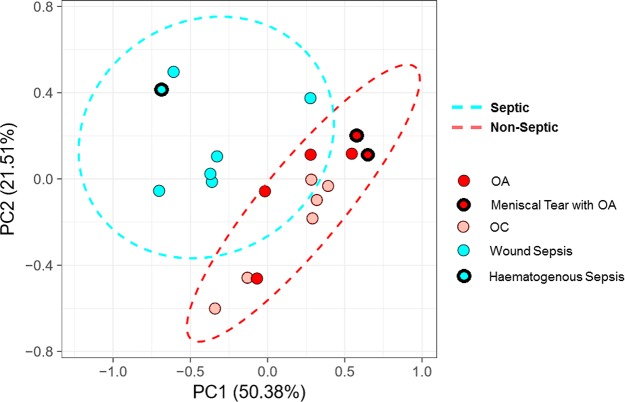
Unsupervised multivariate analysis (PCA) identified clear variance between spectra from septic and nonseptic joint pathologies, forming two distinct clusters (Figure 2). Ninety-five percent of the variance was explained by seven principal components (PCs) with PC1 and PC2 explaining a combined 71.89% of variance within the data. Although the analysis between groups was limited to a binary diagnosis (septic vs nonseptic) to explore the variance in metabolite profile for SF aspirated from horses with differing underlying conditions, the PCA score plots were colored in terms of either main diagnosis (Binary) or subdiagnosis (five groups). Within the nonseptic group, samples obtained from joints with OA and associated meniscal tears were found to cluster together. In addition, despite a separate etiology, SF obtained from a foal with hematogenous sepsis clustered with SF collected from joints diagnosed with sepsis subsequent to penetrating wounds.
Figure 2.
Principal component analysis (PCA) scores plot of septic (blue) and nonseptic (red) SF samples. Samples with meniscal tear or hematogenous sepsis subdiagnoses for OA and sepsis, respectively, are highlighted in each group with a thick black outline, OC are distinguished from OA by highlight shade.
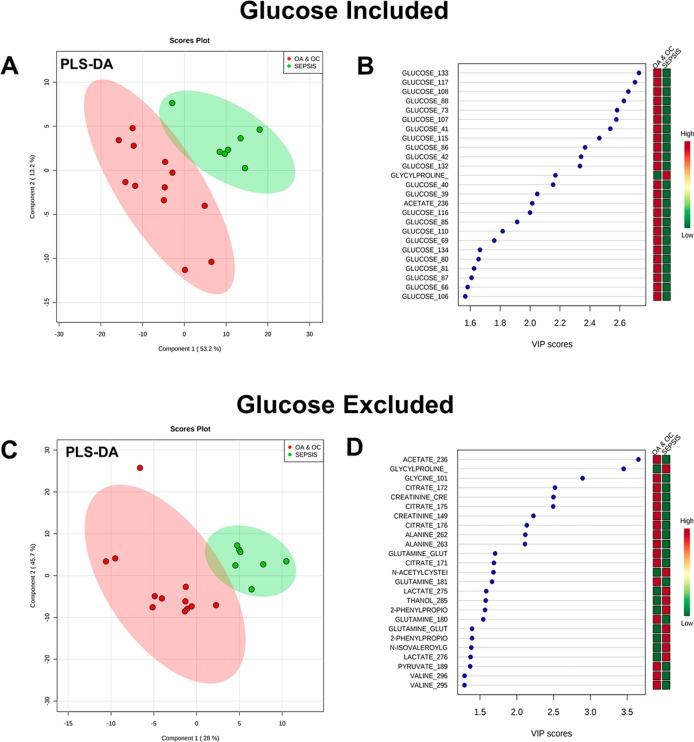
Supervised multivariate discriminant analysis using PLS-DA plots of the known metabolites indicated that the variance was heavily influenced by glucose (Figure 3a,b), with 23 of the 25 most influential buckets attributed to glucose peaks. The optimal model comprised two components with reasonable predictive power (R2 = 0.85, Q2 = 0.72). Omitting glucose signals from the analysis gave rise to a somewhat lower predictive model (R2 = 0.70, Q2 = 0.45 fit with 1 component) (Figure 3c). From the glucose free model, metabolites acetate, glycylproline, glycine, citrate, creatinine, and alanine were among the most influential (Figure 3d).
