SYNOPSIS
Deep brain stimulation (DBS) has preliminary evidence of clinical efficacy, but has been difficult to develop into a robust therapy. This is in part because its mechanisms are incompletely understood. We review evidence from movement and psychiatric disorder studies, with an emphasis on how DBS changes brain networks. From this, we argue for a network-oriented approach to future DBS studies. That network approach, in turn, requires methods for identifying patients with specific circuit/network deficits. We describe how dimensional approaches to diagnoses may aid that identification. Finally, we discuss the use of network/circuit biomarkers to develop self-adjusting “closed loop” systems.
Keywords: Closed-loop DBS, network-oriented DBS, DBS in psychiatry, mechanisms of DBS, dimension-oriented psychiatry
Introduction: The Promise and Frustrations of DBS in Psychiatry
As discussed in the companion article by Dougherty1 in this issue, deep brain stimulation (DBS) has promise in intractable obsessive-compulsive disorder (OCD) and major depression (MDD), but has not fared well in traditional randomized trials. This contrasts with DBS’ success in Parkinson Disease (PD), where it has become part of standard care2. The difference in outcomes arises because PD and other movement disorders arise from well-explored neural circuitry, with well-understood, reliable measures of symptoms. Psychiatric conditions arise from multiple dysfunctional neural circuits, not all of which are known or well-described3,4. Our symptom measures are also less robust, diluting the clinical signal5,6. For example, a meta-analysis of depression questionnaires showed that general factors, such as mood, explain more variance than any specific MDD symptom7. In the DSM-5 field trials, comorbidity was more common than “pure” diagnoses, suggesting that diagnostic criteria and rating scales do not measure separable entities8.
Studies in both psychiatric and PD patients have yielded proposed mechanisms of DBS, leading to new treatment strategies. Some of these proposals emphasize anatomy; others have both functional and anatomical components. We argue that DBS in psychiatry depends on both function and anatomy, viewed at the circuit/network level. Here, we review the functional and network-oriented theories of psychiatric DBS. We begin each section with a review of what is known or strongly suspected, then highlight directions the field may soon take.
Neurophysiologic Mechanisms of DBS
Neural Inhibition
DBS often mimics the clinical effect of a brain lesion at the target site. Most of the PD and MDD/OCD targets were chosen because a lesion at that target was known or expected to ameliorate disease9,10. Several studies reported decreased neural activity at the DBS site11–13. Yet, DBS-like stimulation can also increase neural activity, depending on how the electric field is oriented relative to individual cells14,15. DBS also appears to increase brain metabolism at structures connected to the target16–18. This casts doubt on the inhibition hypothesis.
Informational Lesion
One possible explanation for these contradictions is that DBS may be inhibitory at the level of information flow. The high-frequency pulses (over 100 Hz) used in DBS are above the firing frequency of most neurons, meaning that DBS effectively “takes over” the stimulated axons and cell bodies. Normal brain activity is irregular and variable, and that irregularity conveys information. DBS changes this to regularized, less-variant activity19, reducing the amount of information sent between network nodes in a mathematical sense20. This might make the overall network function better. For instance, in a hemiparkinsonian rat model of PD, the amount of information (i.e., neuronal entropy) in the globus pallidus and substantia nigra increased after the onset of Parkinsonism21. DBS of those regions reduced local information but increased the information transmission between these regions21. The informational lesion theory has only been evaluated in PD, but with good results. A human study showed that pulse sequences optimized for information blockade are as effective as high-frequency DBS but require much less energy delivery to the brain22.
Disruption of Pathological Oscillations
Neural network communication requires coordination of activity within and between areas. When networks are functioning efficiently, coordinated oscillations appear in the local field potential (LFP)23 and scalp electroencephalogram (EEG). Neural network dysfunction may be reflected in abnormal oscillatory activity, and rhythmic DBS might restore normal oscillations. For example, beta band (12–30 Hz) power normally decreases during movement24. In PD, however, cortico-basal circuits remain in synchronized (i.e., coherent) beta oscillation, which is believed to produce PD’s core symptoms of bradykinesia and rigidity25,26. Patients receiving DBS for the first time showed decreased beta-gamma synchrony (cross-frequency coupling) between subthalamic nucleus and motor cortex27. Similarly, the extent to which the power of gamma band activity (above 40 Hz) was nested within alpha/beta band activity decreased with DBS of ventral striatum/ventral capsule in OCD patients28, although this effect did not replicate in an independent sample29. This touches a much broader difficulty with identifying oscillatory biomarkers of psychiatric illness, to which we return below. The beta findings in PD, replicated by multiple groups, led to an important innovation: DBS systems that can record and store electrophysiologic information from human patients as they undergo treatment30. Those systems offer an unparalleled view into brain function31.
