SYNOPSIS
Increased expression of the HER protein family, especially HER2 and HER3, are biomarkers in breast cancer that have become significant targets for molecular imaging and therapy. Imaging modalities that target HER2 and HER3 have the ability to diagnose breast cancer with a specific, biologically relevant target in a non-invasive manner. Repeat tumor biopsies do not address the issue of heterogeneity intratumorally or between primary disease and metastasis. HER2- and HER3- targeted PET is an important tool to diagnose disease state in breast cancer and evaluate response to therapies that target these receptors. PET is a highly sensitive and quantitative imaging technique with subnanomolar detection. PET and SPECT with radiolabeled biomolecules can be used for noninvasive detection and quantification of specific targets, allowing physicians and scientists alike to better understand the behavior and effectiveness of treatment across patient cohorts.
Keywords: PET, SPECT, CT, Imaging, Radiotracers, HER2, HER3, Metastasis, Breast cancer
INTRODUCTION
Breast cancer is the most common cancer in women worldwide, with approximately 12% of women in the United States developing invasive breast cancer in her lifetime.1 Although breast cancer has come a long way in regards to treatment options and overall survival, there are still challenges that occur mostly from intratumoral heterogeneity2 and metastatic disease.3 While significant research has emerged to identify biological processes leading to breast cancer,4 selecting patients that would benefit from targeted therapies is still a major hurdle in the field. Non-invasive tools to stratify patients and facilitate precision medicine are needed to address this issue.5–7
Although breast cancer is typically diagnosed and staged via three main biomarkers, estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2),8 there are other biomarkers of interest that are relevant and may be beneficial to the clinical outcome of breast cancer. Reports of clinical benefit from HER2-targeted therapy in patients with a primary HER2-negative tumor have facilitated to spearhead the concept behind HER2-targeted molecular imaging for unsuspecting metastasis in breast cancer.9–10 An additional biomarker of interest includes human epidermal growth factor receptor 3, or HER3.11 HER3 overexpression has been linked with poor prognosis in multiple cancer subtypes including breast, which has driven interest in HER3 as potential target for both imaging and therapy. Additionally, emerging resistance to HER2-targeted therapy has driven the need to explore additional targets for both imaging and therapy.11 The HER family in general has been extensively studied in breast cancer,12 and there are many HER2-targeted agents for therapy and molecular imaging,13–15 and strategies to target HER3 are also on the rise with clinical trials.16–17
Molecular imaging, particularly with tracers used in positron emission tomography (PET) and single-photon emission computed tomography (SPECT) have the ability to non-invasively detect heterogeneity between primary tumors and metastases in breast cancer.6, 18 PET and SPECT-based measurement of the HER2 and HER3 expression in breast cancer offers several advantages over repeated biopsies in patient cohorts. Several groups have developed antibody, affibody, and nanoparticle-based radioligands for PET imaging preclinically and clinically,19–22 many of which include radiotracers used to image HER2 and HER3 in breast cancer.13, 23 Pairing these targeting moieties with longer-lived radioisotopes such as zirconium-89 (89Zr, t1/2 = 78 h) for PET, along with lutetium-177 (177Lu, t1/2 = 6.7 days) and indium-111 (111In, t1/2 = 67 h) for SPECT can be used to match the biological half-life of antibodies in vivo (t1/2 ~72 h for typical full-length antibodies).24 Isotopes like copper-64 (64Cu, t1/2 = 12 h) or technium-99m (99mTc, t1/2 = 6 h) can be used in PET and SPECT, respectively, for both full length and antibody fragments, as well as affibodies.25–27 Antibody fragments and affibody molecules are often labeled with shorter-lived isotopes such as gallium-68 (68Ga, t1/2 = 68 min) or fluorine-18 (18F, t1/2 = 110 min) to pair appropriately with shorter biological half-lives in vivo.28–31
Many of the PET and SPECT imaging tracers that have made it to the clinic (in Phase I/II trials) to detect HER2-positive breast cancer and metastases, are summarized in our previous review.15 This current review seeks to communicate an update about the current clinical trials for both HER2 and HER3-targeted imaging, which are outlined in Table 1. The current clinical radiotracers used to image HER3+ and HER2+ breast cancers are review within.
Table 1.
Summary of current clinical trials with PET and SPECT radiotracers targeting HER2 and HER3-positive breast cancer in 2017. BC: breast cancer. MBC: metastatic breast cancer. IMPACT: IMaging PAtients for Cancer Drug selection.
|
64Cu-DOTA-Patritumab 89Zr-DFO-Lumretuzumab |
RO, PK, and Treatment Response Treatment Response |
NCT01479023 NCT01482377 |
Washington University School of Medicine Hoffmann-La Roche |
Terminated Completed |
| 89Zr-DFO-Trastuzumab | HER2+ BC HER2+ MBC IMPACT-MBC |
NCT02065609 NCT02286843 NCT01957332 |
Washington University School of Medicine Memorial Sloan Kettering Cancer Center University Medical Center Groningen |
Recruiting Recruiting Recruiting |
| 89Zr-DFO-Pertuzumab | HER2+ MBC HER2+ MBC |
NCT03109977 NCT02286843 |
Memorial Sloan Kettering Cancer Center Memorial Sloan Kettering Cancer Center |
Completed Recruiting |
| 64Cu-DOTA-Trastuzumab | Treatment Response in HER2+ BC HER2+ BC |
NCT02827877 UMIN000017446 |
City of Hope Medical Center P-DIRECT |
Recruiting Completed |
| 177Lu-DOTA-Trastuzumab | HER2+ BC Healthy Patients and HER2+ BC HER2+ MBC |
Bhusari et al. NCT02683083 NCT01304797 |
PGIMER Camel-IDS NV Merrimack Pharmaceuticals |
Completed Recruiting Ongoing, Not Recruiting |
| [131I]-SGMIB Anti-HER2 VHH1 | ||||
| 64Cu-MM-302 |
PET AND SPECT IMAGING WITH MONOCLONAL ANTIBODIES IN HER2-POSITIVE BREAST CANCER
An extensively studied target for imaging in breast cancer is HER2, as clinical trials utilizing this target are happening at cancer centers all over the world.9, 32–33 HER2 imaging can address the issue of tumor heterogeneity and reliably determine both the quantity and the functional status of tumor HER2 in individual lesions in a noninvasive manner. This technique is of critical importance to identify patients who would truly benefit from HER2-targeted therapy, and to monitor the change in HER2 status during immunotherapy.34–35 We have previously reviewed HER2 targeted imaging,15 and provide an update of more recent work here.