Figure 3.
(a) PLS-DA plots of OA/OC versus sepsis using only metabolite annotated buckets: first two components shown out of a total of two components used to fit the model (R2 = 0.85, Q2 = 0.72). (b) VIP scores for the 25 most influential buckets of PLS-DA. (c) PLS-DA plots of OA/OCD versus sepsis using only metabolite annotated/identified buckets with all glucose buckets excluded: first two components shown. It should be noted that only one component was used to fit the model (R2 = 0.70, Q2 = 0.45). (d) VIP scores for the 25 most influential buckets of glucose-excluded PLS-DA.
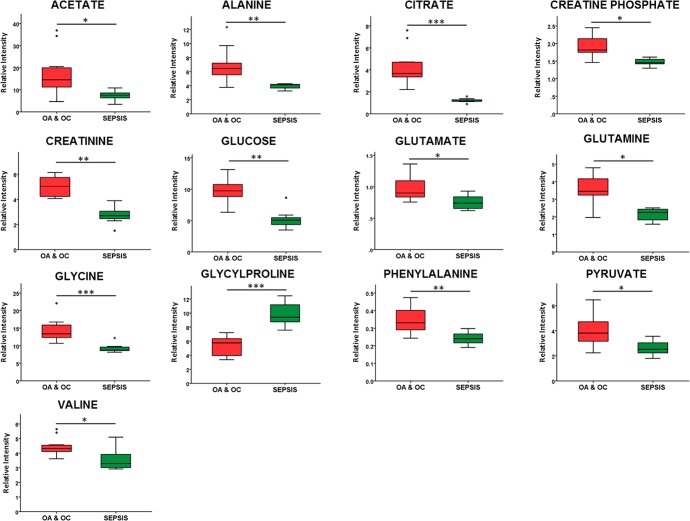
Both univariate and multivariate analysis were compared to identify differentially abundant metabolites. Metabolite peaks identified as either significantly different (p < 0.05) via t test or in the top 25 variables of influence (VIP) via glucose excluded PLS-DA were then attributed to a genuine differentially abundant metabolite change if the individual metabolite peak changes correlated. In total, a panel of 13 metabolites was identified as being differentially abundant between septic and nonseptic groups. Acetate, alanine, citrate, creatine phosphate, creatinine, glucose, glutamate, glutamine, glycine, phenylalanine, pyruvate, and valine were higher in the nonseptic group, while glycylproline was higher in synovial sepsis (Figure 4).
Figure 4.
Boxplots of key metabolites, shown as relative intensities corresponding to the most representative peak for each metabolite. t test: ∗ = p < 0.05, ∗∗ = p < 0.01, and ∗∗∗ = p < 0.001.
PCA analysis of OA and OC subgroups suggested that, bar one sample, they may cluster separately and may therefore demonstrate distinctive metabolomes. However, with this modest group size, no significant differences in metabolites (p < 0.05) were identified by univariate analysis (data not shown).
Discussion
Despite OA, OC, and synovial sepsis having a high prevalence and clinical relevance, diagnosis, staging, monitoring, and determination of an accurate prognosis remain challenging for practising veterinarians. Therefore, to differentiate equine articular joint pathologies, there is a need to identify reliable biomarkers of disease. To aid equine synovial sepsis diagnosis, currently recognized markers of glucose and lactate are considered to be nonspecific and unreliable, respectively.12,15 In addition, clinically assessing response to treatment of septic joints can be challenging, with differentiating horses with continued lameness from a nonresolving sepsis to those with a resolving sepsis but ongoing significant inflammation, difficult. Therefore, reliable metabolic markers of sepsis may aid as part of a longitudinal clinical assessment.