DBS’ effect on oscillations offers the potential for treatment innovation. Stimulation could be aligned to coincide with the phase of frequency of a band of interest, such as frontal theta in anxiety disorders32 or beta band in PD25,33. This approach was taken in a PD DBS study, where phase dependent DBS (i.e., DBS delivered in synchrony with beta band activity) was superior to consistent, high-frequency DBS33. In depression, a transcranial magnetic device operating on similar frequency-locked principles has evidence of possible efficacy34. The authors have launched a trial specifically designed to modify oscillations in cortico-striatal loops of OCD (NCT03184454). As we learn to better identify the oscillatory features of dysfunctional networks, oscillation-based DBS may become useful in psychiatric disorders.
Neuroplasticity
Neuroplasticity underlies the brain’s long-term learning and reorganization capabilities35. Psychiatric DBS changes symptoms over a slow time course consistent with plasticity effects17,26,36, implying that DBS may work through neuroplasticity. This hypothesis is supported by animal studies37–40. Hamani et al.37 found that a single DBS session increased stressed rats’ performance on a working memory task, but only when measured 33 days after the DBS treatment. Chakravarty et al.38 demonstrated that DBS of ventromedial prefrontal cortex, a putative rodent homologue of human subcallosal cingulate, increased synaptic density. Last but not least, Creed and colleagues40 reversed cocaine-induced plasticity in the nucleus accumbens (NAc) of rodents with DBS. They found that DBS successfully suppressed sensitization responses caused by repeated exposure to cocaine, but only when administered with a D1R antagonist that altered local excitability. These findings demonstrate DBS’ potential to induce neuroplasticity and structural alterations of neural networks. This could be a critical mechanism to exploit, given the role of learning and plasticity impairments in psychiatric conditions41,42.
Network Mechanisms and Targets for DBS
Modern neuroscience focuses on networks as units of study43. Psychiatric dysfunctions are commonly believed to be dysfunctions of neural networks, and DBS likely acts at the network level. For example, subthalamic nucleus (STN) and globus pallidus internus (GPi) are both parts of the cortico-basal ganglia network44, and DBS at either site can be effective in PD. DBS of STN is believed to reduce excitation from STN to globus pallidus, leading to higher firing rates in globus pallidus and a variety of downstream effects45. This ultimately normalizes activity throughout the cortico-basal loop, decreasing the motor signs of PD46. Similarly, dysfunctions of cortico-striato-thalamo-cortical10,47,48 circuits are associated with OCD, and nodes in these loops are targeted for OCD neurosurgery47,49,50. With the advent of modern imaging technologies, such as diffusion tensor imaging (DTI) and functional connectivity MRI, researchers can better study structural and functional networks51. This is enabling more rigorous empirical and computational studies of DBS’ mechanisms at a network level52.
Network Studies and Functional Mapping
Network effects of DBS are readily observable in regions connected to a DBS target. In their study of the subgenual anterior cingulate (Cg25), Mayberg and colleagues13 showed reduced cerebral blood flow (CBF) to Cg25 and the neighboring orbitofrontal cortex after DBS. However, long-term responders to DBS also demonstrated CBF changes in other regions involved in depression, such as increases in dorsolateral prefrontal cortex and decreases in hypothalamus.
Similar findings are also seen with acute stimulation. Rauch et al.16 found increased CBF in right medial orbitofrontal cortex (OFC) and right dorsolateral putamen from acute high-frequency (clinically effective) DBS. Similarly, Dougherty and colleagues47 observed increased regional CBF in OCD patients in dorsal anterior cingulate cortex (dACC) when the stimulation DBS contact was more ventral in VC/VS. This effect also significantly correlated with improvements in the depressive symptom severity of the OCD patients. However, with more dorsal stimulation, the network activation changed and rCBF increases were observed in thalamus, striatum, and globus pallidus. Taken together, these results suggest that DBS must influence wide networks to be clinically effective. The wide-network hypothesis is supported by recent DTI studies51,52. For example, Riva-Posse et al. recently identified 4 white matter bundles that were uniquely activated in a cohort of DBS responders51. The researchers then used the identified bundles as DBS targets in a new cohort of MDD patients. This advanced targeting yielded response rates of 73% at six months and 82% at one year in the new prospective (albeit unblinded) cohort51, much higher than those in a recent non-targeted DBS trial54.
Optogenetics, the use of light in modulating neural activity55, is another state-of-the-art technique that informs network-oriented DBS. In animal models, optogenetics allows stimulation of specific connections between brain nuclei, allowing researchers to narrow DBS’ mechanisms to sub-networks. In a recent example, Gradinaru et al.56 tested whether the effect of DBS in PD is due to inhibition of STN, or instead due to disrupted connectivity between STN and motor cortex. They reported that in hemiparkinsonian rats, precise inhibition of STN did not lead to improvements in PD symptoms. The only optogenetic manipulation improving PD symptoms was exciting the afferent motor cortex neurons that projected into STN. Similar studies should be possible in animal models of psychiatric illness; indeed, optogenetic stimulation of specific projections has dramatic effects on a variety of laboratory behaviors that model aspects of mental illness57.
