Synopsis
This article reviews the role of surgical and medical management in patients with Zollinger-Ellison syndrome (ZES) due to a gastrin-secreting neuroendocrine tumor (gastrinoma). It concentrates on the status at present but also briefly reviews the changes over time in treatment approaches. Generally, surgical and medical therapy are complementary today; however, in some cases such as patients with ZES and Multiple Endocrine Neoplasia type 1 the treatment approach remains controversial.
Keywords: Zollinger-Ellison syndrome, gastrinoma, neuroendocrine tumor, acid secretion, proton pump inhibitors, Multiple Endocrine Neoplasia-type 1
The relationship between surgical treatments and medical treatments in the various management aspects of the Zollinger-Ellison syndrome (ZES) has taken many forms. In some aspects of ZES at different times, only one of these approaches has been used, while at other times both are available and used to different extents by different groups and thus they have had a somewhat adversarial relationship, whereas in other cases they are complementary. The latter is the situation at present in most instances; however, there remain management aspects where the exact role of surgery or medical treatment remains unclear and contentious. In this article, these aspects will be discussed showing changes over time, but generally concentrating on the role of each in the current management of ZES.
I. General/Definitions
ZES was first described in 1955 in two patients by two surgeons at Ohio State University, RM Zollinger and EH Ellison, and 6 additional cases were described by other surgeons in the discussion of this article1. A later review of the literature prior to this time concluded at least 4 cases of probable gastrinomas had been described previously 2, but it was Zollinger/Ellison who made the critical hypothesis that the gastric acid hypersecretion was due to secretion of the pancreatic endocrine tumor (panNET)1, 2. At present, it is known that ZES is due to the ectopic secretion of gastrin by a neuroendocrine tumor (NET)(gastrinoma) resulting in gastric acid hypersecretion 3–5, which characteristically causes advanced gastroesophageal reflux(GERD) and/or peptic ulcer disease, often refractory in nature 1, 6. The terms gastrinoma and ZES are frequently used synonymously; however, historically gastrinoma referred to the NET secreting gastrin and ZES to the clinical manifestations of the disease. Numerous tumors, including non-NET neoplasms, synthesize gastrin, and in most it is not fully processed to biologically active gastrin-17–34; however, these do not cause ZES because they do not secrete sufficient amounts of fully processed gastrin, and thus are generally not called gastrinomas by most clinicians and in most classifications of panNETs7, 8.
Gastrinomas like all other functional NETs (F-panNETs) secreting biologically-active peptides causing a functional syndrome (insulinomas, VIPomas, glucagonomas, etc.) differ from other more common neoplasms (colon, pancreatic adenocarcinomas, etc) in presenting to the clinician two different treatment problems 8–11. In each syndrome, the hormone excess-state needs to be controlled (i.e. gastric acid hypersecretion in gastrinomas) and the tumor itself dealt with, because in all cases except insulinomas, F-panNETs are malignant in >50% of cases (i.e. 60–90% for gastrinomas)(Figure 1A) 8–11. Whereas complete surgical resection would treat both of these problems with one approach, as is the usual case with patients with insulinomas10, 12, 13, unfortunately surgical cure in ZES, even today, is seen in <50% of all ZES patients in most series 4, 14–16. Thus, both treatment of the gastric acid hypersecretion and the tumor per se have remained separate treatment problems in most ZES patients, and surgical and medical approaches have played variably important roles in the treatment of each over the years 4, 17, 18.
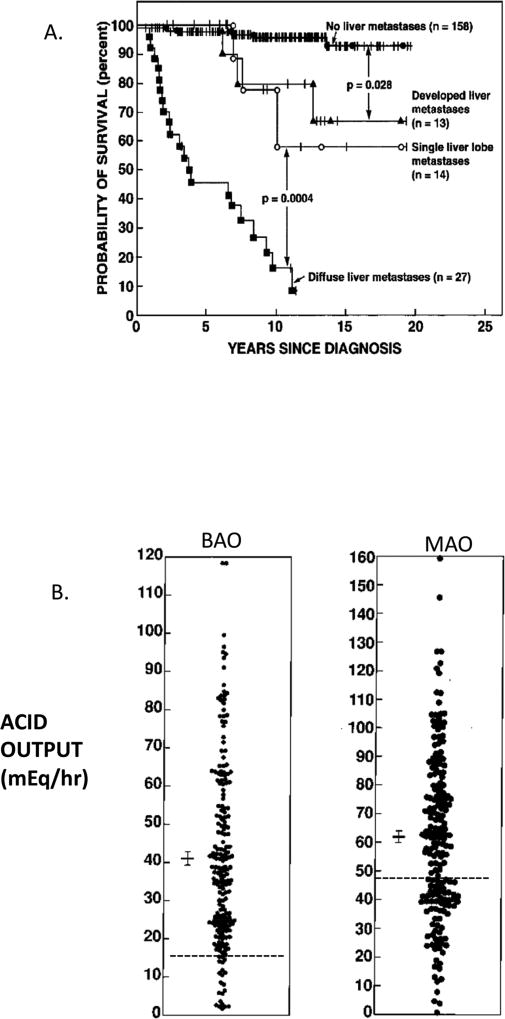
Figure 1.
Extent of disease effect on survival and acid hypersecretion in ZES patients. Panel A. Shown are results from 212 ZES patients prospectively followed.. Panel B. Results are from 205 ZES patients without previous gastric acid recuing surgery. Each point represents data from one patient. The dotted line is the upper limit of normal. The mean ± SEM is shown for each.
II. Roles of medical and surgical treatment in control of gastric acid hypersecretion in ZES patients: Past vs present
II.A. General points: acid hypersecretion
Since the first description of patients with ZES and detailed reports from the original ZES registry and various early series 1, 9, 19, 20, it the morbidity of the devastating effects of uncontrolled acid hypersecretion in ZES patients has become clear. This occurs because ZES patients have on the average a basal acid output (BAO) 4–6-fold elevated and in some patients up to >10 fold increased, combined with an increased maximal ability to secrete acid (MAO) (Figure 1B) due to the stimulatory and trophic effects of chronic hypergastrinemia on the parietal cells, gastric enterochromaffin-like (ECL cells) cells and other gastric mucosal cells 3, 9, 21–23. In almost all cases the initial clinical symptoms of patients with ZES are due to the effects of the acid hypersecretion, with pain due to peptic ulcer disease (73–98%), heartburn (52–56%-recent series), diarrhea (60–75% recent series), weight loss (7–53%) and symptoms due to the complications of the acid hypersecretion (bleeding, strictures, perforation, penetration) 6. These early studies as well as later studies have taught clinicians that ZES patients require control of the gastric acid hypersecretion at all times, both acutely when first seen and long-term 9, 16, 24–27.
II.B. Past-control of acid hypersecretion: medical vs surgical
Initially, medical therapy had no role in the control of acid hypersecretion in ZES patients, with anticholinergic drugs, radiation, and other drugs, being ineffective 9, 28. Surgery, ultimately only total gastrectomy (i.e., removal of the primary target for gastrin) proved to be the only effective treatment in most patients, because it was not possible to surgically cure the patients in most cases by removing the gastrinoma resulting in long-term cure 4, 9, 25, 29, 30. Thus, a surgical approach was the only effective treatment for the gastric acid hypersecretion until the development of histamine H2-receptor antagonists in the 1970’s 9, 31, 32.
The histamine H2 receptor antagonists (metiamide, cimetidine, ranitidine, nizatidine, famotidine, etc.) were all effective in different series at reducing the acid hypersecretion, but results in different series reported a 0–60% failure rate 18, 26, 31–33, which was primarily due to a failure to use established criteria for acid control and to titrate the dose in different patients 18, 24. The NIH studies demonstrated that if sufficient drug was used to control the acid hypersecretion to <10mEq/hr prior to the next drug dose (<5mEq/hr if patient had previous gastric acid-reducing surgery), then in 100% of the patients acid secretion could be controlled, and peptic lesions both healed and the development of new ones prevented 18, 26, 27, 31, 33. Unfortunately, in many patients this took high, frequent doses of the histamine H2 receptor antagonists, and it was true for all members of this class with the dosing only varying by their potency 18, 26, 27, 31, 33. Similarly, during times of surgery and when patients could not take oral medications, parenteral administration of histamine H2 receptor antagonists required relatively high doses given by continuous intravenous administration 18. Furthermore, patients treated long-term with histamine H2 receptor antagonists required yearly reassessment of acid control and on the average required one dosing change (usually an increase) once per year 18, 33, 34. Because of the requirement for dose titration in all patients coupled with the decreasing availability of gastric acid analysis in the US and in other countries until the development and availability of proton pump inhibitors (PPIs) in the 1980s35, both histamine H2 receptor antagonists and the continued use of total gastrectomy and other surgical products such as parietal cell vagotomy coupled with the use of histamine H2 receptor antagonists were used by different groups to control acid hypersecretion in ZES patients from 1970s to 1990s18, 24, 26, 33, 36.
II.C. Present-control of acid hypersecretion-medical vs surgical
At present the pendulum has swung almost 180 degrees from the initial use of only surgical treatments to control the acid hypersecretion in ZES patients to the almost exclusive use of medical therapy. Currently, except for the rare patient (<1%) who cannot or will not reliably take oral medications, PPIs have become the drugs of choice 8, 18, 22, 35, 37. This has occurred because PPIs have a long duration of action allowing once or twice a day dosing in most patients, little tachyphylaxis is seen with <1 dosing change per year, and in most patients acid hypersecretion is adequately controlled without requiring dose titration with measurement of gastric acid secretory rates on the drug 17, 18, 22, 31. All PPIs (omeprazole, esomeprazole, lansoprazole, pantoprazole, rebeprazole) have been shown to be efficacious in ZES for controlling the acid hypersecretion, and PPIs have proven safe and effective for >10 years of treatment and except for low vitamin B12 levels in some patients, no side-effects have limited their use 17, 18, 21, 31, 38, 39. From epidemiological studies on patients without ZES taking long-term PPIs (GERD, idiopathic peptic ulcer disease, etc.) a number of side-effects of PPI have been proposed including bone fractures, dementia, hypomagnesemia, decrease nutrient absorption (vitamin B12, iron, calcium, etc.), interstitial renal disease, various bacterial overgrowths in the gut (clostridia, etc.) and interference with metabolism or absorption of a number of drugs 40, 41. There are no specific reports of these occurring with increased frequency in ZES patients or limiting further PPI treatment.
In cases where patients cannot take oral medications (i.e., during surgery, surgical recovery, etc) parenteral PPIs have also become the agents of choice because of their potency and long duration of action18, 42. They can be given by intermittent intravenous injections which are more convenient than prolonged continuous infusion of histamine H2 receptor antagonists 18, 42.
Histamine H2 receptor antagonists remain effective and can be used in the rare patient who cannot take PPIs; however, they are rarely used today because of the need for high doses, frequent dosing, assessment of acid control to determine proper dosing and for continuous infusion with parenteral administration 8, 18.
Although surgery is not the primary method for acute or long-term control acid hypersecretion in ZES patients, it nevertheless plays a long-term role in its effect on gastric hypersecretion post-curative resection of the gastrinoma. As mentioned earlier, cure is generally not possible in >50% of all patients (Figure 2) (discussed in detail in a later section); nevertheless, a significant proportion of patients can be rendered disease-free post-gastrinoma resection 8, 14, 15, 43–47. There are relatively few studies of the effect of the curative resection on the acid secretory rates, but in a number of reports a proportion of disease-free patients are able to stop or significantly decrease all antisecretory drugs 8, 14, 15, 43–46, 48. Four detailed prospective studies 42, 48–50 of the effect of curative resection of the gastrinoma on disease-free status have been reported and provided some important findings. First, post-curative gastrinoma resection, the mean BAO decreased by 75%, the MAO by 50%, and remained at similar levels for up to 4 years. Second, even though the BAO and MAO markedly decreased post-curative resection, 67% of patients continued to show mild hypersecretion for up to 4 years even though the patients remained disease free (normal fasting serum gastrin levels, negative imaging, negative secretin tests)(Figure 3A). Third, post-curative resection the ranitidine daily dose could be reduce by 66%, and 40% of patients could be removed from all antisecretory drugs. These results demonstrate that curative resection of the gastrinoma has a profound effect on the acid secretory rates, although some patients continue to show mild-moderate hypersecretion and require low doses of antisecretory drugs by an unknown mechanism at present, even though cured.
