Abstract
Background
One of the key recommendations of the Second Panel on Cost-Effectiveness in Health and Medicine is to take a societal perspective when evaluating new technologies—including measuring the productivity benefits of new treatments. Yet relatively little is known about the impact that new treatments have on labor productivity.
Objectives
To examine the relationship between new drug treatments and gains in labor productivity across conditions in the US, and to evaluate which randomized clinical trials (RCTs) collect productivity data.
Methods
We collect data on US-based RCTs with work-ability surveys from searches of Google Scholar, PubMed, Scopus, the Cochrane Central Registry of Clinical Trials, and ClinicalTrails.gov. Combining RCT data with survey data from the Medical Expenditure Panel Survey, we assess productivity changes from new drug treatments.
Results
During the last decade, some disease conditions have seen treatments that improve ability-to-work by as much as 60%. The annual increase in productivity gains attributable to new drug treatments was modest 1.1% (p = 0.53). Of the 5,092 RCTs reviewed, ability-to-work measures were collected in 2% of them. Work productivity surveys are more likely among more prevalent medical conditions that tend to affect individuals who work, earn higher wages, and experience larger reductions in hours worked as a consequence of disease diagnosis.
Conclusions
From our data, we estimate that drug innovation increased productivity by 5.5 million work days per year and $233 billion in wages per year. These labor-sector benefits should be accounted for when assessing the socially optimal cost for new drug innovation.
Keywords: labor productivity, drug value, randomized clinical trials, work ability
Introduction
One of the key recommendations of the Second Panel on Cost-Effectiveness in Health and Medicine is to take a societal perspective when evaluating new technologies.1 When considering the resource costs associated with the use of health care interventions, one should account for societal benefits from increased productivity, a dimension that is not traditionally captured by preference- or health-based measures. This societal perspective is important given that medical innovation is a global public good, and efficiently managing resource both across- and within-countries relies on a complete understanding of the health and non-health welfare impacts.
In the US, non-health considerations are particularly salient since most Americans obtain their health insurance through their employers. In 2015, employers covered on average 72 to 83% of average annual premiums, which totaled $6,251 for single coverage and $17,545 for family coverage.2 Despite the significant subsidies that employers provide, little is known about the impact that medical treatments have on labor productivity. This issue is particularly salient for employees, who often take prescription drugs for primary or secondary prevention, with the goal of maintaining good function.
US-based estimates of the productivity losses due to poor health are large. In 2003, 885 million days were lost due to own- or family-related illnesses that prevented employees from concentrating at work or coming into work.3 An additional 18 million adults ages 19 to 64 remained unemployed due to health reasons. Both workers and firms bear the burden of these health costs: individuals experience the impaired or lost ability to work, and firms face the costs of rehiring and retraining replaced workers, which can include higher wages, lost revenues, and idle assets.4,5 Estimates of health-related productivity losses sum to around $226 to $260 billion every year.3,6,7
However, while the burden is large, it is less clear whether new treatments can alleviate it. Gains in labor productivity are often overlooked when assessing returns to medical innovation. Cost effectiveness studies, especially those on pharmaceuticals, have focused on gains in short- and long-term survival, quality of life, disease progression, consumer surplus, and total health spending.8–13 The few studies that do consider labor productivity gains tend to focus on particular conditions. For example, Thirumurthy et al. (2008) focus on anti-retroviral medication, Berndt et al. (1998, 2000) and Timbie et al. (2006) consider mental health medications, and Garthwaite (2010) examines antiarthritic medication.14–18 Overall, we lack clear, unified evidence on the extent to which medical innovations have improved on-the-job productivity or reduced employee absences.19
In this study, we identify the relationship between new drug treatments and labor productivity across several disease groups. Using evidence from randomized clinical trials (RCTs), we assess when ability-to-work measures are collected and determine how those measures have changed over time.
