Abstract
Lung cancer is the leading cause of cancer-specific death among Canadians, with non-small-cell lung cancer (nsclc) being the most common histologic variant. Despite advances in the understanding of the molecular biology of nsclc, the survival rate for this malignancy is still poor. It is now understood that, to evade detection and immune clearance, nsclc tumours overexpress the immunosuppressive checkpoint protein programmed death ligand 1 (PD-L1). Inhibiting the PD-1/PD-L1 axis with monoclonal antibodies has significantly changed the treatment landscape in nsclc during the last 5 years. Despite evidence of clinical response in some patients, only approximately 20% of patients obtain any durable benefit, and many of the patients who do respond ultimately relapse with drug-resistant disease. The identification of patients who are most likely to benefit from such therapy is therefore important. In the present review, we cover the basics of the PD-1/PD-L1 axis and its clinical significance in nsclc, biomarkers that are predictive of treatment response, relevant clinical trials of PD-1/PD-L1 blockade completed to date, and proposed mechanisms of acquired therapeutic resistance.
Keywords: Lung cancer, nsclc, immunotherapy, immune checkpoints, PD-1/PD-L1
INTRODUCTION
In 2017, it was estimated that lung cancer, the leading cause of cancer-specific death, would claim the lives of more than 21,000 Canadians1. Non-small-cell lung cancer (nsclc), the most common histologic subtype, often presents as locally advanced or metastatic disease2. In the absence of an epidermal growth factor receptor (EGFR) mutation (present in approximately 50% of patients of Asian ethnicity and in 15% of white patients)3 or an anaplastic lymphoma kinase (ALK) fusion (in <5% of the population)4, first-line platinum-based chemotherapy was, until recently, the standard of care. Despite improvements in systemic therapy, the survival rate for patients with stage iv disease is poor, with fewer than 5% living 5 years after diagnosis5. Novel therapeutic strategies are therefore desperately needed.
In the last decade, a paradigm shift has occurred in the understanding of the relationship between the immune system, cancer development, and subsequent disease progression. Avoiding immune destruction is now recognized as a “hallmark” of carcinogenesis6. Although the dysregulation of the host immune system in cancer is multifaceted and complex, aberrancies in the expression of immune check- points—namely, PD-1 and its cognate ligand PD-L1—have risen to the forefront as a therapeutic target of interest. Monoclonal antibodies that target and inhibit the PD-1/PD-L1 axis have been associated with remarkable success in phase iii clinical trials in a number of tumour histologies, ultimately leading Health Canada to approve those agents for melanoma7, renal cell carcinoma8, squamous cell carcinoma of the head and neck9, and nsclc10–12. However, phase iii clinical trials in nsclc have uncovered two crucial issues connected with targeting this immunosuppressive axis: first, only approximately 20% of patients overall have any objective disease response to PD-1 axis blockade, and second, the median duration of response shows significant heterogeneity. It is therefore desirable to identify patients who would be optimal candidates to receive this immunotherapy and to understand what drives resistance in the patients who do respond.
Here, we summarize the current state of PD-1/PD-L1 axis targeting for the treatment of nsclc as it pertains to the Canadian landscape. Because numerous clinical trials are currently evaluating these agents in combination with other chemotherapeutic and immunotherapeutic strategies, we hope to contextualize how PD-1/PD-L1 inhibition has solidified itself in the clinical arena as monotherapy. To that end, we highlight the evidence about predictive biomarkers of therapy response, summarize key clinical trials targeting the PD-1/PD-L1 axis in nsclc that have been completed to date, and explore possible mechanisms of acquired resistance to these agents. It is also important to recognize that other immune checkpoint proteins—including ctla-4, lag-3, tim-3, ido, and ox40—are all of therapeutic interest in nsclc, but because of a lack of current Health Canada approval are not discussed here.