89Zr-Trastuzumab
89Zr-trastuzumab has been vastly studied across patient cohorts and clinical trials for both primary and metastatic breast cancer.9, 36–40 Ulaner and coworkers have expanded the field with 89Zr-trastuzumab to detect unsuspecting metastasis in both HER2+ and HER2− primary breast tumors.9, 41 Multiple studies are being carried out at Memorial Sloan Kettering Cancer Center to image this HER2 expression discordance and guide biopsies.41–42 Another current clinical trial utilizing 89Zr-Trastuzumab is led by Laforest and coworkers at Washington University in St. Louis.43 Twelve women were enrolled in the study; six with primary breast cancer and six with metastatic breast cancer. Eleven of these patients underwent 89Zr-trastuzumab PET/CT during neoadjuvant or adjuvant therapy, and the remaining patient was imaged before neoadjuvant therapy. Laforest et al. found that increasing the dose to 62 ± 2 MBq (vs. the average dose of 37 MBq, which was used in previous studies done by Dijkers et al.44–45 the ZEPHIR trial)33 allowed for optimal images at later time points (Figures 1 and 2). The dose-limiting organ was found to be the liver, with a dose of 1.63 mSv/MBq. The biodistribution of 89Zr-trastuzumab did not significantly change during the 7 days of imaging. Slow blood clearance was observed with a biological half-life of 113 h and an initial level of 58% ID. Overall, this study showed that imaging 89Zr-trastuzumab imaging is safe, has acceptable dosimetry, and provides a noninvasive means of assessing HER2 status of individual lesions in patients with breast cancer.
FIGURE 1.

Anterior and posterior reprojection PET images of a patient with HER2-positive metastatic breast cancer on a day 3 and b day 6 following 89Zr-trastuzumab administration demonstrate greater tracer uptake in the right femoral metastatic lesion without other significant change in tracer biodistribution on day 6 compared to day 3. The area of intense activity in the right chest is related to administration of 89Zr-trastuzumab via a port catheter.
From Laforest R, Lapi SE, Oyama R, Bose R, et al. [89Zr]Trastuzumab: Evaluation of Radiation Dosimetry, Safety, and Optimal Imaging Parameters in Women with HER2-Positive Breast Cancer. Mol Imaging Biol 2016;18(6): 952-959; with permission.
FIGURE 2.

Axial PET (top) and fused PET/CT (bottom) images of a patient with HER2-positive metastatic breast cancer at the chest level on a day 2 and b day 4 following 89Zr-trastuzumab administration demonstrate greater tracer uptake in the right humeral metastatic lesion on day 4 compared to day 2.
From Laforest R, Lapi SE, Oyama R, Bose R, et al. [89Zr]Trastuzumab: Evaluation of Radiation Dosimetry, Safety, and Optimal Imaging Parameters in Women with HER2-Positive Breast Cancer. Mol Imaging Biol 2016;18(6): 952-959; with permission.
89Zr-Pertuzumab
As 89Zr-trastuzumab has been associated with avidity on PET/CT that does not allow correlate with HER2 positivity on pathology, additional HER2 targeted tracers have been investigated. For example, pertuzumab is a newer humanized monoclonal antibody that binds to the HER2 receptor at a site distinct from trastuzumab and appears to be more efficient than trastuzumab.46 A first-in-human trial of 89Zr-pertuzumab imaging in patients with HER2-positive breast cancer has demonstrated safety and dosimetry.47 A potential clinical application of 89Zr-pertuzumab and other HER2-targeting agents was demonstrated in patients with two primary breast malignancies, one HER2+ and the other HER2-, who developed brain metastases. HER2-targeted imaging allowed determination of the HER2-status of the brain metastases, and could assist in the choice of HER2-targeted therapy. In one patient, resection of a symptomatic brain metastasis confirmed a HER2-positive metastasis, as imaged on 89Zr-pertuzumab PET/CT (Figure 3).
FIGURE 3.
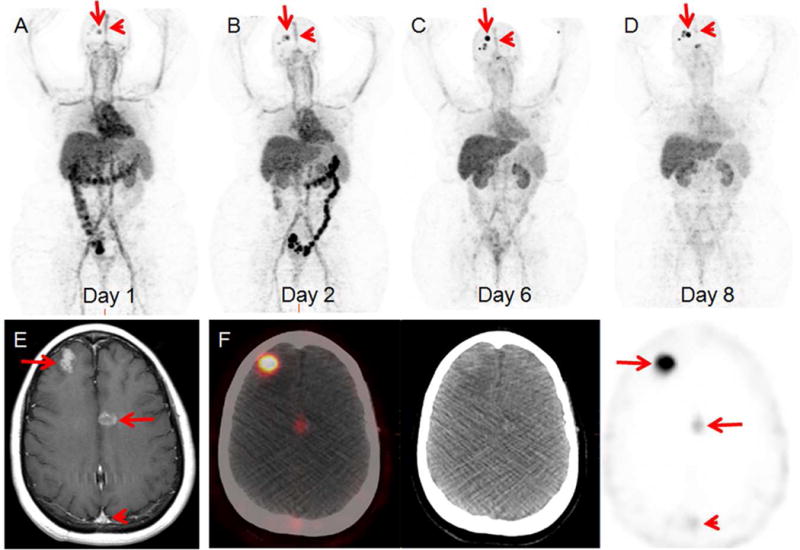
46-year-old woman with both HER2-positive and HER2-negative primary breast malignancies and recently diagnosed brain metastases. Sequential maximum-intensity projection (MIP) images (A) 1 day, (B) 2 days, (C) 6 days, and (D) 8 days following administration of 89Zr-pertuzumab. Blood pool and liver background clears on sequential images. Excreted bowel activity is seen on days 1 and 2. Bilateral kidney activity is visualized on all days. Increasing activity in foci overlying the skull is seen as time progresses (arrows). Decreasing activity is seen in the blood pool of the superior sagittal sinus (arrowheads). (E) Gadolinium-enhanced T1 weighed MR of the brain demonstrates enhancing brain metastases (arrows) and the superior sagittal sinus (arrowhead). (F) Axial fused PET/CT, CT, and PET images 8 days following 89Zr-pertuzumab administration demonstrate avidity in the brain metastases (arrows) and minimal residual avidity in the superior sagittal sinus (arrowhead).
From Ulaner GA, Lyashchenko SK, Riedl C, et al. First-in-human HER2-targeted imaging using 89Zr-pertuzumab PET/CT: Dosimetry and clinical application in patients with breast cancer. J Nucl Med. In Press; with permission.
64Cu-Trastuzumab
64Cu-DOTA-trastuzumab has also been used in multiple clinical trials to date.25–27 The first study to use this radiotracer was to determine the safety, distribution, internal dosimetry, and initial HER2–positive tumor images of 64Cu-DOTA-trastuzumab in humans by Tamura et al.26 Since this study, this tracer has been used for a number of studies to detect both HER2+ primary and metastatic disease and assess therapy response. Tamura and coworkers have expanded upon this work to assess intratumoral heterogeneity in HER2+ breast cancer.48 In a separate study, SUVmax values were correlated with HER2-immunohistochemistry (IHC) scores and assessment for HER2 status by HER2-PET imaging strongly correlated with histologic HER2 expression status. Tamura and coworkers have also previously demonstrated HER2-specific 64Cu-trastuzumab accumulation in a specimen of removed brain metastasis using IHC and autoradiography.25 An image of the month study highlighted the first report describing the visualization of HER2-specific intratumoral heterogeneity (IHC 1+ and 2+) using HER2 PET imaging (Figure 4).48 This interesting finding suggests that HER2 PET imaging could facilitate decision making for clinical treatment strategies. The relationship between HER2 PET imaging and the effects of anti-HER2 therapy, as well as the use of a high-resolution dedicated breast PET scanner, remain to be evaluated.
FIGURE 4.
Visualization of HER2-specific intratumoral heterogeneity (IHC 1+ and 2+) with 64Cu-trastuzumab.