In this study, it has been shown that the metabolite profile of equine SF is able to discriminate between septic and nonseptic joint pathologies. A panel of 13 metabolites was identified which were differentially abundant between these two groups. These metabolites may in turn prove beneficial in an equine clinical setting as a diagnostic aid as well as monitoring response to treatment and assessing prognosis. Ideally, this could be carried out through field tests, similar to those produced and used for equine biofluids for metabolites glucose and lactate, urinalysis, and the protein inflammatory marker serum amyloid A.32−35 No metabolite abundances were identified to be statistically different between OA and OC subgroups. However, the PCA analysis suggested that, bar one sample, OC and OA metabolite profiles were different and thus may demonstrate distinctive metabolomes sufficient to apply robust statistical models such as PLS-DA. This can only be tested with a larger sample size which was not possible on this modest subset of patients.
The PLS-DA VIP scores revealed spectral peaks assigned to glucose were highly represented as a discriminant of joint sepsis (23/25 of the most influential peaks). Reduced levels of glucose in human and equine SF, due to an increase in synovial and neutrophil cell glycolytic activity in severe inflammation or infection, have previously been identified, with a serum-synovial glucose difference of >2.2 mmol/L considered supportive of a diagnosis of synovial sepsis.9,10 Glucose is, however, a nonspecific parameter and can be influenced by multiple other variables.12 However, PLS-DA analysis with all glucose influenced spectral peaks omitted revealed there to still be a modest separation between the groups, thus indicating the differentiated metabolomes are driven by a panel of metabolites and potential septic markers, opposed to glucose alone.
Previous studies have described mixed results as to whether elevated synovial lactate can be used to help distinguish synovial sepsis.11,12,14,15 In this study, we did not identify lactate abundance to be different between the septic and nonseptic groups, therefore providing further evidence that lactate is an unreliable marker for synovial sepsis. 1H NMR spectroscopy is not able to distinguish d-lactate from l-lactate, and consequently, we are unable to draw any conclusions on the specific levels of bacterially derived d-lactate within the groups.
In this study, the majority of differentially abundant metabolites were found to be reduced in sepsis compared to the nonseptic group. However, glycylproline was elevated in synovial sepsis. Glycylproline is a dipeptide end product of the catabolism of collagen.36 Following synovial infection and an insufficient immunological response, collagen catabolism is upregulated through a high cytokine concentration increasing the release of matrix metalloproteinases and other collagen-degrading enzymes from within the host.37 Elevated levels in markers of collagen destruction in synovial sepsis over nonseptic joint pathologies have been identified previously, with one study demonstrating increased synovial levels of the collagen degradation marker cross-linked C-telopeptide fragments of type II collagen (CTX-II) in longitudinal samples of a patient with septic arthritis compared to osteoarthritic patients’ SF.38 Thus, glycylproline, in combination with other metabolite/protein derived markers of collagen catabolism, may aid clinical diagnosis of synovial sepsis. Following on from this study, further investigation using mass spectrometry analysis would complement these results as the identified degradation of extracellular matrix may provide lipid and carbohydrate profiles of interest.
All raw NMR spectral data and associated annotations that contributed to this study have been deposited with the open access online repository Metabolights, curated by the European Bioinformatics Institute (EBI), including acquisition and processing parameters with all metabolites identified clearly reported. The authors feel this transparent, gold standard approach to data submission to independent bioinformatics specialists, such as the EBI, should be further encouraged within the NMR metabolomics field, in line with other “omics” journal submissions.
Study Limitations
Modest sample size prohibited further analysis of metabolome differences between OA and OC or between wound sepsis and hematogenous sepsis. Similarly, SF with OA and associated meniscal tears were found to cluster together although we are unable to conclude that SF collected from femorotibial joints with meniscal tears form a distinct metabolome as only two samples were available for inclusion within this study. This is of particular interest as a diagnostic aid for meniscal tears would have a practical clinical implication within veterinary medicine which could also translate to human medicine. It is our hope that additional studies will provide further information and enable more stringent analyses to be undertaken. Furthermore, because of limited sample size, SF was aspirated from a range of joints, which may lead to variation in data due to biomechanical differences between joints. Again, a larger sample size would enable a greater understanding as to what influence joint location has on the metabolome and a stricter control of site of sample collection (if necessary) and thus enable further information to be extracted from the metabolic profiles. In addition, during the joint pathologies studied, there are likely to be changes to management of the animal, particularly exercise and diet, which may alter the metabolome of SF, although this influence is likely to be minimal compared to the significant metabolic influence of the localized joint pathology.