Methods
Data Sources
Our main data source is a systematic collection of work productivity data from RCTs. Following the literature, we identified 26 instruments that measured the effects of ill health on productivity because of absence from work or reduced performance while at work (Appendix Table 1). Twenty of the listed surveys have been identified in independent, systematic reviews on health-related productivity loss.20–21 Six additional surveys, which have been extensively validated among specific disease groups, included the Life Functioning Questionnaire for psychiatric illness; Occupational Role Questionnaire and Quality and Quantity Method in Productivity for back pain; Work Productivity Survey for rheumatoid arthritis; Work Role Functioning Questionnaire and Workstyle Scale for pain at work.22–25
Using each of these instruments as search terms, we conducted a search through Google Scholar (additionally including “randomized trial” in the search term), PubMed (focusing exclusively on “clinical trial” article types), Scopus (additionally including “randomized trial” in the abstracts), the Cochrane Central Registry of Clinical Trials, and ClinicalTrials.gov. Our inclusion criteria were RCTs among adults in the US between 2000 and 2015 that included measures on work impairment, productivity, presentism, or absenteeism from one of the identified survey instruments. We further restricted included studies to those with either pre-trial ability to work baseline measures or changes in ability to work reported as a percent change.
The last inclusion criterion is important because work productivity surveys use differing scale ranges and directions to measure labor productivity. For example, the Endicott Work Productivity Scale assigns overall scores out of 100, whereas the Work Limitations Questionnaire index ranges from 0 to 28.6. The Work Productivity and Activity Impairment Questionnaire scores have higher numbers corresponding to worsening productivity, whereas the Short-Form Health and Labor Questionnaire defines higher values as corresponding to improvements productivity. By calculating percent changes where positive values reflect improvements in work productivity, we take into account coding idiosyncrasies across surveys. Each survey measures productivity from the same basic definitions of perceived impairment, comparative efficiency with others norm, unproductive time while at work, and absences from work.21 The overall improvement due to a new drug is then calculated as the difference in percent changes between the control and treatment groups. To reduce bias, two researchers independently collected the final data that is analyzed (Appendix Figure 1).
Next, to identify when labor productivity surveys are administered, we rely on a broader search of both published and unpublished trials from ClinicalTrials.gov (Appendix Figure 2). The website, established by the Food and Drug Administration Modernization Act of 1997 and made public in 2000, contains a registry of clinical trials for both federally and privately funded trials conducted under investigational new drug applications from 2000 onward. We again focus on US-based, completed drug-related clinical trials, in phase 3 or 4, with randomized interventions between 2000 and 2015, treatment listed as the primary purpose, and adults being treated. Data variables include drug name, disease condition, trial funding source, enrollment size, gender distribution, and type of randomization (e.g., single or double blind). We constructed an indicator equal to one if the RCT administered a work productivity survey, defined as including any of the 26 work instruments or the term “work productivity” in the trial entry. We also used the “condition” variable to sort the RCTs into one of 14 disease groups: infectious and parasitic diseases, neoplasms, metabolic diseases, diseases of blood organs and the circulatory system, mental disorders, diseases of the nervous system, diseases of the sense organs, diseases of the respiratory system, diseases of the digestive system, diseases of the genitourinary system, complications of pregnancy, diseases of the skin, diseases of the musculoskeletal system, and injuries (Appendix Table 2).
Finally, we utilized survey data from the Medical Expenditure Panel Survey (MEPS). The MEPS data from 2000 to 2015 are nationally representative and the most complete source of data on the cost and use of health care. Importantly, the MEPS provides information on a respondent’s work, including employment status and self-reported wages, which we convert to $2015 dollars using the Consumer Price Index. It also offers details regarding any office, inpatient, outpatient, or emergency room visit that the respondent had within the year, and the ICD-9 diagnosis code associated with each visit. We limit this sample to adults ages 18 to 64 and used the ICD-9 codes to again group individuals into the 14 aforementioned disease groups (Appendix Table 2). For each disease group, we calculated the prevalence of disease, the propensity to work conditional on having a disease, and average wages conditional on having a disease and working. Utilizing the two-year panel design of the MEPS survey, we also calculate the annual per-person change in hours worked among those who are newly diagnosed with a disease (i.e., individuals who do not have the disease diagnosis in the first year and receive it the following year). The change in hours serves as a proxy for diseases where the potential gain in labor productivity is high.
Statistical Analyses
We relied on two types of regression models: linear and logit. Our main analysis on labor productivity gains utilized a linear regression to estimate the trajectory of productivity improvements over time. Next, we considered whether the collection of work productivity information in RCTs was biased. We focused on two sets of potential predictors: RCT-specific and disease-group characteristics. When assessing the predictive power of RCT-specific characteristics, we relied on logit regression models. The logit models included disease group fixed effects to account for variation in disease-specific drug development, and year fixed effects to control for trends in work productivity over time. Utilizing variation within disease groups over time, we determined whether characteristics—such as enrollment size, trial phase, funding source, participant demographics, and trial design—are predictive the RCT having administered a work instrument. For the correlation between administering work productivity surveys and disease-group characteristics, we utilized linear models. We estimated how the probability of tracking work productivity correlates with disease prevalence, employment probability among those with a given disease, and average wages among working individuals with a given disease.
