SYNOPSIS
Molecular imaging using FES and FFNP PET can assess in vivo function of steroid hormone receptors in breast cancer. These experimental agents have been tested in many single center clinical trials and show promise to elucidate prognosis and predict endocrine therapy response. The current multicenter trial of FES PET imaging will help bring this radiotracer closer to clinical use. There is tremendous potential for these tracers to advance drug development, enhance understanding of ER+ tumor biology and personalize treatment.
Keywords: FES-PET, FFNP-PET, Estrogen Receptor imaging, Progesterone Receptor imaging, breast cancer
Introduction
The majority of breast cancers diagnosed are estrogen receptor (ER) and progesterone receptor (PR) positive and early outcomes are better across all stages compared to ER-PR- disease (1). Advances in endocrine therapy, show improved outcomes, for patients with ER+ tumors (2–7). Interestingly, despite the promise of molecularly targeted therapies, ER expression is the only established biomarker of response to cyclin dependent kinase 4/6 inhibitors (8). It is not uncommon for patients with ER+ tumors to have late recurrences or indolent disease several years after diagnosis, or present with de novo metastatic disease (9, 10). The most common site of metastases is the bone, where biopsies may be challenging to obtain and response is difficult to measure by current clinical imaging (11–13).
Positron emission tomography (PET) with 18F-radiolabeled steroid hormones can be used to image tumoral expression of ER and PR in patients with primary and metastatic breast cancer. These radiotracers include 16α-[18F]fluoro-17β-estradiol (FES) for ER imaging and 18F-fluoro-furanyl-norprogesterone (FFNP) for PR imaging.
Tumor uptake from FES-PET imaging has been proven to correlate with ER receptor protein (14, 15) demonstrating the functional presence of ER. Likewise, FFNP uptake as measured by tumor-to-normal breast tissue ratios has been shown to be greater in PR+ breast cancers compared to PR- cancers (16). FES and FFNP PET can be used to both qualitatively and quantitatively measure uptake from all metastatic sites at the same time through whole-body scanning. These molecular imaging agents may also be used in conjunction with dedicated high resolution PET systems for breast imaging or simultaneous breast PET/magnetic resonance imaging (MRI) to better depict primary ER+ tumor biology and assess changes in receptor expression through preoperative window of opportunity drug trials.
As new endocrine therapies and novel biologics are introduced into clinic practices, better strategies are needed to individualize therapy. In this article, the authors review the research of the most commonly used PET tracer for ER, 16α-[18F]fluoro- 17β-estradiol (FES) and 18F-fluoro-furanyl-forprogesterone (FFNP) for PR imaging and their potential use in a clinical setting. For FES, the past studies include use to predict response to endocrine therapy, as a biomarker in pharmacodynamics imaging, as means to help develop ER antagonist therapy, and to identify tumor heterogeneity. Several current and upcoming trials will be discussed for their potential to contribute to the promising future of using estrogen and progesterone receptor imaging in the clinical setting.
FES predicts response to endocrine therapy
Perhaps the most impactful use of FES-PET imaging in the clinic will be the ability to predict the effectiveness of endocrine therapy. Approximately 30% of breast cancer patients develop tumor regression with initial endocrine therapy and only 20% have stable disease (17). Second- and third- line therapies are available, however, and the ability to measure ER expression in all tumor sites can ultimately influence treatment decisions, especially if there is substantial heterogeneity between tumor sites. Several groups have shown that FES predicts whether patients will experience clinical benefit with ER-directed therapies in first line and later line treatment (18–21). Clinical benefit, defined as stable disease, partial response, or complete response, is clinically meaningful, and a logical endpoint in ER+ breast cancer, where indolent disease does not usually show measurable tumor shrinkage. While these data, from single centers, is convincing, and clinically relevant, a multi-center trial is ongoing to confirm these single center results.
Another study tested the ability of FES-PET to predict response to salvage aromatase inhibitor treatment in 47 heavily pre-treated metastatic breast cancer patients with ER+ primary tumors. Treatment selection based on quantitative FES- PET and a maximum standardized uptake value (SUV) cutoff of 1.5 would have increased the rate of response from 23% to 34% overall, and from 29% to 46% in the subset of HER2-negative patients (20). This study found that quantitative FES- PET provides additional information over conventional clinical criteria to predict response to hormonal therapy in the challenging situation of patients who have received multiple prior lines of therapy.
