Introduction
Major updates have been made in the latest (8th) edition of the American Joint Commission on Cancer (AJCC) manual for staging of oropharynx squamous cell cancer (OPSCC)[1, 2] which are based on changes in epidemiology of OPSCC related to the emergence of Human Papilloma Virus (HPV) as the major cause for OPSCC.[3, 4] It is now recognized that patients with HPV positive oropharyngeal cancer (HPV+OPSCC) have superior survival compared to patients with HPV negative oropharynx cancer (HPV-OPSCC).[5] One of the key characteristics of patients with HPV+OPSCC is a markedly improved prognosis despite advanced nodal disease when compared to patients with HPV-disease.[3, 6–8] Traditional risk factors predictive of outcome, such as positive margins and extranodal extension (ENE), are not accurate predictors of outcome in HPV+ patients.[9]
Previous studies have reported that the AJCC 7th edition staging system has limited ability to differentiate prognosis accurately among stages in the HPV+OPSCC population.[6–8] As a result, a new clinical staging system (International Collaboration on Oropharyngeal cancer Network for Staging, ICON-S) and a new pathological staging system (HPV-Path) were introduced in 2016 specifically for HPV+OPSCC patients. The ICON-S staging system was developed from a study of 1907 HPV+OPSCC patients using an adjusted hazard ratio model that considered age, smoking status and use of cytotoxic chemotherapy.[10] This staging model was created from data of patients treated primarily with chemoradiation. In contrast, the HPV-Path system was created from a multi-institutional dataset of HPV+OPSCC patients treated with surgery, and thus the staging system was based on pathological variables that were not available in the ICON-S study.[11] The parameters of both staging systems have been incorporated into the new 8th edition of the AJCC staging system for OPSCC.[1, 2] The ICON-S system is the new AJCC 8th edition clinical staging system and the HPV-Path system the pathological staging system. A recent report has suggested the two staging systems result in discordant staging[12]
The objective of our study was to validate the new clinical and pathological staging systems in an independent cohort of HPV+OPSCC patients who had received surgery as the initial treatment modality. We also determined which staging system was more appropriate for patients treated with surgery and evaluated the degree of discordance between the clinical and pathological staging systems.
Methods
After Institutional Review Board (IRB) approval, we performed a retrospective analysis of our surgical oropharyngeal patient database from 1985 to 2015. Patients with OPSCC who had surgery at our institution as the primary form of treatment were identified. HPV status was determined by p16 immunohistochemistry, which is now accepted as a surrogate marker.[13, 14] The patients also received adjuvant treatment based on current National Comprehensive Cancer Network(NCCN) guidelines or, in some cases, after multidisciplinary team consultations. Patients with distant metastatic disease at presentation, those who were HPV negative or HPV unknown status, those with recurrent disease, and patients that had received any form of neoadjuvant treatment were excluded from the study.
The demographic, clinicopathologic, and outcomes data were extracted from the medical record for eligible patients. The clinical tumor (cT) and nodal status (cN) were verified by reviewing the radiological scans. Histopathological data was reviewed to ascertain the pathological tumor (pT) and pathological nodal (pN) status.
Staging systems
The clinical and pathological staging systems are summarized in Figure 1. For the clinical staging system, the cT category remains the same in both 8th and 7th edition AJCC staging system. However, nodal staging is different in the 8th edition: for the cN status, in HPV+ve patients, cN1, cN2a, and cN2b are combined together and have been reclassified as cN1, cN2c has been reclassified as cN2, and cN3 reclassified as N3. The AJCC 7th edition system determines nodal burden based on size, laterality, and number (single/multiple), whereas the AJCC 8th edition clinical system relies on laterality and size only. The HPV-Path system, on the other hand, is a pathological staging system. The pT categories are the same as AJCC 7th edition, but it has a different nodal status categorization that is based on the number of involved nodes. M stage remains consistent across all three staging systems. Finally, the new clinical and pathological systems stage HPV+ OPSCC patients into only three stages (I-III) which differs from the AJCC 7th edition that has four stages (I-IV).
Figure 1.