Results
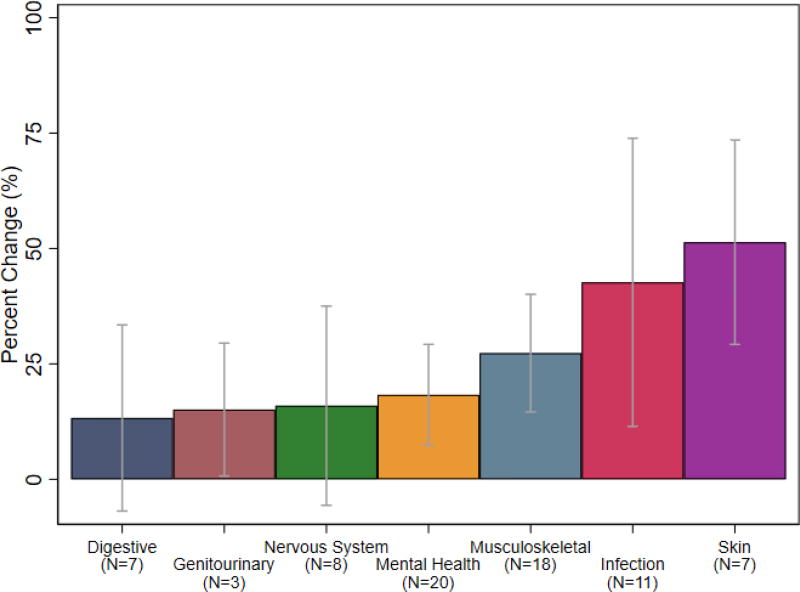
From the systematic data collection, we find that new drug treatments introduced significant gains in work productivity (N = 78). We classify the trials into disease groups and identify the average changes in work productivity (Figure 1). The most common diseases with data on labor productivity data were mental health diagnoses—including major depressive disorder and general anxiety disorder—and musculoskeletal conditions, including arthritis and fibromyalgia. These categories experienced average gains of about 18% to 27%, respectively. The smallest gains in work productivity were among drugs for digestive or gastrointestinal diseases (with an average 13% gain), such as Crohn’s and irritable bowel syndrome, and genitourinary diseases (with an average 15% gain), such as overactive bladders. Larger gains were achieved among infectious diseases (with an average 42.6% gain) and skin diseases (with an average 82.4% gain). In our data, Simeprevir, a drug used to treat chronic hepatitis C, had the largest returns to labor productivity: in addition to its well-known health benefits, Simeprevir improved presenteeism by 142% and overall work productivity by 167%.26 Since chronic hepatitis C has been shown to reduce on-the-job work output and increase work days lost to sickness, affected individuals with access to Simeprevir can experience significant improvements in both health and labor.27
Figure 1. Percent change in work productivity by disease group.
Notes: Data from a systematic literature search. Each bar show shows the average percent change in work productivity, with 95% confidence intervals calculated from the standard error of the mean across studies within the disease category. We omit categories with only one study (i.e., neoplasm and respiratory).
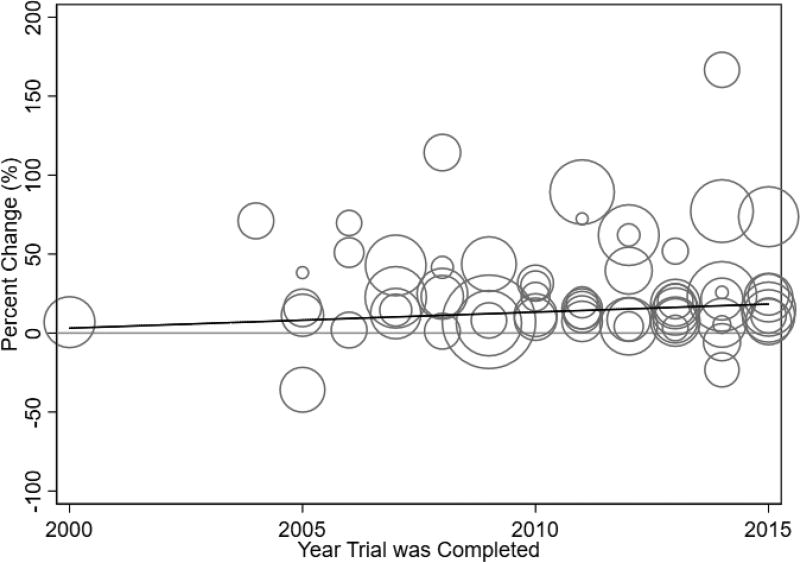
We also consider the overall change in work productivity over time (Figure 2). The universe of our labor productivity data is plotted, with each circle representing the difference-in-difference change in work productivity from a separate RCT and the size of the bubble corresponding to the number of participants in the trial. Almost all drug treatments have improved ability to work, highlighting the productivity gains that have been achieved with effective medical care. The overall trend in work productivity improvements remains relatively flat with a slope of 1.1% (p=0.53). This analysis illustrates that on average, new treatments generated a 30% increase in work productivity, and subsequent innovations have maintained, if not slightly increased, this level of improvement.
