Abstract
BACKGROUND
Calcific aortic stenosis is a chronic inflammatory disease. Pro-inflammatory stimulation via Toll-like receptor 4 (TLR4) causes the aortic valve interstitial cell (AVIC) to undergo phenotypic change. The AVIC assumes an inflammatory phenotype characterized by the production of inflammatory mediators such as intercellular adhesion molecule-1 (ICAM-1), interleukin-8 (IL-8), and monocyte chemoattractant protein-1 (MCP-1). This change has been linked with an osteogenic phenotypic response. Statins have recently been shown to have anti-inflammatory properties. We therefore hypothesized that statins may have an anti-inflammatory effect on human AVICs by down-regulation of TLR4-stimulated inflammatory responses. Our purposes were: (1) to determine the effect of simvastatin on TLR4-induced expression of inflammatory mediators in human AVICs, and (2) to determine the mechanism(s) through which simvastatin exert this effect.
MATERIALS AND METHODS
Human AVICs were isolated from the explanted hearts of four patients undergoing cardiac transplantation. Cells were treated with simvastatin (50μM) for 1 hour prior to stimulation with TLR4 agonist lipopolysaccharide (LPS, 0.2 μg/mL). Immunoblotting (IB) was used to analyze cell lysates for ICAM-1 expression, and ELISA was used to detect IL-8 and MCP-1 in cell culture media. Likewise, lysates were analyzed for TLR4 and nuclear factor-kappa B (NF-kB) activation (IB). After simvastatin treatment, lysates were analyzed for TLR4 levels (IB). Statistics were by ANOVA (p<0.05).
RESULTS
Simvastatin reduced TLR4-induced ICAM-1, IL-8, and MCP-1 expression in AVICs. Simvastatin down-regulated TLR4 levels and suppressed TLR4-induced phosphorylation of NF-kB.
CONCLUSIONS
These data demonstrate the potential of a medical therapy (simvastatin) to impact the pathogenesis of aortic stenosis.
Keywords: aortic stenosis, valve interstitial cell, statin
INTRODUCTION
Calcific aortic stenosis is diagnosed in at least 2% of the US population over the age of 751. It is the third most common cardiovascular disease, behind only coronary artery disease and hypertension, and it is the most common indication for aortic valve replacement2. Heretofore, no pharmacologic therapy has been identified for the prevention or treatment of aortic stenosis.
The pathogenesis of calcific aortic stenosis is not well defined. Although traditionally considered a degenerative disease in which calcium passively accumulates on the aortic valve leaflets, recent data suggest that aortic stenosis may be a chronic inflammatory disease. The aortic valve interstitial cell (AVIC) has been implicated in the pathogenesis of aortic stenosis3,4. In response to toll-like receptor-4 (TLR4) stimulation, AVICs adopt an inflammatory phenotype, characterized by the production of inflammatory proteins like intercellular adhesion molecule-1 (ICAM-1), interleukin-8 (IL-8), and monocyte-chemoattractant protein-1 (MCP-1)5,6. This inflammatory phenotypic change is linked to an osteogenic phenotypic change which is thought to be involved in valve calcification5,7. Prior studies have yet to identify a pharmacologic therapy that could interrupt these pathologic changes. Such a discovery could have an impact on the prevention or treatment of aortic stenosis.
Commonly used for the treatment of hypercholesterolemia, statins have been shown to possess important anti-inflammatory properties8. Given the role of inflammation in the pathogenesis of aortic stenosis, other investigators have explored a possible therapeutic role for of statins in aortic stenosis with mixed results. While some clinical investigators have demonstrated no effect of statin therapy on the progression of aortic stenosis9,10, others have suggested a benefit11. However, at the cellular level, some recent data suggest that statins may reverse pro-calcific pathways in human AVICs12,13. Further, simvastatin has been shown to inhibit calcium nodule formation in porcine AVICs14. These data led us to hypothesize that statins may have anti-inflammatory actions in human AVICs. Given the important role of TLR4 in mediating pro-inflammatory responses in AVICs, we further hypothesized that the anti-inflammatory effects of statins are mediated via the down-regulation of TLR4-stimulated responses in human AVICs.
The purposes of this study were to (1) evaluate the effect of simvastatin therapy on TLR-4 induced inflammatory protein expression in human AVICs, and (2) to determine the mechanism(s) through which this effect is mediated. The results of this study demonstrate that simvastatin can reduce TLR4-induced inflammatory responses in human AVICs, and this effect may be mediated through both down-regulation of total TLR4 expression and modulation of nuclear factor-kappa B (NF-κB) activation.
MATERIALS AND METHODS
This work was approved by the Colorado Multiple Institutional Review Board at the University of Colorado Health Sciences Center.