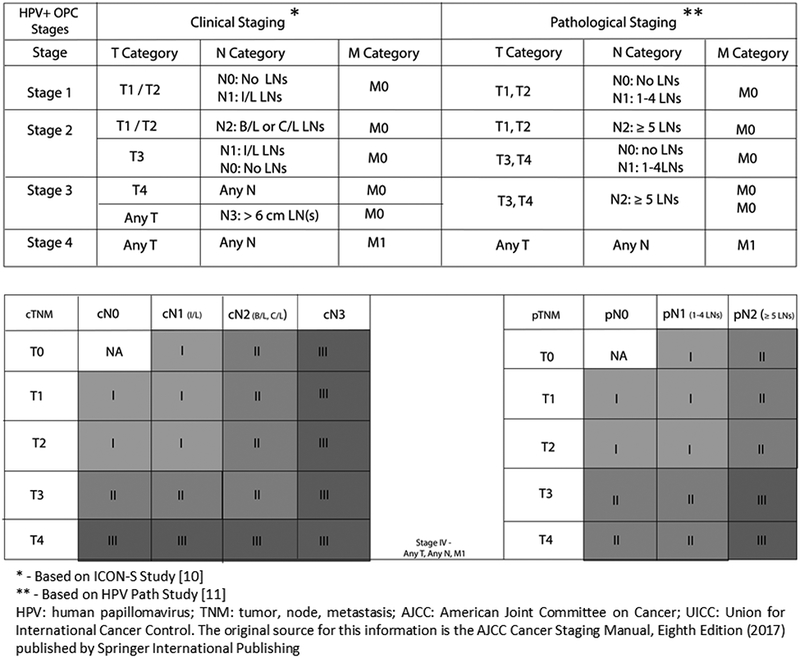
HPV related oropharyngeal carcinoma TNM staging AJCC UICC 2017
Outcomes Data
Overall survival (OS) was calculated from the date of surgery to either the last date the patient was known to be alive, regardless of disease status, or death date. An OS event was defined as death from any cause. Disease-specific survival (DSS) was calculated from the date of surgery to last date of disease assessment or death date. A patient was only considered to have a DSS event if he/she died of disease or if the patient had active disease at the date of last disease assessment. All other patients were censored for DSS at the last date of disease assessment by a medical professional.
Statistical Analysis
Statistical analysis was performed using the SPSS Statistics Software for Windows, V24.0 (IBM, Armonk, NY). The patient baseline characteristics were summarized using descriptive statistics. Variables assessed were age, sex, cT status, cN status, pT and pN status, margin status, depth of invasion, lympho-vascular invasion (LVI), and peri-neural invasion (PNI). The survival outcomes were compared using the Log-Rank method, and a p-value of less than 0.05 was considered statistically significant. Kaplan Meier Survival Curves for OS and DSS were determined for each staging system. The Cox proportional hazard method was used to calculate the hazard ratios for OS across the three staging systems. Unadjusted and adjusted hazard ratios were calculated controlling for other variables. Goodness of fit analysis was carried out to determine which staging system performed the best in terms of model fit to predict overall survival. The Akaike information criterion (AIC) is an estimator of the relative quality of statistical models, which in the case of survival outcomes is a Cox proportional hazard model.[15] Given a collection of models for the data, AIC estimates the quality of each model relative to each of the other models. However, for smaller models, AIC may lead to over-fitting, and thus a modified AIC (AlCc) was used in this study.[16] The R program (RStudio-Version 1.1.383, RStudio, Inc.) with survival, rms and MASS package was utilized to calculate the AICc values for each staging system.
Results
Patient Characteristics
Four hundred and thirty-six OPSCC patients were treated with surgery at our institute between 1985 and 2015. Of these, 230 patients were p16 positive (i.e., HPV+); in total, 218 patients were eligible for the study (Figure 2). The demographic and clinical data are summarized in Table 1. The median age at the time of diagnosis was 56 years (interquartile range, IQR 49–62); 81.2% of patients were male and 64.7% of patients were ever smokers. Tonsil was the most common primary site of disease. Most of the patients in our group presented with early (T1/T2) disease (83.5%) and had N+ disease (83.5%). Differences between our study population and the published datasets of the ICON-S trial and the HPV-Path study are summarized in the Supplementary Table 1. The HPV-Path cohort used a pooled cohort of 704 patients from 5 cancer centers which included a smaller cohort of 99 patients treated at MSKCC from the period 1985–2005. The present MSKCC study is a larger series of 218 patients over a longer time period 1985–2015. Notably, our cohort had more patients in stage I (83.9%) compared to the ICON-S study (stage I- 50.4%). Similarly, the HPV-Path cohort, our patients had more stage I to the HPV-Path study (e.g., stage I: 79.8% in MSKCC cohort vs. 69.5% in HPV-Path cohort).
Figure 2.