Figure 2. Percent Change in work productivity over time.
Notes: Data from a systematic literature search. Each trial with work productivity data is represented by a circle, and the bubble size corresponds to the number of participants in the trial. The line is a fitted regression with diagnosis group fixed effects and slope 1.01 (p-value = 0.53).
Next, we turn to the ClinicalTrials.gov database to glean whether there are systematic differences between trials with and without labor productivity information. We collected information on 5,092 clinical trials, of which 115 had administered a work productivity survey. RCTs with work productivity information tend to have larger log enrollment (5.8 relative to 5.3), more industry funding (79% relative to 68%), and more stringent masking through double blind set-ups (67% relative to 52%), as opposed to single blind set-ups or open labels (Table 1). Only about 21% of RCTs without work data and 30% of trials with work instruments reported trial outcomes. The summary means suggest that there are significant differences across trials with and without work productivity information, but many differences do not persist when we account for the predictive power of these characteristics simultaneously (Table 2). The logit regressions indicate that the collection of work information is correlated with only enrollment size and trial phase (with odds ratios of 0.23 and 0.46, respectively). The remaining RCT characteristics are not predictive of the collection of work information, and controlling for time trends and disease-specific characteristics do not appreciably change the results.
Table 1.
Summary Statistics
| No Work Info | Work Info | P-Value (1)≠(3) (5) |
|||
|---|---|---|---|---|---|
|
|
|||||
| Mean (1) |
SD (2) |
Mean (3) |
SD (4) |
||
| Log(Enrollment) | 5.362 | 1.399 | 5.813 | 1.315 | 0.0006** |
| Trial Phase | 3.349 | 0.476 | 3.330 | 0.472 | 0.673 |
| 1(Industry Funded) | 0.678 | 0.467 | 0.791 | 0.408 | 0.01** |
| 1(Only Male) | 0.033 | 0.180 | 0.043 | 0.205 | 0.551 |
| 1(Has Results) | 0.303 | 0.5 | 0.208 | 0.497 | 0.067* |
| 1(Single-Blind) | 0.363 | 0.483 | 0.248 | 0.437 | 0.029** |
| 1(Double-Blind) | 0.520 | 0.499 | 0.670 | 0.472 | 0.002** |
| Observations | 4,977 | 115 | |||
Notes:
Data from ClinicalTrials.gov. We conduct a two-sided t-test that verifies whether Columns (1) and (3) are different, with p-values shown in column (5), and significantly different at the
5% level or
1% level.
Table 2.
Predictors of Labor Productivity Information among Drug Trials
| All Trials | |||
|---|---|---|---|
|
|
|||
| (1) | (2) | (3) | |
| Log(Enrollment) | 0.228*** (0.0862) | 0.242*** (0.0881) | 0.214** (0.0924) |
| Average Phase | 0.456* (0.236) | 0.465* (0.238) | 0.520** (0.245) |
| 1(Industry Funded) | 0.421 (0.274) | 0.386 (0.277) | 0.323 (0.293) |
| 1(Only Male) | 0.234 (0.466) | 0.239 (0.467) | −0.0693 (0.504) |
| 1(Has Results) | 0.217 (0.200) | 0.134 (0.220) | 0.138 (0.225) |
| 1(Single Blind) | 0.0364 (0.352) | −0.0593 (0.356) | −0.134 (0.370) |
| 1(Double Blind) | 0.584* (0.304) | 0.451 (0.312) | 0.288 (0.322) |
| Year FE | X | X | |
| Disease FE | X | ||
| Observations | 5,189 | 5,101 | 4,757 |
| Dep Var Mean | 0.0226 | 0.0226 | 0.0226 |
Notes:
Data from ClinicalTrials.gov. Logistic regressions with odds ratios reported. Standard errors in parentheses;
5% or
1% significance level. Columns (1) through (3) progressively control for year (15) and disease category (20) fixed effects (FE).