From Sasada S, Kurihara H, Kinoshita T, et al. Visualization of HER2-specific breast cancer intratumoral heterogeneity using 64Cu-DOTA-trastuzumab PET. Eur J Nucl Med Mol Imaging 2017; doi: 10.1007/s00259-017-3781-6; with permission.
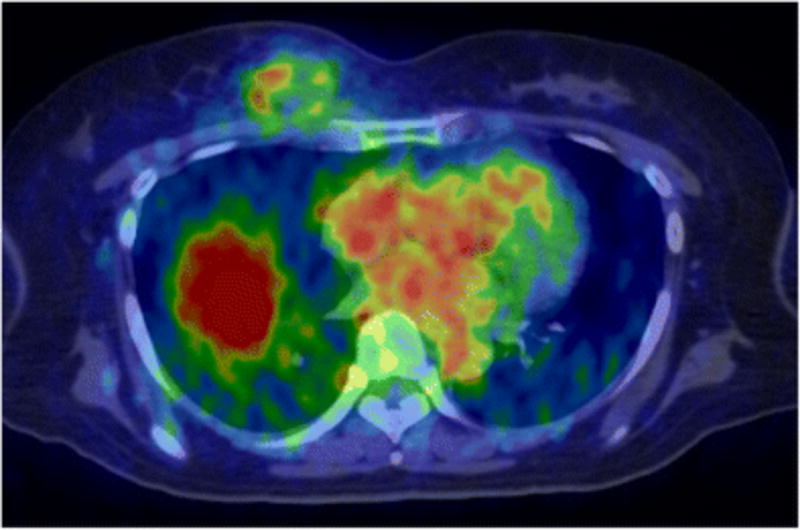
Mortimer and coworkers are also using the 64Cu-trastuzumab to assess metastatic breast cancer.49 Women with biopsy-confirmed metastatic breast cancer and not given trastuzumab for 2 months underwent complete staging, including 18F-FDG PET/CT. Although 18F-FDG has been shown to be avid for some cancers, it does not fully address the heterogeneity in breast cancer.5,50–52 Patients were classified as HER2-positive (HER2+) or negative (HER2−) based on fluorescence in situ hybridization (FISH) and IHC of biopsied tumor tissue (Figure 5). Eighteen patients underwent 64Cu-trastuzumab injection, preceded in 16 cases by trastuzumab infusion (45 mg). PET/CT was performed on 1 and 2 days after h post-injection with 64Cu-trastuzumab (Figure 6).
FIGURE 5.

Tumor uptake (SUVmax) of 64Cu-DOTA-trastuzumab versus patient IHC/FISH score from biopsied tumor. Data are from 64Cu-DOTA-trastuzumab PET/CT scans acquired 1 day (A, B) and 2 days (C, D) post injection. In A and C, the data for individual tumors (black dots) are grouped by patient and IHC/FISH score. Intra-patient means are represented by red horizontal lines. In B and D, SUVmax values for individual lesions (open circles) are combined across patients and grouped by IHC/FISH score (n = number of tumors per group). Intra-group medians are represented as amplitudes of rectangular overlays; errors bars denote 1st and 3rd quartiles. In both analyses, tumor uptake is generally higher for the HER2+ subgroups (3+ or 2+/FISH+) (P < 0.005). Relative variability of uptake was greater for the HER2+ than the HER2− group, both among (P < 0.001) and within (P < 0.05 on Day 2) patients.
From Mortimer JE, Bading JR, Park JM, et al. Tumor Uptake of 64Cu-DOTA-Trastuzumab in Patients with Metastatic Breast Cancer. J Nucl Med 2017; doi: 10.2967/jnumed.117.193888; with permission.
FIGURE 6.

Examples of increased tumor uptake and tumor-to-non-tumor contrast between 1 and 2 days after injection of 64Cu-DOTA-trastuzumab. White and red arrows respectively denote tumors and blood pool as seen in transaxial PET/CT fusion images. The images on the left are from a HER2+ (IHC2+/FISH+) patient. Panels A (Day 1) and C (Day 2) show a large metastasis in a pre-vascular lymph node for which uptake was concentrated at the tumor surface. The upper intensity threshold (white color) corresponds to SUV = 22 g/ml. At the times of the 2 scans (24 and 48 h), measured SUVmax for the tumor was 17 and 27 g/ml, respectively, while the SUV for blood was 15 and 11 g/ml. The right-hand column depicts a metastatic mass in the right breast of a HER2-negative patient (IHC1+). The upper intensity threshold for the images (white color) was set at SUV = 10 g/ml. The SUVmax for the tumor increased from 2.6 to 5.0 g/mL between (B) the Day 1 (25 h post-injection) and the (D) Day 2 (49 h post-injection) scan, while the SUV for blood declined from 12 to 8.8 g/mL.
From Mortimer JE, Bading JR, Park JM, et al. Tumor Uptake of 64Cu-DOTA-Trastuzumab in Patients with Metastatic Breast Cancer. J Nucl Med 2017; doi: 10.2967/jnumed.117.193888; with permission.
The observations made by Mortimer and coworkers show that most tumors are well visualized with PET and uptake is indicative of binding to HER2 within 1 d post injection, even in patients classified as HER2−, which indicates that trastuzumab uptake in metastatic breast cancer is sufficiently rapid, and does not require longer lived isotopes or lag times. This is significant for PET imaging of trastuzumab with respect to both patient radiation dose and clinical applicability.
177Lu-Trastuzumab
Bhusari and coworkers developed a 177Lu-trastuzumab tracer for radioimmunotherapy of HER2 expressing breast cancer and also explored its potential as an imaging agent.53 This study demonstrated that the development, characterization and radiolabeling of 177Lu-trastuzumab can be efficiently achieved in-house and that this radiotracer is safe to be administered to the patients. The HER2 positive primary and metastatic breast lesions specifically take up 177Lu-trastuzumab, with two patient examples shown in Figures 7 (Day 1 and Day 7 time points) and Figure 8 (Day 5). This strategy can be considered as an agent for palliative treatment in combination to other conventional treatments for treatment of HER2 metastatic breast cancer.
FIGURE 7.

(a) Whole body image of a 60-year-old breast cancer patient HER2 (2+) at Day 1 and Day 7 post administration of 370 MBq 177Lu-trastuzumab (5 mg, co administered with 20 mg cold trastuzumab). Tracer uptake can be observed in primary breast tumor (black arrow head) with T/N = 2.9 and 3.2 at Day 1 and Day 7, respectively. The bone metastasis in the acetabulum region was visualized at Day 1 (T/N = 1.2) and Day 7 (T/N = 2.5) (blue arrow heads). (b) SPECT/CT, CT, and SPECT images showing lymph node metastases (T/N = 2.4) which could not be localized on whole body scan (white arrow heads).
From Bhusari P, Vatsa R, Singh G, Parmar M, Bal A, Dhawan DK, Mittal BR, Shukla J. Development of Lu-177-trastuzumab for radioimmunotherapy of HER2 expressing breast cancer and its feasibility assessment in breast cancer patients. Int J Cancer 2017;140(4):938-947; with permission.
FIGURE 8.