FES and FDG PET provide prognostic information
Like ER expression measured using immunohistochemistry, FES PET imaging has the potential to provide prognostic information. Kurland et al evaluated progression-free survival (PFS) in 84 ER+HER2- breast cancer patients receiving endocrine therapy and lesion uptake from FDG and FES PET. Patients were stratified into three response groups: patients with low FDG uptake (suggesting indolent disease) (29%;24/84), FDG-avid tumors with high FES uptake (59%;50/84), and FDG-avid tumors with low FES uptake (12%;10/84). Median PFS in these groups was 26.1 months (95%CI:11.2–49.7 months), 7.9 months (95%CI:5.6–11.8), and 3.3 months (95%CI:1.4-not evaluable), respectively. Figure 1 shows images of representative cases from three classification groups: A) low FDG SULmax3 B) High FDG SULmax3 and low FES SULmean3, and C) High FDG SULmax3 and high FES SULmean3. This study suggested that FDG PET and FES PET may help provide important prognostic information by identifying patients with indolent disease, and aid in deciding when to select targeted and/or cytotoxic chemotherapy (7).
Figure 1.
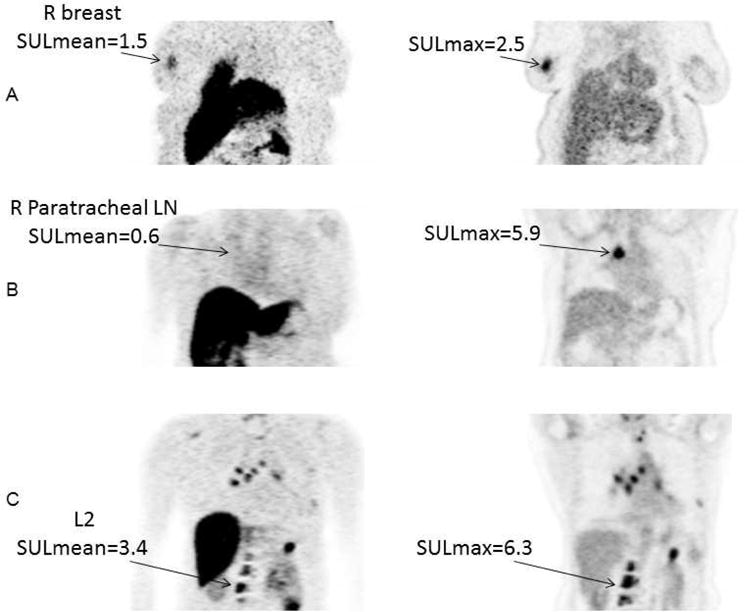
Representative cases for the three classification groups. Coronal view from FES PET and FDG PET scans. Ordered subset expectation maximization (OSEM) reconstruction was used for improved presentation, but not for quantitation. A, Low FDG SULmax3. This 56—year—old woman had 4 lesions in her breast and lymph nodes (LN). Geometric mean FDG SULmax for the 3 hottest lesions was 1.5 (geometric mean FES SULmean was 1.1). She was on anastrozole for 5 months until progression. R, Right. B, High FDG SULmax3 and low FES SULmean3. This 59—year—old woman had 5 lesions in lymph nodes and spine. Geometric mean FDG SULmax for the 3 hottest lesions was 4.4. Geometric mean FES SULmean3 was 0.3. She was on tamoxifen for 3 months until progression. C, High FDG SULmax3 and high FES SULmean3. This 59—year—old woman had lesions in the breast, chest wall, and hilar nodes, as well as multiple bony lesions. Geometric mean FDG SULmax for the 3 hottest lesions was 12.7. Geometric mean FES SULmean was 6.6. She was on exemestane for 9.5 months until progression.
From Kurland BF, Peterson LM, Lee JH, et al. Estrogen Receptor Binding (18F-FES PET) and Glycolytic Activity (18F-FDG PET) Predict Progression-Free Survival on Endocrine Therapy in Patients with ER+ Breast Cancer. Clin Cancer Res. 2017;23:407–415; with permission.