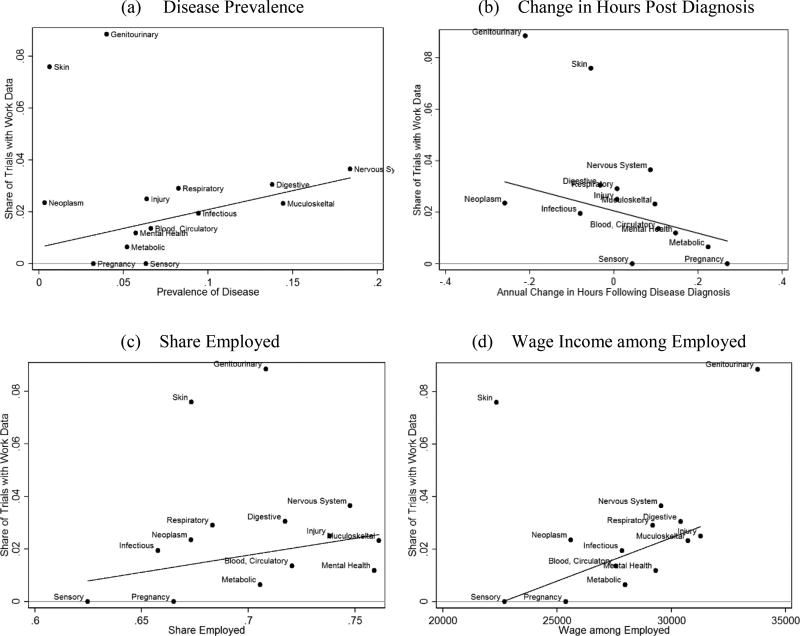
Considering disease-group characteristics, we utilize the MEPS data, which followed 326,596 adults across the 16-year data period. On average, 47% in our sample had at least one doctor visit during the year. We collapsed the MEPS data into disease groups and matched it, by disease group, to the ClinicalTrials.gov RCT data. While work productivity information was most frequently gathered for drugs pertaining to skin and genitourinary conditions, those groups are clear outliers (Figure 3). Excluding them, there is a statistically significant relationship between the probability of reporting work information and the prevalence of the disease (slope of 0.15, p-value 0.03), the change in hours worked following a new disease diagnosis (slope of −0.04, p-value 0.09), share of disease-impacted people who are employed (slope of 0.13, p-value 0.12), and average wages among the disease-impacted employed (slope 3.30e-6, p-value 0.01). Those who are working with medical conditions span all industries and occupations, with approximately 53%, 24% and 22% in white-collar, blue-collar and other (farming, service or military) jobs, respectively. The results suggest that new drugs are more likely to measure work productivity gains when the drug targets more prevalent diseases among working individuals earning high wages. It also suggests that the incorporation of labor market impacts into RCTs is more likely among diseases where the per-person loss in hours worked is higher. We note that the change in work hours reflects several factors, including reduced physical ability but concurrent need for increased income to access treatments.
Figure 3. Availability of work productivity information by disease group.
Notes: Data from ClinicalTrials.gov and MEPS. We consider relevant ICD-9 classifications among office, outpatient, emergency room, and inpatient settings, among adults ages 18 to 65. Skin and genitourinary diseases are clear outliers. We fit a linear line, excluding those two points. The slope and p-values for the fitted regression lines are: 0.15 (p-value = 0.03) for plot (a), −0.4 (p-value = 0.09) for plot (b), 0.13 (p-value = 0.12) for plot (c), 3.30e-6 (p-value = 0.01) for plot (d).