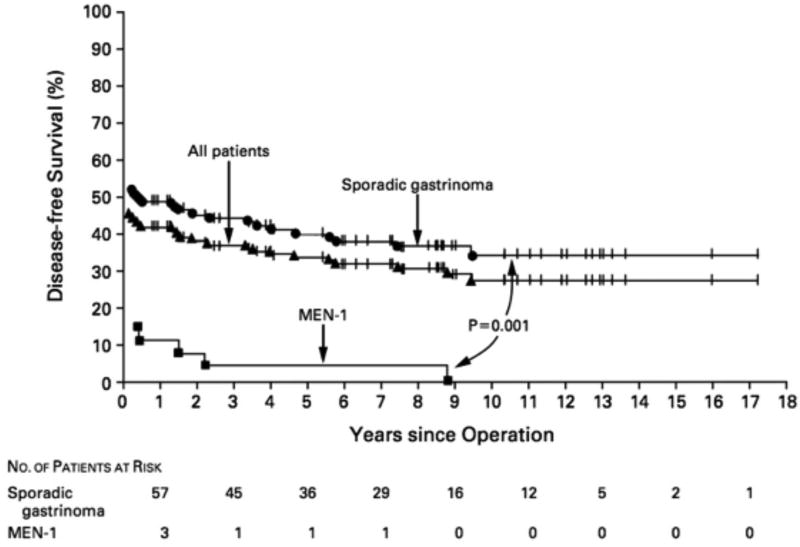
Figure 2.
Disease free survival post-surgery (enucleation, resection) in patients with ZES with or without MEN1. Data are from 123 patients with sporadic ZES and 28 patients with MEN1/ZES. Patents were treated by a fixed protocol involving enucleation of tumor, local tumor resection, and distal pancreatectomy where indicated, but without Whipple resections.
Adapted from Norton JA, Fraker DL, Alexander HR et al. Surgery to cure the Zollinger-Ellison syndrome. N Engl J Med 1999;341:635–644; with permission.
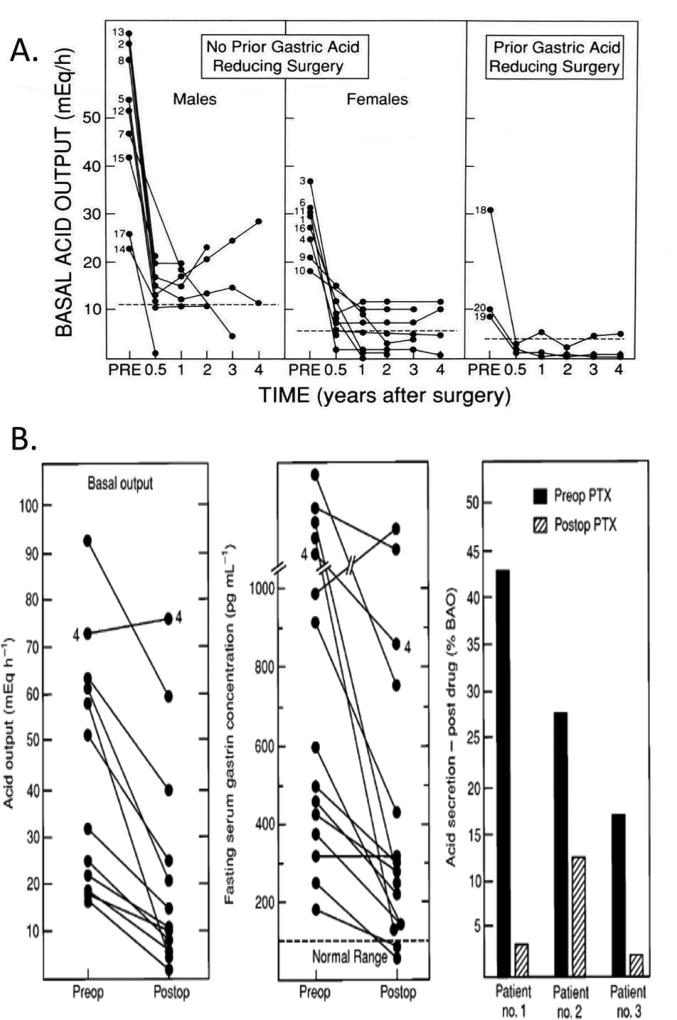
Figure 3.
Effect of curative gastrinoma resection on basal acid hypersecretion(BAO) (Panel A) and effect of parathroidectomy (Panel B) on basal acid hypersecretion, fasting serum gastrin levels and responsiveness to antisecretory drugs in MEN1/ZES patients with hyperparathyroidism.
Panel A. Shown are results from 20 patients surgically rendered disease-free. Mean preoperative BAO was 39 mEq/hr, and the mean serum fasting gastrin 1020 pg/ml (nl<100). By 3–6 mos. postoperatively BAO had decreased 75% and remained unchanged. Dotted lines show upper limit of normal in these studies.
Adapted from Pisegna JR, Norton JA, Slimak G, et al. Effects of curative resection on gastric secretory function and antisecretory drug requirement in the Zollinger-Ellison syndrome. Gastroenterology 1992;102:767–778; with permission.
Panel B. Shown are results from 10 consecutive MEN1/ZES patients with hyperparathyroidism with basal acid output, fasting serum gastrin levels(FSG), and sensitivity to antisecretory drugs (Histamine H2 receptor antagonists [], determined before and a different times post parathyroidectomy. All patients except patient 4 became normocalcemic post parathyroidectomy. Post parathyroidectomy 9/10 (90%) had a decrease in BAO, and 7/10 showed a decrease in FSG including to normal levels in 2 patients. Acid responsiveness was expressed as the percent of the BAO at a given time after taking the same dose of histamine H2 receptor antagonist. In each of the three patients studied the given dose of histamine H2 receptor antagonist caused greater acid suppression post parathyroidectomy.
Adapted from Norton JA, Cornelius MJ, Doppman JL et al. Effect of parathyroidectomy in patients with hyperparathyroidism, Zollinger-Ellison syndrome and multiple endocrine neoplasia Type I: A prospective study. Surgery 1987;102:958–966; with permission.
III. Roles of medical and surgical treatment in treatment of sporadic gastrinoma: Past vs present
III.A. General points: treatment directed at gastrinoma
Most patients with ZES have a sporadic, non-inherited form (75–80%), whereas the remainder (20–25%) have it as part of the Multiple Endocrine Neoplasia type 1 syndrome (MEN1/ZES), an autosomal dominant disorder 51, 52. This distinction is important for many aspects of the treatment, both directed at the acid hypersecretion and the gastrinoma itself 51–53. In this section, treatment directed at the gastrinoma in sporadic cases will be discussed, and in a later section the special aspects in the use of surgical or medical approaches for the treatment of MEN1/ZES will be considered, a number of which are contentious 16, 53–56.
Initially, it was thought that almost all sporadic gastrinomas occurred in the pancreas, similar to insulinomas; however, it is now established that most (60–95%) occur in the duodenum and in recent series they are 3–9-fold more frequent than pancreatic gastrinomas 15, 45. Duodenal and pancreatic gastrinomas differ in their biological behavior in that both are associated with frequent lymph node metastases (30–70%) 16, 57, 58 ; however, the pancreatic tumors have a much higher rate of liver metastases57, 58, which is one of the primary determinants of long-term survival (Figure 1A), with the result that patients with pancreatic gastrinomas have a worse prognosis 57–59. Sporadic gastrinomas as a group are malignant in 60–90% of cases, and approximately 13–53% (mean-34%) of patients have liver metastases at presentation, with the majority being diffuse liver involvement 57–60.
III.B. Past-treatment directed at sporadic gastrinoma: nonsurgical vs surgical approach
With the increased ability to medically control acid hypersecretion in sporadic ZES patients, since the 1980’s attention has shifted increasingly to the possible role of surgery for curative resection. Initially, a number of authorities recommended a non-operative approach in sporadic ZES patients with either small or no tumors imaged 61, 62. This was based on the fact that patients at that time were rarely cured, and in 30–60% of patients no gastrinoma was found at surgery 9, 61, 62 and because these patients generally did very well with long-term acid suppression alone 61, 62. This situation occurred primarily because it was not yet clear that most of the sporadic gastrinomas were in the duodenum, and that only with a careful search for these could they be found (mobilization of duodenum, duodenotomy, transillumination of duodenum) 45, 63–66, because they were often <1 cm in diameter 45, 57, 67(Figure 4A.). Furthermore, it was not appreciated in MEN1/ZES patients the gastrinomas were also in the duodenum, the imaged pancreatic tumors were usually NF-panNETs, and that these patients could rarely be cured because of the multiplicity of the duodenal gastrinomas (Figure 2) without aggressive resections such as a Whipple resection, which was not routinely recommended 8, 14, 15, 53, 56, 68.
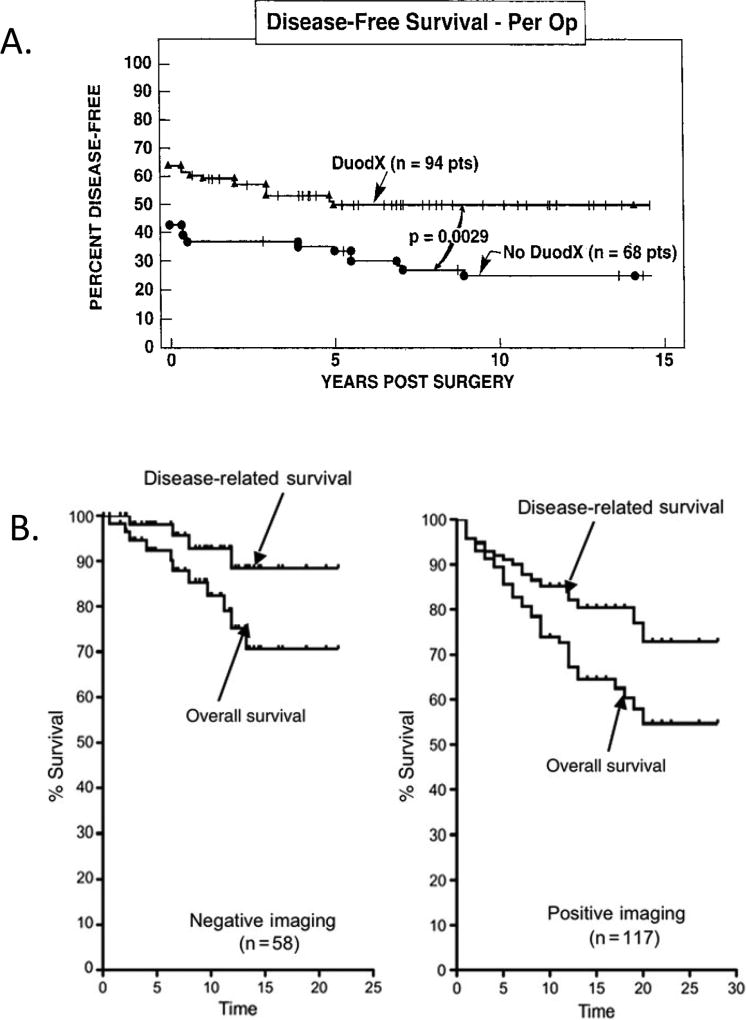
Figure 4.
Results of performing a duodenotomy (Panel A) on disease-free status and results of surgical exploration for possible cure in ZES patients with or without positive pre-operative imaging studies (Panel B). Panel A. Effect of duodenotomy(DUODX) on disease free-status in 142 patients with ZES without MEN1. With DUODX, gastrinomas were found in 98%, duodenal gastrinomas in 62%, and the cure rate postoperatively was 65% compared with patients without DUODX (p<0.01) in whom gastrinomas were found in 76%, 18% had duodenal gastrinomas found and 44% were cured post resection. Modified and drawn from data in 45. Panel B. Surgical results from 117 patients with sporadic ZES with positive imaging are compared to results in 58 patients with sporadic ZES with negative preoperative imaging. Postoperatively 63% of the patients with negative imaging were disease free postoperatively, whereas it was seen in 54% with positive imaging and at a 20-yr follow-up, the negative imaging patients had a better survival (71% vs 58%), and better disease related survival (88% vs 73%, [p=0.15]).