Reagents
Earle’s balanced salt solution (EBSS), culture medium 199, penicillin G, streptomycin, and amphotericin B were all purchased from Lonza (Walkersville, MD). Fetal bovine serum (FBS) was obtained from Aleken chemicals (Nash, TX). Laemmli sample buffer, nitrocellulose membranes, and mini-protean gels (4–20% gradient) were bought from Bio-rad (Hercules, CA). Chemiluminescent substrate (ECL) and LDH assay kits were purchased from Thermo Scientific (Rockford, IL). Rabbit derived antibodies against human ICAM-1 and TLR-4 were obtained from Santa Cruz (Dallas, TX). Rabbit-derived antibodies against human phospho- and total nuclear factor kappa B (NFκB), and rabbit derived antibody against human beta-actin (β-actin) were purchased from Cell Signaling (Danvers, MA). Enzyme linked immunosorbent assay (ELISA) kits were purchased from R&D Systems (Minneapolis, MN). All other reagents, including simvastatin, were purchased from Sigma Aldrich (St. Louis, MO).
Cell Isolation and Culture
Human AVICs were isolated and cultured as described previously5. Preoperative consent was obtained according to institutional review board protocol. At the time of heart transplantation, the recipient’s native heart was removed and the aortic valves were inspected to ensure that no calcium nodules or fibrotic areas were identified. H&E staining is performed on our donors to ensure normal cellular architecture within the valve as described previously5. The aortic valve leaflets were carefully excised and placed into a sterile saline container. This container was transported on ice from the operating room to the laboratory. The leaflets were washed five times with EBSS, and then sectioned into small pieces. The pieces were placed into a 15mL conical tube and digested using collagenase diluted in full strength cell culture medium (medium 199 with penicillin G, streptomycin, amphotericin B, and 10% FBS) to a concentration of 2.5mg/mL. After 30 minutes of digestion with gentle agitation at a temperature of 37 degrees C, the mixture was centrifuged at 500 RPM for 2 minutes. Supernatant containing the stripped endothelial cells was then discarded. The tissue was again mixed with a lower concentration of collagenase (0.8mg/mL) for three hours. After this time, the cells were again centrifuged at 500 RPM for 2 minutes. The supernatant was then centrifuged again for 8 minutes at 1100 RPM. The pellet was re-suspended with culture medium and then placed into a small (25cm^2) flask along with 5 mL of culture media. This flask was placed into an incubator maintained at 37 degrees C and 5% CO2. AVIC phenotype was verified using immunofluorescent staining as described previously5. Cells are stained for actin and vimentin in order to identify them as myofibroblasts (the most common phenotype for valve interstitial cells). Baseline expression of inflammatory proteins such as ICAM-1 is almost negligible in isolated human AVICs as demonstrated by immunofluorescence imaging in a prior study16. In addition, normal human AVICs express low baseline expression of other inflammatory proteins like TGF-β1 as previously demonstrated by western blotting17. Cells were grown to passages 2–6 prior to use for experiments.
Immunoblotting
Cells were plated onto 24-well plates and lysed using laemli buffer (1X) along with β-mercaptoethanol. Lysates were loaded into a 15-well mini Protean TGX gel and run at 180V for 45 minutes. Gels were transferred to nitrocellulose membranes at 100V for 70 minutes. Membranes were removed and rinsed with DI-water then cross-linked twice using a UV Stratalinker (Stratagene, La Jolla, CA). Membranes were then blocked with 5% milk dissolved in 0.1% Tween in phosphate-buffered saline (T-PBS) for 45 minutes. After blocking, membranes were washed with phosphate-buffered saline (PBS) three times for 15 minutes per wash. Then primary antibody was applied overnight. Membranes were washed twice with PBS for 5 minutes per wash, then horseradish peroxidase-conjugated rabbit secondary antibody diluted in T-PBS was added for 1 hour at room temperature. The membranes were washed three times with T-PBS before applying ECL for two minutes at room temperature. Membranes were imaged using a Bio-rad Chemi-Doc (Hercules, CA). NIH Image-J software (Bethesda, MD) was used to perform densitometry analysis.
Simvastatin’s effect on TLR4-induced ICAM-1 expression
Preliminary experiments determined that simvastatin at a dose of 50μM was most effective at reducing inflammatory protein expression (doses of 10μM and 30μM were tested – see supplemental data). Human AVICs were treated with simvastatin (50μM) for 1 hour in cell culture media (5% FBS). Media was removed and replaced with fresh cell culture media (5% FBS). Cells were treated for 24 hours with TLR4 agonist lipopolysaccharide (LPS, 0.2μg/mL). Lysates were analyzed for ICAM-1 using immunoblotting and subsequent densitometry analysis was performed. LPS was dissolved in PBS and diluted using culture media. Dimethyl sulfoxide (DMSO) was utilized as a vehicle for simvastatin. DMSO was therefore utilized as a vehicle control.
Simvastatin’s effect on TLR4 receptor levels during TLR4 stimulation
Cells were plated onto 24 well plates and pre-treated with simvastatin (50 μM) for one hour. Media was replaced, and cells were treated with LPS (0.2μg/mL) for 24 hours. Lysates were analyzed for TLR4 receptor levels using immunoblotting.