(a) Whole Body images of a 30-year-old woman patient with HER2 (3+) breast cancer showing 177Lu-trastuzumab (445 MBq) uptake in primary breast tumor (black arrow head; T/N = 3.1). (b) SPECT/CT and CT sagittal sections of the primary breast tumor showing localization of 177Lu-trastuzumab (T/N = 3.6) (white arrow head). (c) SPECT/CT and CT images showing 177Lu-trastuzumab localization at the metastatic sites: lymph node; T/N = 3.4 and lesion in the sternum; T/N = 1.3 (white arrow heads) and lung metastases (T/N = 1.5) at Day 5 post administration of 444 MBq 177Lu-trastuzumab.
From Bhusari P, Vatsa R, Singh G, Parmar M, Bal A, Dhawan DK, Mittal BR, Shukla J. Development of Lu-177-trastuzumab for radioimmunotherapy of HER2 expressing breast cancer and its feasibility assessment in breast cancer patients. Int J Cancer 2017;140(4):938-947; with permission.
NANOPARTICLES IN HER2-TARGETED IMAGING AND THERANOSTICS
Nanoparticles and other nanomaterials have also been embellished with the HER2 target in mind. Therapeutic nanoparticles are designed to deliver their drug payloads through enhanced permeability and retention (EPR) in solid tumors. The extent of EPR and its variability in human tumors is a controversial issue in the field, as it may be the reason for variable responses to therapeutic nanoparticles in clinical studies. This issue was addressed by Lee and coworkers in an imaging study in metastatic breast cancer with 64Cu-MM-302, a radiolabeled HER2-targeted PEGylated liposomal doxorubicin.54 Nineteen patients with HER2-positive metastatic breast cancer underwent 2 to 3 PET/CT scans post-administration of 64Cu-MM-302 as part of a clinical trial of MM-302 plus trastuzumab with and without cyclophosphamide.55–57 Tumor accumulation of 64Cu-MM-302 at 24 to 48 hours varied from 0.52–18.5 %ID/kg (Figure 9), including deposition in bone and brain lesions, along with significant background uptake of 64Cu-MM-302 in the liver and spleen. Peak liposome circulation was found to be between 24 and 48 h based on computational analysis, and high 64Cu-MM-302 deposition was associated with more favorable treatment outcomes. These findings deliver key evidence and quantification of the EPR effect in HER2+ metastatic tumors, supporting the use of nanoparticle imaging as a tool to stratify patients that would benefit from such therapy.
FIGURE 9.

Biodistribution of 64Cu-MM-302 in patients. Maximum intensity projection PET images of 2 patients with HER2-positive breast cancer injected with 30 mg/m2 of MM-302 and a tracer dose of 64Cu-MM-302 (400 MBq). PET/CT Images were acquired at 0.6 and 19 hours post-injection in patient 02 (A), and 0.7, 24, and 47 hours post-injection in patient 06 (B). Immediately after administration, 64Cu-MM-302 activity was primarily confined in the blood pool because of the extended circulation property of liposomes. On days 2 and 3, 64Cu-MM-302 uptake was evident in normal spleen and liver, as well as in various tumor lesions.
Figure reproduced from H Lee et al., Clin Cancer Res 2017, with permission from the publisher.
Other nanoparticles on the preclinical end that show promise include studies with 99mTc-nanosilica58 and superparamagnetic nanoparticles59 for detection of HER2-positive breast cancer. This emerging realm will bridge the gap between therapies and imaging in the field of nanomedicine, allowing for new subsets of targeted molecules.
PET AND SPECT IMAGING WITH MONOCLONAL ANTIBODIES IN HER3-POSITIVE BREAST CANCER
As there are emerging strategies with monoclonal antibodies to diagnosis disease, assess progression, and interpret specific biomarkers within breast cancer, an up and coming area of this strategy is developing with HER3-targeted antibodies. One of the latest treatments incorporated into clinical trials is patritumab, which binds the extracellular domain of HER3 and promotes receptor internalization, leading to the inhibition of basal and ligand-induced HER3 activation and downstream signaling. Patritumab was labeled with 64Cu and imaging studies were done in patients with multiple tumor types (including breast) that were HER3+, in a recent publication from Lockhart and coworkers.16 Dosimetry, safety, and receptor occupancy (RO) were the main objectives of this clinical trial. The tumor SUVmax was 5.6 ± 4.5, 3.3 ± 1.7 and 3.0 ± 1.1 at 3, 24 and 48 h, respectively, in the dosimetry cohort. The liver was the dose-limiting organ, with a critical dose of 0.46 ± 0.086 mGy/MBq. In the cohort of patients undergoing RO, the tumor-to-blood ratio decreased from 1.00 ± 0.32 at baseline to 0.57 ± 0.17 after patritumab treatment, corresponding to a RO of 42.1 ± 3.9%. Future studies will be needed to improve the characterization of HER3 receptor occupancy by patritumab and its relationship with serum patritumab levels. Additional trials will likely incorporate reduced the time between 89Zr-labeled and unlabeled antibody dosing to reduce liver uptake of the labeled product.
Another antibody-based radiotracer was developed by Bensch and Lamberts et al. with 89Zr-lumretuzumab PET to assess therapy response with lumretuzumab in patients with solid tumors.17 Lumretuzumab is a humanized monoclonal antibody directed against the extracellular domain of HER3, displacing its ligand and inhibiting heterodimerization and downstream signaling. Furthermore, it has been shown that lumretuzumab can cause cell death through antibody-dependent cellular cytotoxicity (ADCC). A phase I study in patients with HER3-positive solid tumors showed that lumretuzumab monotherapy was well tolerated and clinically useful, leading to an imaging study with 89Zr-lumretuzumab. This trial recruited 20 patients with histologically confirmed HER3-expressing tumors that underwent 89Zr-lumretuzumab PET. In one arm of the trial, 89Zr-lumretuzumab was given alongside escalating doses of unlabeled lumretuzumab and scans were performed 2, 4 and 7 days post-injection to determine optimal imaging conditions. In another arm of the trial, patients were scanned following tracer injection before (baseline) and after a pharmacodynamic (PD)-active lumretuzumab dose to determine saturation. HER3 expression was determined via immunohistochemical analysis of skin biopsies.
Optimal PET conditions were found to be 4 and 7 days after administration of 89Zr-lumretuzumab with 100 mg unlabeled antibody. At baseline, post-administration of “cold” lumretuzumab (100 mg), the tumor SUVmax was 3.4 ± 1.9 at 4 days post-injection of 89Zr-lumretuzumab. Tumor uptake decreased by 11.9% ± 8.2, 10.0% ± 16.5, and 24.6% ± 20.9 after lumretuzumab administration (escalating doses of 400, 800 and 1600 mg, respectively) when compared to baseline. HER3 expression was also downregulated in concurrent skin biopsies at the lowest dose (400 mg) lumretuzumab. Overall, this study with 89Zr-lumretuzumab PET showed promising biodistribution and tumor specificity. Despite the correlative trend between increased doses of lumretuzumab and decreased 89Zr-lumretuzumab uptake, the tumor uptake never plateaued, indicating a lack of tumor saturation. Therefore, it is possible that the dosing of the radiotracer will need further optimization to have optimal delineation.