During this study, we were unable to compare the SF of the joint pathologies of interest to a normal group. In equine clinical practice, only pathological joints are aspirated during diagnostic investigations. Post-mortem SF cannot be defined as having a normal metabolome due to the anaerobic changes that take place following death, including artifactual changes in the abundance of glucose and lactate, two key metabolites of interest during sepsis.
Conclusion
This paper is the first to use NMR-led metabolomics to analyze equine SF with multiple pathologies and demonstrates that NMR-led metabolomics is an effective technique for analysis of equine SF collected within a clinical veterinary environment. Furthermore, this study demonstrates a panel of synovial metabolites that can distinguish between septic and nonseptic equine SF, with glucose the principal discriminator. To translate this into a diagnostic aid, a wider study with participants with less common diagnoses is required to improve the diagnostic power.
Acknowledgments
The authors are grateful for the use of R scripts written by Dr. A. Grauslys, Dr. E. Caamano Gutierrez (Computational Biology Facility), and R. Grosman (NMR metabolomics facility) at the University of Liverpool and staff at the Philip Leverhulme Equine Hospital, University of Liverpool, for their involvement with sample collection. The project was funded by a Veterinary School Research Project Support Grant (VSRP VET016) and the Technology Directorate voucher scheme (www.liverpool.ac.uk/technology-directorate), University of Liverpool. M.P. is funded through a Wellcome Trust Intermediate Clinical Fellowship (107471/Z/15/Z) and J.A. through a Horse Trust PhD studentship (G1015). Software licenses for data analysis used in the Shared Research Facility for NMR metabolomics were funded by the MRC Clinical Research Capabilities and Technologies Initiative (MR/M009114/1). Ethics were approved by University of Liverpool Ethics approval ref: VREC175.
Glossary
Abbreviations
- COMP
cartilage oligomeric matrix protein
- CPMG
Carr–Purcell–Meiboom–Gill
- CTX-II
C-telopeptide fragments of type II collagen
- EBI
European Bioinformatics Institute
- FT
femorotibial
- GH
glenohumeral
- MCP
metacarpophalangeal
- MMPs
matrix metalloproteinases
- MSI
Metabolomics Standards Initiative
- MT
meniscal tear and concurrent OA
- MTP
metatarsophalangeal
- NMR
nuclear magnetic resonance
- OA
osteoarthritis
- OC
osteochondrosis
- PCA
principal component analysis
- PC
principal components
- PLS-DA
partial-least-squares discriminant analysis
- SF
synovial fluid
- TC
tarsocrural
- VIP
variables of influence
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.jproteome.8b00190.
