Abstract
Heart failure (HF) patients suffer from exercise intolerance that diminishes their ability to perform normal activities of daily living and hence compromises their quality of life. This is due largely to detrimental changes in skeletal muscle mass, structure, metabolism, and function. This includes an impairment of muscle contractile performance, i.e., a decline in the maximal force, speed, and power of muscle shortening. Although numerous mechanisms underlie this reduction in contractility, one contributing factor may be a decrease in nitric oxide (NO) bioavailability. Consistent with this, recent data demonstrate that acute ingestion of NO3−-rich beetroot juice, a source of NO via the NO synthase-independent enterosalivary pathway, markedly increases maximal muscle speed and power in HF patients. This review discusses the role of muscle contractile dysfunction in the exercise intolerance characteristic of HF, and the evidence that dietary NO3− supplementation may represent a novel and simple therapy for this currently-underappreciated problem.
Keywords: heart failure, dietary nitrate, nitric oxide, muscle speed, muscle power, beetroot juice
Introduction
Heart failure (HF) is a deadly disease affecting nearly 6 million men and women in the United States and millions more worldwide (1). It is also a highly-disabling disease that markedly impairs the ability of patients to perform normal activities of daily living (e.g., walking) and hence significantly reduces their quality of life. Indeed, one of the major systems for characterizing the severity of HF, i.e., the New York Heart Association (NYHA) Functional Classification, categorizes HF patients based largely on their ability to undertake physical activity. Historically, the exercise intolerance accompanying HF was viewed as being strictly a function of the diminished ability of the failing heart to provide adequate blood flow to peripheral tissues (2,3). Over the last three decades, however, considerable evidence has accumulated that deleterious changes within skeletal muscle itself also majorly contribute to the diminished exercise capacity of patients with “classic” HF, i.e., HF with reduced ejection fraction (2,3). Much more recently, similar muscle abnormalities have been reported in patients with HF with preserved ejection faction (3,4,5). In this brief review, we discuss these data, and in particular the evidence that alterations in intrinsic muscle contractile properties, i.e., in muscle strength, speed, and power, play an important role in limiting the functional capacity of patients with HF. From there we turn out attention to the possible significance of reductions in nitric oxide (NO) bioavailability in contributing to these detrimental changes. Finally, we describe recent research indicating that dietary nitrate (NO3−), a source of NO via the NO synthase (NOS) –independent enterosalivary pathway, may provide a simple but effective means of combating this problem.
Causes of exercise intolerance in HF
HF as a “cardiomuscular” disease
As indicated above, it had long been held that decreased perfusion of skeletal muscle was the singular determinant of the reduced exercise capacity of HF patients. Indeed, this seems to be a common misconception among many clinicians even today. It is now abundantly clear, however, that secondary effects of HF on skeletal muscle morphological and metabolic characteristics play a critical, if not dominant, role in this impairment. These HF-induced changes in muscle include, but are not limited to, an increased percentage of type II, or fast-twitch, fibers (7–11), an elevation in glycolytic enzyme activities (7,8), a decrease in capillarization (8,9), and a decline in mitochondrial respiratory capacity (7,8,11) resulting not only from a reduction in total muscle mitochondrial content but also from changes in the quality of mitochondria themselves (12). The sum total of these changes is a more fatigable phenotype characterized by a diminished ability to match aerobic ATP production to ATP demand during contractile activity, resulting in a greater decrease in phosphocreatine (PCr) and greater increases in inorganic phosphate (Pi) and H+ levels during exercise at any given intensity (7,13,14,15). Importantly, these metabolic abnormalities appear to be independent of any reduction in blood flow and hence in O2 delivery to muscle (13–19). In particular, increasing cardiac output and hence bulk limb blood flow via dobutamine infusion does not improve VO2peak or exercise capacity in patients with HF (13,14,19), nor does it correct the abnormalities in muscle metabolism observed during exercise (19). Thus, the primary limitation(s) to sustained exercise performance in HF patients appear(s) to reside outside of the heart, and within the skeletal muscles themselves. Indeed, even what is perhaps the signature complaint of patients with HF – that is, of dyspnea on exertion – has been hypothesized to be due largely, if not entirely, to these changes in skeletal muscle characteristics (20). The resulting abnormal metabolic response to contractile activity is hypothesized to lead to greater stimulation of group III–IV afferent nerves in muscle and hence enhanced feedback stimulation of ventilation during exercise. Notably, the morphological and metabolic abnormalities found in the muscles of patients with HF do not improve following cardiac transplantation, which almost certainly contributes to the fact that even after transplant the exercise capacity of such patients remains significantly diminished compared to that of healthy control subjects (21,22).
Role of diminished muscle contractile function
The changes in skeletal muscle characteristics and thus metabolism and energetics described above are undoubtedly important in accounting for the reduced exercise tolerance found in patients with HF. Other factors, however, are also operative. In particular, a substantial number of studies have demonstrated that the muscles of HF patients are smaller and weaker than those of healthy individuals (23–30), with the plantar flexors in particular seeming to be adversely affected (31,32). Somewhat counterintuitively, given the changes in fiber type mentioned previously, HF also results in a significant decrease in the maximal velocity of muscle contraction, and hence an even greater reduction in maximal muscle power (which is the product of force and velocity) (30,33). These observations are salient for a number of reasons. First, many normal activities of daily living (e.g., getting out of a chair, climbing a flight of stairs, carrying groceries) are heavily dependent upon the ability of muscle to generate significant force and/or power. Second, HF-induced reductions in whole-body exercise capacity are closely associated with these alterations in muscle contractile performance (21,23,26,27,31,34,35). Senden et al. (36), for example, found that maximal power output during an incremental cycle ergometer test to fatigue in HF patients was significantly correlated with the strength and endurance of their knee extensors and flexors as assessed using isokinetic dynamometry. Other studies have demonstrated a significant relationship between the reduction in muscle function in HF patients and the reduction in VO2peak (27) and/or the elevated ventilatory demand during exercise (35). In keeping with such data, resistance exercise training has been found to significantly improve walking ability and VO2peak in HF patients (37,38), as well as to increase both objectively and subjectively measured physical function (39). Third, muscle function has been demonstrated to be a powerful predictor of long-term survival in patients with HF, even more so than VO2peak (39). Clearly, alterations in muscle contractile characteristics play a critical role in the morbidity and mortality of HF.