Next Steps: From Diagnoses to Dimensions
Changes in brain physiology, including information flow, oscillatory synchrony, and synaptic weighting, may each play a role in DBS’ therapeutic effects. Each of these appears to act more at the network level than on any single brain structure. As described above, specific DBS protocols and/or combinations of DBS with targeted pharmacology can produce equally specific physiologic changes. Novel closed-loop and recording systems will soon be able to monitor those changes and adjust stimulation intensity without immediate physician involvement31,58,59. This is a powerful toolbox, and its main limitation is that we do not know which physiologic changes may be beneficial for which mental illness. There is an extensive literature on attempts to find physiologic biomarkers, especially in MDD60. The results are very mixed, and our group’s attempts to independently replicate candidate markers have failed61–63. We argue that this problem arises from the heterogeneity of categorical psychiatric diagnoses64. MDD, OCD, and other DBS-targetable disorders are too phenotypically diverse to arise from only one neurologic impairment. The National Institute of Mental Health’s Research Domain Criteria (RDoC) initiative seeks to re-cast mental illnesses not as diagnoses, but as quantitatively described impairment in specific functional domains4,6. This domain- and circuit-oriented approach to illness may be particularly useful for psychiatric DBS. DBS modulates specific circuits, which in turn might lead to focused behavioral changes that cut across traditional diagnoses64. Multiple groups are now identifying cross-diagnostic network signatures in psychiatric populations65,66, and stimulation based on these signatures may change psychiatrically relevant behaviors59.
Next Steps: Closed-Loop, Activity-Dependent Stimulation
DBS as practiced to date is “open loop”. That is, the physician takes clinical data into account, sets the stimulation parameters, and then a single pattern of stimulation is applied to the patient’s brain for the next several weeks to months9. This practice is substantially based on trial and error67 and the decisions are based on physicians’ subjective evaluations and indirect behavioral assessment4–6. “Closed-loop” DBS is an emerging alternative. In this paradigm, a neural biomarker that captures an essential aspect of disease is identified, such as increased beta band activity in the STN in PD24,25. The DBS system then directly measures the biomarker and utilizes this information to adjust stimulation parameters31. DBS systems currently in production (e.g., Medtronic’s PC+S) can record local field potentials (LFP) from lead contacts at the site of stimulation30. Stimulation parameters may be adjusted by predictive algorithms to achieve a desired neurophysiologic signature59. Preliminary demonstrations of this approach in PD have equaled and, in some cases, exceeded the performance of traditional DBS44,68.
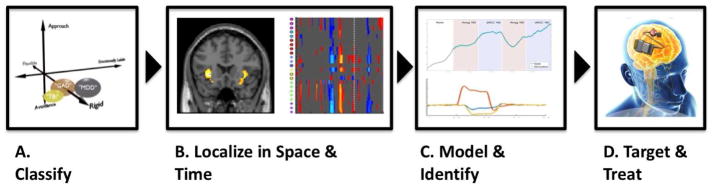
As just noted, biomarker development is a major challenge for closed-loop DBS algorithm development in psychiatry. We suggest a domain-oriented approach applied in four steps (see Figure 1). DSM-based diagnoses (e.g., General Anxiety Disorder and Major Depressive Disorder) may share a common phenotype (e.g., cognitive rigidity). These phenotypes may be identified through a combination of self-report questionnaires (e.g., for the cognitive rigidity example, Brief Inventory of Executive Functioning69), standardized behavioral assessments (e.g., a cognitive interference task70), and imaging techniques. Patients who demonstrate the phenotypic impairment of interest could then be studied with high-temporal-resolution recordings (e.g., LFP and EEG) to identify candidate predictive algorithms31,59. The developed algorithms could then aid the DBS physician in adjusting stimulation settings. With full closed-loop DBS, the adjustment process could be transferred to an automatic controller in the DBS system itself. It should be noted that closed-loop DBS in psychiatry remains more of a vision than a near-term guarantee. There have been successful pilots in Parkinsonism44, and reports of early psychiatric closed-loop demonstrations in lab environments59, but the concept remains to be validated in a clinical setting. The essential test for its efficacy is how it performs in comparison to the current open loop approaches.
Figure 1.
A closed-loop DBS pipeline example. (A) Patients’ dysfunction is individually assessed. The emphasis is in measuring patients’ (dys)function in multiple cross-diagnostic domains. (B) Activity correlated with domain/function impairment is localized to brain structures that are amenable to neurostimulation. (C) Computational modeling quantifies the relationship of behavior to brain activity, and formulates a control relationship between brain and behavior. (D) Based on this quantification, closed-loop treatment specific to individual patients is administered.
From Widge AS, Ellard KK, Paulk AC, et al. Treating refractory mental illness with closed-loop brain stimulation: Progress towards a patient-specific transdiagnostic approach. Exp Neurol 2017;287(Part 4):470; with permission.
A recent demonstration by Wu et al.58 exemplifies the approach. The authors selected a phenotypical component of hypersensitivity to reward, then modeled this phenotype by creating a group of mice prone to binge eating. The LFPs from NAc of the mice had higher delta-band (i.e., 1–4 Hz) power in NAc when these mice anticipated food. This biomarker was used to trigger a DBS-like neurostimulator in the NAc, disrupting the reward hypersensitivity. This closed-loop neurostimulation extinguished animals’ tendency to binge on high-fat chow. Additionally, a similar delta-band signature of reward anticipation was identified in NAc LFPs of a pilot human subject, demonstrating this biomarker’s potential translational relevance. On the basis of this result, the authors hope to implement delta-locked closed-loop DBS in disorders of human reward hypersensitivity, including binge eating and drug addiction.