Statistically significant differentially abundant peaks between septic and nonseptic equine synovial fluid (PDF)
Author Contributions
Wrote the manuscript (J.A., M.M.P.), revised the manuscript (J.A., M.M.P., P.C., M.J.P., L.R.M.), analyzed the data (J.A., M.M.P.), experimental design (J.A., M.M.P., P.C., M.J.P., L.R.M.), collected clinical samples (L.R.M.). All authors read and approved the final manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Summerhays G. E. Treatment of Traumatically Induced Synovial Sepsis in Horses with Gentamicin-Impregnated Collagen Sponges. Vet. Rec. 2000, 147 (7), 184–188. 10.1136/vr.147.7.184. [DOI] [PubMed] [Google Scholar]
- Blewis M. E.; Nugent-Derfus G. E.; Schmidt T. A.; Schumacher B. L.; Sah R. L. A Model of Synovial Fluid Lubricant Composition in Normal and Injured Joints. Eur. Cell Mater. 2007, 13, 26–39. 10.22203/eCM.v013a03. [DOI] [PubMed] [Google Scholar]
- Ruiz-Romero C.; Blanco F. J. Proteomics Role in the Search for Improved Diagnosis, Prognosis and Treatment of Osteoarthritis. Osteoarthr. Cartil. 2010, 18 (4), 500–509. 10.1016/j.joca.2009.11.012. [DOI] [PubMed] [Google Scholar]
- Mateos J.; Lourido L.; Fernandez-Puente P.; Calamia V.; Fernandez-Lopez C.; Oreiro N.; Ruiz-Romero C.; Blanco F. J. Differential Protein Profiling of Synovial Fluid from Rheumatoid Arthritis and Osteoarthritis Patients Using LC-MALDI TOF/TOF. J. Proteomics 2012, 75 (10), 2869–2878. 10.1016/j.jprot.2011.12.042. [DOI] [PubMed] [Google Scholar]
- Sanchez Teran A. F.; Rubio-Martinez L. M.; Villarino N. F.; Sanz M. G. Effects of Repeated Intra-Articular Administration of Amikacin on Serum Amyloid A, Total Protein and Nucleated Cell Count in Synovial Fluid from Healthy Horses. Equine Vet. J. Suppl. 2012, 44 (43), 12–16. 10.1111/j.2042-3306.2012.00637.x. [DOI] [PubMed] [Google Scholar]
- Sanchez-Teran A. F.; Bracamonte J. L.; Hendrick S.; Riddell L.; Musil K.; Hoff B.; Rubio-Martínez L. M. Effect of Repeated Through-and-through Joint Lavage on Serum Amyloid A in Synovial Fluid from Healthy Horses. Vet. J. 2016, 210, 30–33. 10.1016/j.tvjl.2016.01.001. [DOI] [PubMed] [Google Scholar]
- Sanchez-Teran A. F.; Bracamonte J. L.; Hendrick S.; Burguess H. J.; Duke-Novakovski T.; Schott M.; Hoff B.; Rubio-Martínez L. M. Effect of Arthroscopic Lavage on Systemic and Synovial Fluid Serum Amyloid A in Healthy Horses. Vet. Surg. 2016, 45 (2), 223–230. 10.1111/vsu.12439. [DOI] [PubMed] [Google Scholar]
- Robinson C. S.; Singer E. R.; Piviani M.; Rubio-Martinez L. M. Are Serum Amyloid A or D-Lactate Useful to Diagnose Synovial Contamination or Sepsis in Horses?. Vet. Rec. 2017, 181 (16), 425. 10.1136/vr.104386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. E.; McLees B. D.; Kerby G. P. Pathways of Glucose Metabolism in Rheumatoid and Nonrheumatoid Synovial Membrane. J. Lab. Clin. Med. 1967, 70 (3), 503–511. [PubMed] [Google Scholar]
- Krey P. R.; Bailen D. A. Synovial Fluid Leukocytosis. A Study of Extremes. Am. J. Med. 1979, 67 (3), 436–442. 10.1016/0002-9343(79)90790-3. [DOI] [PubMed] [Google Scholar]
- Gobelet C.; Gerster J. C. Synovial Fluid Lactate Levels in Septic and Non-Septic Arthritides. Ann. Rheum. Dis. 1984, 43 (5), 742–745. 10.1136/ard.43.5.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulamo R.-M.; Bramlage L. R.; Gabel A. A. The Influence of Corticosteroids on Sequential Clinical and Synovial Fluid Parameters in Joints with Acute Infectious Arthritis in the Horse. Equine Vet. J. 1989, 21 (5), 332–337. 10.1111/j.2042-3306.1989.tb02682.x. [DOI] [PubMed] [Google Scholar]