Mechanisms
The mechanisms responsible for the HF-induced reductions in muscle force, speed, and power described above have not been fully elucidated. In part, they may be due to a disease-related reduction in habitual physical activity (33), and/or due to the muscle atrophy (“cardiac cachexia”) often found in patients with HF. Seminal research by Toth, Miller, and colleagues (34), however, has demonstrated that HF patients are weaker, slower, and less powerful than healthy control subjects even when both groups are carefully matched for age, sex, level of physical activity, and medication use. Furthermore, such differences persist even when data are statistically adjusted for leg lean mass to account for possible muscle atrophy in the HF patients (34,41). Thus, physical inactivity and/or muscle wasting alone cannot account for the changes in muscle contractile function that are associated with, and contribute significantly to, the exercise intolerance characteristic of HF.
If the reductions in muscle force, speed, and power with HF cannot be fully explained by a more sedentary lifestyle or muscle atrophy, what factor(s) are responsible? The answer to that question would seem to reside at the molecular level itself. For example, using isolated single muscle fibers Szentesi et al. (42) have demonstrated that HF is accompanied by a reduction in isometric force per unit of cross-sectional area, which is paralleled by a decline in ATPase activity. These findings imply that HF results in a reduction in the number and/or rate of actomyosin crossbridge formation. Consistent with this, working in Toth’s laboratory Miller found that HF was associated with a selective loss of myosin in type I, type IIA, and type IIA/X fibers (43). In a follow-up study, these same authors demonstrated a HF-induced slowing of myosin cross-bridge kinetics in both type I and type IIA fibers, as well as a reduction in Ca2+ sensitivity in the latter fiber type (44). These alterations were ascribed, at least in part, to a reduction in Akt phosphorylation, which was significantly correlated with the decline in myosin heavy-chain protein content (45). Studies by others have demonstrated abnormalities in Ca2+-handling protein (i.e., ryanodine receptor type I, sarco(endo)plasmic reticulum Ca2+ ATPase (SERCA) 2a, phospholamban, and dihydropyridine receptor) regulation and/or content in the skeletal muscles of patients with HF (46,47), similar to those found in the failing heart (48). Importantly, changes in anabolic signaling and myosin heavy chain expression precede changes in whole-muscle function in HF patients, providing evidence of their cause-and-effect relationship (49).
Significance of NO
Along with the molecular alterations described above, another factor potentially contributing to the HF-related impairment of skeletal muscle function may be a decrease in NO bioavailability. Although initially identified as a vasodilator, i.e., as “endothelium-derived relaxing factor”, NO is in fact a key cellular signaling molecule with pleiotropic effects in many tissues. These include skeletal muscle, wherein among its other effects NO helps modulate contractile function (50,51). Specifically, during isometric contractions NO may (50,51), or may not (52), slightly suppress maximal force production. This may be due to nitrosation or S-nitrosylation of various proteins (53,54). During concentric activity, however, NO significantly increases the rate of force development, maximal shortening velocity, and maximal power of both single muscle fibers and isolated muscles (50,51). These stimulatory effects are thought to be mediated via activation of the classic NO-soluble guanyl cyclase (sGC)-cycle GMP (cGMP) pathway, and have been euphemistically described by Maréchal and Gailly (50) as a “slow-to-fast” shift qualitatively akin to the chronic transformation of muscle fiber type that occurs with, e.g., prolonged electrical stimulation. In failing cardiac muscle, however, increased production of reactive oxygen species (ROS) leads to more rapid destruction of NO, and hence reduced NO-sGC-cGMP signaling, which in turn is thought to contribute to reduced contractility in HF (55). Given other parallels between the effects of HF on cardiac and skeletal muscle (including, somewhat paradoxically, an increase in inducible NOS, or iNOS, expression in both heart (56) and skeletal muscle (57,58)), it is possible that NO bioavailability is also diminished in the skeletal muscles of patients with HF, thus contributing to their reduced muscle function as described above.
That HF reduces NO signaling in skeletal muscle has not been directly demonstrated. There is, however, considerable evidence that HF leads to a decline in whole-body NO production and/or availability. Katz et al. (59), for example, have demonstrated that HF results in reduced urinary excretion of [15N]nitrate after infusion of L-[15N]arginine, indicative of an overall decline in NOS activity. In keeping with this, a number of studies have demonstrated that breath NO levels are lower in HF patients, both at rest (60) and especially during exercise (61,62,63), which is correlated with the magnitude of the impairment in exercise capacity (60,62). It is also well-established that HF leads to endothelial dysfunction in various tissues, including skeletal muscle, as a result of reduced NO production via endothelial NOS (64). These data, along with the central role that skeletal muscle myocytes are now known to play in NO/nitrite (NO2−)/NO3− metabolism (65,66), strongly suggest that HF reduces NO bioavailability within skeletal muscle, just as it does in the heart.
Dietary NO3− as a source of NO
If an increase in ROS production and/or down-regulation of NOS-mediated NO synthesis with HF reduces skeletal muscle NO levels and thus contributes to muscle contractile dysfunction, what might be done to treat this problem? One intriguing possibility would be to attempt to increase NO production via the NOS-independent enterosalivary pathway (67). This pathway has been reviewed recently in this journal by Chirinos and Zamani (68), and hence will not be discussed in great detail here. In brief, however, in this pathway NO3− in the diet (found primarily in green leafy vegetables, e.g., spinach, arugula, chard, and also beetroot) is reduced to NO2− and then to NO. The initial conversion of NO3− to NO2− is performed by facultative anaerobic bacteria in the oral cavity, partially on first-pass but primarily after NO3− has been taken up via the gastrointestinal tract, circulated via the bloodstream, and then concentrated and secreted by the salivary glands. The NO2− that is formed is then also absorbed and circulated throughout the body, where it can be reduced to NO. A number of molecules have been found capable of catalyzing this second step, with deoxyhemoglobin/deoxymyoglobin, aldehyde oxidase, and xanthine oxidoreductase likely being particular important in skeletal muscle (66,69,70). Of note, production of NO via this pathway does not require O2 and proceeds more rapidly under acidic than neutral/basic conditions, which is precisely the opposite of the more well-known NOS pathway. This direct conversion of NO3− to NO2− and hence to NO therefore represents a very important mechanism for generating NO “on demand” under hypoxic/acidic conditions, such as commonly occur in contracting skeletal muscle. Indeed, recent research using homogenized rat skeletal muscle has directly demonstrated that acute exercise enhances NO production from NO2− (66).