Simvastatin treatment effect on TLR4 receptor levels
Human AVICs were plated onto 24 well plates and treated with simvastatin (50 μM) for 1 hour. Media was then replaced and cells were lysed at different lengths of time (15 minutes, 30 minutes, 1 hour, 2 hour, 4 hours, and 8 hours). Lysates were then analyzed for TLR4 receptor levels using western blotting.
NF-κB activation modulation by simvastatin
After pretreatment with simvastatin for 1 hour, cells were treated with LPS for 1 hour. Lysates were analyzed using immunoblotting for phosphorylated and total nuclear factor kappa B (NFκB).
Enzyme-Linked Immunosorbent Assay (ELISA)
AVICs treatments were performed in 24-well plates. At the completion of treatment, 100 microliters of cell culture media was removed and placed into a well of the ELISA plate. All samples were duplicated. The ELISA assay was performed according to the published protocol from DuoSet ELISA Development (R&D Systems, Minneapolis, MN). Optical densities were obtained with an Eon Biotek plate reader (Winooski, VT). Optical densities were averaged for each sample duplicate, and this value was as used for analysis.
Simvastatin’s impact on TLR4-induced IL-8 and MCP-1 expression in human AVICs
Cells were pre-treated with simvastatin 50 μM) for 1 hour. Media was removed and cells were treated for 24 hours with TLR4 agonist lipopolysaccharide (LPS, 0.2μg/mL) in cell culture media with 5% FBS. Media was removed and ELISA was performed to analyze the expression of secreted cytokines IL-8 and MCP-1.
LDH Assay
LDH assay was performed according to the published instructions for determining chemical compound-mediated cytotoxicity (Thermo Scientific, Rockford, IL). Human AVICs were pre-treated with simvastatin for one hour. Media was collected 24 hours later and analyzed using the chemical compound-mediated cytotoxicity LDH assay according to manufacturer instructions. DMSO was also used as vehicle control.
Statistical Analysis
Data were analyzed using Graphpad Prism software (La Jolla, CA). Data are presented as means +/− standard error of the mean (SEM), Groups were compared using ANOVA with Tukey’s post-hoc test. Statistical significance was achieved at a p-value of 0.05.
RESULTS
Donor Information
Aortic valve leaflets were obtained from four patients undergoing heart transplant at the University of Colorado Hospital. These tissue donors all suffered from idiopathic dilated cardiomyopathy. All of the donors were non-smoking males. Preoperative coronary angiography demonstrated no coronary artery disease, and preoperative echo showed normal aortic valve structure and function. The ages of the donors were 28, 53, 57, and 61 years. At the time of excision, valve leaflets were thin, pliable, and had no gross evidence of calcification or fibrosis.
Simvastatin reduces TLR-4 induced inflammatory protein expression in human AVICs
Pre-treatment with simvastatin (50μM) reduced TLR4-induced ICAM-1 expression (Figure 1). In addition, simvastatin treatment resulted in a decrease in secretion of inflammatory cytokines MCP-1 and IL-8 into the culture media (Figure 2). Simvastatin also demonstrated minimal toxicity to AVICs, as determined by the LDH assay (Figure 3).
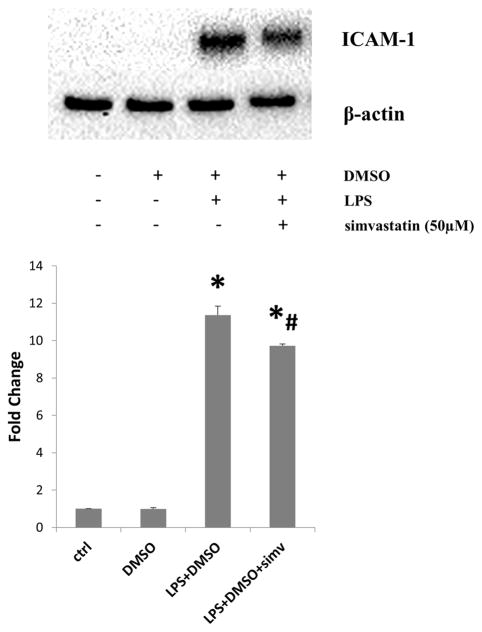
Figure 1.
Simvastatin attenuates toll-like receptor-4 (TLR4)-induced intercellular adhesion moledule-1 (ICAM-1) expression in human aortic valve interstitial cells (AVICs). AVICs were treated with simvastatin (50μM) for 1 hour prior to stimulation with lipopolysaccharide (LPS)(0.2μg/mL) for 24 hours. Lysates were analyzed for ICAM-1 levels using immunoblotting and corresponding densitometry analysis. Treatment with LPS induced the expression of ICAM-1 (LPS+DMSO vs. ctrl, LPS+DMSO vs. DMSO; LPS+DMSO+simv vs. ctrl, LPS+DMSO+simv vs. DMSO; *p<0.05), however pre-treatment with simvastatin (50μM) attenuated TLR4-induced ICAM-1 expression in human AVICs (LPS+DMSO+simv vs. LPS+DMSO; #p<0.05). Four donors were utilized (N=4).