ER as a biomarker in pharmacodynamic imaging
PET imaging also has a unique role in pharmacodynamics, the study of biochemical and physiological effects of a drug on the body. Serial imaging of ER can provide a non-invasive assessment of the effective dosing and delivery of a drug at early time points. In recent studies, FES-PET imaging has been shown to be a pharmacodynamic biomarker for ER-directed therapy. In a study by Lin, et al (22), 15 patients with refractory ER+ metastatic disease were imaged with FES-PET at baseline and 8 of those patients were imaged again 1–5 days after administration of Z-endoxifen (a metabolite of tamoxifen). Decreases in SUVmax were observed (p=0.0078) as early as 1 day after the drug was administered. Earlier studies, such as the study of metabolic flare as an indicator of hormone responsiveness, (18) concluded that the functional status of tumor ER could be predictive of tamoxifen therapy 7–10 days after start of treatment in patients with advanced ER+ breast cancer. In a study by Linden, et al imaging revealed significant differences between agents, including differences in the efficacy of blockade by different ER antagonists. Figure 2 shows images from that study (23). In the clinical setting the use of FES- PET could be used to test ER blockade, both quantitatively and qualitatively, as an indicator that the drug is reaching the correct target. In addition, pharmacodynamic imaging of ER can be used to optimize drug dose and timing and whether or not complete blockade is necessary for drug effectiveness.
Figure 2.
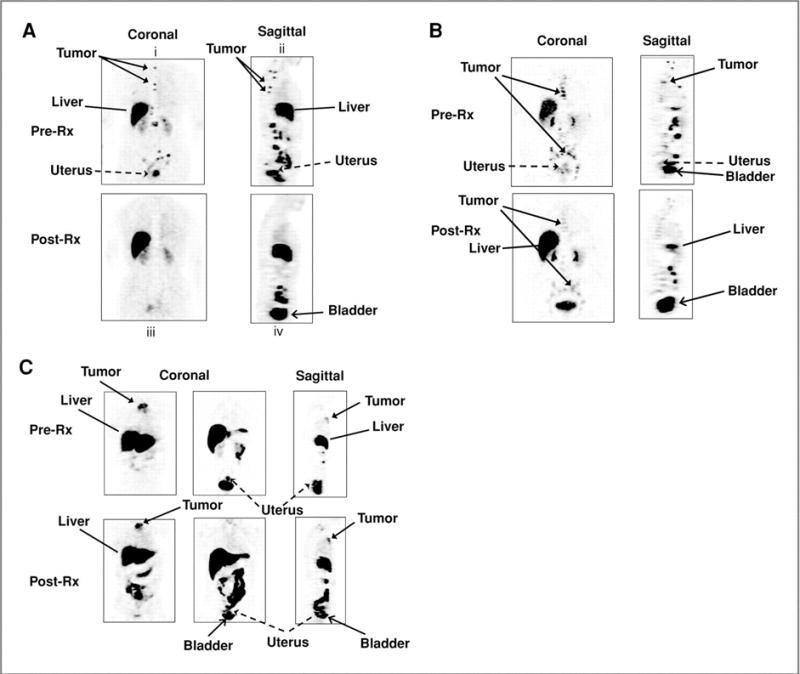
Pre and post treatment images in patients treated with ER— blocking (A. Tamoxifen, B. Fulvestrant) and estrogen lowering therapies (C. aromatase inhibitor). Tumor in the upper spine shows complete ER blockade. Tumor in mediastinal nodes shows incomplete ER blockade. Sternal tumor shows no blockade.
From Linden HM, Kurland BF, Peterson LM, et al. Fluoroestradiol positron emission tomography reveals differences in pharmacodynamics of aromatase inhibitors, tamoxifen, and fulvestrant in patients with metastatic breast cancer. Clin Cancer Res. 2011;17:4799–4805; with permission.