Effect of dietary NO3− on muscle function in HF patients
To test the hypothesis that reduced NO bioavailability contributes to the diminished muscle function found in HF patients, and to evaluate dietary NO3− as a potential treatment, we recently studied nine middle-aged (average age 57±10 y) men (n=5) and women (n=4) with reduced systolic function (average ejection fraction = 28±11%) (71). Based on the NYHA criteria, the distribution of patients was 1/5/3/0 in classes I/II/III/IV, and all were treated with standard HF medications. Each was studied twice in the fasted state, using a double-blind, placebo-controlled, crossover design. In one trial, patients were tested 2 h after ingesting 140 mL of a concentrated beetroot juice (BRJ) supplement (Beet It Sport®, James White Drinks, Ipswich, UK) containing 11.2 mmol of NO3−, whereas in the other they were tested 2 h after ingesting the same volume of an NO3−-depleted BRJ placebo that was indistinguishable in terms of color, texture, taste, and smell. Plasma NO3− and NO2− and breath NO levels along with heart rate and blood pressure were measured throughout each experiment. Muscle contractile function was assessed using isokinetic dynamometry, with each subject performing 3–4 maximal knee extensions using their dominant leg at angular velocities of 0 (isometric), 1.57, 3.14, 4.71, and 6.28 rad/s (0, 90, 180, 270, and 360 °/s, respectively). This range of velocities was chosen to span the ascending limb of the inverse parabolic power-velocity relationship, thus allowing us to determine (via curve fitting) the maximal velocity (Vmax) and power (Pmax) of knee extension based on the peak power (product of peak torque and velocity) achieved at each velocity. Following this testing, the patients also performed 50 maximal knee extensions at a velocity of 3.14 rad/s (180°/s), to determine the possible effects of dietary NO3− on fatigue resistance during repetitive, dynamic muscle contractions.
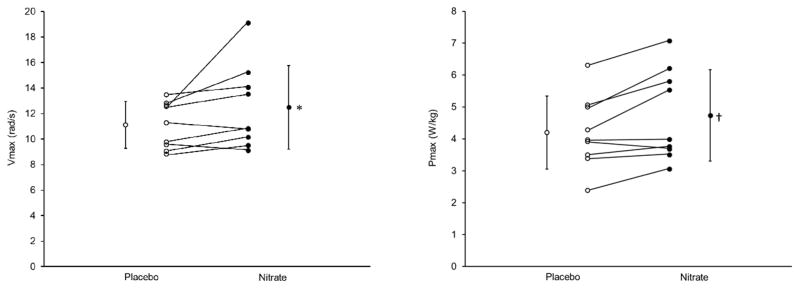
We found that ingestion of BRJ containing 11.2 mmol of NO3− resulted in a significant (P<0.001) elevation in plasma NO3− concentrations (average = 508±143 μmol/L in the NO3− trial vs. 29±11 μmol/L in the placebo trial). Average plasma NO2− concentration also tended to be higher following BRJ ingestion (i.e., 0.57±27 vs. 0.44±0.24 μmol/L), although this difference was not significant due to high variability. On the other hand, the difference in average breath NO levels (i.e., 29±13 vs. 16±5 ppb) was statistically significant (i.e., P<0.01). This increase in whole-body NO bioavailability was accompanied by 9% (P=0.07) and 11% (P<0.05) increases in peak knee extensor torque, and hence power, at the two highest velocities tested, i.e., 4.71 and 6.28 rad/s (270 and 360 °/s), respectively (Table 1). As a result, calculated Vmax and Pmax were 12% (P=0.08) and 13% (P<0.05) higher NO3− ingestion (Figure 1). Six of the nine HF patients were “responders”, with Pmax increasing by 17±10%, whereas the other three showed minimal change (i.e., −1±5%). Although peak power was initially 6% higher (i.e., 1.78±0.46 vs. 1.68±0.39 W/kg; P<0.05) during the 50 contraction fatigue test, no difference was found in overall work performed (i.e., 28.8±9.8 vs. 28.1±9.5 J/kg; P=0.38) because the patients tended to fatigue somewhat more rapidly following NO3− ingestion. We also found no differences in heart rate (69±12 vs. 67±12 beats/min; P=0.40) or systolic (105±9 vs. 103±15 mmHg; P=0.76) or diastolic (66±8 vs. 66±8 mmHg; P=0.91) blood pressures.
Table 1.
Effect of acute ingestion of 11.2 mmol of nitrate on peak knee extensor torque and power at varying angular velocities in patients with HF due to systolic dysfunction.
| Placebo | Nitrate | ||
|---|---|---|---|
| Angular velocity | |||
| Peak torque (Nm/kg) | 0.00 rad/s | 1.98±0.48 | 1.99±0.31 |
| 1.57 rad/s | 1.36±0.32 | 1.38±0.29 | |
| 3.14 rad/s | 1.05±0.25 | 1.09±0.25 | |
| 4.71 rad/s | 0.85±0.17 | 0.93±0.22* | |
| 6.28 rad/s | 0.64±0.22 | 0.71±0.24† | |
| Angular velocity | |||
| Peak power (W/kg) | 0.00 rad/s | n/a | n/a |
| 1.57 rad/s | 2.14±0.51 | 2.17±0.46 | |
| 3.14 rad/s | 3.29±0.77 | 3.42±0.79 | |
| 4.71 rad/s | 4.03±0.80 | 4.38±1.03* | |
| 6.28 rad/s | 4.02±1.41 | 4.48±1.53† |
Figure 1.
Effect of acute ingestion of 11.2 mmol of nitrate on the maximal speed (Vmax) and power (Pmax) of knee extension in patients with HF due to systolic dysfunction. Placebo; open circles. Nitrate; closed circles. Values are mean ± SD; individual results are also shown. *P=0.08; †P<0.05. Data from Ref. 71.