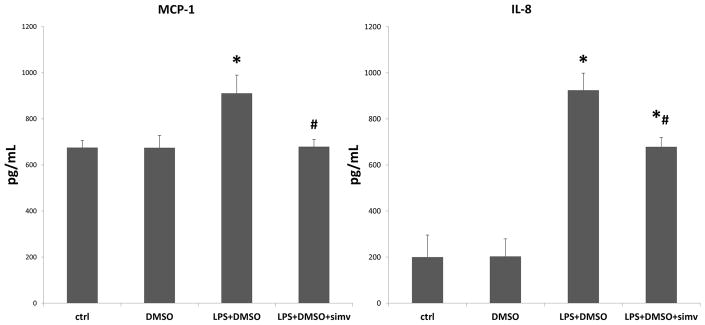
Figure 2.
Simvastatin reduces toll-like receptor-4 (TLR4)-induced expression of inflammatory cytokines in aortic valve interstitial cells (AVICs). Cells were treated with simvastatin (50μM) for 1 hour prior to treatment with lipopolysaccharide (LPS) for 24 hours. Cell culture media was analyzed using enzyme-linked immunosorbent assay (ELISA) for inflammatory cytokines interleukin-8 (IL-8) and monocyte-chemoattractant protein-1 (MCP-1). TLR4 stimulation with LPS induced a significant increase in both IL-8 and MCP-1 secretion in AVICs (LPS+DMSO vs. ctrl, LPS+DMSO vs. DMSO,*p<0.05). However, treatment with simvastatin (50μM) reduced the TLR4-induced expression of both IL-8 and MCP-1 (LPS+DMSO+simv vs. LPS+DMSO, #p<0.05). Four donors were utilized (N=4).
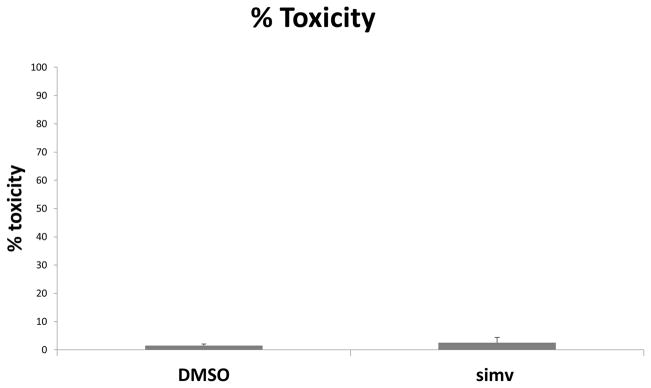
Figure 3.
Simvastatin is non-toxic to human aortic valve interstitial cells (AVICs). AVICs were treated with simvastatin (50μM) or equivalent concentration of vehicle control dimethyl sulfoxide (DMSO) for 1 hour prior. Media was removed 24 hours later and LDH assay was performed. Bar graph shows % toxicities for DMSO and simvastatin. Four donors were utilized (N=4).
Simvastatin and TLR-4 receptor expression and signaling effects
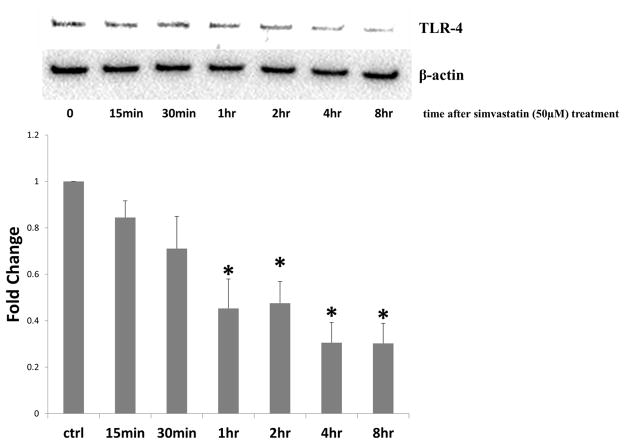
Pre-treatment with simvastatin had specific effects on TLR4 expression and signaling in human AIVCs. During TLR-4 stimulation, simvastatin treatment resulted in a decrease in TLR4 receptor expression (Figure 4). In the next experiment, we removed TLR4 stimulation from the equation by treating cells with simvastatin alone. TLR4 receptor levels were found to decrease significantly after 1 hour of treatment. Receptor levels remained reduced for up to 8 hours after simvastatin treatment (Figure 5). Simvastatin also had an impact on TLR4 signaling. Pre-treatment with simvastatin significantly reduced TLR-4-induced phosphorylation of NF-κB (Figure 6).