FES can be used to help develop novel ER antagonist therapy
In a recent Phase I dose escalation trial, Wang et al showed that FES PET/CT was a useful biomarker of ER occupancy and/or downregulation and helped optimize the dosage of the novel ER antagonist/degrader (GDC-0810) for future Phase II trials (24). In that study, 30 patients with ER+ metastatic breast cancer underwent 2 FES PET/CT scans, one prior to therapy and one at cycle 2 (day 3) of the GDC-0810 therapy. Up to 5 lesions were evaluated and complete ER suppression was defined as ≥ 90% decrease in the aggregate background corrected FES SUVmax. Twenty-four patients (80%) achieved complete ER suppression. Thus, FES PET imaging appears useful in evaluating novel ER antagonist therapies by determining the optimal dose needed to suppress ER while reducing dose-related drug toxicities.
FES can be used to identify tumor heterogeneity
Biopsy of a single metastatic site is indicated to confirm diagnosis at the time of metastatic presentation, but the heterogeneity of ER/PR expression across all metastatic tumor sites may indicate a need for change in therapy. However, it is not feasible to biopsy all metastatic tumor sites. Furthermore, many clinical trials require biopsy prior to treatment to study relevant biomarkers. We recently reviewed ER and HER2 expression from prior evaluations of site-to-site tumor heterogeneity within patients imaged with FES-PET from 3 different studies. Of 46 patients, 6 (13%) had at least one ER- metastatic biopsy. One (2%) biopsy changed from ER+/HER2- to ER-/HER2+, and one (2%) biopsy changed from ER+/HER2+ to ER+/HER2-. Although the FDG-PET or CT scan helped guide the selection of the biopsy site, the FES results correlated with each biopsy finding. Biopsy findings resulted in changes in clinical management due to change in ER/HER2 status; 3 patients were given another type of endocrine therapy, 1 patient was treated with surgery, and 2 patients were switched to chemotherapy plus biologics (25). Figure 3 is an example of one of the patients that switched from endocrine therapy to chemotherapy after a liver biopsy. These results are consistent with pathology studies where receptor discordance was found in approximately 15% of metastatic lesions compared to primary lesions (26, 27). In another case report study, temporal heterogeneity was seen in serial FES scans in a patient who was imaged at four time points: prior to initiation of endocrine therapy; after response to endocrine therapy; at subsequent disease progression on endocrine therapy; and at disease progression on chemotherapy (Figure 4). This study indicated an increase in the tumor’s capability to bind estradiol at the time of disease progression on chemotherapy, signifying increased ER expression (28).
Figure 3.
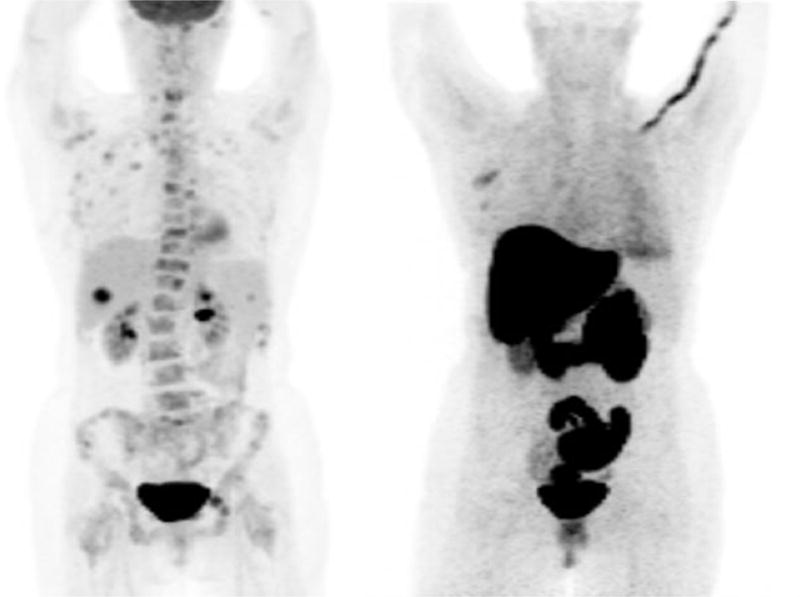
57- year old woman with documented de novo metastatic invasive ductal carcinoma. The primary breast tumor was ER+. FDG-– - PET scan showed multiple lesions with intense uptake. FES-– - PET showed uptake in the right axilla, but not in the bone lesions. Based on an ER−– - biopsy of the liver, the patient was switched from fulvestrant to pertuzumab, trastuzumab and paclitaxel and removed from the study.