Next Steps: Ethical Foundations for DBS in Psychiatry
DBS aims to improve psychiatric outcomes by altering emotion-related brain function. This DBS effect raises concerns around patient autonomy71, decisional capacity72, subject selection73, control over the device’s function, and informed consent74. DBS may alter a patient’s sense of authenticity, create a sense of alienation from that “authentic self”, or change interpersonal dynamics75. For instance, Klein et. al76 conducted a study of MDD and OCD patients who had undergone DBS surgery. While many patients found it a challenge to decipher how much of their emotional state was the direct result of DBS, a few stated that DBS had, indeed, helped them return to their “true self”77. In line with those results, de Haan et al.78 found that the clinical experience of DBS is not limited to psychopathological symptoms. It instead pervades the participants’ sense of self-reliance and basic trust. The authors suggested offering participants options to contact other DBS participants because the unusual nature of the intervention may lead them to experience isolation. Another potential issue with DBS consent is subjects’ impaired ability to make informed decisions. For instance, Fisher et al.79 found that despite an intact decisional capacity in TRD patients, 64% displayed therapeutic misconception, an inability to differentiate between treatment and clinical research. Remedying this issue requires educating participants, preferably, by individuals who are not directly involved in the study79. These considerations will become more important as advanced technologies, including those with some capacity for self-adjustment, become available. The next generation of DBS studies will likely incorporate ethical review and/or research ethicists directly into their design.
Conclusion
Based on the experimental evidence reviewed above13,16,56,67, DBS likely exerts its effect at the network level. Probable mechanisms include affecting information transmission between brain structures21, disrupting pathological oscillations27, and inducing long-term plasticity37,38,40. These mechanisms are all aspects of the phenomenon of inter-neuronal communication. Accordingly, conceiving of DBS as a network therapy may help understand its effects and uses59.
Manual programming of DBS parameters by clinicians may not be an effective way to modulate networks. Closed-loop DBS technology, which uses neural signal-based algorithms to adjust treatment parameters dynamically30,31, has demonstrated early efficacy in movement disorders44. Pilot closed-loop investigations are also underway in psychiatric disorders59. A more dimensional approach to psychiatry should help identify the circuit bases of mental illness, in turn indicating which patients may benefit most from DBS at a given target site. Understanding these mechanisms and the basis for patient-specific DBS is critical to achieve the clinical promise of this innovative, but still nascent therapy.
KEY POINTS.
DBS’ likely mechanism is altered inter-neuronal communication, which may include alterations in neural firing patterns, oscillatory dynamics, or synaptic plasticity.
DBS acts at the network level, not on single brain structures.
Advanced technologies, including closed-loop systems, are rapidly being deployed in movement disorders. Recent progress in novel applications suggests that they may soon be used in psychiatry.
The optimal use of DBS, both the current-generation and next-generation systems, likely requires a dimensional approach to identify patients with treatment-amenable brain circuit impairment.
Acknowledgments
Preparation of this work was not sponsored or supported by any commercial entity. ASW, AG, and MTB were supported by grants to ASW from the Brain & Behavior Research Foundation, National Institutes of Health (MH109722, MH111320, and NS100548), and Defense Advanced Research Projects Agency (Cooperative Agreement W911NF-14-2-0045). The views, opinions, and/or findings expressed are those of the authors and should not be interpreted as representing the official views or policies of any sponsor or funding source.
Footnotes
DISCLOSURE STATEMENT
ASW receives consulting income and device donations from Medtronic, which manufactures DBS systems. ASW has also filed multiple patent applications related to closed-loop deep brain stimulation, none of which is yet commercially licensed.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
M. Taha Bilge, Department of Psychiatry, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA.
Aishwarya Gosai, Department of Psychiatry, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA.
Alik S. Widge, Department of Psychiatry, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA; Picower Institute for Learning & Memory, Massachusetts Institute of Technology, Cambridge, MA, USA.