Although there are a number of preclinical tracers to target HER3 that are on the rise, few have yet to reach the clinic to drive the relevance of this protein in breast cancer imaging. Some of the more recent antibody developments include those that are not only relevant for diagnosis,60–62 but also assessing therapy response,63–65 some of which were actually used in the clinical trials described above. Affibodies are also an up and coming strategy that has been more embellished upon with the HER2 target, but is also slowly on the rise with regards to HER3.66–69 Radiotracers that are successful for both diagnostic purposes and predicting therapy are particularly useful from a breast cancer standpoint, as many chemotherapeutic and targeted therapy options can be toxic and have harmful side effects. The utilization of a specific, biologically relevant radiotracer to detect changes in HER3 expression and assess therapy response serves to improve breast cancer treatment.
CONCLUSIONS
HER receptor status, namely HER2 and HER3, encompass a subset of critical biomarkers in breast cancer patients, and can be essential to making treatment decisions and finalizing prognosis. The heterogeneous expression of HER proteins both intratumorally and between primary and metastatic disease limits the value of tumor biopsies and demonstrates a need for accurate, whole body assessment, which is where targeted molecular imaging arises as a valuable tool to solve this clinical issue. Antibodies, antibody fragments, affibodies, and nanoparticles targeted to HER2 and HER3 can be radiolabeled for PET and SPECT imaging to target HER2-and HER3 lesions and metastases and therapy response. These clinical tools are necessary to make the push towards precision medicine, and will serve to improve the outcomes for breast cancer patients everywhere.
KEY POINTS.
The human epidermal growth factor receptor (HER) family members are of increasing interest to target by small molecules, affibody moieties, and monoclonal antibodies.
Imaging can simultaneously assess HER expression of primary and metastatic sites, which may vary across lesions within any given patient. PET and SPECT imaging of HER2 and HER3 allows for non-invasive diagnosis in breast cancer, has the ability to detect unknown metastatic disease, and addresses the issue of tumor heterogeneity within breast cancer.
Resistance against the epidermal growth factor receptor (EGFR) and HER2-targeting agents is a clinically relevant problem that requires optimization of targeting other members of the HER family, increasing relevance in HER3 from a therapy and imaging standpoint.
HER3 is strongly involved in the development and maintenance of many tumor types, and is emerging to play a significant role in breast cancer.
Acknowledgments
The authors would like to acknowledge the National Institute of Health R01CA204167 (J.S.L., G.A.U.), the Department of Defense BC132676 (G.A.U.), and the MSKCC Radiochemistry and Molecular Imaging Probe Core (NIH grant P30 CA008748). K.E.H. gratefully acknowledges the Center for Molecular Imaging and Technology Tow Fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURE STATEMENT
The authors have nothing to disclose.
References
- 1.[Internet] WHO Breast Cancer: Prevention and Control. Available from: http://www.who.int/cancer/detection/breastcancer/en/. Accessed September 29, 2017.
- 2.Beca F, Polyak K. Intratumor Heterogeneity in Breast Cancer. Adv Exp Med Biol. 2016;886:169–189. doi: 10.1007/978-3-319-22909-6_7. [DOI] [PubMed] [Google Scholar]
- 3.Scully OJ, Bay BH, Yip G, Yu Y. Breast Cancer Metastasis. Cancer Genomics Proteomics. 2012;9(5):311–320. [PubMed] [Google Scholar]
- 4.Mardamshina M, Geiger T. Next-Generation Proteomics and Its Application to Clinical Breast Cancer Research. Am J Pathol. 2017;187(10):2175–2184. doi: 10.1016/j.ajpath.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 5.Henry KE, Dilling TR, Abdel-Atti D, Edwards KJ, Evans MJ, Lewis JS. Non-invasive 89Zr-Transferrin PET Shows Improved Tumor Targeting Compared to 18F-FDG PET in MYC-overexpressing Human Triple Negative Breast Cancer. J Nucl Med. 2017 doi: 10.2967/jnumed.117.192286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vercher-Conejero JL, Pelegrí-Martinez L, Lopez-Aznar D, Cózar-Santiago M del P. Positron Emission Tomography in Breast Cancer. Diagnostics. 2015;5(1):61–83. doi: 10.3390/diagnostics5010061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chudgar A, Clark A, Mankoff D. Applications of PET/CT in breast cancer, NCCN guidelines and beyond. J Nucl Med. 2016;57(supplement 2):1304. [Google Scholar]
- 8.Dunnwald LK, Rossing MA, Li CI. Hormone receptor status, tumor characteristics, and prognosis: a prospective cohort of breast cancer patients. Breast Cancer Res. 2007;9(1):1–10. doi: 10.1186/bcr1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ulaner GA, Hyman D, Ross D, Corben A, Chandarlapaty S, Goldfarb S, McArthur H, Erinjeri J, Solomon S, Kolb H, Lyashchenko S, Lewis JS, Carrasquillo J. Detection of HER2-positive metastases in patients with HER2-negative primary breast cancer using the 89Zr-DFO-trastuzumab PET/CT. J Nucl Med. 2016;57(10):1523–1528. doi: 10.2967/jnumed.115.172031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ulaner GA, Riedl CC, Dickler MN, Jhaveri K, Pandit-Taskar N, Weber W. Molecular Imaging of Biomarkers in Breast Cancer. Journal of Nuclear Medicine. 2016;57(Supplement 1):53S–59S. doi: 10.2967/jnumed.115.157909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kol A, Terwisscha van Scheltinga AGT, Timmer-Bosscha H, Lamberts LE, Bensch F, de Vries EG, Schröder CP. HER3, serious partner in crime: Therapeutic approaches and potential biomarkers for effect of HER3-targeting. Pharmacol Ther. 2014;143(1):1–11. doi: 10.1016/j.pharmthera.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 12.Pool M, de Boer HR, Hooge MNL, van Vugt MATM, de Vries EG. Harnessing Integrative Omics to Facilitate Molecular Imaging of the Human Epidermal Growth Factor Receptor Family for Precision Medicine. Theranostics. 2017;7(7):2111–2133. doi: 10.7150/thno.17934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tolmachev V. Imaging of HER-2 Overexpression in Tumors for Guiding Therapy. Curr Pharm Des. 2008;14(28):2999–3019. doi: 10.2174/138161208786404290. [DOI] [PubMed] [Google Scholar]