THE PD-1/PD-L1 AXIS: THE BASICS
To ensure that the adaptive immune system is capable of defending the host while maintaining self-tolerance and preventing autoimmunity, a delicate interplay between positive and negative regulatory signals is carried on. Although that regulation is multidimensional, one important aspect is the PD-1/PD-L1 axis. PD-1 is a type i transmembrane protein that is transcriptionally induced in activated T cells, B cells, and myeloid cells13. Its two ligands, PD-L1 and PD-L214,15, are members of the B7 family of proteins and have a similar sequence homology16. The major role of PD-1 and its cognate ligands is to limit the activity of T cells in peripheral tissues during an inflammatory response and to limit autoimmunity14,17. That activity contrasts with the activity of cytotoxic ctla-4, which is expressed exclusively on T cells to regulate the degree of their initial activation centrally (at the level of secondary lymphoid organs). As summarized by He et al.18, the PD-1/PD-L1 axis is immunosuppressive in a number of ways:
■ Induction of apoptosis in activated T cells
■ Facilitation of T-cell anergy and exhaustion
■ Enhancement of the immunosuppressive function of regulatory T cells
■ Limitation of T-cell proliferation
■ Restraint of T-cell activation and production of interleukin-2
Specific to cancer, signalling through PD-1 can also prevent the conversion of CD8+ T memory cells into CD8+ central memory cells19, thus reducing long-term adaptive immune memory that might otherwise prevent future recurrent disease.
PD-1 is expressed on a large proportion of tumour-infiltrating lymphocytes (tils) in varying tumour histologies, including carcinoma of the head and neck20, melanoma21, and nsclc22. Not only do tils within the tumour microenvironment express PD-1, but tumour cells themselves are also recognized to have a tendency to overexpress PD-L1 as a way to avoid immune detection23. Tumour PD-L1 expression is induced by a host of pro-inflammatory molecules, with interferon γ being the most potent inducer23,24. As activated type i T cells produce interferon γ, the tumour responds to infiltrating effector T cells by upregulating expression of PD-L1 and thus protecting itself from immune attack25. In this way, the PD-1/PD-L1 axis is a major contributor to “adaptive immune resistance” within the tumour microenvironment.
In contrast, tumoural regulation of PD-L1 expression can be driven through “innate immune resistance,” where- by expression is modulated through constitutive oncogenic signalling pathways within the tumour cell. Aberrant alk signalling, which is implicated in a subset of nsclc patients, has been postulated to increase PD-L1 expression through stat3 signalling26,27. Furthermore, epidermal growth factor receptor (egfr) signalling through its cognate ligands has been implicated in the mediation of PD-L1 upregulation in nsclc28, and other studies have demonstrated an association of PD-L1 overexpression with the presence of activating EGFR mutations29,30. Those data provide the rationale for future combinatorial therapy in nsclc that uses agents targeting oncogenic drivers such as alk and egfr, and inhibitors of the PD-1/PD-L1 axis. However, it is interesting to note that retrospective analyses of clinical trials involving PD-1 axis inhibition have demonstrated low objective response rates in patients with mutated EGFR or ALK translocations31. Further studies are therefore needed to draw clinically meaningful conclusions about the implications of oncogenic driver mutations for PD-L1 expression in nsclc and response to PD-1/PD-L1 axis blockade.
THE CLINICAL SIGNIFICANCE OF PD-1/PD-L1 IN NSCLC
As discussed, the PD-1/PD-L1 axis is just one way in which nsclc evades the immune system. In addition, it has long been acknowledged that most patients with nsclc present with alterations in the counts of peripheral and tumour effector lymphocytes32 and of immunosuppressive regulatory T cells33. From a clinical perspective, those data are interesting, because the levels of tils34,35 and regulatory T cells36,37 have both been shown to be significant prognosticators of overall survival (os) in patients with nsclc.
In the same way, tumour expression of PD-L1 has been implicated as prognostic for os. However, the data in the various studies are inconsistent. For example, studies by Mu et al.38 and Zhang et al.39 both found PD-L1 expression to be a poor prognosticator of os, but that finding was not corroborated by other groups40,41. Two main meta-analyses have therefore been conducted to assess the clinical implications of tumour PD-L1 expression in nsclc42,43.