In summary, the results of this study clearly show that dietary NO3− ingestion can increase maximal muscle speed and power in patients with systolic HF. In fact, compared to healthy, somewhat younger subjects that we studied in parallel (72), the HF patients had lower breath NO levels, both at rest and after BRJ ingestion, but demonstrated approximately twice as large of an increase in Vmax and Pmax after NO3− intake, consistent with the hypothesis that the muscle dysfunction accompanying cardiac failure is due, at least in part, to a decline in NO bioavailability. Indeed, based on the study of Toth et al. (34) this improvement in muscle power resulting from dietary NO3− intake would have been sufficient to immediately erase approximately one-third of the deficit normally associated with HF. Stated another way, the magnitude of the improvement that we observed is comparable to that resulting from several months of resistance exercise training in HF patients (73,74), which has been shown to result in significant improvement in Minnesota Living with Heart Failure Questionnaire score (37). Importantly, these improvements occurred in patients already receiving standard HF medical therapy, which by itself does nothing to enhance muscle contractile function (75–79). The results of our study compliment other recent studies demonstrating that dietary NO3− (or NO2−) supplementation enhances physiological responses and/or performance during aerobic exercise in patients with HF with either reduced (80) or maintained (81,82,83) ejection fraction. Nonetheless, it should be emphasized that, mechanistically, different factors are likely responsible for these benefits. In particular, while NO-mediated effects on the cardiovascular system are likely largely responsible for the improvements reported in these other studies, they would not explain the significant increases in muscle function we have observed during individual muscle contractions lasting <1 s. This interpretation is consistent with the fact that we found no overall enhancement of performance during the 50 contraction fatigue test, which takes ~1 min to complete. Somewhat along the same lines, the improvements in muscle function we have observed in response to acute dietary NO3− intake cannot be explained by the increases in muscle calsequestrin 1 and dihydropyridine receptor content that have been observed in rodent muscle following chronic NO3− supplementation (84). We have therefore hypothesized that our findings are due to activation of sGC by NO, and hence an elevation in muscle cGMP levels. Studies to directly test this hypothesis are presently underway.
Summary and Conclusions
HF is an all-too-common disease with that severely compromises both the quantity and quality of life. The latter is due, in part, to exercise intolerance that reduces the patient’s ability to perform ordinary activities of daily living. This diminished exercise capacity is not simply the result of impaired cardiac output, but instead is caused by numerous changes in the mass, structure, metabolism, and function of skeletal muscle – in other words, in terms of exercise tolerance HF should really be considered a cardiomuscular disease. Among the HF-induced changes in muscle, one of the most prominent is a decline in the maximal force, speed, and power of muscle shortening. This decline in contractile performance, which is closely related to the reductions in whole-body exercise capacity and VO2peak, is due in part to changes in muscle protein content and/or function. Reductions in NO-sGC-cGMP signaling, however, may also play a role. In support of the latter hypothesis, we have recently demonstrated in a small number of HF patients that dietary NO3−, a source of NO via the NOS-independent enterosalivary pathway, significantly increases the maximal speed and power of muscle contraction. Larger, multi-center trials are needed to confirm these observations and to determine the effects of dietary NO3− on physical activity levels and quality of life in patients with HF. If proven effective in such studies, dietary NO3− supplementation would represent a novel therapy for muscle dysfunction in HF, an important symptom of the disease not presently addressed by any standard medications or therapies.
Footnotes
Compliance with Ethical Guidelines
Conflict of Interest
Dr. Coggan reports grants from the Institute for Clinical and Translational Sciences and the Barnes-Jewish Hospital Foundation during the conduct of the study.
Dr. Peterson reports grants from the Institute of Clinical and Translational Sciences and the Barnes-Jewish Hospital Foundation during the conduct of the study; and other relevant financial activies outside the submitted work from Merck, Johnson and Johnson, Medronic, and Gilead.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Després JP, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER, 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics--2015 Update: A Report From the American Heart Association. Circulation. 131:e29–e322. doi: 10.1161/CIR.0000000000000152. 20150. [DOI] [PubMed] [Google Scholar]
- 2.Zizola C, Schulze PC. Metabolic and structural impairment of skeletal muscle in heart failure. Heart Fail Rev. 2013;18:623–630. doi: 10.1007/s10741-012-9353-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3•.Okita K, Kinugawa S, Tsutsui H. Exercise intolerance in chronic heart failure. Skeletal muscle dysfunction and potential therapies. Circ J. 2013;77:293–300. doi: 10.1253/circj.cj-12-1235. This article summarizes research demonstrating the importance of skeletal muscle abnormalities to the exercise intolerance that accompanies HF. [DOI] [PubMed] [Google Scholar]