Discussion
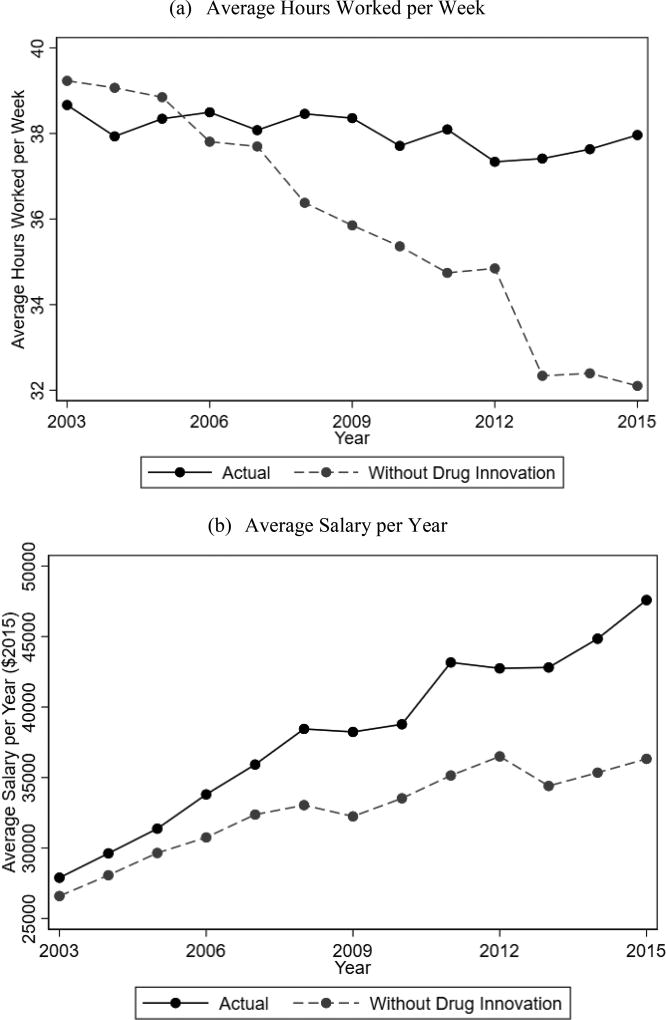
We examined over 5,000 clinical trials over a 15-year period but could only identify 115 (2%) that evaluated treatment effects on labor productivity. However, some interesting insights did emerge. Importantly, we found that among RCTs with work productivity data, new drug developments have introduced large gains in work ability, and the gain in labor productivity remains consistently large over time. To understand the cumulative impact of new drug treatments, we used the MEPS to calculate the counterfactual hours worked per week and annual wages if the relevant population did not benefit from any of these new drug developments (Figure 5). Specifically, for each trial for which we have labor productivity information, we identified the 3-digit ICD-9 code of the disease for which the RCT targeted. For individuals affected by those 3-digit ICD-9 codes, we calculate the work hours and wages from 2003 to 2015 had the cumulative gains in disease-specific productivity not been introduced (N=26,742). For unaffected individuals (N=173,806), the counterfactual hours and wages equal the actual reported values. Respondents reported that average hours worked per week did not change from 2003 to 2015, and the average wage increased from $26,592 to $44,473. However, if the respondents had not benefited from new drugs developments, the counterfactual hours worked would have fallen from 39.23 hours to 32.10 hours, and the group would have experienced a stunted wage growth from $26,592 in 2013 to only $36,323 in 2015. These differences are magnified when accounting for disease prevalence. With about 203.9 million working adults in 2016, 13.33% of whom are affected by a disease for which we have documented drug-related improvements in ability-to-work, we find a total gain of approximately $210 billion in annual wages and 4.5 million work days (assuming 40-hours worked per week).
These implied gains in productivity are large, though we recognize that our analysis is limited to labor productivity changes as measured by RCTs. As shown, the collection of productivity data in the RCTs is not completely idiosyncratic; ability-to-work improvements are more often measured in larger trials considering drug treatments that impact workers earning higher wages. Moreover, while RCTs are often considered the gold-standard for comparative effectiveness research, real-world magnitudes may differ. There are several reasons why RCTs may overstate real-world returns in work productivity.28 The control group in RCTs often receive placebo treatments, as opposed to the next best alternative, so changes in labor productivity will appear larger in RCTs. Moreover, patients in RCTs tend to be healthier than those who ultimately receive treatment, and drug-adherence is usually higher. More comorbidities and poorer medication adherence will dampen actual returns. However, arguments can also be made in favor of real-world returns being higher than those measured in the RCTs. Although RCTs tend to focus on younger populations, older adults tend to earn more, so real-world wage increases are potentially larger. Alternatively, patients who experience the biggest gains in work productivity may be those with blue-collar jobs requiring manual labor, and the underrepresentation of socioeconomically disadvantaged groups in RCTs may understate work gains. Finally, the average trial length in our data is approximately 2.5 years, whereas true treatment effects, particularly for chronic conditions, can continue to produce benefits years after treatment is initiated. In light of these various factors, it is difficult to discern how magnitudes in the real-world compare to RCTs.