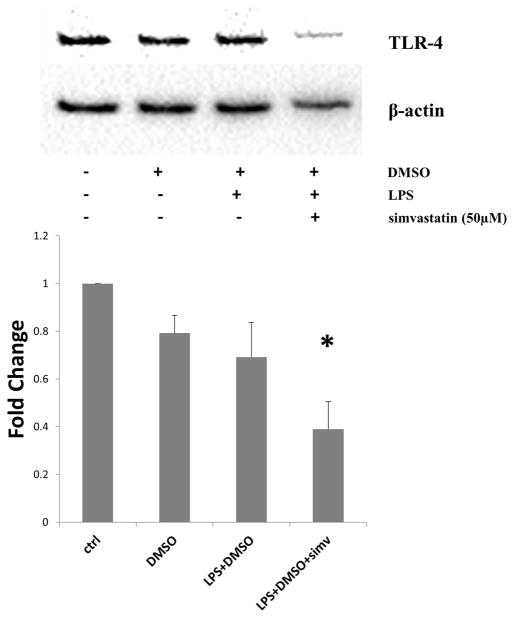
Figure 4.
Simvastatin reduces toll-like receptor 4 (TLR4) expression during lipopolysaccharide (LPS) stimulation in human aortic valve interstitial cells (AVICs). Cells were treated with simvastatin (50μM) for 1 hour prior to TLR4 stimulation with LPS (0.2μg/mL) for 24 hours. Lysates were analyzed for TLR4 expression using western blotting with subsequent densitometry analysis. TLR-4 expression was significantly reduced by using pre-treatment with simvastatin (50μM) (LPS+DMSO+simv vs. ctrl, LPS+DMSO+simv vs. DMSO *p<0.05). Four donors were utilized (N=4).
Figure 5.
Simvastatin treatment alone reduces TLR-4 expression in human aortic valve interstitial cells (AVICs). Cells were treated with simvastatin (50μM) for 1 hour, then AVICs were lysed at 15 minutes, 30 minutes, 1 hour, 2 hours, 4 hours, and 8 hours after treatment. Lysates were analyzed using immunoblotting and densitometry analysis. Simvastatin treatment reduced total cellular TLR-4 expression after 1 hour of treatment, and TLR4 expression remained reduced at 2, 4, and 8 hours (1 hr, 2 hr, 4 hr, and 8 hr treatments vs. ctrl, *p<0.05). Four donors were utilized (N=4).
Figure 6.
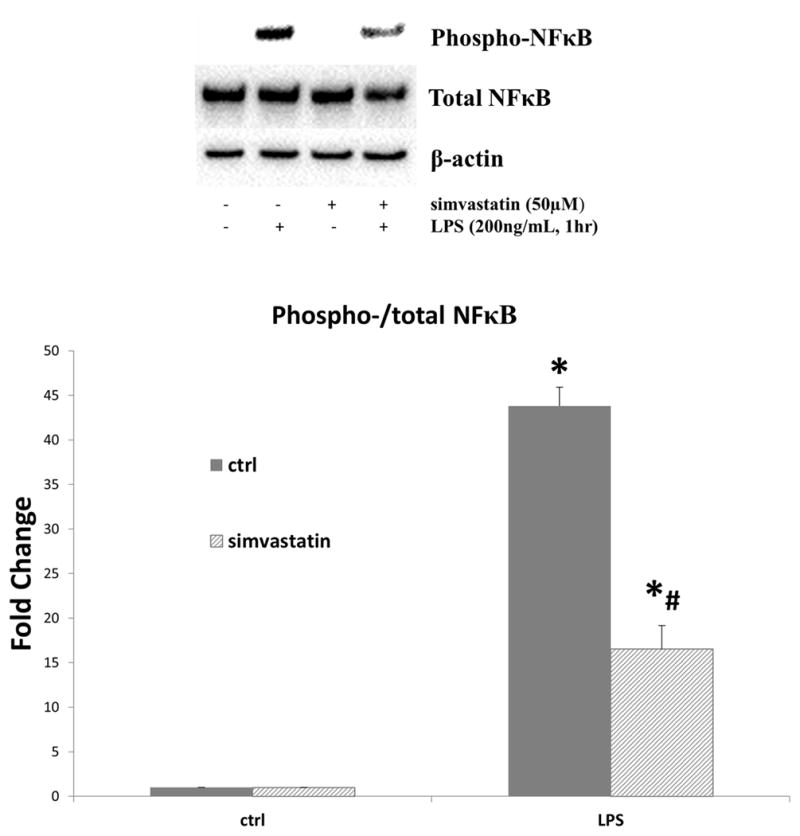
In human aortic valve interstitial cells (AVICs), simvastatin reduces toll-like receptor-4 (TLR-4) induced activation of nuclear factor kappa B (NF-κB). AVICs were treated with simvastatin (50μM) for 1 hour prior to stimulation with TLR4 agonist lipopolysaccharide (LPS, 0.2μg/mL). After 1 hour of LPS stimulation, cell lysates were analyzed for phospho- and total NF-κB using immunoblotting and densitometry analysis. TLR4 stimulation resulted in an increase in phosphorylation of NF-κB in both control and simvastatin pre-treated AVICs (LPS vs. ctrl columns, *p<0.05). However, simvastatin treatment reduced the TLR4-induced phosphorylation of NF-κB (LPS+simvastatin [striped bar] vs. LPS [solid bar], #p<0.05). Four donors were utilized (N=4).