- 14.Elias SG, Adams A, Wisner DJ, Esserman LJ, van’t Veer LJ, Mali WP, Gilhuijs KG, Hylton NM. Imaging Features of HER2 Overexpression in Breast Cancer: A Systematic Review and Meta-analysis. Cancer Epidemiol Biomarkers Prev. 2014;23(8):1464–1483. doi: 10.1158/1055-9965.EPI-13-1170. [DOI] [PubMed] [Google Scholar]
- 15.Henry KE, Ulaner GA, Lewis JS. HER2-Targeted PET/SPECT Imaging of Breast Cancer: Non-Invasive Measurement of a Biomarker Integral to Tumor Treatment and Prognosis. PET Clinics. 2017;12(3):269–288. doi: 10.1016/j.cpet.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lockhart AC, Liu Y, Dehdashti F, Laforest R, Picus J, Frye J, Trull L, Belanger S, Desai M, Mahmood S, Mendell J, Welch MJ, Siegel BA. Phase 1 Evaluation of (64)Cu-DOTA-Patritumab to Assess Dosimetry, Apparent Receptor Occupancy, and Safety in Subjects with Advanced Solid Tumors. Mol Imaging Biol. 2016;18(3):446–453. doi: 10.1007/s11307-015-0912-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bensch F, Lamberts LE, Smeenk MM, Jorritsma-Smit A, Lub-de Hooge MN, Terwisscha van Scheltinga AGT, de Jong JR, Gietema JA, Schröder CP, Thomas M, Jacob W, Abiraj K, Adessi C, Meneses-Lorente G, James I, Weisser M, Brouwers AH, de Vries EG. Zr-lumretuzumab PET imaging before and during HER3 antibody lumretuzumab treatment in patients with solid tumors. Clin Cancer Res. 2017 doi: 10.1158/1078-0432.CCR-17-0311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bénard F, Turcotte É. Imaging in breast cancer: Single-photon computed tomography and positron-emission tomography. Breast Cancer Res. 2005;7(4):153–162. doi: 10.1186/bcr1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Warram JM, de Boer E, Sorace AG, Chung TK, Kim H, Pleijhuis RG, van Dam GM, Rosenthal EL. Antibody Based Imaging Strategies of Cancer. Cancer Metastasis Rev. 2014;33(2–3):809–822. doi: 10.1007/s10555-014-9505-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lamberts LE, Williams SP, Terwisscha van Scheltinga AGT, Lub-de Hooge MN, Schröder CP, Gietema JA, Brouwers AH, de Vries EG. Antibody Positron Emission Tomography Imaging in Anticancer Drug Development. J Clin Oncol. 2015;33(13):1491–1504. doi: 10.1200/JCO.2014.57.8278. [DOI] [PubMed] [Google Scholar]
- 21.Boerman OC, Oyen WJ. Immuno-PET of Cancer: A Revival of Antibody Imaging. J Nucl Med. 2011;52(8):1171–1172. doi: 10.2967/jnumed.111.089771. [DOI] [PubMed] [Google Scholar]
- 22.van Dongen GA, Visser GW, Lub-de Hooge MN, de Vries EG, Perk LR. Immuno-PET: A Navigator in Monoclonal Antibody Development and Applications. The Oncologist. 2007;12(12):1379–1389. doi: 10.1634/theoncologist.12-12-1379. [DOI] [PubMed] [Google Scholar]
- 23.Capala J, Bouchelouche K. Molecular imaging of HER2-positive breast cancer - a step toward an individualized “Image and Treat” strategy. Current Opin Oncol. 2010;22(6):559–566. doi: 10.1097/CCO.0b013e32833f8c3a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deri MA, Zeglis BM, Francesconi LC, Lewis JS. PET Imaging with (89)Zr: From Radiochemistry to the Clinic. Nucl Med Biol. 2013;40(1):3–14. doi: 10.1016/j.nucmedbio.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kurihara H, Hamada A, Yoshida M, Shimma S, Hashimoto J, Yonemori K, Tani H, Miyakita Y, Kanayama Y, Wada Y, Kodaira M, Yunokawa M, Yamamoto H, Shimizu C, Takahashi K, Watanabe Y, Fujiwara Y, Tamura K. 64Cu-DOTA-trastuzumab PET imaging and HER2 specificity of brain metastases in HER2-positive breast cancer patients. EJNMMI Res. 2015;5(1):1–8. doi: 10.1186/s13550-015-0082-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tamura K, Kurihara H, Yonemori K, Tsuda H, Suzuki J, Kono Y. 64Cu-DOTA-trastuzumab PET imaging in patients with HER2-positive breast cancer. J Nucl Med. 2013;54(11):1869–1875. doi: 10.2967/jnumed.112.118612. [DOI] [PubMed] [Google Scholar]
- 27.Mortimer JE, Bading JR, Colcher DM, Conti PS, Frankel PH, Carroll MI, Tong S, Poku E, Miles JK, Shively JE, Raubitschek AA. Functional imaging of human epidermal growth factor receptor 2–positive metastatic breast cancer using 64Cu-DOTA-trastuzumab PET. J Nucl Med. 2014;5(1):23–29. doi: 10.2967/jnumed.113.122630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sandström M, Lindskog K, Velikyan I, Wennborg A, Feldwisch J, Sandberg D, Tolmachev V, Orlova A, Sörensen J, Carlsson J, Lindman H, Lubberink M. Biodistribution and Radiation Dosimetry of the Anti-HER2 Affibody Molecule 68Ga-ABY-025 in Breast Cancer Patients. J Nucl Med. 2016;57(6):867–871. doi: 10.2967/jnumed.115.169342. [DOI] [PubMed] [Google Scholar]
- 29.Sörensen J, Sandberg D, Sandström M, Wennborg A, Feldwisch J, Tolmachev V, Åström G, Lubberink M, Garske-Román U, Carlsson J, Lindman H. First-in-Human Molecular Imaging of HER2 Expression in Breast Cancer Metastases Using the 111In-ABY-025 Affibody Molecule. J Nucl Med. 2014;55(5):730–735. doi: 10.2967/jnumed.113.131243. [DOI] [PubMed] [Google Scholar]
- 30.Tolmachev V, Velikyan I, Sandström M, Orlova A. A HER2-binding Affibody molecule labelled with 68Ga for PET imaging: direct in vivo comparison with the 111In-labelled analogue. Eur J Nucl Med. 2010;37(7):1356–1367. doi: 10.1007/s00259-009-1367-7. [DOI] [PubMed] [Google Scholar]
- 31.Sörensen J, Velikyan I, Sandberg D, Wennborg A, Feldwisch J, Tolmachev V, Orlova A, Sandström M, Lubberink M, Olofsson H, Carlsson J, Lindman H. Measuring HER2-Receptor Expression In Metastatic Breast Cancer Using [(68)Ga]ABY-025 Affibody PET/CT. Theranostics. 2016;6(2):262–271. doi: 10.7150/thno.13502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wong JY, Raubitschek A, Yamauchi D, Williams LE, Wu AM, Yazaki P, Shively JE, Colcher D, Somlo G. A Pretherapy Biodistribution and Dosimetry Study of Indium-111-Radiolabeled Trastuzumab in Patients with Human Epidermal Growth Factor Receptor 2-Overexpressing Breast Cancer. Cancer Biother Radiopharm. 2010;25(4):387–394. doi: 10.1089/cbr.2010.0783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gebhart G, Lamberts LE, Wimana Z, Garcia C, Emonts P, Ameye L, Stroobants S, Huizing M, Aftimos P, Tol J, Oyen WJ, Vugts DJ, Hoekstra OS, Schröder CP, Menke-van der Houven van Oordt CW, Guiot T, Brouwers AH, Awada A, de Vries EG, Flamen P. Molecular imaging as a tool to investigate heterogeneity of advanced HER2-positive breast cancer and to predict patient outcome under trastuzumab emtansine (T-DM1): the ZEPHIR trial. Ann Oncol. 2016;27(4):619–624. doi: 10.1093/annonc/mdv577. [DOI] [PubMed] [Google Scholar]