- 4.Bhella PS, Prasad A, Heinicke K, Hastings JL, Arbab-Zadeh A, Adams-Heut B, Pacini EL, Shibata S, Palmer MD, Newcomer BR, Levine BD. Abnormal hemodynamic response to exercise in heart failure with preserved ejection fraction. Eur J Heart Fail. 2011;13:1296–1304. doi: 10.1093/eurjhf/hfr133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haykowsky MJ, Kouba EJ, Brubaker PH, Nicklas BJ, Eggebeen J, Kitzman DW. Skeletal muscle composition and its relation to exercise tolerance in older patients with heart failure and preserved ejection fraction. Am J Cardiol. 2014;113:1211–1216. doi: 10.1016/j.amjcard.2013.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kitzman DW, Nicklas B, Kraus WE, Lyles MF, Eggebeen J, Morgan TW, Haykowsky M. Skeletal muscle abnormalities and exercise intolerance in older patients with heart failure and preserved ejection fraction. Am J Heart Circ Physiol. 2014;306:H1364–H1370. doi: 10.1152/ajpheart.00004.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mancini DM, Coyle EF, Coggan AR, Beltz J, Ferraro N, Montain S, Wilson JR. Contribution of intrinsic skeletal muscle changes to 31P NMR abnormalities in patients with chronic heart failure. Circulation. 1989;80:1338–1346. doi: 10.1161/01.cir.80.5.1338. [DOI] [PubMed] [Google Scholar]
- 8.Sullivan MJ, Green HJ, Cobb FR. Skeletal muscle biochemistry and histology in ambulatory patients with long term heart failure. Circulation. 1990;81:518–527. doi: 10.1161/01.cir.81.2.518. [DOI] [PubMed] [Google Scholar]
- 9.Schaufelberger M, Eriksson BO, Grimby G, Held P, Swedberg K. Skeletal muscle fiber composition and capillarization in patients with chronic heart failure: relation to exercise capacity and central hemodynamics. J Card Fail. 1995;1:267–272. doi: 10.1016/1071-9164(95)90001-2. [DOI] [PubMed] [Google Scholar]
- 10.Massie BM, Simoni A, Sahgal P, Wells L, Dudley GA. Relation of systemic and local muscle exercise capacity to skeletal muscle characteristics in men with congestive heart failure. J Am Coll Cardiol. 1996;27:140–145. doi: 10.1016/0735-1097(95)00416-5. [DOI] [PubMed] [Google Scholar]
- 11.Szentesi P, Bekedam MA, van Beek-Harmsen BJ, van der Laarse WJ, Zaremba R, Boonstra A, Visser FC, Stienen GJ. Depression of force production and ATPase activity in different types of human skeletal muscle fibers from patients with chronic heart failure. J Appl Physiol. 2005;99:2189–2195. doi: 10.1152/japplphysiol.00542.2005. [DOI] [PubMed] [Google Scholar]
- 12.Drexler H, Riede U, Munzel T, Konig H, Funke E, Just H. Alterations of skeletal muscle in chronic HF. Circulation. 1992;85:1751–1759. doi: 10.1161/01.cir.85.5.1751. [DOI] [PubMed] [Google Scholar]
- 13.Wiener DH, Fink LI, Maris J, Jones RA, Chance B, Wilson JR. Abnormal skeletal muscle bioenergetics during exercise in patients with heart failure: role of reduced muscle blood flow. Circulation. 1986;73:1127–1136. doi: 10.1161/01.cir.73.6.1127. [DOI] [PubMed] [Google Scholar]
- 14.Maskin CS, Forman R, Sonnenblick EH, Frishman WH, LeJemtel TH. Failure of dobutamine to increase exercise capacity despite hemodynamic improvement in severe chronic heart failure. Am J Cardiol. 1983;51:177–182. doi: 10.1016/s0002-9149(83)80032-0. [DOI] [PubMed] [Google Scholar]
- 15.Okita K, Yonezawa K, Nishijima H, Hanada A, Ohtsubo M, Kohya T, Murakami T, Kitabatake A. Skeletal muscle metabolism limits exercise capacity in patients with chronic heart failure. Circulation. 1998;98:1886–1891. doi: 10.1161/01.cir.98.18.1886. [DOI] [PubMed] [Google Scholar]
- 16.Wilson JR, Martin JL, Ferraro N. Impaired skeletal muscle nutritive flow during exercise in patients with congestive heart failure: role of cardiac pump dysfunction as determined by the effect of dobutamine. Am J Cardiol. 1984;53:1308–1315. doi: 10.1016/0002-9149(84)90085-7. [DOI] [PubMed] [Google Scholar]
- 17.Minotti JR, Christoph I, Oka R, Weiner MW, Wells L, Massie BM. Impaired skeletal muscle function in patients with congestive heart failure. Relationship to systemic exercise performance. J Clin Invest. 1991;88:2077–2082. doi: 10.1172/JCI115537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hanada A, Okita K, Yonezawa K, Ohtsubo M, Kohya T, Murakami T, Nishijima H, Tamura M, Kitabatake A. Dissociation between muscle metabolism and oxygen kinetics during recovery from exercise in patients with chronic heart failure. Heart. 2000;83:161–166. doi: 10.1136/heart.83.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mancini DM, Schwartz M, Ferraro N, Seestedt R, Chance B, Wilson JR. Effect of dobutamine on skeletal muscle metabolism in patients with congestive heart failure. Am J Cardiol. 1990;65:1121–1126. doi: 10.1016/0002-9149(90)90325-u. [DOI] [PubMed] [Google Scholar]
- 20.Olson TP, Joyner MJ, Eisenach JH, Curry TB, Johnson BD. Influence of locomotor muscle afferent inhibition on the ventilator response to exercise in heart failure. Exp Physiol. 2014;99:414–426. doi: 10.1113/expphysiol.2013.075937. [DOI] [PubMed] [Google Scholar]
- 21.Schaufelberger M, Eriksson BO, Lönn L, Rundqvist, Sunnerhagen KS, Swedbert K. Skeletal muscle characteristics, muscle strength and thigh muscle area in patients before and after cardiac transplantation. Eur J Heart Fail. 2001;3:59–67. doi: 10.1016/s1388-9842(00)00114-8. [DOI] [PubMed] [Google Scholar]
- 22.Quittan M, Sturm B, Wiesinger GF, Fialka-Moser V, Pacher R, Rödler S. Skeletal muscle strength following orthotopic heart transplantation. Wien Klin Wochenschr. 1999;111:467–483. [PubMed] [Google Scholar]
- 23.Buller NP, Jones D, Poole-Wilson PA. Direct measurement of skeletal muscle fatigue in patients with chronic heart failure. Br Heart J. 1991;65:20–24. doi: 10.1136/hrt.65.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Magnusson G, Isberg B, Karlberg K, Sylvén C. Skeletal muscle strength and endurance in chronic congestive heart failure secondary to idiopathic dilated cardiomyopathy. Am J Cardiol. 1994;73:307–309. doi: 10.1016/0002-9149(94)90239-9. [DOI] [PubMed] [Google Scholar]
- 25.Harridge SDR, Magnusson G, Gordon A. Skeletal muscle contractile characteristics and fatigue resistance in patients with chronic heart failure. Eur Heart J. 1996;17:896–901. doi: 10.1093/oxfordjournals.eurheartj.a014971. [DOI] [PubMed] [Google Scholar]