Wang et al.42, analyzed six studies involving 1157 nsclc patients. They found that PD-L1 expression was significantly associated with tumour differentiation [poorly differentiated vs. well-differentiated odds ratio (or):1.91; 95% confidence interval (ci): 1.33 to 2.75; p = 0.001] and with poor patient os (pooled hr: 1.75; 95% ci: 1.40 to 2.20; p < 0.001). A second meta-analysis by Zhang et al.43 analyzed forty-seven studies with a total of 11,444 patients having lung cancer of varying histology. Pooled results for all the histologic sub-types demonstrated that increased PD-L1 expression was associated with poor prognosis (hr: 1.40; 95% ci: 1.19 to 1.65; p < 0.001)—a result that was echoed in the subgroup analysis of nsclc (hr: 1.26; 95% ci: 1.05 to 1.52; p = 0.01). Further, their results indicated that PD-L1 expression was significantly associated with male sex (or: 1.46; 95% ci: 1.24 to 1.71; p < 0.001), a clinically relevant smoking history (or: 1.57; 95% ci: 1.28 to 1.93; p < 0.001), and EGFR wild-type status (or: 0.61; 95% ci: 0.42 to 0.90; p = 0.01).
Despite the fact that those two robust meta-analyses demonstrated a correlation of PD-L1 expression with poor os in nsclc, it remains important to recognize the variability in that correlation between the individual studies. Possible contributing factors include variations in immunohistochemistry techniques and the antibody used, the cut-off used to determine PD-L1 positivity, the timing of tumour analysis (pre- or post-treatment), any prior treatments received, and variations in tumour histology. Those factors also have implications in the use of tumour PD-L1 expression as a predictive biomarker of response to PD-1/PD-L1 blockade, as will be discussed shortly.
PREDICTIVE BIOMARKERS OF RESPONSE TO CHECKPOINT INHIBITION
In the age of targeted therapies, appropriate selection of patients for treatment is of crucial importance to lower costs, maximize efficacy, and avoid missed treatment opportunities. A prime example comes from the landmark phase iii trial published by Mok et al.44 that evaluated gefitinib against carboplatin–paclitaxel for the first-line treatment of advanced nsclc. Their study demonstrated that, compared with carboplatin–paclitaxel, gefitinib was associated with superior progression-free survival (pfs) in patients who harboured an EGFR mutation, but with inferior pfs in the absence of a mutation—thus highlighting the importance of having a reliable predictive biomarker of treatment response. A biomarker with similar predictive ability could be useful for the optimal use of PD-1/PD-L1 inhibitors, which have been associated with heterogeneous responses in clinical trials completed to date10–12. Further, although those agents are generally regarded to be less toxic than traditional cytotoxic chemotherapy, their use is still associated with a small but serious risk of immunemediated adverse events45. That risk, in conjunction with the substantial economic burden that these novel therapies impose46, makes identifying the patients most likely to benefit a priority. With respect to potential predictive biomarkers of treatment response to PD-1/PD-L1 inhibitors, the most mature data available relate to tumour PD-L1 expression and tumour mutational burden, which are reviewed next. Although not reviewed here, other potential predictive biomarkers are being investigated, including interferon gene signatures47,48, expression of class ii major histocompatibility antigens49, the microbiome50,51, and on-treatment til accumulation52. However, in the context of nsclc, those markers remain distant from routine clinical implementation.
PD-L1 Expression
Almost all clinical studies of PD-1/PD-L1 modulating agents in nsclc have investigated the possible correlation between tumour PD-L1 expression and therapeutic efficacy. Most of the studies have demonstrated that PD-L1 overexpression is associated with significantly higher objective response rates (orrs)53–56, but others have not demonstrated the same correlation10,57.