- 34.Baselga J. Phase I and II clinical trials of trastuzumab. Ann Oncol. 2001;12(suppl 1):S49–S55. doi: 10.1093/annonc/12.suppl_1.s49. [DOI] [PubMed] [Google Scholar]
- 35.Swain SM, Baselga J, Kim SB, Ro J, Semiglazov V, Campone M, Ciruelos E, Ferrero JM, Schneeweiss A, Heeson S, Clark E, Ross G, Benyunes MC, Cortés J. Pertuzumab, Trastuzumab, and Docetaxel in HER2-Positive Metastatic Breast Cancer. N Engl J Med. 2015;372(8):724–734. doi: 10.1056/NEJMoa1413513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oude Munnink TH, Korte MA, Nagengast WB, Timmer-Bosscha H, Schröder CP, de Jong JR, Dongen GAMS, Jensen MR, Quadt C, Hooge MN, de Vries EG. 89Zr-trastuzumab PET visualises HER2 downregulation by the HSP90 inhibitor NVP-AUY922 in a human tumour xenograft. Eur J Cancer. 2010;46(3):678–684. doi: 10.1016/j.ejca.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 37.Gaykema SB, Brouwers AH, Hovenga S, Lub-de Hooge MN, de Vries EG. Zirconium-89-Trastuzumab Positron Emission Tomography As a Tool to Solve a Clinical Dilemma in a Patient With Breast Cancer: A Case Report. J Clin Oncol. 2012;30(6):e74–e75. doi: 10.1200/JCO.2011.38.0204. [DOI] [PubMed] [Google Scholar]
- 38.Dijkers EC, Oude Munnink TH, Kosterink JG, Brouwers AH, Jager PL, de Jong JR, van Dongen GA, Schröder CP, Lub-de Hooge MN, de Vries EG. Biodistribution of 89Zr-trastuzumab and PET Imaging of HER2-Positive Lesions in Patients With Metastatic Breast Cancer. Clin Pharmacol Ther. 2010;87(5):1532–6535. doi: 10.1038/clpt.2010.12. [DOI] [PubMed] [Google Scholar]
- 39.Washington University School of Medicine, National Cancer Institute (NCI) ClinicalTrials.gov [Internet] St. Louis (MO): Washington University School of Medicine (US); 2016. 89Zr-Trastuzumab Breast Imaging With Positron Emission Tomography. [cited 2016, Nov 3]. Available from: https://clinicaltrials.gov/ct2/show/NCT02065609. NLM Identifier: NCT02065609. Accessed September 29, 2017. [Google Scholar]
- 40.Jules Bordet Institute. ClinicalTrials.gov [Internet] Brussels, Belgium: Institut Jules Bordet; 2016. Pilot Imaging Study With 89Zr-Trastuzumab in HER2-positive Metastatic Breast Cancer Patients (IJBMNZrT003) [cited 2016, Nov 3]. Available from: https://clinicaltrials.gov/ct2/show/NCT01420146. NLM Identifier: NCT01420146. [Google Scholar]
- 41.Ulaner GA, Hyman DM, Lyashchenko SK, Lewis JS, Carrasquillo JA. 89Zr-Trastuzumab PET/CT for Detection of Human Epidermal Growth Factor Receptor 2-Positive Metastases in Patients With Human Epidermal Growth Factor Receptor 2-Negative Primary Breast Cancer. Clin Nucl Med. 2017 doi: 10.1097/RLU.0000000000001820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cornelis FH, Durack J, Pandit-Taskar N, Ulaner GA, Lewis JS, Morris MJ, Solomon SB. Long Half-life 89Zr Labeled Radiotracers Can Guide In Suite Percutaneous Molecular Imaging PET/CT-guided Biopsies Without Reinjection of Radiotracer. J Nucl Med. 2017 doi: 10.2967/jnumed.117.194480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Laforest R, Lapi SE, Oyama R, Bose R, Tabchy A, Marquez-Nostra BV, Burkemper J, Wright BD, Frye J, Frye S, Siegel BA, Dehdashti F. [89Zr]Trastuzumab: Evaluation of Radiation Dosimetry, Safety, and Optimal Imaging Parameters in Women with HER2-Positive Breast Cancer. Mol Imaging Biol. 2016;18(6):952–959. doi: 10.1007/s11307-016-0951-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dijkers EC, Kosterink JG, Rademaker AP, Perk LR, van Dongen GA, Bart J, de Jong JR, de Vries EG, Lub-de Hooge MN. Development and Characterization of Clinical-Grade 89Zr-Trastuzumab for HER2/neu ImmunoPET Imaging. J Nucl Med. 2009;50(6):974–981. doi: 10.2967/jnumed.108.060392. [DOI] [PubMed] [Google Scholar]
- 45.Dijkers EC, Oude Munnink TH, Kosterink JG, Brouwers AH, Jager PL, de Jong JR. Biodistribution of 89Zr-trastuzumab and PET imaging of HER2-positive lesions in patients with metastatic breast cancer. Clin Pharmacol Ther. 2010;87(5):586–592. doi: 10.1038/clpt.2010.12. [DOI] [PubMed] [Google Scholar]
- 46.Hudis CA. Trastuzumab — Mechanism of Action and Use in Clinical Practice. N Engl J Med. 2007;357(1):39–51. doi: 10.1056/NEJMra043186. [DOI] [PubMed] [Google Scholar]
- 47.Ulaner GA, Lyashchenko SK, Riedl C, Ruan S, Zanzonico PB, Lake D, Jhaveri K, Zeglis BM, Lewis JS, O’Donoghue JA. First-in-human HER2-targeted imaging using 89Zr-pertuzumab PET/CT: Dosimetry and clinical application in patients with breast cancer. J Nucl Med. doi: 10.2967/jnumed.117.202010. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sasada S, Kurihara H, Kinoshita T, Yoshida M, Honda N, Shimoi T, Shimomura A, Yonemori K, Shimizu C, Hamada A, Kanayama Y, Watanabe Y, Fujiwara Y, Tamura K. Visualization of HER2-specific breast cancer intratumoral heterogeneity using 64Cu-DOTA-trastuzumab PET. Eur J Nucl Med Mol Imaging. 2017 doi: 10.1007/s00259-017-3781-6. [DOI] [PubMed] [Google Scholar]
- 49.Mortimer JE, Bading JR, Park JM, Frankel PH, Carroll MI, Tran TT, Poku EK, Rockne RC, Raubitschek AA, Shively JE, Colcher DM. Tumor Uptake of 64Cu-DOTA-Trastuzumab in Patients with Metastatic Breast Cancer. J Nucl Med. 2017 doi: 10.2967/jnumed.117.193888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Scheidhauer K, Scharl A, Pietrzyk U, Wagner R, Gohring UJ, Schomacker K, Schicha H. Qualitative [18F]FDG positron emission tomography in primary breast cancer: clinical relevance and practicability. Eur J Nucl Med. 1996;23(6):618–623. doi: 10.1007/BF00834522. [DOI] [PubMed] [Google Scholar]
- 51.Moon D, Maddahi J, Silverman DH, Glaspy JA, Phelps ME, Hoh CK. Accuracy of whole-body FDG PET for the detection of recurrent or metastatic breast carcinoma. J Nucl Med. 1998;39(3):431–435. [PubMed] [Google Scholar]