Adapted from Norton JA, Fraker DL, Alexander HR et al. Value of surgery in patients with negative imaging and sporadic zollinger-ellison syndrome. Ann Surg 2012;256:509–517; with permission.
III.C. Present-treatment directed at sporadic gastrinoma: nonsurgical vs surgical approach
In direct contrast to the treatment of the gastric acid hypersecretion in sporadic ZES which has changed from surgical to largely medical, the approach to the tumor has changed in most centers, and became increasingly surgical, with decreasing numbers of patients with potentially resectable sporadic disease followed medically with control of acid hypersecretion only. All existing guidelines including ENETs, NANETs, ESMO’s and the NCCN 8, 12, 13, 69, 70 recommend that in sporadic ZES surgical resection should be carried out, if possible complete tumor removal can be performed, and there are no accompanying medical conditions limiting life expectancy or increasing surgical risks to unacceptable levels.
This change in approach to the sporadic gastrinoma to an increasing surgical option, whenever possible, has occurred for a number of reasons. First, acid hypersecretion can now be well-controlled throughout the surgical period, whereas in the past, the lack of effective medical therapies for this, resulted in mortalities as high as 30% 9, 25, 30. Second, tumor imaging modalities (discussed below), have markedly improved in sensitivity making it possible to better localize the primary and stage the disease, preventing unnecessary surgery 8, 54, 71. Third, more recent surgical studies have demonstrated increasing disease-free rates approaching 40–63% of patients operated on without Whipple resections 44–47, 58(Figure 2), and higher in patients with Whipple resections 15, 72. Fourth, importantly, two studies 73, 74 have provided evidence that surgical resection in sporadic ZES leads to a decreased rate of the development of liver metastases, which are the most important determinate of long-term survival 57–59 (Figure 1A), while also one study 74 demonstrated increased disease-related survival with surgery. Fifth, the surgical approach to find duodenal gastrinomas has been studied and demonstrated that specific techniques are needed to find this tumor (duodenotomy, mobilization of duodenum, intra-operative transillumination of duodenum in some cases) 45, 63–66(Figure 4A). Sixth, a recent study demonstrates that even in patients with sporadic ZES with negative preoperative imaging studies, an experienced surgeon will find gastrinoma in 98% of the patients with 50% rendered disease-free, which is not different from the results in patients with positive imaging preoperatively 44 (Figure 4B). Seventh, the importance of routine lymphadenectomy in sporadic ZES has been emphasized in number of studies and is now routinely recommended. The presence of ‘lymph node primary gastrinomas’ is controversial, even though a number of studies have reported long-term (up to 20 years) with disease free survival post resection of only a lymph node 75–78. Studies have reported increased disease-free survival when lymph nodes are routinely resected in sporadic ZES patients 47. Furthermore, in two studies14, 77 the recurrence or relapse rate after achieving disease-free status post-resection of a primary lymph node gastrinoma was lower than that after resection of a duodenal or pancreatic primary. Therefore, routine removal of lymph nodes can not only increase the disease-free survival rate, but the number of positive lymph nodes or the lymph node ratio has important prognostic significance in gastrinomas and other panNETs12, 79–83.
III.D. Present-surgical treatment directed at sporadic gastrinoma-controversies
Despite the general recommendation that patients with sporadic ZES should undergo surgical resection if possible, there are a number of specific areas in the surgical management that are controversial. In addition to the question of primary lymph node gastrinomas, which were discussed in the previous paragraph, other areas of controversy include: the role of Whipple resection (cephalic pancreaticoduodenectomy); the role of laparoscopic surgery in patients with sporadic gastrinomas; the role of surgical resections in patients with advanced disease or disease possibly involve mesenteric blood vessels; and the extent/timing of imaging in patients without tumors on cross-sectional imaging pre- or post-surgical procedures.
Currently, in the different guidelines the preferred approach if possible is enucleation, local resection for pancreatic head lesions or distal pancreatectomy when necessary for distal pancreatic lesions 8, 12, 13, 69, 70. Whipple resections are generally reserved for large pancreatic head or duodenal lesions which are unable to be adequately removed with enucleation 8, 12, 13, 15, 69, 70. One of the main problems with rendering patients disease-free is that lymph node metastases are found in 30–70% of cases and are therefore often missed without more extensive surgery such as a Whipple resection. Studies support an increased disease-free rate with Whipple resection 15, 72, but because of possible long-term complications, coupled with the excellent prognosis of patients who are not cured but with small residual disease, more aggressive general use of Whipple resections is currently not generally recommended 8, 12, 13, 69, 70.
Laparoscopic surgery is increasingly being used in patients with panNETs, especially those with localized insulinomas, NF-panNETs and in MEN1 patients with panNETs 84–89. In contrast to these other panNETs, only a small number of patients with gastrinomas have been treated with laparoscopic surgery 16, 84, 87, 90–92. This is in large part due to the need for an extensive exploration with a Kocher maneuver, duodenotomy (Figure 4A), routine lymphadenectomy, exploration of the gastrinoma triangle and liver as well as biliary system, required in patients with ZES 16, 55.
Similar to other advanced NETs, the role of surgical resection in ZES patients with advanced metastatic disease or even with extensive invasive localized disease is not well-defined. Unfortunately, most patients presenting with hepatic metastases with gastrinomas have metastases in both hepatic lobes, with only 5–15% have localized hepatic metastases 57, 58, 60. If imaging studies support the resectability of the metastases, then surgical resection is generally recommende, if the patient is an operative candidate without other medical conditions precluding surgery 8, 12, 43. Similarly, if most or all imaginable disease is thought surgically resectable, surgery is generally recommended 8, 12, 43. Patients with gastrinomas as well as other malignant panNETs/NETs not uncommonly present with local invasion and/or vessel encasement or possible involvement, which has led them to frequently not being considered surgical candidates 93. A few recent studies have challenged this thinking93–95. One recent study 93 demonstrated 17% of all gastrinomas fall into this category demonstrating possible major vascular involvement (Figure 5), and in 42 patients a panNETs could be resected with only 9 patients requiring vascular reconstruction, 30% showed long term disease-free status and post-resection the patients had a 10-year survival of 60%. This result 93 led the authors to conclude that surgical resection of panNETs with vascular abutment/invasion is indicated and generally successful without requiring vascular reconstruction, and thus should not be a contraindication to surgery.
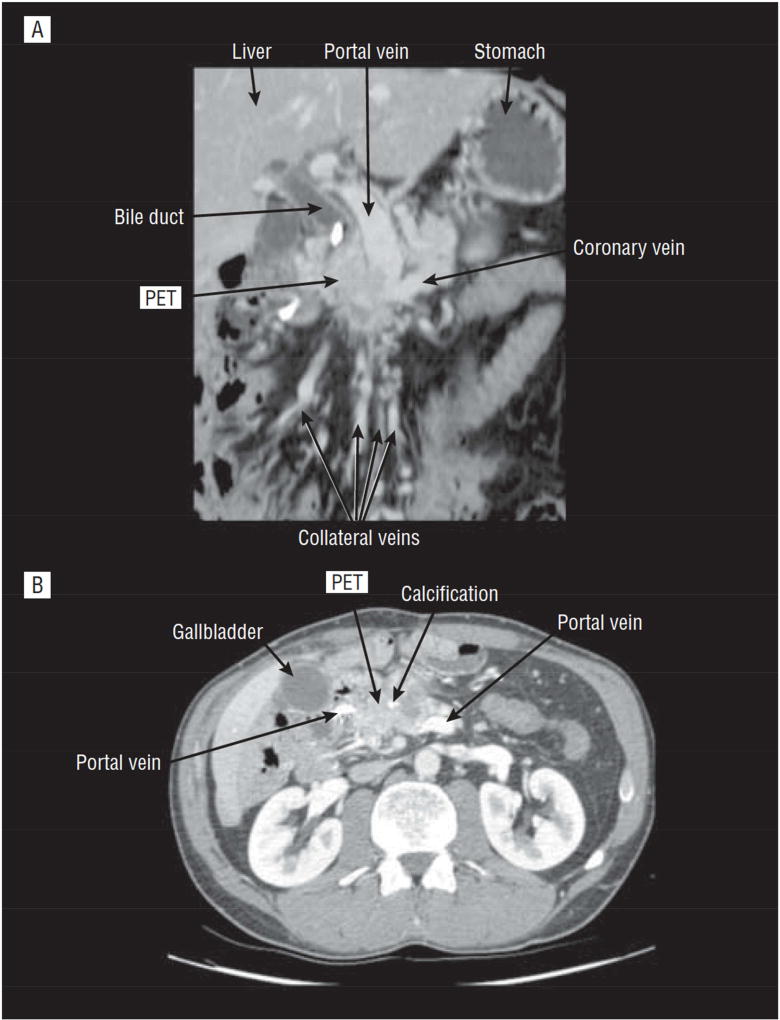
Figure 5.
Imaging results in a patient with a panNET obstructing the proximal portal vein. Panel A shows a coronal planar view and Panel B shows a transverse view of the CT scan. The label PET shows the location of a panNET obstructing the proximal portal vein and with the development of extensive collateral veins. This patient had the tumor and a portion if the portal vein resected with venous reconstruction. This patient is representative af a subgroup of gastrinomas and other PanNET that are thought by many to be unresectable because of the vascular involvement, however a recent study93 shows most are resectable.
Adapted from Norton JA, Harris EJ, Chen Y et al. Pancreatic endocrine tumors with major vascular abutment, involvement, or encasement and indication for resection. Arch Surg 2011;146:724–732; with permission.
Originally, cross-sectional imaging studies (CT, MRI, ultrasound) were primarily used to attempt to localize the primary tumor and establish the extent of the tumor involvement in ZES patients. Even with dramatic improvements in sensitivity, most small gastrinomas (<1–1.5cm) were missed10, 60, 96, 97 and other approaches were increasingly used such as functional studies assessing gastrin gradients either after selective portal venous sampling or hepatic venous sampling after selective intra-arterial secretin injection 60, 98, 99 which had sensitivities of 71–86%. The development of somatostatin receptor imaging (SRI) utilizing the fact that gastrinomas, similar to most NETs, overexpress somatostatin receptors (primarily sst 2), has largely replaced functional imaging studies such as gastrin sampling 8, 16. Initially, 111In-penetreotide was used with SPECT/CT imaging and shown to be more sensitive that any cross-sectional imaging study to allow whole body scans at one time and to be the most sensitive modality for localizing distant metastases in patients with advanced ZES 12, 16, 100–102. Recently, this is being replaced by 68Ga-DOTA-somatostain peptide PET/CT which has even greater sensitivity and is now approved for use in both the US and Europe 71, 96, 103, 104. Endoscopic ultrasound (EUS) is the most sensitivity modality for detecting intra-pancreatic lesion, but its utility is limited in patients with sporadic ZES because the majority of the gastrinomas are duodenal and these are missed on EUS 8, 15. In general, almost all patients with sporadic ZES undergo a conventional imaging study and it is recommended that they also should have SRI, preferably with 68Ga-DOTA-somatostain peptide PET/CT, especially if a surgical procedure is considered.12, 16, 71, 96. At present, the best timing of imaging tests post-surgically is not established or whether they will be more sensitive than functional studies (i.e., assessing serum gastrin, secretin testing) in detecting recurrences postoperatively in ZES patients.