Figure 4.
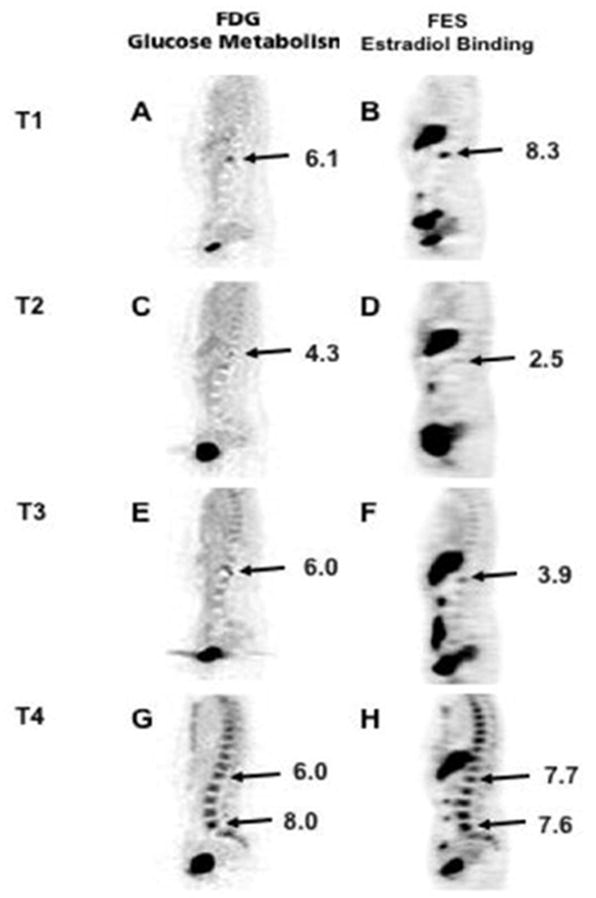
FDG and FES PET images at four time points: T1: prior to initiation of endocrine therapy; T2: after response to endocrine therapy; T3: at subsequent disease progression on endocrine therapy; and T4: at disease progression on chemotherapy. This suggested an increase in the tumor’s capability to bind estradiol at the time of disease progression on chemotherapy, suggesting increased ER expression.
From Currin E, Peterson LM, Schubert EK, et al. Temporal Heterogeneity of Estrogen Receptor Expression in Bone-Dominant Breast Cancer: 18F-Fluoroestradiol PET Imaging Shows Return of ER Expression. J Natl Compr Canc Netw. 2016;14:144–147; with permission.
Progesterone imaging with FFNP may measure response to therapy after endocrine therapy or early response to anti-estrogen therapy
As discussed earlier, measuring decreases in FES uptake after initiation of ER antagonist therapy (e.g., tamoxifen, fulvestrant) can confirm that the drug hit the target appropriately since this reflects preferential occupancy of the receptor ligand binding pocket by the antagonist rather than FES. Antagonist binding to the receptor however does not guarantee that ER transcriptional function will be completely inhibited, particularly in situations of ESR1 gene mutations (29). This has prompted investigations into imaging estrogen-regulated target genes downstream of ER binding as a method for functionally monitoring therapy response. PR is a classic estrogen-regulated ER target gene. There are few clinical studies using progestin-based radioligands for PR imaging (16, 30) 18F-Fluorofuranyl-norprogesterone (FFNP) is the most commonly used PET agent for PR imaging, notably used in a first-in-human study to identify PR+ breast cancer (16) and in preclinical studies to predict response to both fulvestrant therapy (31) and estrogen-deprivation therapy (32). The clinical potential of PR PET imaging is likely to focus on the response to therapy after endocrine therapy or early response to anti-estrogen therapy.