- 26.Harrington D, Anker SD, Chua TP, Webb-Peploe KM, Ponikowski PP, Poole-Wilson PA, Coats AJS. Skeletal muscle function and its relation to exercise tolerance in chronic heart failure. JACC. 1997;30:1758–1764. doi: 10.1016/s0735-1097(97)00381-1. [DOI] [PubMed] [Google Scholar]
- 27.Clark A, Rafferty D, Arbuthnott K. Relationship between isokinetic muscle strength and exercise capacity in chronic heart failure. Int J Cardiol. 1997;59:145–148. doi: 10.1016/s0167-5273(97)02934-3. [DOI] [PubMed] [Google Scholar]
- 28.Sunnerhagen KS, Cider A, Shcaufelberger M, Hedberg M, Grimby G. Muscular performance in heart failure. J Card Fail. 1998;4:97–104. doi: 10.1016/s1071-9164(98)90249-4. [DOI] [PubMed] [Google Scholar]
- 29.Carrington CA, Fisher WK, Davies MK, White MJ. Is there a relationship between muscle fatigue resistance and cardiovascular responses to isometric exercise in mild chronic heart failure? Eur J Heart Fail. 2001;3:53–58. doi: 10.1016/s1388-9842(00)00112-4. [DOI] [PubMed] [Google Scholar]
- 30.Brunjes DL, Dunlop M, Wu C, Jones M, Kato TS, Kennel PJ, Armstrong HF, Choo TH, Bartels MN, Forman DE, Mancini DM, Schulze PC. Analysis of skeletal muscle torque capacity and circulating ceramides in patients with advanced heart failure. J Card Fail. 2016 Feb 12; doi: 10.1016/j.cardfail.2016.02.002. pii: S1071-9164(16)00039-7. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pannizolo FA, Mairorana AJ, Naylor LH, Dembo L, Lloyd DG, Green DJ, Rubenson J. Gait analysis in chronic heart failure: The calf as a locus of impaired walking capacity. J Biomech. 2014;47:3719–3725. doi: 10.1016/j.jbiomech.2014.09.015. [DOI] [PubMed] [Google Scholar]
- 32.Panizzolo FA, Maiorana AJ, Naylor LH, Lichtwark GA, Dembo L, Lloyd DG, Green DJ, Rubenson J. Is the soleus a sentinel muscle for impaired aerobic capacity in heart failure? Med Sci Sports Exerc. 2015;47:498–508. doi: 10.1249/MSS.0000000000000431. [DOI] [PubMed] [Google Scholar]
- 33.Rehn TA, Munkvik M, Lunde PK, Sjaastad I, Sejersted OM. Intrinsic skeletal muscle alterations in chronic heart failure: a disease-specific myopathy or a result of deconditioning? Heart Fail Rev. 2012;17:421–436. doi: 10.1007/s10741-011-9289-4. [DOI] [PubMed] [Google Scholar]
- 34.Toth MJ, Shaw AO, Miller MS, VanBuren P, LeWinter MM, Maughan DW, Ades PA. Reduced knee extensor function in heart failure is not explained by inactivity. Int J Cardiol. 2010;143:276–282. doi: 10.1016/j.ijcard.2009.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suzuki K, Omiya K, Yamada S, Kobayashi T, Suzuki N, Osada N, Miyake F. Relations between strength and endurance of leg skeletal muscle and cardiopulmonary exercise testing parameters in patients with chronic heart failure. J Cardiol. 2004;43:59–68. [PubMed] [Google Scholar]
- 36.Senden PJ, Sabelis LWE, Zonderland ML, van de Kolk R, Meiss L, de Vries WR, Bol E, Mosterd WL. Determinants of maximal exercise performance in chronic heart failure. Eur J Cardiovasc Prevention Rehad. 2004;11:41–47. doi: 10.1097/01.hjr.0000116825.84388.eb. [DOI] [PubMed] [Google Scholar]
- 37.Levinger I, Bronks R, Cody DV, Linton I, Davie A. Resistance training for chronic heart failure patients on beta blocker medications. Int J Cardiol. 2005;102:493–499. doi: 10.1016/j.ijcard.2004.05.061. [DOI] [PubMed] [Google Scholar]
- 38.Delagardelle C, Feiereisen P, Krecke R, Essamri B, Beissel J. Objective of 6 months’ endurance and strength training program in outpatients with congestive heart failure. Med Sci Sports Exerc. 1999;31:1102–1107. doi: 10.1097/00005768-199908000-00004. [DOI] [PubMed] [Google Scholar]
- 39.Savage P, Shaw AO, Miller MS, VanBuren P, LeWinter M, Ades PA, Toth MJ. Effect of resistance training on physical disability in chronic heart failure. Med Sci Sports Exerc. 2011;43:1379–1386. doi: 10.1249/MSS.0b013e31820eeea1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hülsmann M, Quittan M, Berger R, Crevenna R, Springer C, Nuhr M, Mörtl D, Moser P, Pacher R. Muscle strength as a predictor of long-term survival in severe congestive heart failure. Eur J Heart Fail. 2004;6:101–107. doi: 10.1016/j.ejheart.2003.07.008. [DOI] [PubMed] [Google Scholar]
- 41.Toth MJ, Ades PA, Tischler MD, Tracy RP, LeWinter MM. Immune activation is associated with reduced skeletal muscle mass and physical function in chronic heart failure. Int J Cardiol. 2006;109:179–187. doi: 10.1016/j.ijcard.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 42.Szentesi P, Bekedam MA, van Beek-Harmsen BJ, van der Laarse WJ, Zaremba R, Boonstra A, Visser FC, Stienen GJ. Depression of force production and ATPase activity in different types of human skeletal muscle fibers from patients with chronic heart failure. J Appl Physiol. 2005;99:2189–2195. doi: 10.1152/japplphysiol.00542.2005. [DOI] [PubMed] [Google Scholar]
- 43.Miller MS, vanBuren P, LeWinter MM, Lecker SH, Selby DE, Palmer BM, Maughan DW, Ades PA, Toth MJ. Mechanisms underlying skeletal muscle weakness in human heart failure. Alterations in single fiber myosin protein content and function. Circ Heart Fail. 2009;2:700–706. doi: 10.1161/CIRCHEARTFAILURE.109.876433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miller MS, VanBuren P, LeWinter MM, Braddock JM, Ades PA, Maughan DW, Palmer BM, Toth MJ. Chronic heart failure decreases cross-bridge kinetics in single muscle fibers from humans. J Physiol. 2010;588:4039–4053. doi: 10.1113/jphysiol.2010.191957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Toth MJ, Ward K, van der Velden J, Miller MS, VanBuren P, LeWinter MM, Ades PA. Chronic heart failure reduces Akt phosphorylation in human skeletal muscle: relationship to muscle size and function. J Appl Physiol. 2011;110:892–900. doi: 10.1152/japplphysiol.00545.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rullman E, Andersson DC, Melin M, Reiken S, Mancini DM, Marks AR, Lund LH, Gustafsson T. Modifications of skeletal muscle ryanodine receptor type I and exercise intolerance in heart failure. J Heart Lung Transplant. 