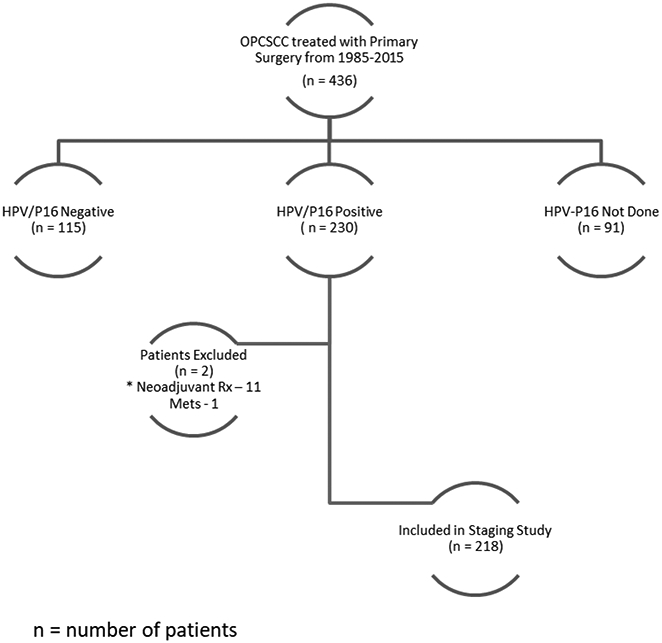
Study Cohort
Table 1.
Baseline Characteristics of 218 Patients Included in this Study.
| Patient Variables | N (%) |
|---|---|
| Mean Age (SD), years | 56 (9.68) |
| Sex | |
| Male | 177(81%) |
| Female | 41(19%) |
| Smoking history | |
| Never | 77(35%) |
| Ever | 141(65%) |
| Subsite | |
| Tonsil | 75(34%) |
| Base of tongue | 135(62%) |
| Other | 8(4%) |
| T-classification | |
| 1 | 110(50%) |
| 2 | 87(40%) |
| 3 | 10(5%) |
| 4 | 11(5%) |
| N-classification (AJCC 7th) | |
| N0 | 27(13%) |
| N1 | 32(15%) |
| N2a | 37(18%) |
| N2b | 111(53%) |
| N2c | 3(1%) |
| N3 | 0 |
| Nx | 8 |
| Extranodal 15xtensión (pathological) | |
| Negative | 114(52%) |
| Positive | 68(31%) |
| Unknown | 36(17%) |
| Margins | |
| Negative | 71(32%) |
| Close (< 5mm) | 78(36%) |
| Positive | 58(27%) |
| Unknown | 11(5%) |
| Perineural invasion | |
| Negative | 156(72%) |
| Positive | 36(16%) |
| Unknown | 26(12%) |
| Lympho-vascular invasion | |
| Negative | 141(65%) |
| Positive | 52(24%) |
| Unknown | 25(11%) |
| Treatment | |
| Surgery only | 34(15%) |
| Surgery + radiation | 150(69%) |
| Surgery + chemoradiation | 34(15%) |
| Surgical Modality | |
| TORS | 152 (70%) |
| Non-TORS | 66 (30%) |
| AJCC 7th stage | |
| I | 7(3%) |
| II | 18(8%) |
| III | 60(28%) |
| IV | 133(61%) |
| ICON-S stage | |
| I | 183(84%) |
| II | 24(11%) |
| III | 11(5%) |
| HPV-Path stage | |
| I | 174(80%) |
| II | 34(15%) |
| III | 10(5%) |
Abbreviations: SD, standard deviation; TORS, transoral robotic surgery.
Outcomes
The median follow-up was 48 months with a median survival of 39.5 months (IQR 11–109). Kaplan Meier survival analysis was performed to identify the association between the various staging systems and the patient outcomes in terms of OS (Figure 3). Using the AJCC 7th edition staging system, patients were divided into four stages, and OS curves were plotted (Figure 3A). There was significant overlap of the Kaplan Meier curves for early (stage I/II) and late (stage III/IV) stage patients. There was no significant difference in the five-year OS for early (77.7%) and late stages (79.9%) (log rank p=0.051).
Figure 3.