IV. Roles of medical and surgical treatment in treatment of MEN1/ZES: Past vs present
IV.A. General points: Treatment of MEN1/ZES
The 20–25% of patients with ZES due to MEN1(MEN1/ZES) have a number of specific problems due to the presence of the MEN1 that affect the medical and surgical treatments51–53, 55. MEN1 patients characteristically develop hyperplasia/tumors of multiple endocrine organs with 98–100% developing multiple parathyroid adenomas with hyperparathyroidism, 80–100% developing panNETs and 50–60% pituitary adenomas 51–53, 55. For the panNETs, 80–100% develop microscopic NF-panNETs (0–13%-symptomatic), and 54% develop MEN1/ZES (range 20–61%), while insulinomas occur in 18%(range 7–31%) with the other F-panNET syndromes occurring <3% 51–53, 55. These patients also developed tumors in other organs to a lesser extent including adrenal adenomas/carcinomas (27–36%), carcinoid tumors [bronchial/lung (0–8%), gastric (7–35%), thymic (0–8%%)], nonendocrine tumors of the skin [angiofibromas/collagenomas (60–90%)], central nervous system tumors [meningiomas, schwannomas, ependymonas](0–8%), and smooth muscle tumors (1–7%-leiomyomas/leiomyosarcomas) 51, 55, 105. Characteristically, these patients present with hyperparathyroidism; however, in some recent series up to 33% present with F-panNETS 52, 106, 107.
The specific features of MEN1 create a number of unique problems in the medical and/or surgical management of the ZES in these patients. First, the hyperparathyroidism can affect the activity of the hormone excess state of the F-panNET such as gastrin/acid secretion and the control of the acid hypersecretion in MEN1/ZES 18, 108–110(Figure 3B). Second, microscopic NF-panNETs are present in all MEN1 patients and in up to 80% larger sizes, and thus not only may require treatment but their presence, complicates the localization of the gastrinoma 51, 53, 54, 111. In 85–100% of MEN1/ZES patients in different series the gastrinomas occur in the duodenum; however, in a minority of patients (0–15%) they are reported in the pancreas 51, 53, 112–114. Third, in MEN1/ZES patients the duodenal gastrinomas are almost always multiple, frequently small (<0.5cm), and associated with lymph node metastases in 40–60% 53, 113, 115. The result of this is MEN1/ZES patients cannot be cured of all their NF-pNETs or gastrinomas (Figure 2) without aggressive resections such as Whipple resection 51, 53, 54. In contrast, other F-panNETs in MEN1 patients (insulinomas, glucagonomas,etc) are generally curable 51, 53, 116. Fourth, the natural history of MEN1 patients is changing; however, it is at present largely unknown although the mean age at death is still shortened at 55–60 years old in a number of studies 53, 105, 117. MEN1 patients are now rarely dying of acid hypersecretion due to MEN1/ZES, which was a major cause of death in early series 52, 53, 105, 117. However, other tumors such as thymic carcinoids are now increasingly described in MEN1 patients, especially males, and are aggressive and an increasing cause of death 53, 105, 117. The lack of the long term natural history is particularly important for NF-panNETs and gastrinomas, because in many cases these patients are treated without surgery, as discussed below.
IV.B. Present-specific aspects of control of acid hypersecretion in MEN1/ZES-medical vs surgical
In general, the management of acid secretion in MEN1/ZES patients follows the patterns discussed in paragraphs II.A-II.C above in patients with sporadic ZES, with initially only a surgical approach with total gastrectomy being effective, to later, where an increasing medical approach has been used, with first histamine H2 receptor antagonists and still later PPIs were increasingly used. At present, as for sporadic ZES, the drugs of choice both for control of the acid hypersecretion in MEN1/ZES patients are PPIs 8, 11, 13, 18.
However, surgery still can play an important role in facilitating control of the acid hypersecretion in MEN1/ZES patients. In contrast to the situation in patients with sporadic ZES where acid hypersecretion could be markedly altered by curing a significant number of patients, cure is rare in MEN1/ZES without aggressive resections, which are not routinely recommended in any current guidelines (ENETs, NANATES) 12, 51, 69. Surgery can play a role in facilitating the control of acid hypersecretion in MEN1/ZES patients by correcting the hyperparathyroidism by an appropriate parathyroidectomy (i.e. 3.5 gland or 4-gland removal with a parathyroid implant) 51, 52, 108, 110(Figure 2B). From acid secretory studies in patients with MEN1/ZES with hyperparathyroidism, increased relative resistance to the effects of antisecretory drugs have been reported, and higher drug doses than in sporadic ZES patients are frequently required 118. Calcium is a potent stimulating of gastrin release from gastrinomas 119, and number of studies report that in patients with MEN1/ZES with hypercalcemia due to the hyperparathyroidism, when the hyperparathyroidism is corrected they have a decreased magnitude of hypergastrinemia (sometimes gastrin levels return to normal), a decrease in secretin-stimulated gastrin release, and an increase in sensitivity to anti-secretion drugs (Figure 2B) 108, 112, 120.
IV.D. Present-treatment directed at MEN1/panNET/gastrinoma: nonsurgical vs surgical approach
At present this is an area of considerable disagreement between a surgical and nonsurgical approach. Whereas all agree that patients with F-panNETs with MEN1, excluding gastrinomas, should undergo routinely surgical exploration because of their high (>90–100%) cure rate, this is not the case with patients with gastrinomas or with NF-panNETs 12, 13, 51, 53, 116. As stated above, gastrinomas/NF-PanNETs are almost always multiple and often small in size (<0.5 cm) and thus they are rarely curable unless aggressive resections are performed, such as Whipple resections or even total pancreatectomy with NF-panNETs 15, 51, 53, 121, 122. This fact, coupled with increasing evidence that patients with PanNETs or with MEN1/ZES with small tumors (<1.5–2cm) have an excellent long-term prognosis without surgery 8, 51, 53, 54, 121, 123, 124(Figure 6), has led to the current controversy on their treatment. Additional points that contribute to this controversy is that these patients frequently present at younger ages than seen with sporadic forms of these tumors, and are often asymptomatic in the case of NF-panNETs. Not only are these resections associated with morbidity/mortality, long-term complications can occur. Also, recent studies report patients with MEN1 have an increased incidence of diabetes/glucose intolerance which are not uncommon after pancreatic resections, reported in 24–86% of MEN1 patients post-resection and 17–25% after pancreaticoduodenectomy53.
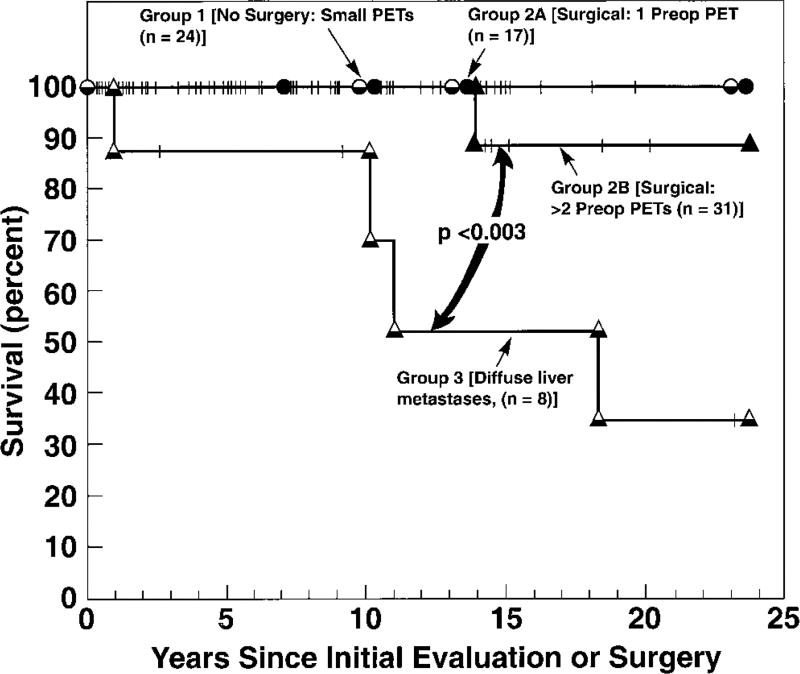
Figure 6.
Survival of different groups of MEN1/ZES patients. Data are for 81 MEN1/ZES patients of which 17 were in group 1(all panNETs imaged preoperative <2.5 cm diameter-no surgery); and Group 3 (n=8) with diffuse liver metastases and no surgical resection. Group 2 consisted of 17 patients in Group 2A with a single panNET (2.5–6 cm in diameter) and Group 2B (n=31) with two or more lesions >2.5 cm, who underwent laparotomy. Group 1, 2A and 2B had similar 15-year survival rates of 89–100%, which was better than patients with diffuse liver metastases in Group 3 (52%). This study concluded that patients with small panNETs<2.5cm with MEN1/ZES can be followed without surgery (15-yr survival=100%) and that patients with larger lesions should have them resected if possible 124.
Adapted from Norton JA, Alexander HR, Fraker DL et al. Comparison of surgical results in patients with advanced and limited disease with multiple endocrine neoplasia type 1 and Zollinger-Ellison syndrome. Ann Surg 2001;234:495–506; with permission.
Current recommendations from a number of societies including ENET, NANETs and the Endocrine Society 8, 12, 13, 125 recommend that small panNETs (<1.5–2m) in patients with NF-pNETs or MEN1/ZES be treated conservatively. All agree that if patients with these small panNETs are treated conservatively it is important that they be closely monitored. How to best monitor these small pancreatic NETs in MEN1 patients is also an area of contention 54. Cross-sectional imaging studies will miss >50% of lesions <1.5 cm; however, SRI has greater sensitivity, but it is not established to be reliable for assessing serial changes in tumor size, and the issue of repeated radiation exposure can be a factor in these patients 53, 54. Numerous studies show that for intrapancreatic NETs such as NF-panNETs, EUS is the most sensitive modality for their detection, and allows accurate assessment of changes in NET size on repeated examinations 54, 89, 126–128. Serial studies show that most panNETs in MEN1 patients <2 cm are relatively stable and uncommonly increase rapidly in size 54, 127, 129–131. In MEN1/ZES with imaged NETs <2cm the role of EUS is much more limited because these NETs are not intrapancreatic and often missed by EUS, and thus they are usually followed by repeated cross-sectional imaging studies 54. Although recent studies demonstrate that 68GaDOTATOC-PET/CT is much more sensitive for detecting lesions in MEN1 patients than cross-sectional imaging, at present its exact role initially and in follow-up is unclear and controversial in MEN1 patients 54, 132–135.
One of the most pressing problems is to identify predictors for which patients with NF-panNETs or gastrinomas will pursue an aggressive course in MEN1 patients 53, 105, 136. Although numerous factors (both clinical and tumoral features) have been reported to have prognostic value in MEN1 patients for the development of a panNETs or their aggressive behavior, similar to patients with sporadic panNETs, in general they are not particularly helpful in a given patient 51, 59, 105, 136–139. In sporadic panNETs, the WHO grading has been shown to have important prognostic value12, 140, 141. Preliminary studies in MEN1 patients suggest that the histological grade of the tumors has predictive value; however, the majority (>80%) are G1 and some of these can also pursue an aggressive course 142. Recently, the predictive value of using 18F-FDG-PET/CT has been proposed for patients with MEN1 with panNETs 143. Numerous studies demonstrate that in most well differentiated NETs (G1, G2) the 18F-FDG PET/CT is negative, but in a proportion it is positive and this correlates with aggressive behavior 71. In the above recent study143 in 49 patients with MEN1 undergoing 18F-FDG PET, 6/8 patients (75%) with FDG-avid panNETs harbored aggressive or metastatic NETs, compared to only 1/41(2.4%) without FDG avidity for a sensitivity of 86%, specificity of 95% for identify aggressive panNETs. Although a few genotype-phenotype correlations with prognostic value have been reported in patients with MEN1 with panNETs including: mutations in JunD, CHES1, truncation mutations in the N- or C-terminal of the MEN1 gene, missense mutations in the MEN1 gene) and the CDNK1B V109G polymorphism, they have not well studied prospectively and are not widely used at present131, 139, 144–148.