- Proot J. L. J.; Vicente F. de.; Sheahan D. E. Analysis of Lactate Concentrations in Canine Synovial Fluid. Vet. Comp. Orthop. Traumatol. 2015, 28 (05), 301–305. 10.3415/VCOT-15-01-0007. [DOI] [PubMed] [Google Scholar]
- Washington W. L.Role of Synovial Fluid Lactate in the Immediate Diagnosis of Septic Arthritis (Abstract). Clin Res. 1980, 28 ( (791A), ). [Google Scholar]
- Arthur R. E.; Stern M.; Galeazzi M.; Baldassare A. R.; Weiss T. D.; Rogers J. R.; Zuckner J. Synovial Fluid Lactic Acid in Septic and Nonseptic Arthritis. Arthritis Rheum. 1983, 26 (12), 1499–1505. 10.1002/art.1780261212. [DOI] [PubMed] [Google Scholar]
- Balakrishnan L.; Nirujogi R. S.; Ahmad S.; Bhattacharjee M.; Manda S. S.; Renuse S.; Kelkar D. S.; Subbannayya Y.; Raju R.; Goel R.; et al. Proteomic Analysis of Human Osteoarthritis Synovial Fluid. Clin Proteomics 2014, 11 (1), 6. 10.1186/1559-0275-11-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zrimsek P.; Kadunc Kos V.; Mrkun J.; Kosec M. Diagnostic Value of Matrix Metalloproteinases MMP-2 and MMP-9 in Synovial Fluid for Identifying Osteoarthritis in the Distal Interphalangeal Joint in Horses. Acta Vet. Brno 2007, 76, 87–95. 10.2754/avb200776010087. [DOI] [Google Scholar]
- Taylor S. E.; Weaver M. P.; Pitsillides A. A.; Wheeler B. T.; Wheeler-Jones C. P. D.; Shaw D. J.; Smith R. K. W. Cartilage Oligomeric Matrix Protein and Hyaluronan Levels in Synovial Fluid from Horses with Osteoarthritis of the Tarsometatarsal Joint Compared to a Control Population. Equine Vet. J. 2006, 38 (6), 502–507. 10.2746/042516406X156073. [DOI] [PubMed] [Google Scholar]
- Laverty S.; Ionescu M.; Marcoux M.; Bouré L.; Doizé B.; Poole A. R. Alterations in Cartilage Type-Ii Procollagen and Aggrecan Contents in Synovial Fluid in Equine Osteochondrosis. J. Orthop. Res. 2000, 18 (3), 399–405. 10.1002/jor.1100180311. [DOI] [PubMed] [Google Scholar]
- Billinghurst R. C.; Brama P. A. J.; van Weeren P. R.; Knowlton M. S.; McIlwraith C. W. Evaluation of Serum Concentrations of Biomarkers of Skeletal Metabolism and Results of Radiography as Indicators of Severity of Osteochondrosis in Foals. Am. J. Vet. Res. 2004, 65 (2), 143–150. 10.2460/ajvr.2004.65.143. [DOI] [PubMed] [Google Scholar]
- Donabedian M.; Weeren P. R.; Perona G.; Fleurance G.; Robert C.; Leger S.; Bergero D.; Lepage O.; Martin-Rosset W. Early Changes in Biomarkers of Skeletal Metabolism and Their Association to the Occurrence of Osteochondrosis (OC) in the Horse. Equine Vet. J. 2008, 40 (3), 253–259. 10.2746/042516408X273657. [DOI] [PubMed] [Google Scholar]
- Wang X.; Zhang A.; Han Y.; Wang P.; Sun H.; Song G.; Dong T.; Yuan Y.; Yuan X.; Zhang M.; et al. Urine Metabolomics Analysis for Biomarker Discovery and Detection of Jaundice Syndrome in Patients with Liver Disease. Mol. Cell. Proteomics 2012, 11 (8), 370–380. 10.1074/mcp.M111.016006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jukarainen N.NMR Metabolomics Techniques and Mathematical Tools as an Aid in Neurological Diagnosis; University of Kuopio, 2009. [Google Scholar]
- Keun H. C.; Athersuch T. J. Nuclear Magnetic Resonance (NMR)-Based Metabolomics. Methods Mol. Biol. 2011, 708, 321–334. 10.1007/978-1-61737-985-7_19. [DOI] [PubMed] [Google Scholar]