Conclusions
We have used RCTs to study gains in labor productivity from new drug developments. Although RCTs offer the benefit of randomization, allowing us to recover causal estimates between new drug treatments and changes in labor productivity, we recognize that RCTs are not without limitations. RCTs with labor productivity information disproportionately reflect diseases that affect individuals who are employed and earn higher wages. Despite these limitations, RCTs have demonstrated that labor productivity can be dramatically improved with the quality of health care and medical research. Our results indicate the increases in labor productivity due to new drug innovation is large, and a solitary focus on health benefits will miss important labor gains for the working age populations. With continually rising drug costs, it is paramount to take a societal perspective that includes labor productivity to better estimate the returns to innovation.
Supplementary Material
Figure 4. Impact of Drug Innovation Counterfactual.
Notes: Data from MEPS and a systematic literature search. Plot (a) shows the actual average hours worked per week (solid) and the counterfactual hours worked without any of the drug-induced work productivity changes (dashed). Plot (b) shows a similar plot focused on the average salary per year in $2015 dollars.
Acknowledgments
We thank Alicia Gonzalez for research assistance.
Support: Research reported in this publication was supported by the Leonard D. Schaeffer Center for Health Policy and Economics and the National Institute On Aging of the National Institutes of Health under Awards Numbered P30AG024968 and P01AG033559. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Neumann PJ, Sanders GD, Russell LB, Siegel JE, Ganiats TG. Cost-effectiveness in health and medicine. 2. New York: Oxford University Press; 2016. [Google Scholar]
- 2.Claxton G, Rae M, Panchal N, et al. Health benefits in 2015: Stable trends in the employer market. Health Affairs. 2015;34(10):1779–88. doi: 10.1377/hlthaff.2015.0885. [DOI] [PubMed] [Google Scholar]
- 3.Mattke S, Balakrishnan A, Bergamo G, Newberry SJ. A review of methods to measure health-related productivity loss. American Journal of Managed Care. 2007;13(4):211–7. [PubMed] [Google Scholar]
- 4.Pauly MV, Nicholson S, Polsky D, Berger ML, Sharda C. Valuing reductions in on-the-job illness: ‘presenteeism’ from managerial and economic perspectives. Health Economics. 2008;17(4):469–85. doi: 10.1002/hec.1266. [DOI] [PubMed] [Google Scholar]
- 5.Strömberg C, Aboagye E, Hagberg J, Bergström G, Lohela-Karlsson M. Estimating the effect and economic impact of absenteeism, presenteeism, and work environment-related problems on reductions in productivity from a managerial perspective. Value in Health. 2017;20(8):1058–1064. doi: 10.1016/j.jval.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 6.Davis K, Collins SR, Doty MM, Ho A, Holmgren A. Health and productivity among U.S. workers. The Commonwealth Fund Issue Brief. 2005;856 [PubMed] [Google Scholar]
- 7.Stewart WF, Ricci JA, Chee E, Morganstein D. Lost productive work time costs from health conditions in the United States: Results from the American productivity audit. Journal of Occupational and Environmental Medicine. 2003;45(12):1234–46. doi: 10.1097/01.jom.0000099999.27348.78. [DOI] [PubMed] [Google Scholar]
- 8.Chandra A, Jena AB, Skinner JS. The pragmatist’s guide to comparative effectiveness research. Journal of Economic Perspectives. 2011;25(2):27–46. doi: 10.1257/jep.25.2.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cutler DM. The lifetime costs and benefits of medical technology. Journal of Health Economics. 2007;23(6):1081–1100. doi: 10.1016/j.jhealeco.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 10.Grosse SD, Teutsch SM, Haddix AC. Lessons from cost-effectiveness research of United States public health policy. Annual Review of Public Health. 2007;28:365–91. doi: 10.1146/annurev.publhealth.28.021406.144046. [DOI] [PubMed] [Google Scholar]