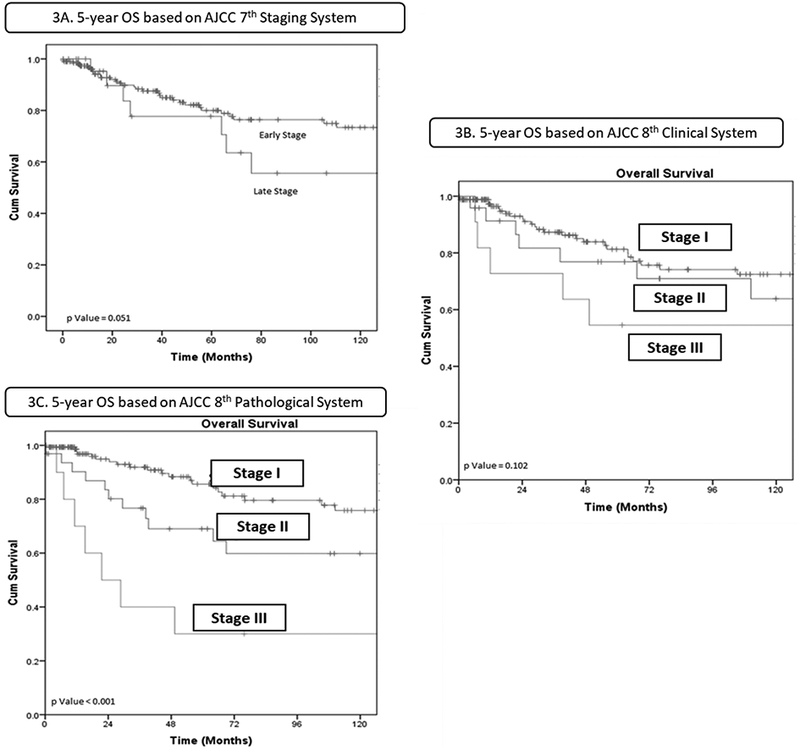
Kaplan Meier survival analysis for overall survival for each staging system.
Classification with the AJCC 8th edition clinical system changed staging in our patient cohort significantly (p <0.01). This was mainly due to patients with N2a and N2b disease in AJCC 7th edition being reclassified as N1 disease, resulting in most patients being reclassified into stage I (183/218). There was a significant difference in OS across all stages based on the clinical staging system. However, as shown in Figure 3B, stage I and stage II patients had very similar outcomes early in the disease history only to diverge at later time points. Despite better stratification compared to the AJCC 7th edition, the confidence intervals of stage I and stage II overlapped with each other and thus were not significant. The 5-year OS for AJCC 8th clinical stages I, II, and III were 82.4%, 76.7% and 54.5%, respectively (log rank p=0.102)
The AJCC 8th edition pathological system stages patients based on the pathological characteristics. The survival curves based on this system for our patient cohort were widely spaced (Figure 3C), and there was a progressive decline in OS across all stages. The 5-year OS for AJCC 8th pathological stages I, II, and III were 86.9%, 67.2%, and 30%, respectively (p<0.01).
Kaplan Meier survival analysis was also carried out for DSS for each staging system, and results are shown in Supplementary Figure 1. Again, there was poor differentiation of stages (Early/Late) with the AJCC 7th edition staging system. The AJCC 8th edition clinical staging system performed poorly with overlap of stages II and III. In contrast, with the AJCC 8th edition pathological system survival curves were all appropriately spaced with no overlap of the 95% confidence intervals.
Comparison of the AJCC 8th edition Clinical and Pathological Staging Systems
The unadjusted and adjusted hazard ratios (HR) for the clinical and pathological staging systems are shown in Table 2. Comparing the two systems, the pathological system showed a better stage discrimination based on a significant increase in HR (Stage II - 1.65 and Stage III - 5.69) compared to the clinical system (Stage II - 1.72 and Stage III - 1.96). These differences remained significant after adjusting for age and smoking status (Table 2, Adjusted Hazard Ratio). Further, there was a significant difference in outcomes when patients were classified with clinical and pathological systems (log rank test for equality of survival function pathological p<0.001 compared to clinical p=0.102).
Table 2.
Risk Stratification by the Staging Systems.
| Unadjusted Hazard Ratio* | Adjusted Hazard Ratio* (Controlling for Age and Smoking Status) |
|
|---|---|---|
| AJCC 7th System | ||
| Stage I | Ref | Ref |
| Stage II | 0.28 (0.07–1.08), 0.064 | 0.45 (0.11–1.77), 0.253 |
| Stage III | 0.19 (0.05–0.67), 0.01 | 0.27 (0.07–0.95), 0.042 |
| Stage IV | 0.18 (0.05–0.6), 0.005 | 0.28 (0.08–0.93), 0.039 |
| AJCC 8th (ICON-S) Clinical System | ||
| Stage I | Ref | Ref |
| Stage II | 1.72 (0.89–3.31), 0.104 | 1.25 (0.64–2.45), 0.515 |
| Stage III | 1.96 (0.87–4.43), 0.103 | 2.09 (0.92–4.73), 0.077 |
| AJCC 8th (HPV-Path) Pathological System | ||
| Stage I | Ref | Ref |
| Stage II | 1.65 (0.9–3.04), 0.108 | 2.35 (1.22–4.52), 0.01 |
| Stage III | 5.69 (2.6–12.46), <0.001 | 8.34 (3.66–18.99), <0.001 |
Hazard Ratio (95% CI), p-value.