Current and Upcoming Clinical Trials
Eighteen studies using FES-PET are currently listed in clinicaltrials.gov as of September 2017. Seven studies are currently enrolling patients. All of the studies currently enrolling patients include using FES-PET to measure ER status in patients with metastatic breast cancer, and one of the studies also allows patients with ER+ primary breast cancer. The outcomes include determining ER status, predicting response to therapy, and using FES-PET to guide fulvestrant therapy. There is only one multi-center study (NCT02398773) currently recruiting patients. The primary objective of the study is to determine the negative predictive value (NPV) of FES uptake for clinical benefit of endocrine therapy at 6 months. Secondary study aims are to determine the test-retest reproducibility of quantitative assessment of tumor FES uptake by SUVs; evaluate the accuracy of FES PET for predicting response in patients treated with first line endocrine therapy for metastatic breast cancer; evaluate the accuracy of FES PET for predicting progression-free survival (PFS) in patients treated with first line endocrine therapy for metastatic breast cancer; and evaluate FES SUVmax < 1.5 as the optimal cut-point for predicting progression-free survival (PFS) to first line endocrine therapy for metastatic breast cancer. For FFNP-PET imaging, there are two studies listed in clinicaltrials.gov that are currently recruiting patients. One study (NCT02455453) aims to determine if changes in FFNP uptake measured using PET/CT in postmenopausal patients with metastatic ER+ breast cancer after one day of estradiol administration (“estrogen challenge”) predicts response to endocrine therapy. The other study (NCT03212170) aims to compare FFNP uptake measured using PET/MRI in patients with newly diagnosed primary breast cancer with PR immunohistochemistry to determine the accuracy of this approach for subsequent studies investigating its predictive value for neoadjuvant endocrine therapy response.
Summary
Steroid hormone receptor imaging provides a virtual assessment of receptor binding at multiple tumor sites and can identify tumor heterogeneity. There is encouraging data from many centers to suggest clinical utility of imaging to help clinicians select treatment, and aid in drug development. Studies are underway to bring these tracers closer to clinical use, in a field of endocrine therapy which remains an area of growth and improvement in patient outcomes. One impactful goal of imaging is to guide patients toward the most effective and least toxic therapies in this era of precision medicine.
KEY POINTS.
FES–PET is likely to become clinically available to predict clinical benefit from endocrine therapy given alone or in combination with new cyclin-dependent kinase 4/6 inhibitors
FES-PET could help predict therapy response at multiple points in time along the course of treatment when endocrine resistance may arise
FES-PET could be used for pharmacodynamic imaging to help develop new ER antagonist drug therapies
FES-PET can identify tumor heterogeneity and may facilitate selection of optimal biopsy site or confirm metastatic diagnosis if biopsy is not possible
Progesterone receptor imaging is being investigated as a method to measure response to therapy after endocrine therapy or early response to anti-estrogen therapy.
Acknowledgments
This work was supported, in part, by P01CA42045, R01CA72064, R01CA148131, P30CA015704, N01CM-37008, SAC140060, DOD W81XWH-04-01-0675, P30CA047904, U01CA148131, Mary Kay Ash Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURE STATEMENT
The authors have nothing to disclose
References