DISCUSSION
Calcification of the aortic valve is a disease of aging, and the burdens placed on patients by this disease are a significant problem in the United States18. Risk factors for calcific aortic valve disease include smoking, hypertension, hyperlipidemia, diabetes, bicuspid valve, and disturbances in mineral metabolism. Other risk factors are genetic, including links to dyslipidemia, NOTCH signaling, and other gene polymorphisms such as apolipoproteins1. This disease process is complex, involving gene networks, gene re-expression, mechanoreceptors, mechanotransducers, hemodynamic/myocardial stresses, positive and negative feedback, and specific signaling molecules and pathways19,20. The results of this study demonstrate that simvastatin down-regulates TLR4-induced inflammatory responses in isolated human AVICs. Thus, simvastatin may have an anti-inflammatory role in the cellular pathobiology of the aortic valve.
This study has several limitations. First, it examined isolated human AVICs in-vitro, and such analysis is unable to fully replicate the in vivo environment of the human aortic valve interstitial cell. Even though we have previously demonstrated that cells in later passages express similar receptor levels compared to freshly isolated cells5, it is still possible that cells from later passages do not behave like cells isolated primarily from the aortic valve tissue. Second, the aortic valve tissue utilized for these experiments was isolated from patients who suffered from idiopathic dilated cardiomyopathy. Thus, even though the valves had no echocardiographic abnormalities and had no abnormalities on direct visual inspection, the cells within the valve may not have been completely phenotypically “normal.” Despite this limitation, our lab has previously demonstrated that these AVICs behave in a manner that is not different from cells isolated from normal donor hearts that could not be used for transplantation. Third, it was a difficult task choosing the appropriate cellular concentration for statin treatments. Cellular uptake of lipophilic statins such as simvastatin occurs through passive diffusion. This allows the drugs to be widely distributed in different tissues, and therefore the concentrations of statins in aortic valve tissue are unknown. We must acknowledge that these concentrations are difficult to convert to oral dosing. Many factors affect this conversion including bioavailability, bioactivity, and patient characteristics8. Proper oral dosing would have to be worked out in further in vivo and clinical studies. For the present study, we chose statin concentrations that were consistent with prior studies21 and considered to be reasonable approximations to those seen in vivo. Impurities in the commercial LPS product were also not specifically examined by this study and could have affected results22. This study also did not examine the effects of simvastatin on TLR2 expression or pathway signaling. Since LPS has been found to directly stimulate TLR2 in other cell lines23,24, this could have affected results. However, prior work from our lab has demonstrated that majority of LPS-induced expression of inflammatory proteins such as ICAM-1 in human AVICs is mediated by TLR425. Further work is needed to determine the contribution of TLR2 to LPS signaling and statin signaling inhibition in these cells.
The effects of statins on TLR4 responses and receptor mRNA expression have been evaluated in other cell lines26,27. Methe and colleagues28 demonstrated that simvastatin could suppress TLR4 expression in CD14+ monocytes. Other investigators have also examined the effects of statins on AVIC pathobiology. Wu et al29 demonstrated that statins may inhibit calcification in porcine aortic valve myofibroblasts. Osman and colleagues12 demonstrated that atorvastatin inhibits ATP-induced alkaline phosphatase activity in human AVICs. The present study extends the findings of these investigators. To our knowledge, this study is the first to examine an interaction between simvastatin and TLR4 receptor expression in isolated human AVICs.
To date, the clinical trials with statins and aortic stenosis have had mixed results. These studies evaluated patients who already had developed significant (moderate-severe aortic stenosis) calcification of the aortic valve. It is possible that at that point, the disease in such patients has already advanced beyond a point where the anti-inflammatory actions of statins could demonstrate a benefit. At a certain point in the progression of the disease of aortic stenosis, down-regulation of the TLR4 receptor levels may no longer be possible. The results of this study do suggest that simvastatin may have a preventative role in the treatment of aortic stenosis by inhibiting the pathological cascade of inflammation and osteogenesis. In any event, more bench and clinical research will be required to establish a link between simvastatin and the potential prevention of calcific aortic valve disease.
In summary, the results of the present study demonstrate that simvastatin, reduced TLR4-induced inflammatory responses in human AVICs. The effects of simvastatin were accomplished through down-regulation of total TLR4 expression and/or modulation of NF-κB activation. These data offer mechanistic insight into a possible therapeutic role for simvastatin in the treatment and/or prevention of aortic stenosis.
Supplementary Material
Acknowledgments
The authors would like to acknowledge Lihua Ao for her expertise and assistance with the immunoblotting techniques used in this project.
FUNDING SOURCES
This work was funded by grants from the American Heart Association (AHA:11GRNT7900016) and the National Institutes of Health (NIH RO1 HL 106582-01).