The staging systems were then analyzed as goodness of fit models to identify which model best described the dataset for overall survival. The AICc values were calculated using the extractAIC function (RStudio version 1.1.383) for models with the clinical and pathological AJCC adjusted for age and smoking. The pathological staging system had the lowest AICc values suggesting the best fit (AJCC pathological = 482 vs. AJCC clinical = 491) (Figure 4A).
Figure 4.
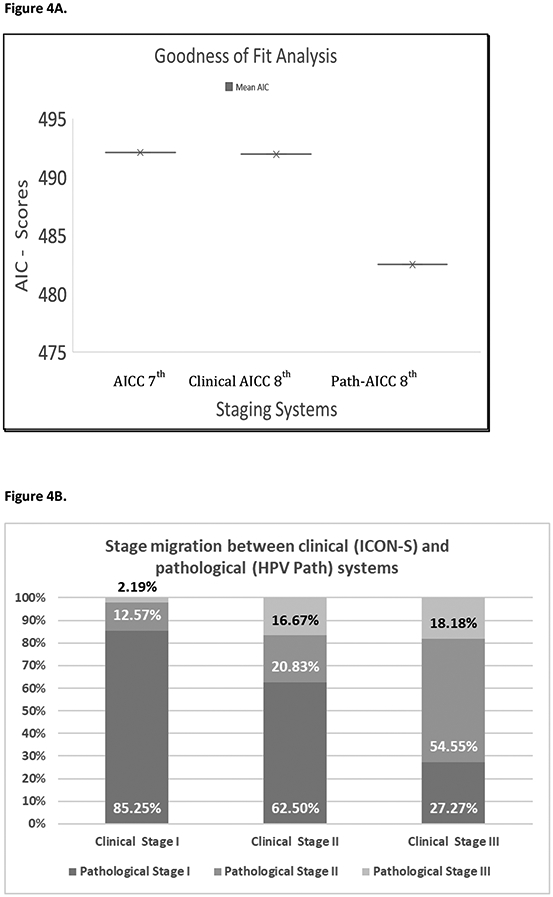
4A. Goodness of fit analysis. 4B. Stage migration between clinical (ICON-S) and pathological (HPV Path) systems.
Concordance between clinical and pathological staging systems
We found considerable discordance between the AJCC clinical and pathological staging systems. Figure 4B shows the stage migration between clinical and pathological Systems. By comparing the stages obtained for AJCC clinical and AJCC pathological staging for individual patients, we found the highest concordance for stage I patients (82.5%), but there was a high degree of disagreement for the other stages. Of patients classified as clinical stage II by AJCC clinical, 62.5% were downstaged to stage I. Further, 81% patients classified as clinical stage III by AJCC clinical were downstaged to a lesser stage (54% Stage II and 27% Stage I).
Discussion
The new AJCC 8th edition includes a separate staging system for OPSCC to account for the improved prognosis of HPV+ patients compared to HPV- patients. This staging system now includes distinct clinical and pathological staging systems based on recent recommendations from the ICON-S and HPV-Path studies.[2] The patient cohorts used in these studies differed in terms of the primary treatment: the ICON-S cohort was comprised mainly of patients treated with chemoradiation (only 2% treated with primary surgery), whereas the HPV-Path cohort was entirely made up of surgically treated patients. The objective of our study was to validate and compare the new AJCC 8th edition staging systems (ICON-S and HPV-Path) in a cohort of HPV+OPSCC patients who had received surgery as the initial treatment modality.
Our results show that both the AJCC 8th edition clinical and pathological staging systems were better able to discriminate between stages than the AJCC 7th edition staging system in HPV+OPSCC patients. This improvement can likely be attributed to earlier staging based on lymph node status by the new systems, staging that is more consistent with the clinical behavior of HPV+OPSCC disease.[17–20] Similar to previous reports, our data showed that the AJCC 7th edition staging system failed to accurately predict outcome and there was substantial overlap for overall survival among stages in our HPV+OPSCC cohort.[21–24] Similar to the findings by O’Sullivan et al, it was noted that although the T status is a predictive factor, the nodal staging is not as predictive.[10] In our study T4 was identified as an adverse risk variable irrespective of N category and formed the highest risk category within the AJCC staging system.