- 1.Jemal A, Ward EM, Johnson CJ, et al. Annual Report to the Nation on the Status of Cancer, 1975-2014, Featuring Survival. J Natl Cancer Inst. 2017;109 doi: 10.1093/jnci/djx030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ellis MJ, Llombart-Cussac A, Feltl D, et al. Fulvestrant 500 mg Versus Anastrozole 1 mg for the First-Line Treatment of Advanced Breast Cancer: Overall Survival Analysis From the Phase II FIRST Study. J Clin Oncol. 2015;33:3781–3787. doi: 10.1200/JCO.2015.61.5831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mehta RS, Barlow WE, Albain KS, et al. Combination anastrozole and fulvestrant in metastatic breast cancer. N Engl J Med. 2012;367:435–444. doi: 10.1056/NEJMoa1201622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baselga J, Campone M, Piccart M, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med. 2012;366:520–529. doi: 10.1056/NEJMoa1109653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jordan VC. Endoxifen: The End, or Are We at the Beginning? J Clin Oncol. 2017 doi: 10.1200/JCO.2017.74.9325. JCO2017749325. [DOI] [PubMed] [Google Scholar]
- 6.Finn RS, Crown JP, Lang I, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol. 2015;16:25–35. doi: 10.1016/S1470-2045(14)71159-3. [DOI] [PubMed] [Google Scholar]
- 7.Kurland BF, Peterson LM, Lee JH, et al. Estrogen Receptor Binding (18F-FES PET) and Glycolytic Activity (18F-FDG PET) Predict Progression-Free Survival on Endocrine Therapy in Patients with ER+ Breast Cancer. Clin Cancer Res. 2017;23:407–415. doi: 10.1158/1078-0432.CCR-16-0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ingham M, Schwartz GK. Cell-Cycle Therapeutics Come of Age. J Clin Oncol. 2017;35:2949–2959. doi: 10.1200/JCO.2016.69.0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jatoi I, Anderson WF, Jeong JH, Redmond CK. Breast cancer adjuvant therapy: time to consider its time-dependent effects. J Clin Oncol. 2011;29:2301–2304. doi: 10.1200/JCO.2010.32.3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lim E, Metzger-Filho O, Winer EP. The natural history of hormone receptor-positive breast cancer. Oncology (Williston Park) 2012;26:688–694. 696. [PubMed] [Google Scholar]
- 11.Collignon J, Gennigens C, Jerusalem G. Assessment of Response to Therapy for Bone Metastases: Is it Still a Challenge in Oncology? PET Clin. 2010;5:311–326. doi: 10.1016/j.cpet.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 12.Cook GJ, Azad GK, Goh V. Imaging Bone Metastases in Breast Cancer: Staging and Response Assessment. J Nucl Med. 2016;57(Suppl 1):27S–33S. doi: 10.2967/jnumed.115.157867. [DOI] [PubMed] [Google Scholar]
- 13.Woolf DK, Padhani AR, Makris A. Assessing response to treatment of bone metastases from breast cancer: what should be the standard of care? Ann Oncol. 2015;26:1048–1057. doi: 10.1093/annonc/mdu558. [DOI] [PubMed] [Google Scholar]
- 14.Mintun MA, Welch MJ, Siegel BA, et al. Breast cancer: PET imaging of estrogen receptors. Radiology. 1988;169:45–48. doi: 10.1148/radiology.169.1.3262228. [DOI] [PubMed] [Google Scholar]
- 15.Peterson LM, Mankoff DA, Lawton T, et al. Quantitative imaging of estrogen receptor expression in breast cancer with PET and 18F-fluoroestradiol. J Nucl Med. 2008;49:367–374. doi: 10.2967/jnumed.107.047506. [DOI] [PubMed] [Google Scholar]
- 16.Dehdashti F, Laforest R, Gao F, et al. Assessment of progesterone receptors in breast carcinoma by PET with 21-18F-fluoro-16alpha,17alpha-[(R)-(1′-alpha-furylmethylidene)dioxy]-19-norpregn- 4-ene-3,20-dione. J Nucl Med. 2012;53:363–370. doi: 10.2967/jnumed.111.098319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Osborne CK, Schiff R. Mechanisms of endocrine resistance in breast cancer. Annu Rev Med. 2011;62:233–247. doi: 10.1146/annurev-med-070909-182917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mortimer JE, Dehdashti F, Siegel BA, Trinkaus K, Katzenellenbogen JA, Welch MJ. Metabolic flare: indicator of hormone responsiveness in advanced breast cancer. J Clin Oncol. 2001;19:2797–2803. doi: 10.1200/JCO.2001.19.11.2797. [DOI] [PubMed] [Google Scholar]