A laparoscopic approach is being increasingly used in MEN1 patients with insulinomas, other localized nongastrinoma panNETs and NF-panNETs, but is not generally used in patients with MEN1/ZES, except the occasional patient with a gastrinoma limited to the pancreatic tail 86, 89, 149. In a meta-analysis 150 i of pancreatic distal resection for all indications, the laparoscopic approach resulted in a lower complication rate, less blood loss, and shorter hospital stays; however, the rate of development of postoperative fistulas was similar. Whereas the results in MEN1 patients are more limited, the available results support the conclusions that minimally-invasive approaches in MEN1 patients with the panNETs listed above is safe and feasible 86, 89.
V. Roles of medical and surgical treatment in treatment of MEN1/ZES patients with advanced disease
As discussed in section IV above, unfortunately most patients with advanced metastatic disease with MEN1/ZES present with diffuse hepatic metastases and in only a minority (<15%) is surgical resection (i.e., generally, removal of least 90% of the disease) possible and recommended 13, 57, 75, 124, 151. The patients with advanced metastatic disease that is nonresectable and that is progressive have a decreased survival (Figure 1, Figure 6) and thus require treatment which involves a number of possible anti-tumor nonsurgical approaches. These include: medical therapy (everolimus, or tyrosine kinase inhibitors such as sunitinib), peptide radioreceptor therapy (PRRT) with 177Lu-labeled somatostatin analogues (which will likely be approved by the FDA this coming year based on a recent successful phase 3 trial in GI midgut NETs152), chemotherapy or liver-directed therapies (embolization, chemoembolization, radioembolization). These treatments are similar to that in other advanced NETs and are not specific for MEN1/ZES and have been recently reviewed in other publications 153–157, so will not be dealt with further in this article.
Key Points.
Zollinger-Ellison syndrome (ZES) is caused by a gastrin-secreting neuroendocrine tumor that results in marked acid hypersecretion.
All patients with ZES have two management problems which must both be dealt with: control of the acid hypersecretion which causes refractory peptic disease, and control of the gastrinoma which is malignant in 60–90% of cases.
20–25% of patients with ZES have it as part of the MEN1 syndrome that needs to be recognized as its management differs from sporadic cases (75–80%).
Over the years surgical and medical approaches have played varying roles in the treatment of each aspect of ZES.
Presently, the roles of medical and surgical approaches are generally complementary; however, in several areas the selective use of one over the other is controversial.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: The authors have nothing to disclose.
References
- 1.Zollinger RM, Ellison EH. Primary peptic ulcerations of the jejunum associated with islet cell tumors of the pancreas. Ann Surg. 1955;142:709–728. [PMC free article] [PubMed] [Google Scholar]
- 2.Stabile BE. Gastrinoma before Zollinger and Ellison. Am J Surg. 1997;174:232–236. doi: 10.1016/s0002-9610(97)00131-1. [DOI] [PubMed] [Google Scholar]
- 3.Roy PK, Venzon DJ, Feigenbaum KM, et al. Gastric secretion in Zollinger-Ellison syndrome: correlation with clinical expression, tumor extent and role in diagnosis - A prospective NIH study of 235 patients and review of the literature in 984 cases. Medicine(Baltimore) 2001;80:189–222. doi: 10.1097/00005792-200105000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Ellison EC, Johnson JA. The Zollinger-Ellison syndrome: a comprehensive review of historical, scientific, and clinical considerations. Curr Probl Surg. 2009;46:13–106. doi: 10.1067/j.cpsurg.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 5.Berna MJ, Hoffmann KM, Serrano J, et al. Serum gastrin in Zollinger-Ellison syndrome: I. Prospective study of fasting serum gastrin in 309 patients from the National Institutes of Health and comparison with 2229 cases from the literature. Medicine (Baltimore) 2006;85:295–330. doi: 10.1097/01.md.0000236956.74128.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roy PK, Venzon DJ, Shojamanesh H, et al. Zollinger-Ellison syndrome: clinical presentation in 261 patients. Medicine (Baltimore) 2000;79:379–411. doi: 10.1097/00005792-200011000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Rehfeld JF, van Solinge WW. The tumor biology of gastrin and cholecystokinin. Adv Cancer Res. 1994;63:295–347. doi: 10.1016/s0065-230x(08)60403-0. [DOI] [PubMed] [Google Scholar]
- 8.Jensen RT, Niederle B, Mitry E, et al. Gastrinoma (duodenal and pancreatic) Neuroendocrinology. 2006;84:173–182. doi: 10.1159/000098009. [DOI] [PubMed] [Google Scholar]
- 9.Jensen RT, Gardner JD. Gastrinoma. In: Go VLW, DiMagno EP, Gardner JD, Lebenthal E, Reber HA, Scheele GA, editors. The Pancreas: Biology, Pathobiology and Disease. New York: Raven Press Publishing Co.; 1993. pp. 931–978. [Google Scholar]
- 10.Metz DC, Jensen RT. Gastrointestinal neuroendocrine tumors:; Pancreatic endocrine tumors. Gastroenterology. 2008;135:1469–1492. doi: 10.1053/j.gastro.2008.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ito T, Igarashi H, Jensen RT. Pancreatic neuroendocrine tumors: clinical features, diagnosis and medical treatment: Advances. Best Pract Res Clin Gastroenterol. 2012;26:737–753. doi: 10.1016/j.bpg.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Falconi M, Eriksson B, Kaltsas G, et al. ENETS Consensus Guidelines Update for the Management of Patients with Functional Pancreatic Neuroendocrine Tumors and Non-Functional Pancreatic Neuroendocrine Tumors. Neuroendocrinology. 2016;103:153–171. doi: 10.1159/000443171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jensen RT, Cadiot G, Brandi ML, et al. ENETS Consensus Guidelines for the Management of Patients with Digestive Neuroendocrine Neoplasms: Functional Pancreatic Endocrine Tumor Syndromes. Neuroendocrinology. 2012;95:98–119. doi: 10.1159/000335591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Norton JA, Fraker DL, Alexander HR, et al. Surgery to cure the Zollinger-Ellison syndrome. N Engl J Med. 1999;341:635–644. doi: 10.1056/NEJM199908263410902. [DOI] [PubMed] [Google Scholar]
- 15.Norton JA, Jensen RT. Resolved and unresolved controversies in the surgical management of patients with Zollinger-Ellison syndrome. Ann Surg. 2004;240:757–773. doi: 10.1097/01.sla.0000143252.02142.3e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ito T, Igarashi H, Jensen RT. Zollinger-Ellison syndrome: Recent advances and controversies. Current Opinion in Gastroenterology. 2013;29:650–661. doi: 10.1097/MOG.0b013e328365efb1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Metz DC, Strader DB, Orbuch M, et al. Use of omeprazole in Zollinger-Ellison: A prospective nine-year study of efficacy and safety. Aliment Pharmacol Ther. 1993;7:597–610. doi: 10.1111/j.1365-2036.1993.tb00140.x. [DOI] [PubMed] [Google Scholar]
- 18.Ito T, Igarashi H, Uehara H, et al. Pharmacotherapy of Zollinger-Ellison syndrome. Expert Opin Pharmacotherapy. 2013;14:307–321. doi: 10.1517/14656566.2013.767332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ellison EC, O'Dorisio TM, Woltering EA, et al. Suppression of gastrin and gastric acid secretion in the Zollinger-Ellison syndrome by long-acting somatostatin (SMS 201-995) Scand J Gastroenterol. 1986;21(Suppl 119):206–211. [Google Scholar]
- 20.Guida PM, Todd JE, Moore SW, et al. Zollinger-Ellison syndrome with interesting variations. Am J Surg. 1966;112:807–817. doi: 10.1016/0002-9610(66)90130-9. [DOI] [PubMed] [Google Scholar]
- 21.Maton PN, Lack EE, Collen MJ, et al. The effect of Zollinger-Ellison syndrome and omeprazole therapy on gastric oxyntic endocrine cells. Gastroenterology. 1990;99:943–950. doi: 10.1016/0016-5085(90)90611-4. [DOI] [PubMed] [Google Scholar]
- 22.Jensen RT. Consequences of long-term proton pump blockade: Highlighting insights from studies of patients with gastrinomas. Basic Clin Pharmacol Toxicol. 2006;98:4–19. doi: 10.1111/j.1742-7843.2006.pto_378.x. [DOI] [PubMed] [Google Scholar]
- 23.Peghini PL, Annibale B, Azzoni C, et al. Effect of chronic hypergastrinemia on human enterochromaffin-like cells: insights from patients with sporadic gastrinomas. Gastroenterology. 2002;123:68–85. doi: 10.1053/gast.2002.34231. [DOI] [PubMed] [Google Scholar]
- 24.Jensen RT. Basis for failure of cimetidine in patients with Zollinger- Ellison syndrome. Dig Dis Sci. 1984;29:363–366. doi: 10.1007/BF01318525. [DOI] [PubMed] [Google Scholar]
- 25.Ellison EH, Wilson SD. The Zollinger-Ellison syndrome: Re-appraisal and evaluation of 260 registered cases. Ann Surg. 1964;160:512–530. doi: 10.1097/00000658-196409000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Metz DC, Pisegna JR, Fishbeyn VA, et al. Control of gastric acid hypersecretion in the management of patients with Zollinger-Ellison syndrome. World J Surg. 1993;17:468–480. doi: 10.1007/BF01655106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raufman JP, Collins SM, Pandol SJ, et al. Reliability of symptoms in assessing control of gastric acid secretion in patients with Zollinger-Ellison syndrome. Gastroenterology. 1983;84:108–113. [PubMed] [Google Scholar]
- 28.Jensen RT, Gardner JD, Raufman JP, et al. Zollinger-Ellison syndrome: current concepts and management. Ann Intern Med. 1983;98:59–75. doi: 10.7326/0003-4819-98-1-59. [DOI] [PubMed] [Google Scholar]
- 29.Maton PN, Mackem SM, Norton JA, et al. Ovarian carcinoma as a cause of Zollinger-Ellison syndrome. Natural history, secretory products and response to provocative tests. Gastroenterology. 1989;97:468–471. doi: 10.1016/0016-5085(89)90085-1. [DOI] [PubMed] [Google Scholar]
- 30.Fox PS, Hofmann JW, Wilson SD, et al. Surgical management of the Zollinger-Ellison syndrome. Surg Clin North Am. 1974;54:395–407. doi: 10.1016/s0039-6109(16)40287-2. [DOI] [PubMed] [Google Scholar]
- 31.Jensen RT. Use of omeprazole and other proton pump inhibitors in the Zollinger-Ellison syndrome. In: Olbe L, editor. Milestones in Drug Therapy. Basel, Switzerland: Birkhauser Verlag AG Publish. Co.; 1999. pp. 205–221. [Google Scholar]
- 32.McCarthy DM, Olinger EJ, May RJ, et al. H2-histamine receptor blocking agents in the Zollinger-Ellison syndrome. Experience in seven cases and implications for long-term therapy. Ann Intern Med. 1977;87:668–675. doi: 10.7326/0003-4819-87-6-668. [DOI] [PubMed] [Google Scholar]