- 52.Shreve PD, Anzai Y, Wahl RL. Pitfalls in Oncologic Diagnosis with FDG PET Imaging: Physiologic and Benign Variants. RadioGraphics. 1999;19(1):61–77. doi: 10.1148/radiographics.19.1.g99ja0761. [DOI] [PubMed] [Google Scholar]
- 53.Bhusari P, Vatsa R, Singh G, Parmar M, Bal A, Dhawan DK, Mittal BR, Shukla J. Development of Lu-177-trastuzumab for radioimmunotherapy of HER2 expressing breast cancer and its feasibility assessment in breast cancer patients. Int J Cancer. 2017;140(4):938–947. doi: 10.1002/ijc.30500. [DOI] [PubMed] [Google Scholar]
- 54.Lee H, Shields AF, Siegel BA, Miller KD, Krop I, Ma CX, LoRusso PM, Munster PN, Campbell K, Gaddy DF, Leonard SC, Geretti E, Blocker SJ, Kirpotin DB, Moyo V, Wickham TJ, Hendriks BS. Cu-MM-302 Positron Emission Tomography Quantifies Variability of Enhanced Permeability and Retention of Nanoparticles in Relation to Treatment Response in Patients with Metastatic Breast Cancer. Clin Cancer Res. 2017;23(15):4190–4202. doi: 10.1158/1078-0432.CCR-16-3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.University of California, San Francisco. ClinicalTrials.gov [Internet] San Francisco (CA): University of California, San Francisco (US); 2016. 64-Cu Labeled Brain PET/MRI for MM-302 in Advanced HER2+ Cancers With Brain Mets. [cited 2016, Nov 3]. Available from: https://clinicaltrials.gov/ct2/show/NCT02735798. NLM Identifier: NCT02735798. Accessed September 29, 2017. [Google Scholar]
- 56.Merrimack Pharmaceuticals. ClinicalTrials.gov [Internet] Cambridge (MA): Merrimack Pharmaceuticals (US); 2016. MM-302 Plus Trastuzumab vs. Chemotherapy of Physician’s Choice Plus Trastuzumab in HER2-Positive Locally Advanced/Metastatic Breast Cancer Patients (HERMIONE) [cited 2016, Nov 3]. Available from: https://clinicaltrials.gov/ct2/show/NCT02213744. NLM Identifier: NCT02213744. Accessed September 29, 2017. [Google Scholar]
- 57.Merrimack Pharmaceuticals. ClinicalTrials.gov [Internet] Cambridge (MA): Merrimack Pharmaceuticals (US); 2016. Safety and Pharmacokinetic Study of MM-302 in Patients With Advanced Breast Cancer. [cited 2016, Nov 3]. Available from: https://clinicaltrials.gov/ct2/show/NCT01304797. NLM Identifier: NCT01304797. 2016. Accessed September 29, 2017. [Google Scholar]
- 58.Rainone P, Riva B, Belloli S, Sudati F, Ripamonti M, Verderio P, Colombo M, Colzani B, Gilardi MC, Moresco RM, Prosperi D. Development of (99m)Tc-radiolabeled nanosilica for targeted detection of HER2-positive breast cancer. Int J Nanomedicine. 2017;12:3447–3461. doi: 10.2147/IJN.S129720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li DL, Tan JE, Tian Y, Huang S, Sun PH, Wang M, Han YJ, Li HS, Wu HB, Zhang XM, Xu YK, Wang Multifunctional superparamagnetic nanoparticles conjugated with fluorescein-labeled designed ankyrin repeat protein as an efficient HER2-targeted probe in breast cancer. Biomaterials. 2017;147:86–98. doi: 10.1016/j.biomaterials.2017.09.010. [DOI] [PubMed] [Google Scholar]
- 60.Terwisscha van Scheltinga AGT, Lub-de Hooge MN, Abiraj K, Schröder CP, Pot L, Bossenmaier B, Thomas M, Hölzlwimmer G, Friess T, Kosterink JG, de Vries EG. ImmunoPET and biodistribution with human epidermal growth factor receptor 3 targeting antibody 89Zr-RG7116. MAbs. 2014;6(4):1051–1058. doi: 10.4161/mabs.29097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Warnders FJ, Terwisscha van Scheltinga AGT, Knuehl C, van Roy M, de Vries EFJ, Kosterink JGW, de Vries EG, Lub-de Hooge MN. Human Epidermal Growth Factor Receptor 3– Specific Tumor Uptake and Biodistribution of 89Zr-MSB0010853 Visualized by Real-Time and Noninvasive PET Imaging. J Nucl Med. 2017;58(8):1210–1215. doi: 10.2967/jnumed.116.181586. [DOI] [PubMed] [Google Scholar]
- 62.Wehrenberg-Klee E, Turker NS, Chang B, Heidari P, Mahmood U. Development of a HER3 PET probe for breast cancer imaging. J Nucl Med. 2014;55(supplement 1):550. [Google Scholar]
- 63.Wehrenberg-Klee E, Turker NS, Heidari P, Larimer B, Juric D, Baselga J, Scaltriti M, Mahmood U. Differential Receptor Tyrosine Kinase PET Imaging for Therapeutic Guidance. J Nucl Med. 2016;57(9):1413–1419. doi: 10.2967/jnumed.115.169417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pool M, Kol A, de Jong JR, de Vries EG, Lub-de Hooge MN. Terwisscha van Scheltinga AGT, 89Zr-mAb3481 PET for HER3 tumor status assessment during lapatinib treatment. MAbs. 2017:1–9. doi: 10.1080/19420862.2017.1371382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Alsaid H, Skedzielewski T, Rambo MV, Hunsinger K, Hoang B, Fieles W, Long ER, Tunstead J, Vugts DJ, Cleveland M, Clarke N, Matheny C, Jucker BM. Non invasive imaging assessment of the biodistribution of GSK2849330, an ADCC and CDC optimized anti HER3 mAb, and its role in tumor macrophage recruitment in human tumor-bearing mice. PLoS One. 2017;12(4):e0176075. doi: 10.1371/journal.pone.0176075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Orlova A, Malm M, Rosestedt M, Varasteh Z, Andersson K, Selvaraju RK, Altai M, Honarvar H, Strand J, Ståhl S, Tolmachev V, Löfblom J. Imaging of HER3-expressing xenografts in mice using a 99mTc(CO)3-HEHEHE-ZHER3:08699 affibody molecule. Eur J Nucl Med. 2014;41(7):1450–1459. doi: 10.1007/s00259-014-2733-7. [DOI] [PubMed] [Google Scholar]
- 67.Rosestedt M, Andersson KG, Mitran B, Tolmachev V, Löfblom J, Orlova A, Ståhl S. Affibody-mediated PET imaging of HER3 expression in malignant tumours. Sci Rep. 2015;5:15226. doi: 10.1038/srep15226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Andersson KG, Rosestedt M, Varasteh O, Malm M, Sandström M, Tolmachev V, Löfblom J, Ståhl S, Orlova A. Comparative evaluation of 111In-labeled NOTA-conjugated affibody molecules for visualization of HER3 expression in malignant tumors. Oncol Rep. 2015;34(2):1042–1048. doi: 10.3892/or.2015.4046. [DOI] [PubMed] [Google Scholar]
- 69.Da Pieve C, Allott L, Martins CD, Vardon A, Ciobota DM, Kramer-Marek G, Smith G. Efficient [18F]AlF Radiolabeling of ZHER3:8698 Affibody Molecule for Imaging of HER3 Positive Tumors. Bioconjug Chem. 2016;27(8):1839–1849. doi: 10.1021/acs.bioconjchem.6b00259. [DOI] [PubMed] [Google Scholar]