References
- 1.Dougherty DD. Deep brain stimulation in psychiatry. Psychiatr Clin. 2018 doi: 10.1016/j.psc.2018.04.004. Current Issue. [DOI] [PubMed] [Google Scholar]
- 2.Krack P, Martinez-Fernandez R, del Alamo M, Obeso JA. Current applications and limitations of surgical treatments for movement disorders: surgical treatments for movement disorders. Mov Disord. 2017;32(1):36–52. doi: 10.1002/mds.26890. [DOI] [PubMed] [Google Scholar]
- 3.Lichtman JW, Denk W. The big and the small: challenges of imaging the brain’s circuits. Science. 2011;334(6056):618–623. doi: 10.1126/science.1209168. [DOI] [PubMed] [Google Scholar]
- 4.Cuthbert BN, Insel TR. Toward the future of psychiatric diagnosis: the seven pillars of RDoC. BMC Med. 2013;11(1):126. doi: 10.1186/1741-7015-11-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kramer M. Cross-national study of diagnosis of the mental disorders: origin of the problem. Am J Psychiatry. 1969;125(10S):1–11. doi: 10.1176/ajp.125.10s.1. [DOI] [PubMed] [Google Scholar]
- 6.Cuthbert B, Insel T. The data of diagnosis: new approaches to psychiatric classification. Psychiatry Interpers Biol Process. 2010;73(4):311–314. doi: 10.1521/psyc.2010.73.4.311. [DOI] [PubMed] [Google Scholar]
- 7.Shafer AB. Meta-analysis of the factor structures of four depression questionnaires: Beck, CES-D, Hamilton, and Zung. J Clin Psychol. 2006;62(1):123–146. doi: 10.1002/jclp.20213. [DOI] [PubMed] [Google Scholar]
- 8.Regier DA, Narrow WE, Clarke DE, et al. DSM-5 field trials in the United States and Canada, Part II: test-retest reliability of selected categorical diagnoses. American Journal of Psychiatry. 2013;170(1):59–70. doi: 10.1176/appi.ajp.2012.12070999. [DOI] [PubMed] [Google Scholar]
- 9.Dougherty DD, Widge AS. Neurotherapeutic interventions for psychiatric illness. Harv Rev Psychiatry. 2017;25(6):253–255. doi: 10.1097/HRP.0000000000000174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Widge AS, Dougherty DD. Deep brain stimulation for treatment-refractory mood and obsessive-compulsive disorders. Curr Behav Neurosci Rep. 2015;2(4):187–197. doi: 10.1007/s40473-015-0049-y. [DOI] [Google Scholar]
- 11.Vitek JL. Mechanisms of deep brain stimulation: Excitation or inhibition. Mov Disord. 2002;17(S3):S69–S72. doi: 10.1002/mds.10144. [DOI] [PubMed] [Google Scholar]
- 12.Welter ML, Houeto JL, Bonnet AM, et al. Effects of high-frequency stimulation on subthalamic neuronal activity in parkinsonian patients. Arch Neurol. 2004;61(1):89–96. doi: 10.1001/archneur.61.1.89. [DOI] [PubMed] [Google Scholar]
- 13.Mayberg HS, Lozano AM, Voon V, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45(5):651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 14.McIntyre CC, Grill WM, Sherman DL, Thakor NV. Cellular effects of deep brain stimulation: Model-based analysis of activation and inhibition. J Neurophysiol. 2004;91(4):1457–1469. doi: 10.1152/jn.00989.2003. [DOI] [PubMed] [Google Scholar]
- 15.Florence G, Sameshima K, Fonoff ET, Hamani C. Deep brain stimulation: More complex than the inhibition of cells and excitation of fibers. The Neuroscientist. 2016;22(4):332–345. doi: 10.1177/1073858415591964. [DOI] [PubMed] [Google Scholar]
- 16.Rauch SL, Dougherty DD, Malone D, et al. A functional neuroimaging investigation of deep brain stimulation in patients with obsessive–compulsive disorder. J Neurosurg. 2006;104(4):558–565. doi: 10.3171/jns.2006.104.4.558. [DOI] [PubMed] [Google Scholar]
- 17.Dougherty DD, Rezai AR, Carpenter LL, et al. A randomized sham-controlled trial of deep brain stimulation of the ventral capsule/ventral striatum for chronic treatment-resistant depression. Biol Psychiatry. 2015;78(4):240–248. doi: 10.1016/j.biopsych.2014.11.023. [DOI] [PubMed] [Google Scholar]
- 18.Mayberg HS. Targeted electrode-based modulation of neural circuits for depression. J Clin Invest. 2009;119(4):717–725. doi: 10.1172/JCI38454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grill WM, Snyder AN, Miocinovic S. Deep brain stimulation creates an informational lesion of the stimulated nucleus. NeuroReport. 2004;15(7):1137–1140. doi: 10.1097/00001756-200405190-00011. [DOI] [PubMed] [Google Scholar]
- 20.Dorval AD, Russo GS, Hashimoto T, Xu W, Grill WM, Vitek JL. Deep brain stimulation reduces neuronal entropy in the MPTP-primate model of Parkinson’s disease. J Neurophysiol. 2008;100(5):2807–2818. doi: 10.1152/jn.90763.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dorval AD, Grill WM. Deep brain stimulation of the subthalamic nucleus reestablishes neuronal information transmission in the 6-OHDA rat model of parkinsonism. J Neurophysiol. 2014;111(10):1949–1959. doi: 10.1152/jn.00713.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brocker DT, Swan BD, So RQ, Turner DA, Gross RE, Grill WM. Optimized temporal pattern of brain stimulation designed by computational evolution. Sci Transl Med. 2017;9(371):eaah3532. doi: 10.1126/scitranslmed.aah3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buzsáki G, Draguhn A. Neuronal oscillations in cortical networks. Science. 2004;304(5679):1926–1929. doi: 10.1126/science.1099745. [DOI] [PubMed] [Google Scholar]