- de Sousa E. B.; Dos Santos G. C.; Duarte M. E. L.; Moura V.; Aguiar D. P.; Neto; Aguiar D. P. Metabolomics as a Promising Tool for Early Osteoarthritis Diagnosis. Braz. J. Med. Biol. Res. 2017, 50 (11), e6485. 10.1590/1414-431x20176485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugle T.; Kovacs H.; Heijnen I. A.; Daikeler T.; Baisch U.; Hicks J. M.; Valderrabano V. Synovial Fluid Metabolomics in Different Forms of Arthritis Assessed by Nuclear Magnetic Resonance Spectroscopy. Clin. Exp. Rheumatol. 2012, 30 (2), 240–245. [PubMed] [Google Scholar]
- Lacitignola L.; Fanizzi F. P.; Francioso E.; Crovace A. 1H NMR Investigation of Normal and Osteo-Arthritic Synovial Fluid in the Horse. Vet. Comp. Orthop. Traumatol. 2008, 21 (1), 85–88. 10.3415/VCOT-06-12-0101. [DOI] [PubMed] [Google Scholar]
- Sumner L. W.; Amberg A.; Barrett D.; Beale M. H.; Beger R.; Daykin C. A.; Fan T. W.-M.; Fiehn O.; Goodacre R.; Griffin J. L.; et al. Proposed Minimum Reporting Standards for Chemical Analysis Chemical Analysis Working Group (CAWG) Metabolomics Standards Initiative (MSI). Metabolomics 2007, 3 (3), 211–221. 10.1007/s11306-007-0082-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salek R. M.; Steinbeck C.; Viant M. R.; Goodacre R.; Dunn W. B. The Role of Reporting Standards for Metabolite Annotation and Identification in Metabolomic Studies. GigaScience 2013, 2 (1), 13. 10.1186/2047-217X-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia J.; Wishart D. S.; Xia J.; Wishart D. S.. Using MetaboAnalyst 3.0 for Comprehensive Metabolomics Data Analysis. In Current Protocols in Bioinformatics; John Wiley & Sons, Inc.: Hoboken, NJ, 2016; pp 14.10.1–14.10.91. [DOI] [PubMed] [Google Scholar]
- Benjamini Y.; Hochberg Y.. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society. Series B (Methodological); WileyRoyal Statistical Society: 1995; pp 289–300. [Google Scholar]
- Hackett E. S.; McCue P. M. Evaluation of a Veterinary Glucometer for Use in Horses. J. Vet. Intern. Med. 2010, 24 (3), 617–621. 10.1111/j.1939-1676.2010.0481.x. [DOI] [PubMed] [Google Scholar]
- Kobayashi M. Simple Lactate Measurement in Horses Using a Portable Lactate Analyzer with Lancet Skin Punctures under Field Conditions. J. Equine Sci. 2007, 18 (1), 5–11. 10.1294/jes.18.5. [DOI] [Google Scholar]
- Savage C. J. Urinary Clinical Pathologic Findings and Glomerular Filtration Rate in the Horse. Vet. Clin. North Am. Equine Pract. 2008, 24 (2), 387–404. 10.1016/j.cveq.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Stack J. D.; Walshe K.; Steele E.; Cousty M.; Lechartier A.; David F. Synovial Serum Amyloid A (SAA) Point-of-Care Test - A Valuable Aid to Immediate Diagnosis of Synovial Sepsis in Horses. Vet. Surg. 2014, 43, E202. [Google Scholar]
- Le J.; Perier C.; Peyroche S.; Rascle F.; Blanchon M. A.; Gonthier R.; Frey J.; Chamson A. Urine Glycyl-L-Proline Increase and Skin Trophicity. Amino Acids 1999, 17 (3), 315–322. 10.1007/BF01366930. [DOI] [PubMed] [Google Scholar]
- Shirtliff M. E.; Mader J. T. Acute Septic Arthritis. Clin. Microbiol. Rev. 2002, 15 (4), 527–544. 10.1128/CMR.15.4.527-544.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohmander L. S.; Atley L. M.; Pietka T. A.; Eyre D. R. The Release of Crosslinked Peptides from Type II Collagen into Human Synovial Fluid Is Increased Soon after Joint Injury and in Osteoarthritis. Arthritis Rheum. 2003, 48 (11), 3130–3139. 10.1002/art.11326. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.