GLOSSARY OF ABBREVIATIONS
- TLR4
Toll-like receptor-4
- AVIC
Aortic valve interstitial cell
- ICAM-1
Intracellular adhesion molecule-1
- IL-8
Interleukin-8
- MCP-1
Monocyte chemoattractant protein-1
- LPS
Lipopolysaccharide
- IB
Immunoblotting
- ELISA
Enzyme-linked immunosorbent assay
- NF-κB
Nuclear factor kappa-B
Footnotes
Author contributions: Neil Venardos (conception and design, acquisition of data, analysis and interpretation, drafting, revising), Xin-Sheng Deng (acquisition of data, analysis of data), Quinzhou Yao (acquisition of data, analysis of data), Michael J Weyant (analysis/interpretation of data, final approval of submitted version), Brett Reece (analysis/interpretation of data, final approval of submitted version), Xianzhong Meng (conception/design of the study, interpretation of data, revising the article, final approval of submitted version), David A Fullerton (conception and design, analysis/interpretation of data, revising the article, approval of the final version to be submitted).
The authors have no disclosures.
DISCLOSURE
The authors report no proprietary or commercial interest in any product mentioned or concept discussed in this article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stewart BF, Siscovick D, Lind BK, Gardin JM, Gottdiener JS, Smith VE, et al. Clinical Factors Associated With Calcific Aortic Valve Disease. J Am Coll Cardiol. 1997;29(3):630–634. doi: 10.1016/s0735-1097(96)00563-3. [DOI] [PubMed] [Google Scholar]
- 2.Clark MA, Duhay FG, Thompson AK, Keyes MJ, Svensson LG, Bonow RO, et al. Clinical and economic outcomes after surgical aortic valve replacement in Medicare patients. Risk Manag Healthc Policy. 2012:117–126. doi: 10.2147/RMHP.S34587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leopold JA. Cellular mechanisms of aortic valve calcification. Circ Cardiovasc Interv. 2012;5(4):605–614. doi: 10.1161/CIRCINTERVENTIONS.112.971028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu AC, Joag VR, Gotlieb AI. The emerging role of valve interstitial cell phenotypes in regulating heart valve pathobiology. Am J Pathol. 2007;171(5):1407–1418. doi: 10.2353/ajpath.2007.070251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meng X, Ao L, Song Y, Babu A, Yang X, Wang M, et al. Expression of functional Toll-like receptors 2 and 4 in human aortic valve interstitial cells: potential roles in aortic valve inflammation and stenosis. Am J Physiol Cell Physiol. 2008;294(1):C29–C35. doi: 10.1152/ajpcell.00137.2007. [DOI] [PubMed] [Google Scholar]
- 6.Babu AN, Meng X, Zou N, Yang X, Wang M, Song Y, et al. Lipopolysaccharide stimulation of human aortic valve interstitial cells activates inflammation and osteogenesis. Ann Thorac Surg. 2008;86(1):71–76. doi: 10.1016/j.athoracsur.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 7.Wang D, Zeng Q, Song R, Ao L, Fullerton DA, Meng X. Ligation of ICAM-1 on human aortic valve interstitial cells induces the osteogenic response: A critical role of the Notch1-NF-kB pathway in BMP-2 expression. Biochim Biophys Acta. 2014 Nov;1843(11):2744–53. doi: 10.1016/j.bbamcr.2014.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Golia E, Limongelli G, Natale F, Fimiani F, Maddaloni V, Pariggiano I, et al. Inflammation and cardiovascular disease: From pathogenesis to therapeutic target. Curr Atheroscler Rep. 2014;16(9) doi: 10.1007/s11883-014-0435-z. [DOI] [PubMed] [Google Scholar]
- 9.Rossebø AB, Pedersen TR, Boman K, Brudi P, Chambers JB, Egstrup K, et al. Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. N Engl J Med. 2008;359(13):1343–1356. doi: 10.1056/NEJMoa0804602. [DOI] [PubMed] [Google Scholar]
- 10.Cowell SJ, Newby DE, Prescott RJ, Bloomfield P, Reid J, Northridge DB, et al. A randomized trial of intensive lipid-lowering therapy in calcific aortic stenosis. N Engl J Med. 2005;352(23):2389–2397. doi: 10.1056/NEJMoa043876. [DOI] [PubMed] [Google Scholar]
- 11.Moura LM, Ramos SF, Zamorano JL, Barros IM, Azevedo LF, Rocha-Gonçalves F, et al. Rosuvastatin affecting aortic valve endothelium to slow the progression of aortic stenosis. J Am Coll Cardiol. 2007;49(5):554–561. doi: 10.1016/j.jacc.2006.07.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Osman L, Chester AH, Amrani M, Yacoub MH, Smolenski RT. A novel role of extracellular nucleotides in valve calcification: A potential target for atorvastatin. Circulation. 2006;114(SUPPL 1) doi: 10.1161/CIRCULATIONAHA.105.001214. [DOI] [PubMed] [Google Scholar]