- 24.Cassidy M, Mazzone P, Oliviero A, et al. Movement-related changes in synchronization in the human basal ganglia. Brain. 2002;125:1235–1246. doi: 10.1093/brain/awf135. [DOI] [PubMed] [Google Scholar]
- 25.Little S, Pogosyan A, Kuhn AA, Brown P. Beta band stability over time correlates with Parkinsonian rigidity and bradykinesia. Exp Neurol. 2012;236(2):383–388. doi: 10.1016/j.expneurol.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Herrington TM, Cheng JJ, Eskandar EN. Mechanisms of deep brain stimulation. J Neurophysiol. 2016;115(1):19–38. doi: 10.1152/jn.00281.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Hemptinne C, Swann NC, Ostrem JL, et al. Therapeutic deep brain stimulation reduces cortical phase-amplitude coupling in Parkinson’s disease. Nat Neurosci. 2015;18(5):779–786. doi: 10.1038/nn.3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bahramisharif A, Mazaheri A, Levar N, Richard Schuurman P, Figee M, Denys D. Deep brain stimulation diminishes cross-frequency coupling in obsessive-compulsive disorder. Biol Psychiatry. 2016;80(7):e57–e58. doi: 10.1016/j.biopsych.2015.05.021. [DOI] [PubMed] [Google Scholar]
- 29.Widge AS, Zorowitz S, Link K, et al. Ventral capsule/Ventral striatum deep brain stimulation does not consistently diminish occipital cross-frequency coupling. Biol Psychiatry. 2016;80(7):e59–e60. doi: 10.1016/j.biopsych.2015.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stanslaski S, Afshar P, Cong P, et al. Design and validation of a fully implantable, chronic, closed-loop neuromodulation device with concurrent sensing and stimulation. IEEE Trans Neural Syst Rehabil Eng. 2012;20(4):410–421. doi: 10.1109/TNSRE.2012.2183617. [DOI] [PubMed] [Google Scholar]
- 31.Lo M-C, Widge AS. Closed-loop neuromodulation systems: next-generation treatments for psychiatric illness. Int Rev Psychiatry. 2017;29(2):191–204. doi: 10.1080/09540261.2017.1282438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cavanagh JF, Shackman AJ. Frontal midline theta reflects anxiety and cognitive control: Meta-analytic evidence. J Physiol-Paris. 2015;109(1–3):3–15. doi: 10.1016/j.jphysparis.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cagnan H, Pedrosa D, Little S, et al. Stimulating at the right time: phase-specific deep brain stimulation. Brain. 2017;140(1):132–145. doi: 10.1093/brain/aww286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jin Y, Phillips B. A pilot study of the use of EEG-based synchronized Transcranial Magnetic Stimulation (sTMS) for treatment of Major Depression. BMC Psychiatry. 2014;14(1):13–19. doi: 10.1186/1471-244X-14-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bennett EL, Diamond MC, Krech D, Rosenzweig MR. Chemical and anatomical plasticity of brain. Science. 1964;146(3644):610–619. doi: 10.1126/science.146.3644.610. [DOI] [PubMed] [Google Scholar]
- 36.Greenberg BD, Gabriels LA, Malone DA, Jr, et al. Deep brain stimulation of the ventral internal capsule/ventral striatum for obsessive-compulsive disorder: worldwide experience. Mol Psychiatry. 2010;15(1):64–79. doi: 10.1038/mp.2008.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hamani C, Stone SS, Garten A, Lozano AM, Winocur G. Memory rescue and enhanced neurogenesis following electrical stimulation of the anterior thalamus in rats treated with corticosterone. Exp Neurol. 2011;232(1):100–104. doi: 10.1016/j.expneurol.2011.08.023. [DOI] [PubMed] [Google Scholar]
- 38.Chakravarty MM, Hamani C, Martinez-Canabal A, et al. Deep brain stimulation of the ventromedial prefrontal cortex causes reorganization of neuronal processes and vasculature. NeuroImage. 2016;125:422–427. doi: 10.1016/j.neuroimage.2015.10.049. [DOI] [PubMed] [Google Scholar]
- 39.Do-Monte FH, Rodriguez-Romaguera J, Rosas-Vidal LE, Quirk GJ. Deep brain stimulation of the ventral striatum increases BDNF in the fear extinction circuit. Front Behav Neurosci. 2013;7:1–9. doi: 10.3389/fnbeh.2013.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Creed M, Pascoli VJ, Lüscher C. Refining deep brain stimulation to emulate optogenetic treatment of synaptic pathology. Science. 2015;347(6222):659–664. doi: 10.1126/science.1260776. doi:1126/science.1260776. [DOI] [PubMed] [Google Scholar]