In recognition of the poor performance of the AJCC 7th edition in HPV+OPSCC cases, Huang et al. proposed changes to the clinical staging system.[25] The new approach was first validated by the ICON-S trial that included data from 1907 patients[10]; subsequently, the ICON-S clinical staging system was validated in several other cohorts (Supplementary Table 2)[11, 21, 22, 24, 26–28]. However, these validation studies mainly included patients treated with chemoradiation[22, 26, 27]. Only Haughey et al.[11] and Wurdemann et al[24] validated ICON-S in patients treated with surgery. Using our surgical cohort, we were also able to validate the ICON-S system and show a consistent decline in OS. Comparing the outcomes of our cohort and the ICON-S cohort [10], we notice similar outcomes for stage I and stage II disease, thus suggesting that patients with early stage disease had similar prognosis with either surgical or non-surgical treatment respectively (Supplementary Table 1). However, we found significant overlap of the 95% CI between the stage I and Stage II OS (Stage I: 78% and Stage II: 76%), which suggests that this group of patients cannot be prognostically stratified based on clinical staging alone.
Surgical management is considered the gold standard to assess the true loco-regional extent of the disease. In the HPV-Path system, Haughey et al. emphasized the prognostic significance of the total number of pathologically proven positive nodes for staging of surgically managed patients. It was also reported that in surgical patients there was significant overlap in outcome curves for ICON-S stage II and stage III tumors, underscoring the need for a new pathological staging system. Compared to the HPV-Path cohort, our patients had a consistent decline in OS thus validating the new AJCC 8th edition pathological staging system. We also showed that the discrimination of stages was superior in the pathological staging system compared to the clinical staging system. This result was further supported by goodness of fit analysis and lower AIC scores with pathological staging system compared to clinical. We conclude that the new pathological staging system improves prognostication over the clinical staging system and the 7th edition system for surgically treated HPV+OPSCC patients
With the inherent differences in how the clinical and pathological system have been formulated, it is not surprising that there is significant discordance between them. In our cohort, most of the patients with AJCC 8th clinical stage II and stage III were over-staged based on the AJCC 8th pathological system. In patients with clinical Stage I, there were significant number of patients who were upstaged by the pathological system. The importance of this discordance was recently emphasized by an editorial by Fakhry et al.[12] The authors raised the concern of different survival estimates from clinical and pathological overall stage and its effect on the prognostic information conveyed 2013 this has several implications for clinical practice. It is possible that the presence of two staging systems may lead to considerable confusion among oncologists. It is extremely important that clinicians realize that these two staging systems are not interchangeable and that they clearly state which staging system is being used when reporting patient outcomes. This also impacts clinical trial design and reporting; when comparing outcomes from different clinical trials, it is essential that the same staging system is used. It is also especially important that clinicians clearly state what staging system is used when providing staging information to patients. In addition, clinicians should not use these staging systems to make clinical decisions on adjuvant treatment. The outcomes that these staging systems were developed from were based on adjuvant treatment using previously recognized prognostic variables, such as margin status, advanced T stage, and presence of extranodal extension (ENE). Thus, the new staging systems should not be used to alter the current management.
Our study provides a strong justification for validation of the AJCC 8th pathological staging system. Its ability to discriminate outcomes in a purely surgical cohort is evident. However, similar conclusion cannot be drawn for the clinical staging criteria. Though smaller numbers in stage III clinical and pathological system does affect the power of analysis, the clinical staging system had a poor discriminant function between the early stages of the disease where we had sufficient numbers to come at the above-mentioned conclusion.
This study has some limitations. It is a retrospective design and is therefore susceptible to limitations attributed to retrospective data collection. There is obvious physician- and patient-related selection bias in management decisions that cannot be fully accounted for. Our study was over a long time period during which time surgical technique as well choice of adjuvant treatment has changed. There is also selection bias introduced due to the large number of patients who did not have paraffin blocks available for p16 analysis and this reduced our numbers overall. It is possible our small patient cohort (218 patients), that included a small number of patients in the AJCC 8th edition pathological stage II and stage III, limited the power of our statistical analysis. Nevertheless, the fact that we see such clear separation of the survival curves with statistical significance is a powerful observation. Further, our sample size is larger than the number of patients that underwent primary surgery included in the ICON-S trial.[10] Lastly, at present there is conflicting evidence whether or not other variables such as PNI, LVI, ECS which are prognostic in HPV negative patients, have any importance in HPV positive patients. These factors do affect clinical decision making and therefore further studies on a larger multi-institutional series of patients is necessary to address this question. Should these be prognostic then the new AJCC pathological staging system could be further refined to accommodate this in the future.