- 33.Collen MJ, Howard JM, McArthur KE, et al. Comparison of ranitidine and cimetidine in the treatment of gastric hypersecretion. Ann Intern Med. 1984;100:52–58. doi: 10.7326/0003-4819-100-1-52. [DOI] [PubMed] [Google Scholar]
- 34.Wolfe MM, Jensen RT. Zollinger-Ellison syndrome: Current concepts in diagnosis and management. N Engl J Med. 1987;317:1200–1209. doi: 10.1056/NEJM198711053171907. [DOI] [PubMed] [Google Scholar]
- 35.Lamers CBHW, Lind T, Moberg S, et al. Omeprazole in Zollinger-Ellison syndrome: effects of a single dose and of long term treatment in patients resistant to histamine H2-receptor antagonists. N Engl J Med. 1984;310:758–761. doi: 10.1056/NEJM198403223101205. [DOI] [PubMed] [Google Scholar]
- 36.Brennan MF, Sloan AP, Friesen SR, et al. Is total gastrectomy still acceptable in the treatment of the Zollinger-Ellison syndrome. Langenbecks Arch Chir. 1986;367:215–221. doi: 10.1007/BF01258940. [DOI] [PubMed] [Google Scholar]
- 37.Nieto JM, Pisegna JR. The role of proton pump inhibitors in the treatment of Zollinger-Ellison syndrome. Expert Opin Pharmacother. 2006;7:169–175. doi: 10.1517/14656566.7.2.169. [DOI] [PubMed] [Google Scholar]
- 38.Hirschowitz BI, Simmons J, Mohnen J. Clinical outcome using lansoprazole in acid hypersecretors with and without Zollinger-Ellison syndrome: a 13-year prospective study. Clin Gastroenterol Hepatol. 2005;3:39–48. doi: 10.1016/s1542-3565(04)00606-8. [DOI] [PubMed] [Google Scholar]
- 39.Termanini B, Gibril F, Sutliff VE, III, et al. Effect of long-term gastric acid suppressive therapy on serum vitamin B12 levels in patients with Zollinger-Ellison syndrome. Am J Med. 1998;104:422–430. doi: 10.1016/s0002-9343(98)00087-4. [DOI] [PubMed] [Google Scholar]
- 40.Ito T, Jensen RT. Association of long-term proton pump inhibitor therapy with bone fractures and effects on absorption of calcium, vitamin b(12), iron, and magnesium. Curr Gastroenterol Rep. 2010;12:448–457. doi: 10.1007/s11894-010-0141-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eusebi LH, Rabitti S, Artesiani ML, et al. Proton pump inhibitors: Risks of long-term use. J Gastroenterol Hepatol. 2017;32:1295–1302. doi: 10.1111/jgh.13737. [DOI] [PubMed] [Google Scholar]
- 42.Metz DC, Benya RV, Fishbeyn VA, et al. Prospective study of the need for long-term antisecretory therapy in patients with Zollinger-Ellison syndrome following successful curative gastrinoma resection. Aliment Pharmacol Ther. 1993;7(3):247–257. doi: 10.1111/j.1365-2036.1993.tb00095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krampitz GW, Norton JA. Current management of the Zollinger-Ellison syndrome. Adv Surg. 2013;47:59–79. doi: 10.1016/j.yasu.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 44.Norton JA, Fraker DL, Alexander HR, et al. Value of surgery in patients with negative imaging and sporadic zollinger-ellison syndrome. Ann Surg. 2012;256:509–517. doi: 10.1097/SLA.0b013e318265f08d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Norton JA, Alexander HR, Fraker DL, et al. Does the use of routine duodenotomy (DUODX) affect rate of cure, development of liver metastases or survival in patients with Zollinger-Ellison syndrome (ZES)? Ann Surg. 2004;239:617–626. doi: 10.1097/01.sla.0000124290.05524.5e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Atema JJ, Amri R, Busch OR, et al. Surgical treatment of gastrinomas: a single-centre experience. HPB (Oxford) 2012;14:833–838. doi: 10.1111/j.1477-2574.2012.00551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bartsch DK, Waldmann J, Fendrich V, et al. Impact of lymphadenectomy on survival after surgery for sporadic gastrinoma. Br J Surg. 2012;99:1234–1240. doi: 10.1002/bjs.8843. [DOI] [PubMed] [Google Scholar]
- 48.Pisegna JR, Norton JA, Slimak GG, et al. Effects of curative resection on gastric secretory function and antisecretory drug requirement in the Zollinger-Ellison syndrome. Gastroenterology. 1992;102:767–778. doi: 10.1016/0016-5085(92)90157-t. [DOI] [PubMed] [Google Scholar]
- 49.Fraker DL, Norton JA, Saeed ZA, et al. A prospective study of perioperative and postoperative control of acid hypersecretion in patients with Zollinger-Ellison syndrome. Surgery. 1988;104:1054–1063. [PubMed] [Google Scholar]
- 50.Ojeaburu JV, Ito T, Crafa P, et al. Mechanism of Acid hypersecretion post curative gastrinoma resection. Dig Dis Sci. 2011;56:139–154. doi: 10.1007/s10620-010-1234-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jensen RT, Berna MJ, Bingham MD, et al. Inherited pancreatic endocrine tumor syndromes: advances in molecular pathogenesis, diagnosis, management and controversies. Cancer. 2008;113(7 suppl):1807–1843. doi: 10.1002/cncr.23648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gibril F, Schumann M, Pace A, et al. Multiple endocrine neoplasia type 1 and Zollinger-Ellison syndrome. A prospective study of 107 cases and comparison with 1009 patients from the literature. Medicine (Baltimore) 2004;83:43–83. doi: 10.1097/01.md.0000112297.72510.32. [DOI] [PubMed] [Google Scholar]
- 53.Jensen RT, Norton JA. Treatment of Pancreatic Neuroendocrine Tumors in Multiple Endocrine Neoplasia Type 1: Some Clarity But Continued Controversy. Pancreas. 2017;46:589–594. doi: 10.1097/MPA.0000000000000825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ito T, Jensen RT. Imaging in multiple endocrine neoplasia type 1: recent studies show enhanced sensitivities but increased controversies. Int J Endocr Oncol. 2016;3:53–66. doi: 10.2217/ije.15.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Norton JA, Krampitz G, Jensen RT. Multiple Endocrine Neoplasia: Genetics and Clinical Management. Surg Oncol Clin N Am. 2015;24:795–832. doi: 10.1016/j.soc.2015.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lopez CL, Waldmann J, Fendrich V, et al. Long-term results of surgery for pancreatic neuroendocrine neoplasms in patients with MEN1. Langenbecks Arch Surg. 2011;396:1187–1197. doi: 10.1007/s00423-011-0828-1. [DOI] [PubMed] [Google Scholar]
- 57.Weber HC, Venzon DJ, Lin JT, et al. Determinants of metastatic rate and survival in patients with Zollinger-Ellison syndrome: a prospective long-term study. Gastroenterology. 1995;108:1637–1649. doi: 10.1016/0016-5085(95)90124-8. [DOI] [PubMed] [Google Scholar]
- 58.Yu F, Venzon DJ, Serrano J, et al. Prospective study of the clinical course, prognostic factors and survival in patients with longstanding Zollinger-Ellison syndrome. J Clin Oncol. 1999;17:615–630. doi: 10.1200/JCO.1999.17.2.615. [DOI] [PubMed] [Google Scholar]
- 59.Jensen RT. Natural history of digestive endocrine tumors. In: Mignon M, Colombel JF, editors. Recent advances in pathophysiology and management of inflammatory bowel diseases and digestive endocrine tumors. Paris, France: John Libbey Eurotext Publishing Co.; 1999. pp. 192–219. [Google Scholar]
- 60.Jensen RT. Zollinger-Ellison syndrome. In: Doherty GM, Skogseid B, editors. Surgical Endocrinology: Clinical Syndromes. Philadelphia: Lippincott Williams & Wilkins; 2001. pp. 291–344. [Google Scholar]
- 61.McCarthy DM. The place of surgery in the Zollinger-Ellison syndrome. N Engl J Med. 1980;302:1344–1347. doi: 10.1056/NEJM198006123022404. [DOI] [PubMed] [Google Scholar]
- 62.Hirschowitz BI. Clinical course of nonsurgically treated Zollinger-Ellison syndrome. In: Mignon M, Jensen RT, editors. Endocrine Tumors of the Pancreas: Recent advances in research and management. Frontiers of Gastrointestinal Research. Basel, Switzerland: S. Karger; 1995. pp. 360–371. [Google Scholar]
- 63.Norton JA, Doppman JL, Jensen RT. Curative resection in Zollinger-Ellison syndrome: Results of a 10-year prospective study. Ann Surg. 1992;215:8–18. doi: 10.1097/00000658-199201000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Frucht H, Norton JA, London JF, et al. Detection of duodenal gastrinomas by operative endoscopic transillumination: a prospective study. Gastroenterology. 1990;99:1622–1627. doi: 10.1016/0016-5085(90)90466-e. [DOI] [PubMed] [Google Scholar]
- 65.Sugg SL, Norton JA, Fraker DL, et al. A prospective study of intraoperative methods to diagnose and resect duodenal gastrinomas. Ann Surg. 1993;218:138–144. doi: 10.1097/00000658-199308000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Thompson NW, Pasieka J, Fukuuchi A. Duodenal gastrinomas, duodenotomy, and duodenal exploration in the surgical management of Zollinger-Ellison syndrome. World J Surg. 1993;17:455–462. doi: 10.1007/BF01655104. [DOI] [PubMed] [Google Scholar]
- 67.Thom AK, Norton JA, Axiotis CA, et al. Location, incidence and malignant potential of duodenal gastrinomas. Surgery. 1991;110:1086–1093. [PubMed] [Google Scholar]
- 68.Thompson NW, Bondeson AG, Bondeson L, et al. The surgical treatment of gastrinoma in MEN I syndrome patients. Surgery. 1989;106:1081–1085. [PubMed] [Google Scholar]
- 69.Kunz PL, Reidy-Lagunes D, Anthony LB, et al. Consensus Guidelines for the Management and Treatment of Neuroendocrine Tumors. Pancreas. 2013;42:557–577. doi: 10.1097/MPA.0b013e31828e34a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Oberg K, Knigge U, Kwekkeboom D, et al. Neuroendocrine gastro-entero-pancreatic tumors: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23(Suppl 7):vii124–vii130. doi: 10.1093/annonc/mds295. [DOI] [PubMed] [Google Scholar]
- 71.Ito T, Jensen RT. Molecular imaging in neuroendocrine tumors: recent advances, controversies, unresolved issues, and roles in management. Curr Opin Endocrinol Diabetes Obes. 2017;24:15–24. doi: 10.1097/MED.0000000000000300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Giovinazzo F, Butturini G, Monsellato D, et al. Lymph nodes metastasis and recurrences justify an aggressive treatment of gastrinoma. Updates Surg. 2013;65:19–24. doi: 10.1007/s13304-013-0201-8. [DOI] [PubMed] [Google Scholar]
- 73.Fraker DL, Norton JA, Alexander HR, et al. Surgery in Zollinger-Ellison syndrome alters the natural history of gastrinoma. Ann Surg. 1994;220:320–330. doi: 10.1097/00000658-199409000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Norton JA, Fraker DL, Alexander HR, et al. Surgery increases survival in patients with gastrinoma. Ann Surg. 2006;244:410–419. doi: 10.1097/01.sla.0000234802.44320.a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Norton JA, Alexander HA, Fraker DL, et al. Possible primary lymph node gastrinomas: occurrence, natural history and predictive factors: A prospective study. Ann Surg. 2003;237:650–659. doi: 10.1097/01.SLA.0000064375.51939.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Arnold WS, Fraker DL, Alexander HR, et al. Apparent lymph node primary gastrinoma. Surgery. 1994;116:1123–1130. [PubMed] [Google Scholar]