- 41.Vinogradov S, Fisher M, de Villers-Sidani E. Cognitive training for impaired neural systems in neuropsychiatric illness. Neuropsychopharmacology. 2012;37(1):43–76. doi: 10.1038/npp.2011.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Subramaniam K, Luks TL, Garrett C, et al. Intensive cognitive training in schizophrenia enhances working memory and associated prefrontal cortical efficiency in a manner that drives long-term functional gains. NeuroImage. 2014;99:281–292. doi: 10.1016/j.neuroimage.2014.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McClelland JL, Rumelhart DE. Distributed memory and the representation of general and specific information. J Exp Psychol Gen. 1985;114(2):159. doi: 10.1037//0096-3445.114.2.159. [DOI] [PubMed] [Google Scholar]
- 44.Rosin B, Slovik M, Mitelman R, et al. Closed-loop deep brain stimulation is superior in ameliorating parkinsonism. Neuron. 2011;72(2):370–384. doi: 10.1016/j.neuron.2011.08.023. [DOI] [PubMed] [Google Scholar]
- 45.Hashimoto T, Elder CM, Okun MS, Patrick SK, Vitek JL. Stimulation of the subthalamic nucleus changes the firing pattern of pallidal neurons. J Neurosci. 2003;23(5):1916–1923. doi: 10.1523/JNEUROSCI.23-05-01916.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Davis KD, Taub E, Houle S, et al. Globus pallidus stimulation activates the cortical motor system during alleviation of parkinsonian symptoms. Nat Med. 1997;3(6):671–674. doi: 10.1038/nm0697-671. [DOI] [PubMed] [Google Scholar]
- 47.Dougherty DD, Chou T, Corse AK, et al. Acute deep brain stimulation changes in regional cerebral blood flow in obsessive-compulsive disorder. J Neurosurg. 2016;125(5):1087–1093. doi: 10.3171/2015.9.JNS151387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Milad MR, Rauch SL. Obsessive-compulsive disorder: beyond segregated cortico-striatal pathways. Trends Cogn Sci. 2012;16(1):43–51. doi: 10.1016/j.tics.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kohl S, Schönherr DM, Luigjes J, et al. Deep brain stimulation for treatment-refractory obsessive compulsive disorder. BMC Psychiatry. 2014;14(214):1–10. doi: 10.1186/s12888-014-0214-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Malone DA, Dougherty DD, Rezai AR, et al. Deep brain stimulation of the ventral capsule/ventral striatum for treatment-resistant depression. Biol Psychiatry. 2009;65(4):267–275. doi: 10.1016/j.biopsych.2008.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Riva-Posse P, Choi KS, Holtzheimer PE, et al. A connectomic approach for subcallosal cingulate deep brain stimulation surgery: prospective targeting in treatment-resistant depression. Mol Psychiatry. 2017 Apr;:1–7. doi: 10.1038/mp.2017.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McIntyre CC, Hahn PJ. Network perspectives on the mechanisms of deep brain stimulation. Neurobiol Dis. 2010;38(3):329–337. doi: 10.1016/j.nbd.2009.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Holtzheimer PE. Subcallosal cingulate deep brain stimulation for treatment-resistant unipolar and bipolar depression. Arch Gen Psychiatry. 2012;69(2):150–158. doi: 10.1001/archgenpsychiatry.2011.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Holtzheimer PE, Husain MM, Lisanby SH, et al. Subcallosal cingulate deep brain stimulation for treatment-resistant depression: a multisite, randomised, sham-controlled trial. Lancet Psychiatry. 2017;4:839–849. doi: 10.1016/S2215-0366(17)30371-1. [DOI] [PubMed] [Google Scholar]
- 55.Deisseroth K, Feng G, Majewska AK, Miesenbock G, Ting A, Schnitzer MJ. Next-generation optical technologies for illuminating genetically targeted brain circuits. J Neurosci. 2006;26(41):10380–10386. doi: 10.1523/JNEUROSCI.3863-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gradinaru V, Mogri M, Thompson KR, Henderson JM, Deisseroth K. Optical deconstruction of parkinsonian neural circuitry. science. 2009;324(5925):354–359. doi: 10.1126/science.1167093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Steinberg EE, Christoffel DJ, Deisseroth K, Malenka RC. Illuminating circuitry relevant to psychiatric disorders with optogenetics. Curr Opin Neurobiol. 2015;30:9–16. doi: 10.1016/j.conb.2014.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu H, Miller KJ, Blumenfeld Z, et al. Closing the loop on impulsivity via nucleus accumbens delta-band activity in mice and man. Proc Natl Acad Sci. 2017 Dec; doi: 10.1073/pnas.1712214114. 201712214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Widge AS, Ellard KK, Paulk AC, et al. Treating refractory mental illness with closed-loop brain stimulation: Progress towards a patient-specific transdiagnostic approach. Exp Neurol. 2017;287:461–472. doi: 10.1016/j.expneurol.2016.07.021. [DOI] [PubMed] [Google Scholar]