- 11.Jena AB, Philipson TJ. Cost-effectiveness analysis and innovation. Journal of Health Economics. 2008;27(5):1224–36. doi: 10.1016/j.jhealeco.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 12.Lichtenberg F. Do (more and better) drugs keep people out of hospitals? American Economic Review Papers and Proceedings. 1996;86:384–8. [PubMed] [Google Scholar]
- 13.Murphy KM, Topel RH. The value of health and longevity. Journal of Political Economy. 2006;114(5):871–904. [Google Scholar]
- 14.Thirumurthy H, Graff Zivin J, Goldstein M. The economic impact of AIDS treatment: Labor supply in Western Kenya. Journal of Human Resources. 2008;43:511–552. [PMC free article] [PubMed] [Google Scholar]
- 15.Berndt E, Bailit H, Keller M, Verner J, Finkelstein S. Health care use and at-work productivity among employees with mental disorders. Health Affairs. 2000;19:244–256. doi: 10.1377/hlthaff.19.4.244. [DOI] [PubMed] [Google Scholar]
- 16.Berndt E, Finkelstein S, Greenberg P, et al. Workplace performance effects from chronic depression and its treatment. Journal of Health Economics. 1998;17:511–535. doi: 10.1016/s0167-6296(97)00043-x. [DOI] [PubMed] [Google Scholar]
- 17.Timbie JW, Horvitz-Lennon M, Frank RG, Normand SL. A meta-analysis of labor supply effects of interventions for major depressive disorder. Psychiatric Services. 2006;57:212–8. doi: 10.1176/appi.ps.57.2.212. [DOI] [PubMed] [Google Scholar]
- 18.Garthwaite C. The economic benefits of pharmaceutical innovations: The case of Cox-2 inhibitors. American Economic Journal: Applied Economics. 2012;4(3):116–37. [Google Scholar]
- 19.Garthwaite C, Duggan M. Empirical evidence on the value of pharmaceuticals. In: Danzon PM, Nicholson S, editors. The Oxford Handbook of the Economics of the Biopharmaceutical Industry. New York: Oxford University Press; 2012. [Google Scholar]
- 20.Despiégel N, Danchenko N, François C, Lensberg B, Drummond MF. The use and performance of productivity scales to evaluate presenteeism in mood disorders. Value in Health. 2012;15(8):1148–61. doi: 10.1016/j.jval.2012.08.2206. [DOI] [PubMed] [Google Scholar]
- 21.Mattke S, Balakrishnan A, Bergamo G, Newberry S. A review of methods to measure health-related productivity loss. The American Journal of Managed Care. 2007;13(4):211–7. [PubMed] [Google Scholar]
- 22.Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. Pharmacoeconomics. 1993;4:353–365. doi: 10.2165/00019053-199304050-00006. [DOI] [PubMed] [Google Scholar]
- 23.Reilly MC, Bracco A, Ricci J, Santoro J, Stevens T. The validity and accuracy of the Work Productivity and Activity Impairment questionnaire -Irritable bowel syndrome version (WPAI:IBS) Alimentary Pharmacology and Therapeutics. 2004;20:459–467. doi: 10.1111/j.1365-2036.2004.02091.x. [DOI] [PubMed] [Google Scholar]
- 24.Reilly MC, Gooch KL, Wong RL, Kupper H, van der Heijde D. Validity, reliability and responsiveness of the Work Productivity and Activity Impairment Questionnaire in ankylosing spondylitis. Rheumatology. 2010;49:812–819. doi: 10.1093/rheumatology/kep457. [DOI] [PubMed] [Google Scholar]
- 25.Reilly MC, Gerlier L, Brabant Y, Brown M. Validity, reliability, and responsiveness of the work productivity and activity impairment questionnaire in Crohn’s disease. Clin Ther. 2008;30:393–404. doi: 10.1016/j.clinthera.2008.02.016. [DOI] [PubMed] [Google Scholar]
- 26.Forns X, Lawitz E, Zeuzem S, et al. Simeprevir with Peginterferon and Ribavirin leads to high rates of SVR in patients with HCV Genotype 1 who relapsed after previous therapy: A phase 3 trial. Gastroenterology. 2014;146(7):166–1679. doi: 10.1053/j.gastro.2014.02.051. [DOI] [PubMed] [Google Scholar]
- 27.Su J, Brook RA, Kleinman NL, Corey-Lisle P. The impact of hepatitis C virus infection on work absence, productivity and healthcare benefit cost. Hepatology. 2010;52(2):436–42. doi: 10.1002/hep.23726. [DOI] [PubMed] [Google Scholar]
- 28.Bothwell LE, Greene JA, Podolsky SH, Jones DS. Assessing the gold standard-- Lessons from the history of RCTs. N Engl J Med. 2016;374:2175–2181. doi: 10.1056/NEJMms1604593. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.