- 77.Chen Y, Deshpande V, Ferrone C, et al. Primary lymph node gastrinoma: A single institution experience. Surgery. 2017;162:1088–1094. doi: 10.1016/j.surg.2017.05.017. [DOI] [PubMed] [Google Scholar]
- 78.Harper S, Carroll RW, Frilling A, et al. Primary lymph node gastrinoma: 2 cases and a review of the literature. J Gastrointest Surg. 2015;19:651–655. doi: 10.1007/s11605-014-2729-4. [DOI] [PubMed] [Google Scholar]
- 79.Krampitz GW, Norton JA, Poultsides GA, et al. Lymph nodes and survival in duodenal and pancreatic neuroendocrine tumors. Arch Surg. 2012;147:820–827. doi: 10.1001/archsurg.2012.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Conrad C, Kutlu OC, Dasari A, et al. Prognostic Value of Lymph Node Status and Extent of Lymphadenectomy in Pancreatic Neuroendocrine Tumors Confined To and Extending Beyond the Pancreas. J Gastrointest Surg. 2016;20:1966–1974. doi: 10.1007/s11605-016-3243-7. [DOI] [PubMed] [Google Scholar]
- 81.Zhang X, Lu L, Shang Y, et al. The number of positive lymph node is a better predictor of survival than the lymph node metastasis status for pancreatic neuroendocrine neoplasms: A retrospective cohort study. Int J Surg. 2017;48:142–148. doi: 10.1016/j.ijsu.2017.10.064. [DOI] [PubMed] [Google Scholar]
- 82.Curran T, Pockaj BA, Gray RJ, et al. Importance of lymph node involvement in pancreatic neuroendocrine tumors: impact on survival and implications for surgical resection. J Gastrointest Surg. 2015;19:152–160. doi: 10.1007/s11605-014-2624-z. [DOI] [PubMed] [Google Scholar]
- 83.Liu P, Zhang X, Shang Y, et al. Lymph node ratio, but not the total number of examined lymph nodes or lymph node metastasis, is a predictor of overall survival for pancreatic neuroendocrine neoplasms after surgical resection. Oncotarget. 2017;8:89245–89255. doi: 10.18632/oncotarget.19184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Heniford BT, Arca MJ, Iannitii DA, et al. Laparoscopic cryoablation of hepatic metastases. Semin Surg Oncol. 1998;15:194–201. doi: 10.1002/(sici)1098-2388(199810/11)15:3<194::aid-ssu9>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 85.Shoji H, Kuroki M, Nakano E, et al. An enucleated duodenal gastrinoma with multiple type 1 endocrine neoplasia located by selective arterial calcium injection. Nihon Shokakibyo Gakkai Zasshi. 2011;108:80–87. [PubMed] [Google Scholar]
- 86.Lopez CL, Albers MB, Bollmann C, et al. Minimally Invasive Versus Open Pancreatic Surgery in Patients with Multiple Endocrine Neoplasia Type 1. World J Surg. 2016 doi: 10.1007/s00268-016-3456-7. [DOI] [PubMed] [Google Scholar]
- 87.Fernandez-Cruz L, Blanco L, Cosa R, et al. Is laparoscopic resection adequate in patients with neuroendocrine pancreatic tumors? World J Surg. 2008;32:904–917. doi: 10.1007/s00268-008-9467-2. [DOI] [PubMed] [Google Scholar]
- 88.Fernandez Ranvier GG, Shouhed D, Inabnet WB., III Minimally Invasive Techniques for Resection of Pancreatic Neuroendocrine Tumors. Surg Oncol Clin N Am. 2016;25:195–215. doi: 10.1016/j.soc.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 89.Nell S, Brunaud L, Ayav A, et al. Robot-assisted spleen preserving pancreatic surgery in MEN1 patients. J Surg Oncol. 2016;114:456–461. doi: 10.1002/jso.24315. [DOI] [PubMed] [Google Scholar]
- 90.Haugvik SP, Marangos IP, Rosok BI, et al. Long-term outcome of laparoscopic surgery for pancreatic neuroendocrine tumors. World J Surg. 2013;37:582–590. doi: 10.1007/s00268-012-1893-5. [DOI] [PubMed] [Google Scholar]
- 91.Atalar K, Warren OJ, Jacyna M, et al. Laparoscopic resection for primary lymph node gastrinoma. Pancreas. 2013;42:723–725. doi: 10.1097/MPA.0b013e31826dcd52. [DOI] [PubMed] [Google Scholar]
- 92.Murase N, Uchida H, Tainaka T, et al. Laparoscopic-assisted pancreaticoduodenectomy in a child with gastrinoma. Pediatr Int. 2015;57:1196–1198. doi: 10.1111/ped.12715. [DOI] [PubMed] [Google Scholar]
- 93.Norton JA, Harris EJ, Chen Y, et al. Pancreatic endocrine tumors with major vascular abutment, involvement, or encasement and indication for resection. Arch Surg. 2011;146:724–732. doi: 10.1001/archsurg.2011.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Haugvik SP, Labori KJ, Waage A, et al. Pancreatic surgery with vascular reconstruction in patients with locally advanced pancreatic neuroendocrine tumors. J Gastrointest Surg. 2013;17:1224–1232. doi: 10.1007/s11605-013-2221-6. [DOI] [PubMed] [Google Scholar]
- 95.Prakash L, Lee JE, Yao J, et al. Role and Operative Technique of Portal Venous Tumor Thrombectomy in Patients with Pancreatic Neuroendocrine Tumors. J Gastrointest Surg. 2015;19:2011–2018. doi: 10.1007/s11605-015-2914-0. [DOI] [PubMed] [Google Scholar]
- 96.Sundin A, Arnold R, Baudin E, et al. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: Radiological, Nuclear Medicine & Hybrid Imaging. Neuroendocrinology. 2017;105:212–244. doi: 10.1159/000471879. [DOI] [PubMed] [Google Scholar]
- 97.Krudy AG, Doppman JL, Jensen RT, et al. Localization of islet cell tumors by dynamic CT: Comparison with plain CT, arteriography, sonography and venous sampling. Am J Roentgenol. 1984;143:585–589. doi: 10.2214/ajr.143.3.585. [DOI] [PubMed] [Google Scholar]
- 98.Cherner JA, Doppman JL, Norton JA, et al. Selective venous sampling for gastrin to localize gastrinomas. A prospective study. Ann Intern Med. 1986;105:841–847. doi: 10.7326/0003-4819-105-6-841. [DOI] [PubMed] [Google Scholar]
- 99.Doppman JL, Miller DL, Chang R, et al. Gastrinomas: localization by means of selective intraarterial injection of secretin. Radiology. 1990;174:25–29. doi: 10.1148/radiology.174.1.2294556. [DOI] [PubMed] [Google Scholar]
- 100.Gibril F, Reynolds JC, Doppman JL, et al. Somatostatin receptor scintigraphy: its sensitivity compared with that of other imaging methods in detecting primary and metastatic gastrinomas: a prospective study. Ann Intern Med. 1996;125:26–34. doi: 10.7326/0003-4819-125-1-199607010-00005. [DOI] [PubMed] [Google Scholar]
- 101.Gibril F, Doppman JL, Reynolds JC, et al. Bone metastases in patients with gastrinomas: a prospective study of bone scanning, somatostatin receptor scanning, and MRI in their detection, their frequency, location and effect of their detection on management. J Clin Oncol. 1998;16:1040–1053. doi: 10.1200/JCO.1998.16.3.1040. [DOI] [PubMed] [Google Scholar]
- 102.Gibril F, Jensen RT. Diagnostic uses of radiolabelled somatostatin-receptor analogues in gastroenteropancreatic endocrine tumors. Dig Liver Dis. 2004;36:S106–S120. doi: 10.1016/j.dld.2003.11.024. [DOI] [PubMed] [Google Scholar]
- 103.Oberg K, Sundin A. Imaging of Neuroendocrine Tumors. Front Horm Res. 2016;45:142–151. doi: 10.1159/000442331. [DOI] [PubMed] [Google Scholar]
- 104.Naswa N, Sharma P, Soundararajan R, et al. Diagnostic performance of somatostatin receptor PET/CT using (68)Ga-DOTANOC in gastrinoma patients with negative or equivocal CT findings. Abdom Imaging. 2013;38:552–560. doi: 10.1007/s00261-012-9925-z. [DOI] [PubMed] [Google Scholar]
- 105.Ito T, Igarashi H, Uehara H, et al. Causes of Death and Prognostic Factors in Multiple Endocrine Neoplasia Type 1: A Prospective Study: Comparison of 106 MEN1/Zollinger-Ellison Syndrome Patients With 1613 Literature MEN1 Patients With or Without Pancreatic Endocrine Tumors. Medicine (Baltimore) 2013;92:135–181. doi: 10.1097/MD.0b013e3182954af1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Benya RV, Metz DC, Venzon DJ, et al. Zollinger-Ellison syndrome can be the initial endocrine manifestation in patients with multiple endocrine neoplasia-type 1. Am J Med. 1994;97:436–444. doi: 10.1016/0002-9343(94)90323-9. [DOI] [PubMed] [Google Scholar]
- 107.Goudet P, Dalac A, Le BA, et al. MEN1 disease occurring before 21 years old. A 160-patient cohort study from the GTE (Groupe d'etude des Tumeurs Endocrines) J Clin Endocrinol Metab. 2015;100:1568–1577. doi: 10.1210/jc.2014-3659. [DOI] [PubMed] [Google Scholar]
- 108.Norton JA, Venzon DJ, Berna MJ, et al. Prospective study of surgery for primary hyperaparathyroidism (HPT) in Multiple Endocrine Neoplasia type 1 (MEN1), and Zollinger-Ellison syndrome (ZES): longterm outcome of a more virulent form of HPT. Ann Surgery. 2008;247:501–510. doi: 10.1097/SLA.0b013e31815efda5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Metz DC, Pisegna JR, Fishbeyn VA, et al. Currently used doses of omeprazole in Zollinger-Ellison syndrome are too high. Gastroenterology. 1992;103:1498–1508. doi: 10.1016/0016-5085(92)91170-9. [DOI] [PubMed] [Google Scholar]
- 110.Norton JA, Cornelius MJ, Doppman JL, et al. Effect of parathyroidectomy in patients with hyperparathyroidism, Zollinger-Ellison syndrome and multiple endocrine neoplasia Type I: A prospective study. Surgery. 1987;102:958–966. [PubMed] [Google Scholar]
- 111.Polenta V, Slater EP, Kann PH, et al. Preoperative Imaging Overestimates the Tumor Size in Pancreatic Neuroendocrine Neoplasms Associated with Multiple Endocrine Neoplasia Type 1. World J Surg. 2017 doi: 10.1007/s00268-017-4317-8. [DOI] [PubMed] [Google Scholar]
- 112.Jensen RT. Management of the Zollinger-Ellison syndrome in patients with multiple endocrine neoplasia type 1. J Intern Med. 1998;243:477–488. doi: 10.1046/j.1365-2796.1998.00281.x. [DOI] [PubMed] [Google Scholar]
- 113.MacFarlane MP, Fraker DL, Alexander HR, et al. A prospective study of surgical resection of duodenal and pancreatic gastrinomas in multiple endocrine neoplasia-Type 1. Surgery. 1995;118:973–980. doi: 10.1016/s0039-6060(05)80102-3. [DOI] [PubMed] [Google Scholar]
- 114.Pipeleers-Marichal M, Somers G, Willems G, et al. Gastrinomas in the duodenums of patients with multiple endocrine neoplasia type 1 and the Zollinger-Ellison syndrome. N Engl J Med. 1990;322:723–727. doi: 10.1056/NEJM199003153221103. [DOI] [PubMed] [Google Scholar]
- 115.Anlauf M, Garbrecht N, Henopp T, et al. Sporadic versus hereditary gastrinomas of the duodenum and pancreas: distinct clinico-pathological and epidemiological features. World J. 2006;12:5440–5446. doi: 10.3748/wjg.v12.i34.5440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Vezzosi D, Cardot-Bauters C, Bouscaren N, et al. Long-term results of the surgical management of insulinoma patients with MEN1: a Groupe d'etude des Tumeurs Endocrines (GTE) retrospective study. Eur J Endocrinol. 2015;172:309–319. doi: 10.1530/EJE-14-0878. [DOI] [PubMed] [Google Scholar]
- 117.Gibril F, Chen Y-J, Schrump DS, et al. Prospective study of thymic carcinoids in patients with Multiple Endocrine Neoplasia Type 1. J Clin Endocrinol Metab. 2003;88:1066–1081. doi: 10.1210/jc.2002-021314. [DOI] [PubMed] [Google Scholar]