Two recent meta-analyses have demonstrated a correlation between tumour PD-L1 expression and orr to PD-1/PD-L1 inhibitors58,59. Passiglia et al.58 analyzed seven studies with a total of 914 patients. Pooled analysis showed that the orr was significantly higher in patients with PD-L1–positive tumours (>1% staining via immunohistochemistry) than in patients with PD-L1–negative tumours (or: 2.44; 95% ci: 1.61 to 3.68). The meta-analysis by Abdel-Rahman59 included twelve studies with a total of 3790 patients. In patients treated with PD-1/PD-L1 inhibitors, the orr was improved when patients had tumour PD-L1 expression exceeding 1% than when they had expression less than 1% (or: 2.18; 95% ci: 1.45 to 3.29; p = 0.0002). Furthermore, the or increased as the PD-L1 cut-off value comparison was increased (>5% vs. <5%, >10% vs. <10%, and >50% vs. <50%). Taken together, those data suggest that tumour PD-L1 expression of more than 1% offers some benefit when nsclc patients are treated with PD-1/PD-L1 inhibitors and that a possible dose–effect relationship might exist between the intensity of PD-L1 staining and orr. However, it is currently unclear whether baseline tumour PD-L1 expression has any clinical relationship with the duration of treatment response.
Despite the role of tumour PD-L1 expression as a validated predictive biomarker of response to PD-1/PD-L1 inhibitors, relying solely on that expression is controversial. For example, the 4 PD-L1 antibodies commonly used as companion diagnostic tests for nivolumab (Dako 28-8 pharmDx: Dako Corporation, Glostrup, Denmark), pembrolizumab (Dako 22C3 pharmDx), atezolizumab (Ventana SP142: Ventana Medical Systems, Tucson, AZ, U.S.A.), and durvalumab (Ventana SP263) have shown variability in staining intensity and patterns. Studies are therefore underway to compare the reliability of the various assays. Early results from the phase i Blueprint PD-L1 IHC Assay Comparison Project60 demonstrated that the percentage of PD-L1–stained tumour cells was comparable when using the 22C3, 28-8, and SP263 assays, but that the SP142 assay for atezolizumab resulted in fewer stained tumour cells overall. It is also important to note that the scoring system for the SP142 assay includes PD-L1–positive immune cells in addition to tumour cells. The authors of Blueprint i concluded that interchanging assays and cut-offs for positivity will lead to the misclassification of PD-L1 status for some patients, which could ultimately affect treatment decisions. More research in this area is needed if PD-L1 expression is to be reliably used when determining a patient’s treatment eligibility.
Furthermore, even if consistency in PD-L1 testing is achieved, it is still important to consider that most clinical trials have demonstrated that a subset of PD-L1–negative patients will experience meaningful objective and durable responses to PD-1/PD-L1 inhibition. The question that remains is what is the definition of PD-L1–positive? PD-L1 can be expressed both by tumour cells and by immune cell populations within the tumour microenvironment, but the significance of that expression is currently unclear52. Further, PD-L1 is an inducible and dynamic biomarker, and thus should be considered differently from the more established oncologic predictive biomarkers such as EGFR and ALK mutations. For example, if PD-L1 expression is transiently elevated because of innate immune resistance rather than because of an antitumour immune response, disrupting the axis might not be effective, because there is no antitumour immune response to restore.
Finally, given that PD-L1 expression is dynamic, the expression level can potentially vary as a result of treatments. For example, it has been demonstrated that, in a subset of patients, tumour PD-L1 expression markedly increases after treatment with the egfr modulator gefitinib61. Because the ways in which various treatment modalities influence PD-L1 expression are currently unclear, future studies should evaluate the utility of tumour rebiopsy when using PD-L1 as a biomarker. It might therefore be necessary to view PD-L1 expression as a continuous variable rather than a binary one (that is, positive vs. negative) and to use it as one component of a broader predictive algorithm for PD-1/PD-L1 inhibitor response. As summarized by Grigg and Rizvi62, the findings from keynote-00154 demonstrated that orr trends were related to stratifications of PD-L1 positivity in the study patients. Having stringent cut-offs would increase the proportion of responders, but would fail to identify a substantial proportion of potential responders. A more lenient cut-off would increase the absolute number of responders, but decrease the predictive power of the biomarker. Uncovering other clinical or pathologic variables that could be predictive of treatment response will therefore be important.