2013;32:925–929. doi: 10.1016/j.healun.2013.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Middlekauf HR, Vigna C, Verity MA, Fonarow GC, Horwich TB, Hamilton MA, Shieh P, Tupling AR. Abnormalities of calcium handling proteins in skeletal muscle mirror those the heart in humans with heart failure: a shared mechanism? J Card Fail. 2012;18:724–733. doi: 10.1016/j.cardfail.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Andersson DC, Marks AR. Fixing ryanodine receptor Ca2+ leak – a novel therapeutic strategy for contractile failure in heart and skeletal muscle. Drug Discov Today Dis Mech. 2010;7:e151–e157. doi: 10.1016/j.ddmec.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Godard MP, Whitman SA, Song Y-H, Delafontaine P. Skeletal muscle molecular alterations precede whole-muscle dysfunction in NYHA Class II heart failure patients. Clin Interven Aging. 2012;7:489–497. doi: 10.2147/CIA.S37879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maréchal G, Gaily P. Effects of nitric oxide on the contraction of skeletal muscle. Cell Mol Life Sci. 1999;55:1088–1102. doi: 10.1007/s000180050359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kaminski HJ, Andrade FH. Nitric oxide: biologic effects on muscle and role in muscle diseases. Neuromuscular Disord. 2001;11:517–524. doi: 10.1016/s0960-8966(01)00215-2. [DOI] [PubMed] [Google Scholar]
- 52.Fulford J, Winyard PG, Vanhatalo A, Bailey SJ, Blackwell JR, Jones AM. Influence of dietary nitrate supplementation on human skeletal muscle metabolism and force production during maximum voluntary contractions. Pflugers Arch. 2013;465:517–28. doi: 10.1007/s00424-013-1220-5. [DOI] [PubMed] [Google Scholar]
- 53.Evangelista AM, Rao VS, Filo AR, Marozkina NV, Doctor A, Jones DR, Gaston B, Guilford WH. Direct regulation of striated muscle myosins by nitric oxide and endogenous nitrosothiols. PLOS One. 2010;5:e11209. doi: 10.1371/journal.pone.0011209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lamb GD, Westerblad H. Acute effects of reactive oxygen and nitrogen species on the contractile function of skeletal muscle. J Physiol. 2011;589:2119–2127. doi: 10.1113/jphysiol.2010.199059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hare JM, Stamler JS. NO/redox disequilibrium in the failing heart and cardiovascular system. J Clin Invest. 2005;115:509–517. doi: 10.1172/JCI200524459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Haywood GA, Tsao PS, von der Leyen HE, Mann MJ, Keeling PJ, Trindade PT, Lewis NP, Byrne CD, Rickenbacher PR, Bishopric NH, Cooke JP, McKenna WJ, Fowler MB. Expression of inducible nitric oxide synthase in human heart failure. Circulation. 1996;93:1087–1094. doi: 10.1161/01.cir.93.6.1087. [DOI] [PubMed] [Google Scholar]
- 57.Adams V, Yu J, Möbius-Winkler S, Linke A, Weigl C, Hilbrich L, Schuler G, Hambrecht R. Inducible nitric oxide synthase in skeletal muscle biopsies from patients with chronic heart failure. Biochem Mol Med. 1997;61:152–160. doi: 10.1006/bmme.1997.2598. [DOI] [PubMed] [Google Scholar]
- 58.Riede UN, Förstermann U, Drexler H. Inducible nitric oxide synthase in skeletal muscle of patients with chronic heart failure. J Am Coll Cardiol. 1998;32:964–969. doi: 10.1016/s0735-1097(98)00335-0. [DOI] [PubMed] [Google Scholar]
- 59.Katz SD, Khan T, Zeballos GA, Mathew L, Potharlanka P, Knecht M, Whelan J. Decreased activity of the L-arginine-nitric oxide metabolic pathway in patients with congestive heart failure. Circ. 1999;99:2113–2117. doi: 10.1161/01.cir.99.16.2113. [DOI] [PubMed] [Google Scholar]
- 60.Clini E, Volterrani M, Pagani M, Bianchi L, Porta R, Gile LS, Giordano A, Ambrosino N. Endogenous nitric oxide in patients with chronic heart failure (CHF): relation to functional impairment and nitrate-containing therapies. Int J Cardiol. 2000;73:123–130. doi: 10.1016/s0167-5273(00)00211-4. [DOI] [PubMed] [Google Scholar]
- 61.Adachi H, Nguyen PH, Belardinelli R, Hunter D, Jung T, Wasserman K. Nitric oxide production during exercise in heart failure. Am Heart J. 1997;133:196–202. doi: 10.1016/s0002-8703(97)70124-8. [DOI] [PubMed] [Google Scholar]
- 62.Busotti M, Andreini D, Agostoni P. Exercise-induced changes in exhaled nitric oxide in heart failure. Eur J Heart Fail. 2004;6:551–554. doi: 10.1016/j.ejheart.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 63.Lovell SL, Stevenson H, Young IS, McDowell G, McEneaney D, Riley MS, Nicholls DP. Exhaled nitric oxide during incremental and constant workload exercise in chronic cardiac failure. Eur J Clin Invest. 2000;30:181–187. doi: 10.1046/j.1365-2362.2000.00613.x. [DOI] [PubMed] [Google Scholar]
- 64.Katz SD. The role of endothelium-derived vasoactive substances in the pathophysiology of exercise intolerance in patients with congestive heart failure. Prog Cardiovasc Dis. 1995;38:23–50. doi: 10.1016/s0033-0620(05)80012-x. [DOI] [PubMed] [Google Scholar]
- 65.Piknova B, Park JW, Swanson KM, Dey S, Noguchi CT, Schechter AN. Skeletal muscle as an endogenous nitrate reservoir. Nitric Oxide. 2015;47:10–16. doi: 10.1016/j.niox.2015.02.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Piknova B, Park JW, Kwan K, Schecter AN. Nitrate as a source of nitrite and nitric oxide during exercise hyperemia in rat skeletal muscle. Nitric Oxide. 2016;55–56:54–61. doi: 10.1016/j.niox.2016.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67•.Omar SA, Webb AJ, Lundberg JO, Weitzberg E. Therapeutic effects of inorganic nitrate and nitrite in cardiovascular and metabolic diseases. J Intern Med. 2016;279:315–326. doi: 10.1111/joim.12441. This comprehensive review summarizes recent animal and humans studies of the cardiovascular and metabolic effects of dietary nitrate. [DOI] [PubMed] [Google Scholar]