- 118.Metz DC, Forsmark C, Lew EA, et al. Replacement of oral proton pump inhibitors with intravenous pantoprazole to effectively control gastric acid hypersecretion in patients with Zollinger-Ellison syndrome. Am J Gastroenterol. 2001;96:3274–3280. doi: 10.1111/j.1572-0241.2001.05325.x. [DOI] [PubMed] [Google Scholar]
- 119.Berna MJ, Hoffmann KM, Long SH, et al. Serum gastrin in Zollinger-Ellison syndrome: II. Prospective study of gastrin provocative testing in 293 patients from the National Institutes of Health and comparison with 537 cases from the literature. evaluation of diagnostic criteria, proposal of new criteria, and correlations with clinical and tumoral features. Medicine (Baltimore) 2006;85:331–364. doi: 10.1097/MD.0b013e31802b518c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.McCarthy DM, Peikin SR, Lopatin RN, et al. Hyperparathyroidism a reversible cause of cimetidine-resistant gastric hypersecretion. Br Med J. 1979;1:1765–1766. doi: 10.1136/bmj.1.6180.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bartsch DK, Albers MB. Controversies in surgery for multiple endocrine neoplasia type-1-associated Zollinger-Ellison syndrome. Int J Endo Oncol. 2015;2:263–271. [Google Scholar]
- 122.Lopez CL, Falconi M, Waldmann J, et al. Partial pancreaticoduodenectomy can provide cure for duodenal gastrinoma associated with multiple endocrine neoplasia type 1. Ann Surg. 2013;257:308–314. doi: 10.1097/SLA.0b013e3182536339. [DOI] [PubMed] [Google Scholar]
- 123.Triponez F, Goudet P, Dosseh D, et al. Is surgery beneficial for MEN1 patients with small (< or = 2 cm), nonfunctioning pancreaticoduodenal endocrine tumor? An analysis of 65 patients from the GTE. World J Surg. 2006;30:654–662. doi: 10.1007/s00268-005-0354-9. [DOI] [PubMed] [Google Scholar]
- 124.Norton JA, Alexander HR, Fraker DL, et al. Comparison of surgical results in patients with advanced and limited disease with multiple endocrine neoplasia type 1 and Zollinger-Ellison syndrome. Ann Surg. 2001;234:495–506. doi: 10.1097/00000658-200110000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Thakker RV, Newey PJ, Walls GV, et al. Clinical Practice Guidelines for Multiple Endocrine Neoplasia Type 1 (MEN1) J Clin Endocrinol Metab. 2012;97:2990–3011. doi: 10.1210/jc.2012-1230. [DOI] [PubMed] [Google Scholar]
- 126.Donegan D, Singh Ospina N, Rodriguez-Gutierrez R, et al. Long-term outcomes in patients with multiple endocrine neoplasia type 1 and pancreaticoduodenal neuroendocrine tumours. Clin Endocrinol (Oxf) 2016 doi: 10.1111/cen.13264. [DOI] [PubMed] [Google Scholar]
- 127.D'souza SL, Elmunzer BJ, Scheiman JM. Long-term follow-up of asymptomatic pancreatic neuroendocrine tumors in multiple endocrine neoplasia type I syndrome. J Clin Gastroenterol. 2014;48:458–461. doi: 10.1097/MCG.0000000000000062. [DOI] [PubMed] [Google Scholar]
- 128.van Asselt SJ, Brouwers AH, van Dullemen HM, et al. EUS is superior for detection of pancreatic lesions compared with standard imaging in patients with multiple endocrine neoplasia type 1. Gastrointest Endosc. 2015;81:159–167. doi: 10.1016/j.gie.2014.09.037. [DOI] [PubMed] [Google Scholar]
- 129.Kann PH, Balakina E, Ivan D, et al. Natural course of small, asymptomatic neuroendocrine pancreatic tumours in multiple endocrine neoplasia type 1: an endoscopic ultrasound imaging study. Endocr Relat Cancer. 2006;13:1195–1202. doi: 10.1677/erc.1.01220. [DOI] [PubMed] [Google Scholar]
- 130.Kappelle WF, Valk GD, Leenders M, et al. Growth rate of small pancreatic neuroendocrine tumors in multiple endocrine neoplasia type 1: results from an endoscopic ultrasound based cohort study. Endoscopy. 2017;49:27–34. doi: 10.1055/s-0042-119402. [DOI] [PubMed] [Google Scholar]
- 131.Pieterman CRC, de Laat JM, Twisk JWR, et al. Long-Term Natural Course of Small Nonfunctional Pancreatic Neuroendocrine Tumors in MEN1-Results From the Dutch MEN1 Study Group. J Clin Endocrinol Metab. 2017;102:3795–3805. doi: 10.1210/jc.2017-00372. [DOI] [PubMed] [Google Scholar]
- 132.Albers MB, Librizzi D, Lopez CL, et al. Limited Value of Ga-68-DOTATOC-PET-CT in Routine Screening of Patients with Multiple Endocrine Neoplasia Type 1. World J Surg. 2017;41:1521–1527. doi: 10.1007/s00268-017-3907-9. [DOI] [PubMed] [Google Scholar]
- 133.Lastoria S, Marciello F, Faggiano A, et al. Role of Ga-DOTATATE PET/CT in patients with multiple endocrine neoplasia type 1 (MEN1) Endocrine. 2016;52:488–494. doi: 10.1007/s12020-015-0702-y. [DOI] [PubMed] [Google Scholar]
- 134.Sadowski SM, Millo C, Cottle-Delisle C, et al. Results of (68)Gallium-DOTATATE PET/CT Scanning in Patients with Multiple Endocrine Neoplasia Type 1. J Am Coll Surg. 2015;221:509–517. doi: 10.1016/j.jamcollsurg.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Morgat C, Velayoudom-Cephise FL, Schwartz P, et al. Evaluation of Ga-DOTA-TOC PET/CT for the detection of duodenopancreatic neuroendocrine tumors in patients with MEN1. Eur J Nucl Med Mol Imaging. 2016 doi: 10.1007/s00259-016-3319-3. [DOI] [PubMed] [Google Scholar]
- 136.Gibril F, Venzon DJ, Ojeaburu JV, et al. Prospective study of the natural history of gastrinoma in patients with MEN1: Definition of an aggressive and a nonaggressive form. J Clin Endocrinol Metab. 2001;86:5282–5293. doi: 10.1210/jcem.86.11.8011. [DOI] [PubMed] [Google Scholar]
- 137.Maton PN, Gardner JD, Jensen RT. Cushing's syndrome in patients with Zollinger-Ellison syndrome. N Engl J Med. 1986;315:1–5. doi: 10.1056/NEJM198607033150101. [DOI] [PubMed] [Google Scholar]
- 138.Mignon M, Cadiot G. Natural history of gastrinoma: lessons from the past. Ital J Gastroenterol Hepatol. 1999;31:S98–S103. [PubMed] [Google Scholar]
- 139.Bartsch DK, Langer P, Wild A, et al. Pancreaticoduodenal endocrine tumors in multiple endocrine neoplasia type 1: Surgery or surveillance? Surgery. 2000;128:958–966. doi: 10.1067/msy.2000.109727. [DOI] [PubMed] [Google Scholar]
- 140.van Vliet EI, Teunissen JJ, Kam BL, et al. Treatment of Gastroenteropancreatic Neuroendocrine Tumors with Peptide Receptor Radionuclide Therapy. Neuroendocrinology. 2013;97:74–85. doi: 10.1159/000335018. [DOI] [PubMed] [Google Scholar]
- 141.Conemans EB, Nell S, Pieterman CRC, et al. PROGNOSTIC FACTORS FOR SURVIVAL OF MEN1 PATIENTS WITH DUODENOPANCREATIC TUMORS METASTATIC TO THE LIVER: RESULTS FROM THE DMSG. Endocr Pract. 2017;23:641–648. doi: 10.4158/EP161639.OR. [DOI] [PubMed] [Google Scholar]
- 142.Conemans EB, Brosens LAA, Raicu-Ionita GM, et al. Prognostic value of WHO grade in pancreatic neuro-endocrine tumors in Multiple Endocrine Neoplasia type 1: Results from the DutchMEN1 Study Group. Pancreatology. 2017;17:766–772. doi: 10.1016/j.pan.2017.07.196. [DOI] [PubMed] [Google Scholar]
- 143.Kornaczewski Jackson ER, Pointon OP, Bohmer R, et al. Utility of FDG-PET Imaging for Risk Stratification of Pancreatic Neuroendocrine Tumors in MEN1. J Clin Endocrinol Metab. 2017;102:1926–1933. doi: 10.1210/jc.2016-3865. [DOI] [PubMed] [Google Scholar]
- 144.Kouvaraki MA, Shapiro SE, Cote GJ, et al. Management of pancreatic endocrine tumors in multiple endocrine neoplasia type 1. World J Surg. 2006;30:643–653. doi: 10.1007/s00268-006-0360-y. [DOI] [PubMed] [Google Scholar]
- 145.Bartsch DK, Slater EP, Albers M, et al. Brief Report: Higher Risk of Aggressive Pancreatic Neuroendocrine Tumors in MEN1 Patients With MEN1 Mutations Affecting the CHES1 Interacting MENIN Domain. J Clin Endocrinol Metab. 2014;99:E2387–E2391. doi: 10.1210/jc.2013-4432. [DOI] [PubMed] [Google Scholar]
- 146.Thevenon J, Bourredjem A, Faivre L, et al. Higher risk of death among MEN1 patients with mutations in the JunD interacting domain: a Groupe d'etude des Tumeurs Endocrines (GTE) cohort study. Hum Mol Genet. 2013;22:1940–1948. doi: 10.1093/hmg/ddt039. [DOI] [PubMed] [Google Scholar]
- 147.Circelli L, Ramundo V, Marotta V, et al. Prognostic role of the CDNK1B V109G polymorphism in multiple endocrine neoplasia type 1. J Cell Mol Med. 2015;19:1735–1741. doi: 10.1111/jcmm.12552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Christakis I, Qiu W, Hyde SM, et al. Genotype-phenotype pancreatic neuroendocrine tumor relationship in multiple endocrine neoplasia type 1 patients: A 23-year experience at a single institution. Surgery. 2017 doi: 10.1016/j.surg.2017.04.044. [DOI] [PubMed] [Google Scholar]
- 149.Kulke MH, Anthony LB, Bushnell DL, et al. NANETS Treatment Guidelines: Well-Differentiated Neuroendocrine Tumors of the Stomach and Pancreas. Pancreas. 2010;39:735–752. doi: 10.1097/MPA.0b013e3181ebb168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Nigri GR, Rosman AS, Petrucciani N, et al. Metaanalysis of trials comparing minimally invasive and open distal pancreatectomies. Surg Endosc. 2011;25:1642–1651. doi: 10.1007/s00464-010-1456-5. [DOI] [PubMed] [Google Scholar]
- 151.Norton JA, Warren RS, Kelly MG, et al. Aggressive surgery for metastatic liver neuroendocrine tumors. Surgery. 2003;134:1057–1065. doi: 10.1016/j.surg.2003.07.025. [DOI] [PubMed] [Google Scholar]
- 152.Strosberg J, El-Haddad G, Wolin E, et al. Phase 3 Trial of 177Lu-Dotatate for Midgut Neuroendocrine Tumors. N Engl J Med. 2017;376:125–135. doi: 10.1056/NEJMoa1607427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ito T, Igarashi H, Jensen RT. Therapy of metastatic pancreatic neuroendocrine tumors (pNETs): recent insights and advances. J Gastroenterol. 2012;47:941–960. doi: 10.1007/s00535-012-0642-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.von Schrenck T, Howard JM, Doppman JL, et al. Prospective study of chemotherapy in patients with metastatic gastrinoma. Gastroenterology. 1988;94:1326–1334. doi: 10.1016/0016-5085(88)90670-1. [DOI] [PubMed] [Google Scholar]
- 155.Pavel M, O'Toole D, Costa F, et al. ENETS Consensus Guidelines Update for the Management of Distant Metastatic Disease of Intestinal, Pancreatic, Bronchial Neuroendocrine Neoplasms (NEN) and NEN of Unknown Primary Site. Neuroendocrinology. 2016;103:172–185. doi: 10.1159/000443167. [DOI] [PubMed] [Google Scholar]
- 156.Do Minh D, Chapiro J, Gorodetski B, et al. Intra-arterial therapy of neuroendocrine tumour liver metastases: comparing conventional TACE, drug-eluting beads TACE and yttrium-90 radioembolisation as treatment options using a propensity score analysis model. Eur Radiol. 2017;27:4995–5005. doi: 10.1007/s00330-017-4856-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Cives M, Strosberg J. Treatment Strategies for Metastatic Neuroendocrine Tumors of the Gastrointestinal Tract. Curr Treat Options Oncol. 2017;18:14. doi: 10.1007/s11864-017-0461-5. [DOI] [PubMed] [Google Scholar]