Global Tumour Mutational Burden
Early clinical studies of PD-1/PD-L1 inhibitors demonstrated the highest efficacy in melanoma and nsclc63,64, two malignancies that are known to have a high rate of somatic mutations as a result of exposure to ultraviolet radiation and tobacco smoke respectively65,66. The mutations in the tumour cells produce “neoantigens” (tumour-specific T-cell epitopes), and T-cell reactivity against the neoantigens can be boosted by targeting PD-1/PD-L1 axis proteins. Studies have therefore been conducted to evaluate the correlation of mutational burden with the efficacy of PD-1/PD-L1 inhibitors.
A landmark paper by Rizvi et al.67 demonstrated that a higher nonsynonymous mutation burden in tumours (defined as a level above the median burden of the cohort) was associated with an improved orr (63% vs. 0%, p = 0.03) and pfs (14.5 months vs. 3.7 months, p = 0.01; hr: 0.19; 95% ci: 0.05 to 0.70) with pembrolizumab treatment. In addition, the efficacy of pembrolizumab correlated with neoantigen burden—a factor also associated with higher nonsynonymous mutation rates. The candidate neoantigen burden was significantly higher in patients who experienced a durable treatment benefit with pembrolizumab than in those who did not experience a durable benefit, and high candidate neoantigen burden was associated with improved pfs (14.5 months vs. 3.5 months, p = 0.002). Furthermore, the authors demonstrated that, in patients with tumour PD-L1 expression of 1%–49%, a high nonsynonymous mutation burden was associated with a durable clinical response in 75% of patients, and a low burden was associated with a durable clinical response in only 11% of patients. Given that the sample sizes in the patient groups were small (4 and 9 respectively), larger-scale studies will be needed to determine if there are relations between PD-L1 intensity, mutational burden, and therapeutic response.
McGranahan et al.68 found that high neoantigen burden and low neoantigen intratumoural heterogeneity in nsclc are associated with significantly longer pfs. Compared with other tumours, tumours harbouring a large clonal neoantigen burden and low neoantigen heterogeneity were seen to have greater PD-L1 expression (p = 0.0017). The authors also demonstrated that, in certain patients with a poor response to PD-1 blockade, cytotoxic chemotherapy–induced subclonal neoantigens were enriched. Those data suggest that neoantigen heterogeneity, in addition to global neoantigen burden, might influence the therapeutic outcome of PD-1/PD-L1 inhibition.
CLINICAL TRIALS OF PD-1/PD-L1 INHIBITORS
Numerous clinical trials have recently compared inhibitors of the PD-1/PD-L1 axis with traditional cytotoxic chemotherapies for the treatment of nsclc (Table i). Positive results from those trials ultimately led to Health Canada (hc) approval of 3 PD-1 inhibitors: nivolumab, pembrolizumab, and atezolizumab (Table ii). Durvalumab, another PD-L1 inhibitor, has recently shown promising results in clinical trials and will likely soon be under review for use in Canada.
TABLE I.
Selected clinical trials of PD-1/PD-L1 inhibitors as monotherapy in non-small-cell lung cancer (NSCLC)
| Trial name | Study drug | Phase | Indication | Population | Survival (months) | Response rate (%) | |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Progression-free | Overall | ||||||