Conclusion
In conclusion, analysis of patients with HPV+OPSCC treated with surgery shows improved risk stratification and prognostic value of predicting survival for the new AJCC 8th edition clinical and pathological staging systems over the AJCC 7th edition. However, the pathological system appears to be better able to predict OS and stratify based on risk for surgically managed HPV+OPCSCC patients. Importantly, clinicians should be aware that the two systems are not interchangeable due to the considerable discordance between them.
Supplementary Material
Highlights:
Both the clinical and pathological staging system of the AJCC 8th edition performed better than the 7th edition in predicting 5-year OS
There was significant discordance between the AJCC 8th clinical and pathological staging systems
Pathological staging had a higher discriminant function and served as a better model fit to predict outcomes
The new staging systems should not be used to alter current management
Acknowledgements:
None
Funding: This report was supported in part by the National Institutes of Health under award number P30-CA008748
Abbreviations
- AJCC
American Joint Commission on Cancer
- OPSCC
manual for staging of oropharynx squamous cell cancer
- HPV
Human Papilloma Virus
- OS
Overall Survival
- DSS
Disease Specific Survival
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement: None declared.
References
- [1].Lydiatt WM, Patel SG, O’Sullivan B, Brandwein MS, Ridge JA, Migliacci JC, et al. Head and Neck cancers-major changes in the American Joint Committee on cancer eighth edition cancer staging manual. CA: a cancer journal for clinicians. 2017;67:122–37. [DOI] [PubMed] [Google Scholar]
- [2].Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, et al. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA: a cancer journal for clinicians. 2017;67:93–9. [DOI] [PubMed] [Google Scholar]
- [3].Sturgis EM, Ang KK. The epidemic of HPV-associated oropharyngeal cancer is here: is it time to change our treatment paradigms? Journal of the National Comprehensive Cancer Network: JNCCN. 2011;9:665–73. [DOI] [PubMed] [Google Scholar]
- [4].Keane FK, Chen YH, Neville BA, Tishler RB, Schoenfeld JD, Catalano PJ, et al. C hanging prognostic significance of tumor stage and nodal stage in patients with squamous cell carcinoma of the oropharynx in the human papillomavirus era. Cancer. 2015;121:2594–602. [DOI] [PubMed] [Google Scholar]
- [5].Chaturvedi AK, Engels EA, Pfeiffer RM, Hernandez BY, Xiao W, Kim E, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2011;29:4294–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Rischin D, Young RJ, Fisher R, Fox SB, Le QT, Peters LJ, et al. Prognostic significance of p16INK4A and human papillomavirus in patients with oropharyngeal cancer treated on TROG 02.02 phase III trial. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2010;28:4142–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Posner MR, Lorch JH, Goloubeva O, Tan M, Schumaker LM, Sarlis NJ, et al. Survival and human papillomavirus in oropharynx cancer in TAX 324: a subset analysis from an international phase III trial. Ann Oncol. 2011;22:1071–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Nguyen-Tan PF, Zhang Q, Ang KK, Weber RS, Rosenthal DI, Soulieres D, et al. Randomized phase III trial to test accelerated versus standard fractionation in combination with concurrent cisplatin for head and neck carcinomas in the Radiation Therapy Oncology Group 0129 trial: long-term report of efficacy and toxicity. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2014;32:3858–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Iyer NG, Dogan S, Palmer F, Rahmati R, Nixon IJ, Lee N, et al. Detailed Analysis of Clinicopathologic Factors Demonstrate Distinct Difference in Outcome and Prognostic Factors Between Surgically Treated HPV-Positive and Negative Oropharyngeal Cancer. Annals of surgical oncology. 2015;22:4411–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].O’Sullivan B, Huang SH, Su J, Garden AS, Sturgis EM, Dahlstrom K, et al. Development and validation of a staging system for HPV-related oropharyngeal cancer by the International Collaboration on Oropharyngeal cancer Network for Staging (ICON-S): a multicentre cohort study. The Lancet Oncology. 2016;17:440–51. [DOI] [PubMed] [Google Scholar]