- 19.Peterson LM, Kurland BF, Schubert EK, et al. A phase 2 study of 16alpha-[18F]-fluoro-17beta-estradiol positron emission tomography (FES-PET) as a marker of hormone sensitivity in metastatic breast cancer (MBC) Mol Imaging Biol. 2014;16:431–440. doi: 10.1007/s11307-013-0699-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Linden HM, Stekhova SA, Link JM, et al. Quantitative fluoroestradiol positron emission tomography imaging predicts response to endocrine treatment in breast cancer. J Clin Oncol. 2006;24:2793–2799. doi: 10.1200/JCO.2005.04.3810. [DOI] [PubMed] [Google Scholar]
- 21.van Kruchten M, Glaudemans AW, de Vries EF, Schroder CP, de Vries EG, Hospers GA. Positron emission tomography of tumour [(18)F]fluoroestradiol uptake in patients with acquired hormone-resistant metastatic breast cancer prior to oestradiol therapy. Eur J Nucl Med Mol Imaging. 2015;42:1674–1681. doi: 10.1007/s00259-015-3107-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin FI, Gonzalez EM, Kummar S, et al. Utility of 18F-fluoroestradiol (18F-FES) PET/CT imaging as a pharmacodynamic marker in patients with refractory estrogen receptor-positive solid tumors receiving Z-endoxifen therapy. Eur J Nucl Med Mol Imaging. 2017;44:500–508. doi: 10.1007/s00259-016-3561-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Linden HM, Kurland BF, Peterson LM, et al. Fluoroestradiol positron emission tomography reveals differences in pharmacodynamics of aromatase inhibitors, tamoxifen, and fulvestrant in patients with metastatic breast cancer. Clin Cancer Res. 2011;17:4799–4805. doi: 10.1158/1078-0432.CCR-10-3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Y, Ayres KL, Goldman DA, et al. 18F-Fluoroestradiol PET/CT Measurement of Estrogen Receptor Suppression during a Phase I Trial of the Novel Estrogen Receptor-Targeted Therapeutic GDC-0810: Using an Imaging Biomarker to Guide Drug Dosage in Subsequent Trials. Clin Cancer Res. 2017;23:3053–3060. doi: 10.1158/1078-0432.CCR-16-2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peterson LM, Kurland BF, Romine P, et al. ASCO. Chicago, IL: 2017. The uses of 18F-fluoroestradial (FES) PET with and without 18F-fluorodeoxyglucose (FDG) PET in breast cancer evaluation. [Google Scholar]
- 26.Karagoz Ozen DS, Ozturk MA, Aydin O, Turna ZH, Ilvan S, Ozguroglu M. Receptor expression discrepancy between primary and metastatic breast cancer lesions. Oncol Res Treat. 2014;37:622–626. doi: 10.1159/000368312. [DOI] [PubMed] [Google Scholar]
- 27.Qu Q, Zong Y, Fei XC, et al. The importance of biopsy in clinically diagnosed metastatic lesions in patients with breast cancer. World J Surg Oncol. 2014;12:93. doi: 10.1186/1477-7819-12-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Currin E, Peterson LM, Schubert EK, et al. Temporal Heterogeneity of Estrogen Receptor Expression in Bone-Dominant Breast Cancer: 18F-Fluoroestradiol PET Imaging Shows Return of ER Expression. J Natl Compr Canc Netw. 2016;14:144–147. doi: 10.6004/jnccn.2016.0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jeselsohn R, Yelensky R, Buchwalter G, et al. Emergence of constitutively active estrogen receptor-alpha mutations in pretreated advanced estrogen receptor-positive breast cancer. Clin Cancer Res. 2014;20:1757–1767. doi: 10.1158/1078-0432.CCR-13-2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dehdashti F, McGuire AH, Van Brocklin HF, et al. Assessment of 21-[18F]fluoro-16 alpha-ethyl-19-norprogesterone as a positron-emitting radiopharmaceutical for the detection of progestin receptors in human breast carcinomas. J Nucl Med. 1991;32:1532–1537. [PubMed] [Google Scholar]
- 31.Fowler AM, Clark AS, Katzenellenbogen JA, Linden HM, Dehdashti F. Imaging Diagnostic and Therapeutic Targets: Steroid Receptors in Breast Cancer. J Nucl Med. 2016;57(Suppl 1):75s–80s. doi: 10.2967/jnumed.115.157933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chan SR, Fowler AM, Allen JA, et al. Longitudinal noninvasive imaging of progesterone receptor as a predictive biomarker of tumor responsiveness to estrogen deprivation therapy. Clin Cancer Res. 2015;21:1063–1070. doi: 10.1158/1078-0432.CCR-14-1715. [DOI] [PMC free article] [PubMed] [Google Scholar]