- 60.Wade EC, Iosifescu DV. Using electroencephalography for treatment guidance in major depressive disorder. Biol Psychiatry Cogn Neurosci Neuroimaging. 2016;1(5):411–422. doi: 10.1016/j.bpsc.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 61.Widge AS, Avery DH, Zarkowski P. Baseline and treatment-emergent EEG biomarkers of antidepressant medication response do not predict response to repetitive transcranial magnetic stimulation. Brain Stimulat. 2013;6(6):929–931. doi: 10.1016/j.brs.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Widge AS, Bilge MT, Montana R, et al. Electroencephalographic biomarkers for treatment response prediction in major depressive illness: a meta-analysis. doi: 10.1176/appi.ajp.2018.17121358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McLoughlin G, Makeig S, Tsuang MT. In search of biomarkers in psychiatry: EEG-based measures of brain function. Am J Med Genet B Neuropsychiatr Genet. 2014;165(2):111–121. doi: 10.1002/ajmg.b.32208. [DOI] [PubMed] [Google Scholar]
- 64.Widge AS, Deckersbach T, Eskandar EN, Dougherty DD. Deep brain stimulation for treatment-resistant psychiatric illnesses: what has gone wrong and what should we do next? Biol Psychiatry. 2016;79(4):e9–e10. doi: 10.1016/j.biopsych.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 65.Drysdale AT, Grosenick L, Downar J, et al. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat Med. 2016;23(1):28–38. doi: 10.1038/nm.4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Grisanzio KA, Goldstein-Piekarski AN, Wang MY, Rashed Ahmed AP, Samara Z, Williams LM. Transdiagnostic symptom clusters and associations with brain, behavior, and daily function in mood, anxiety, and trauma disorders. JAMA Psychiatry. 2017 Dec; doi: 10.1001/jamapsychiatry.2017.3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Choi KS, Riva-Posse P, Gross RE, Mayberg HS. Mapping the “depression switch” during intraoperative testing of subcallosal cingulate deep brain stimulation. JAMA Neurol. 2015;72(11):E1–E9. doi: 10.1001/jamaneurol.2015.2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Herron JA, Thompson MC, Brown T, Chizeck HJ, Ojemann JG, Ko AL. Cortical brain–computer interface for closed-loop deep brain stimulation. IEEE Trans Neural Syst Rehabil Eng. 2017;25(11):2180–2187. doi: 10.1109/TNSRE.2017.2705661. [DOI] [PubMed] [Google Scholar]
- 69.Gioia GA, Isquith PK, Guy SC, Kenworthy L. Test review behavior rating inventory of executive function. Child Neuropsychol. 2000;6(3):235–238. doi: 10.1076/chin.6.3.235.3152. [DOI] [PubMed] [Google Scholar]
- 70.Bush G, Shin LM, Holmes J, Rosen BR, Vogt BA. The Multi-Source Interference Task: validation study with fMRI in individual subjects. Mol Psychiatry. 2003;8(1):60–70. doi: 10.1038/sj.mp.4001217. [DOI] [PubMed] [Google Scholar]
- 71.Johansson V, Garwicz M, Kanje M, Schouenborg J, Tingström A, Görman U. Authenticity, depression, and deep brain stimulation. Front Integr Neurosci. 2011;5:1–3. doi: 10.3389/fnint.2011.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rabins P, Appleby BS, Brandt J, et al. Scientific and ethical issues related to deep brain stimulation for disorders of mood, behavior, and thought. Arch Gen Psychiatry. 2009;66(9):931–937. doi: 10.1001/archgenpsychiatry.2009.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lipsman N, Bernstein M, Lozano AM. Criteria for the ethical conduct of psychiatric neurosurgery clinical trials. Neurosurg Focus. 2010;29(2):E9. doi: 10.3171/2010.4.FOCUS09327. [DOI] [PubMed] [Google Scholar]
- 74.Schermer M. Ethical issues in deep brain stimulation. Front Integr Neurosci. 2011;5:1–5. doi: 10.3389/fnint.2011.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kraemer F. Me, myself and my brain implant: deep brain stimulation raises questions of personal authenticity and alienation. Neuroethics. 2013;6(3):483–497. doi: 10.1007/s12152-011-9115-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Klein E, Goering S, Gagne J, et al. Brain-computer interface-based control of closed-loop brain stimulation: attitudes and ethical considerations. Brain-Comput Interfaces. 2016;3(3):140–148. doi: 10.1080/2326263X.2016.1207497. [DOI] [Google Scholar]
- 77.Nyholm S, O’Neill E. Deep brain stimulation, continuity over time, and the true self. Camb Q Healthc Ethics. 2016;25(4):647–658. doi: 10.1017/S0963180116000372. [DOI] [PubMed] [Google Scholar]
- 78.de Haan S, Rietveld E, Stokhof M, Denys D. Effects of deep brain stimulation on the lived experience of obsessive-compulsive disorder patients: In-depth interviews with 18 patients. PloS One. 2015;10(8):e0135524. doi: 10.1371/journal.pone.0135524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fisher CE, Dunn LB, Christopher PP, et al. The ethics of research on deep brain stimulation for depression: decisional capacity and therapeutic misconception. Ann N Y Acad Sci. 2012;1265(1):69–79. doi: 10.1111/j.1749-6632.2012.06596.x. [DOI] [PMC free article] [PubMed] [Google Scholar]