- 68•.Chirinos JA, Zamani P. The nitrate-nitrite-NO pathway and its implications for heart failure and preserved ejection fraction. Curr Heart Fail Rep. 2016;13:47–59. doi: 10.1007/s11897-016-0277-9. This recent review discusses the potential role of dietary nitrate as well as other compounds that target the nitrate-nitrite-NO pathway as a possible treatment for HF (particularly HF with preserved ejection fraction) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li H, Kundu TK, Zweier JL. Characterization of the magnitude and mechanism of aldehyde oxidase-mediated nitric oxide production from nitrite. J Biol Chem. 2009;284:33850–33858. doi: 10.1074/jbc.M109.019125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cantu-Medellin N, Kelley EE. Xanthine oxidoreductase-catalyzed reduction of nitrite to nitric oxide: insights regarding where, when and how. Nitric Oxide. 2013;34:19–26. doi: 10.1016/j.niox.2013.02.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71••.Coggan AR, Leibowitz JL, Anderson Spearie C, Kadkhodayan A, Thomas DP, Ramamurthy S, Mahmood K, Park S, Waller S, Farmer M, Peterson LR. Acute dietary nitrate intake improves muscle contractile function in patients with heart failure: a double-blind, placebo-controlled, randomized trial. Circ Heart Fail. 2015;8:914–920. doi: 10.1161/CIRCHEARTFAILURE.115.002141. This small clinical trial demonstrated significant improvements in maximal muscle speed and power in HF patients following acute ingestion of dietary nitrate in the form of a concentrated beetroot juice supplement. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72•.Coggan AR, Leibowitz JL, Kadkhodayan A, Thomas DT, Ramamurthy S, Anderson Spearie C, Waller S, Farmer M, Peterson LR. Effect of acute dietary nitrate intake on maximal knee extensor speed and power in healthy men and women. Nitric Oxide. 2015;48:16–21. doi: 10.1016/j.niox.2014.08.014. This was the first study to demonstrate that dietary nitrate enhances the contractile properties of human muscle during voluntary exercise. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Degache F, Garet M, Calmels P, Costes F, Barthélémy JC, Roche F. Enhancement of isokinetic muscle strength with a combined training programme in chronic heart failure. Clin Physiol Funct Imaging. 2007;25:225–230. doi: 10.1111/j.1475-097X.2007.00741.x. [DOI] [PubMed] [Google Scholar]
- 74.Delagardelle C, Feiereisen P, Autier P, Shita R, Krecke R, Beissel J. Strength/endurance training versus endurance training in congestive heart failure. Med Sci Sports Exerc. 2002;34:1868–1872. doi: 10.1097/00005768-200212000-00002. [DOI] [PubMed] [Google Scholar]
- 75.Metra M, Giubbini R, Nodari S, Boldi E, Modena MG, Dei Cas L. Differential effects of beta-blockers in patients with heart failure: A prospective, randomized, double-blind comparison of the long-term effects of metoprolol versus carvedilol. Circulation. 2000;102:546–551. doi: 10.1161/01.cir.102.5.546. [DOI] [PubMed] [Google Scholar]
- 76.Schellenbaum GD, Smith NL, Heckbert SR, Lumley T, Rea TD, Furberg CD, Lyles MF, Psaty BM. Weight loss, muscle strength, and angiotensin-converting enzyme inhibitors in older adults with congestive heart failure or hypertension. J Am Geriatr Soc. 2005;53:1996–2000. doi: 10.1111/j.1532-5415.2005.53568.x. [DOI] [PubMed] [Google Scholar]
- 77.Kinugawa T, Osaki S, Kato M, Ogino K, Shimoyama M, Tomikura Y, Igawa O, Hisatome I, Shigemasa C. Effects of the angiotensin-converting enzyme inhibitor alacepril on exercise capacity and neurohormonal factors in patients with mild-to-moderate heart failure. Clin Exp Pharmacol Physiol. 2002;29:1060–1065. doi: 10.1046/j.1440-1681.2002.03779.x. [DOI] [PubMed] [Google Scholar]
- 78.Edelmann F, Wachter R, Schmidt AG, Kraigher-Krainer E, Colantonio C, Kamke W, Duvinage A, Stahrenberg R, Durstewitz K, Loffler M, Dungen HD, Tschope C, Herrmann-Lingen C, Halle M, Hasenfuss G, Gelbrich G, Pieske B. Effect of spironolactone on diastolic function and exercise capacity in patients with heart failure with preserved ejection fraction: The Aldo-Dhf Randomized Controlled Trial. JAMA. 2013;309:781–791. doi: 10.1001/jama.2013.905. [DOI] [PubMed] [Google Scholar]
- 79.Harrington D, Chua TP, Coats AJ. The effect of salbutamol on skeletal muscle in chronic heart failure. Int J Cardiol. 2000;73:257–265. doi: 10.1016/s0167-5273(00)00233-3. [DOI] [PubMed] [Google Scholar]
- 80.Kerley CP, O’Neill JO, Bijjam R, Blaine C, James PE, Comican L. Dietary nitrate increased exercise tolerance in patients with non-ischemic, dilated cardiomyopathy – a double-blind, randomized, placebo-controlled, crossover trial. J Heart Lung Transplant. 2016 Jan 16; doi: 10.1016/j.healun.2016.01.018. pii: S1053-2498(16)00065-6. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 81.Zamani P, Rawat D, Shiva-Kumar P, Geraci S, Bhuva R, Konda P, Doulias P-T, Ischiropoulus H, Townsend RR, Margulies KB, Cappola TP, Poole DC, Chirinos JA. Effect of inorganic nitrate on exercise capacity in heart failure with preserved ejection fraction. Circulation. 2015;131:371–380. doi: 10.1161/CIRCULATIONAHA.114.012957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Borlaug BA, Koepp KE, Melenovsky V. Sodium nitrite improves exercise hemodynamics and ventricular performance in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2015;66:1672–1682. doi: 10.1016/j.jacc.2015.07.067. [DOI] [PubMed] [Google Scholar]
- 83.Eggebeen J, Kim-Shapiro DB, Haykowsky M, Morgan TM, Basu S, Brubaker P, Rejeski J, Kitzman DW. One week of daily dosing with beetroot juice improves submaximal endurance and blood pressure in older patients with heart failure and preserved ejection fraction. J Am Coll Cardiol HF. 2016 Feb 2; doi: 10.1016/j.jchf.2015.12.013. pii: S2213-1779(15)00835-5. Epub ahead of print. [DOI] [Google Scholar]
- 84.Hernández A, Schiffer TA, Ivarsson N, Cheng AJ, Bruton JD, Lundberg JO, Weitzberg E, Westerblad H. Dietary nitrate increases tetanic [Ca2+]I and contractile force in mouse fast-twitch muscle. J Physiol. 2012;590:3575–3583. doi: 10.1113/jphysiol.2012.232777. [DOI] [PMC free article] [PubMed] [Google Scholar]