- 13.Benton JAa, Kern HB, Leinwand LA, Mariner PD, Anseth KS. Statins block calcific nodule formation of valvular interstitial cells by inhibiting α-smooth muscle actin expression. Arterioscler Thromb Vasc Biol. 2009;29(11):1950–1957. doi: 10.1161/ATVBAHA.109.195271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Monzack EL, Gu X, Masters KS. Efficacy of simvastatin treatment of valvular interstitial cells varies with the extracellular environment. Arterioscler Thromb Vasc Biol. 2009;29(2):246–253. doi: 10.1161/ATVBAHA.108.179218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li F, Song R, Ao L, Reece TB, Cleveland JC, Jr, Dong N, Fullerton DA, Meng X. ADAMTS5 deficiency in calcified aortic valves is associated with elevated pro-osteogenic activity in valvular interstitial cells. Arterioscler Thromb Vasc Biol. 2017;37(7):1339–1351. doi: 10.1161/ATVBAHA.117.309021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song Y, Fullerton DA, Mauchley D, Su X, Ao L, Yang X, Cleveland JC, Meng X. Microfilaments facilitate TLR-4-Mediated ICAM-1 Expression in Human Aortic Valve Interstitial Cells. J Surg Res. 2011;166(1):52–8. doi: 10.1016/j.jss.2009.03.101. [DOI] [PubMed] [Google Scholar]
- 17.Song R, Ao L, Zhao KS, Zheng D, Venardos N, Fullerton DA, Meng X. Soluble biglycan induces the production of ICAM-1 and MCP-1 in human aortic valve interstitial cells through TLR2/4 and the ERK1/2 pathway. Inflamm Res. 2014;63(9):703–10. doi: 10.1007/s00011-014-0743-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet. 2006;368(9540):1005–1011. doi: 10.1016/S0140-6736(06)69208-8. [DOI] [PubMed] [Google Scholar]
- 19.Pasipoularides A. Calcific Aortic Valve Disease: Part 1—Molecular Pathogenetic Aspects, Hemodynamics, and Adaptive Feedbacks. J Cardiovasc Transl Res. 2016 Apr;9(2):102–18. doi: 10.1007/s12265-016-9679-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pasipoularides A. Calcific Aortic Valve Disease: Part 2—Morphomechanical Abonrmalities, gene reexpression, and gender effects on Ventricular Hypertrophy and Its Reversibility. J Cardiovasc Transl Res. 2016 Aug;9(4):374–99. doi: 10.1007/s12265-016-9695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sadaria MR, Reppert AE, Yu JA, Meng X, Fullerton DA, Reece TB, et al. Statin therapy attenuates growth and malignant potential of human esophageal adenocarcinoma cells. J Thorac Cardiovasc Surg. 2011;142(5):1152–1160. doi: 10.1016/j.jtcvs.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 22.Staub AM. Bacterial Lipido-proteinopolysaccharides (O somatic antigens). Extraction with tricloro-acetic acid. In: Whistler RL, Wolfan ML, editors. Methods in Carbohydrate Chemistry. New York: Academic press; 1965. pp. 92–93. [Google Scholar]
- 23.Yang RB, Mark MR, Gray A, et al. Toll-like receptor-2 mediates lipopolysaccharide-induced cellular signalling. Nature. 1998;395(6699):284–288. doi: 10.1038/26239. [DOI] [PubMed] [Google Scholar]
- 24.Kirschning CJ, Wesche H, Merrill Ayres T, Rothe M. Human toll-like receptor 2 confers responsiveness to bacterial lipopolysaccharide. J Exp Med. 1998;188(11):2091–2097. doi: 10.1084/jem.188.11.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Song Y, Fullerton DA, Mauchley D, et al. Microfilaments facilitate TLR4-mediated ICAM-1 expression in human aortic valve interstitial cells. J Surg Res. 2011;166(1):52–58. doi: 10.1016/j.jss.2009.03.101. [DOI] [PubMed] [Google Scholar]
- 26.Liu D, Cui W, Liu B, Hu H, Liu J, Xie R, et al. Atorvastatin protects vascular smooth muscle cells from TGF-β1-stimulated calcification by inducing autophagy via suppression of the β-catenin pathway. Cell Physiol Biochem. 2014;33(1):129–141. doi: 10.1159/000356656. [DOI] [PubMed] [Google Scholar]
- 27.Niessner A, Steiner S, Speidl WS, Pleiner J, Seidinger D, Maurer G, et al. Simvastatin suppresses endotoxin-induced upregulation of toll-like receptors 4 and 2 in vivo. Atherosclerosis. 2006;189(2):408–413. doi: 10.1016/j.atherosclerosis.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 28.Methe H, Kim JO, Kofler S, Nabauer M, Weis M. Statins decrease Toll-like receptor 4 expression and downstream signaling in human CD14+ monocytes. Arterioscler Thromb Vasc Biol. 2005;25(7):1439–1445. doi: 10.1161/01.ATV.0000168410.44722.86. [DOI] [PubMed] [Google Scholar]
- 29.Wu B, Elmariah S, Kaplan FS, Cheng G, Mohler ER. Paradoxical effects of statins on aortic valve myofibroblasts and osteoblasts: Implications for end-stage valvular heart disease. Arterioscler Thromb Vasc Biol. 2005;25(3):592–597. doi: 10.1161/01.ATV.0000154278.01871.64. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.