- [11].Haughey BH, Sinha P, Kallogjeri D, Goldberg RL, Lewis JS Jr., Piccirillo JF, et al. Pathology-based staging for HPV-positive squamous carcinoma of the oropharynx. Oral oncology. 2016;62:11–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Fakhry C, Zevallos JP, Eisele DW. Imbalance Between Clinical and Pathologic Staging in the Updated American Joint Commission on Cancer Staging System for Human Papillomavirus-Positive Oropharyngeal Cancer. Journal of Clinical Oncology. 2018;36:217–9. [DOI] [PubMed] [Google Scholar]
- [13].Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tan PF, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. The New England journal of medicine. 2010;363:24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lewis JS. p16 Immunohistochemistry as a standalone test for risk stratification in oropharyngeal squamous cell carcinoma. Head and neck pathology. 2012;6:75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Akaike H A new look at the statistical model identification. IEEE transactions on automatic control. 1974;19:716–23. [Google Scholar]
- [16].De Ridder F, Pintelon R, Schoukens J, Gillikin DP. Modified AIC and MDL model selection criteria for short data records. IEEE Transactions on Instrumentation and Measurement. 2005;54:144–50. [Google Scholar]
- [17].De Almeida JR, Li R, Magnuson JS, Smith RV, Moore E, Lawson G, et al. Oncologic outcomes after transoral robotic surgery: a multi-institutional study. JAMA Otolaryngology-Head & Neck Surgery. 2015;141:1043–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Calais G, Alfonsi M, Bardet E, Sire C, Germain T, Bergerot P, et al. Randomized trial of radiation therapy versus concomitant chemotherapy and radiation therapy for advanced-stage oropharynx carcinoma. Journal of the National Cancer Institute. 1999;91:2081–6. [DOI] [PubMed] [Google Scholar]
- [19].Adelstein DJ, Ridge JA, Brizel DM, Holsinger FC, Haughey BH, O’sullivan B, et al. Transoral resection of pharyngeal cancer: summary of a National Cancer Institute Head and Neck Cancer Steering Committee clinical trials planning meeting, November 6–7, 2011, Arlington, Virginia. Head & neck. 2012;34:1681–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Mydlarz WK, Chan JY, Richmon JD. The role of surgery for HPV-associated head and neck cancer. Oral oncology. 2015;51:305–13. [DOI] [PubMed] [Google Scholar]
- [21].Cramer JD, Hicks KE, Rademaker AW, Patel UA, Samant S. Validation of the eighth edition American Joint Committee on Cancer staging system for human papillomavirus-associated oropharyngeal cancer. Head & neck. 2018;40:457–66. [DOI] [PubMed] [Google Scholar]
- [22].Malm IJ, Fan CJ, Yin LX, Li DX, Koch WM, Gourin CG, et al. Evaluation of proposed staging systems for human papillomavirus-related oropharyngeal squamous cell carcinoma. Cancer. 2017;123:1768–77. [DOI] [PubMed] [Google Scholar]
- [23].Sano D, Yabuki K, Arai Y, Tanabe T, Chiba Y, Nishimura G, et al. The applicability of new TNM classification for humanpapilloma virus-related oropharyngeal cancer in the 8th edition of the AJCC/UICC TNM staging system in Japan: A single-centre study. Auris, nasus, larynx. 2017. [DOI] [PubMed] [Google Scholar]
- [24].Wurdemann N, Wagner S, Sharma SJ, Prigge ES, Reuschenbach M, Gattenlohner S, et al. Prognostic Impact of AJCC/UICC 8th Edition New Staging Rules in Oropharyngeal Squamous Cell Carcinoma. Frontiers in oncology. 2017;7:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Huang SH, Xu W, Waldron J, Siu L, Shen X, Tong L, et al. Refining American Joint Committee on Cancer/Union for International Cancer Control TNM stage and prognostic groups for human papillomavirus-related oropharyngeal carcinomas. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2015;33:836–45. [DOI] [PubMed] [Google Scholar]
- [26].Horne ZD, Glaser SM, Vargo JA, Ferris RL, Balasubramani GK, Clump DA, et al. Confirmation of proposed human papillomavirus risk-adapted staging according to AJCC/UICC TNM criteria for positive oropharyngeal carcinomas. Cancer. 2016;122:2021–30. [DOI] [PubMed] [Google Scholar]
- [27].Porceddu SV, Milne R, Brown E, Bernard A, Rahbari R, Cartmill B, et al. Validation of the ICON-S staging for HPV-associated oropharyngeal carcinoma using a pre-defined treatment policy. Oral oncology. 2017;66:81–6. [DOI] [PubMed] [Google Scholar]
- [28].Husain ZA, Chen T, Corso CD, Wang Z, Park H, Judson B, et al. A Comparison of Prognostic Ability of Staging Systems for Human Papillomavirus-Related Oropharyngeal Squamous Cell Carcinoma. JAMA oncology. 2017;3:358–65. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