| CheckMate 01269 | Nivolumab | I | First line | 52 Advanced NSCLC | 3.6 (PD-L1≥50%: 8.4) | 21.8 | 23 (PD-L1≥50%: 50) |
| CheckMate 01710 | Nivolumab Docetaxel |
III | Second line | 272 Advanced squamous NSCLC | 4.2 3.5 (HR: 0.62; p<0.001) |
9.2 6.0 (HR: 0.59; p<0.001) |
20 9 (p=0.008) |
| CheckMate 05756 | Nivolumab Docetaxel |
III | Second line | 582 Advanced nonsquamous NSCLC | 2.3 4.2 (HR: 0.92; p=0.39) |
12.2 9.4 (HR: 0.72; p<0.001) |
19 12 (p=0.02) |
| CheckMate 02670 | Nivolumab Platinum-based chemotherapy (up to 6 cycles) |
III | First line | 423 Advanced NSCLC, PD-L1–positive, no EGFR or ALK mutation | 4.2 5.9 (HR: 1.15; p=0.25) |
Not reported | Not reported |
| KEYNOTE 00171 | Pembrolizumab | I | Second line | 495 Advanced NSCLC, PD-L1–positive, no EGFR mutation | 3.7 | 12.0 | 19.4 |
| KEYNOTE 01012 | Pembrolizumab (2 mg/kg) Pembrolizumab (10 mg/kg) Docetaxel |
II/III | Second line | 1034 Advanced NSCLC, PD-L1–positive | 3.9 vs. control (HR: 0.88; p=0.07) 4.0 vs. control (HR: 0.79; p<0.004) vs. 2 mg/kg (HR: 1.09) 4.0 |
10.4 vs. control (HR: 0.71; p=0.0008) 12.7 vs. control (HR: 0.61; p<0.0001) vs. 2mg/kg (HR: 1.17) 8.5 |
18 vs. control (p=0.0005) 18.5 vs. control (p=0.0002) 9.3 |
| KEYNOTE 02411 | Pembrolizumab Platinum-based chemotherapy (4–6 cycles) |
III | First line | 305 Advanced NSCLC PD-L1–positive, no EGFR or ALK mutation | 10.3 6.0 (HR: 0.50; p<0.001) |
6-Month rate: 80.2% 6-Month rate: 72.4% (HR: 0.60; p=0.005) |
44.8 27.8 |
| POPLAR48 | Atezolizumab Docetaxel |
II | Second line | 287 Advanced NSCLC | 2.7 3.0 (HR: 0.94) |
12.6 9.7 (HR: 0.73; p=0.04) |
15 15 |
| OAK72 | Atezolizumab Docetaxel |
III | Second line | 850 Advanced NSCLC | 2.8 4.0 (HR: 0.95; p=0.49) |
13.8 9.6 (HR: 0.73; p=0.0003) |
13.6 13.4 |
| BIRCH73 | Atezolizumab | II | First line | 142 PD-L1–positive, | 7.3 | 13.8 | 13.6 |
| PACIFIC74 | Durvalumab Placebo |
III | Second line (consolidation) | 709 stage III NSCLC with ≥2 rounds of platinum-based chemotherapy and radiotherapy with no progression | 16.8 5.6 (HR: 0.52; p<0.001) |
23.2a 14.6a (HR: 0.52; p<0.001) |
28.4 16.0 (p<0.001) |
Death or distant metastasis.
TABLE II.
Canadian landscape of PD-1/PD-L1 inhibitors in non-small-cell lung cancer (NSCLC)
| Drug | Indication | Approvals | Provincial funding | |
|---|---|---|---|---|
|
| ||||
| Health Canada | pCODR | |||
| Atezolizumab | Locally advanced or metastatic NSCLC with progression on or after systemic chemotherapy | 6 Apr 2018 | Under review | Not applicable |
| Nivolumab | Locally advanced or metastatic NSCLC with progression on or after systemic chemotherapy | 26 Feb 2016 | 3 Jun 2016 | Funded: BC, AB, SK, MB, ON, NS, NB, NL Under review: PEI |
| Pembrolizumab | Previously untreated metastatic NSCLC with tumour expression of PD-L1 and without sensitizing EGFR mutation or ALK translocation. Funding requested for TPS of PD-L1 ≥50%. |
12 Jul 2017 | 23 Aug 2017 | Funded: SK, MB, ON Under review: BC, AB, NS, NB, NL, PEI |
| Pembrolizumab | For treatment of metastatic NSCLC with tumour expression of PD-L1 and previous progression on or after systemic chemotherapy. Funding requested for TPS of PD-L1 ≥1%. |
15 Apr 2016 | 3 Nov 2016 | Funded: SK, MB, ON Under review: BC, AB, NS, NB, NL, PEI |
TPS = tumour proportion score.
Nivolumab
Nivolumab is a monoclonal antibody to PD-1 and has shown efficacy in nsclc in multiple phase iii clinical trials, ultimately leading to hc approval. The phase iii clinical trials CheckMate 01710 and CheckMate 05756 showed the effectiveness of nivolumab (compared with docetaxel chemotherapy) as a second-line therapy in nsclc. CheckMate 017 enrolled patients with advanced nsclc of squamous cell histology. The median os was 9.2 months with nivolumab (95% ci: 7.3 months to 13.3 months) compared with 6.0 months with docetaxel (95% ci: 5.1 months to 7.3 months). Further, the median pfs was 3.5 months with nivolumab compared with 2.8 months with docetaxel (hr: 0.62; 95% ci: 0.47 to 0.81; p < 0.001). A 20% orr was achieved in the nivolumab group, and no associations with PD-L1 tumour expression were observed in subgroup analyses. CheckMate 057 enrolled patients with advanced nsclc of nonsquamous histology. Compared with docetaxel, nivolumab was associated with a significant improvement (os: 12.2 months; response rate: 19%). Subgroup analyses demonstrated significant positive associations of tumour PD-L1 expression (≥5%) with both pfs and os in the nivolumab treatment group. The reasons for the difference in pfs benefit and association with tumour PD-L1 expression in the two studies are not clear, but it has been postulated that the histology difference might be associated with mutational burden and tumour biology75.
In the multi-cohort phase i CheckMate 012 trial69, nivolumab was used as first-line monotherapy in advanced nsclc. That approach resulted in an orr of 23%, a median pfs of 3.6 months, and a median os of 21.8 months. A non-significant association of improved response with 50% or greater PD-L1 expression in tumour cells in 12 patients was observed. Those 12 patients experienced a 50% response rate and 8.4 months of pfs. Those results led to the phase iii clinical trials of nivolumab as first-line monotherapy for advanced nsclc.
CheckMate 02670 is the only completed phase iii clinical trial that has evaluated nivolumab as first-line therapy. That trial compared nivolumab with a traditional platinum-based chemotherapy regimen in patients with advanced nsclc who showed PD-L1 tumour cell expression of 5% or greater, with no EGFR mutation or ALK translocation.
No significant differences in pfs or os between the two study groups were observed. In the nivolumab group, the orr was 26%, and the median pfs and os were 4.2 months and 14.4 months respectively. In comparison, patients randomized to the control arm experienced a response rate of 33%, with a median pfs of 5.9 months and an os of 13.2 months. The lack of a survival benefit was attributed partly to imbalances in post-discontinuation treatment (40% nivolumab vs. 60% control) and rates of strong PD-L1 expression (53.2% nivolumab vs. 74.1% control). Based on the negative results of the study, nivolumab has not been approved for first-line use in nsclc.
Pembrolizumab
Pembrolizumab, another monoclonal antibody to PD-1, has been studied in multiple clinical trials that led to its approval by hc. The phase iii keynote-010 trial12 compared pembrolizumab monotherapy with docetaxel in advanced nsclc. Pembrolizumab was given at 2 doses: 2 mg/kg and 10 mg/kg. The study enrolled nsclc patients who had disease progression after platinum-based chemotherapy or tyrosine kinase inhibitors, and 1% or greater PD-L1 expression by tumour cells. The os and orr were significantly greater for both pembrolizumab groups than for the docetaxel group, but no significant difference in pfs between the two pembrolizumab groups was observed. The lower dose of pembrolizumab was associated with an orr of 18%, a median pfs of 3.9 months, and a median os of 10.4 months. The higher dose of pembrolizumab was associated with an orr of 18.5%, a median pfs of 4.0 months, and a median os of 12.7 months. No significant difference between the two pembrolizumab groups for any outcome and no significant associations in subgroup analysis were observed. The overall quality of the trial was considered moderate for all outcomes75.
In the phase i keynote-001 trial71, pembrolizumab was used as first-line monotherapy for advanced nsclc. The result was an orr of 24.8%, a median pfs of 6 months, and a median os of 22.1 months. In patients having 50% or greater PD-L1 expression in tumour cells, median pfs increased to 12.5 months.
In the pivotal phase iii keynote-02411 trial, pembrolizumab monotherapy was compared with a platinum-based chemotherapy in the first line. The trial enrolled patients with advanced nsclc having PD-L1 tumour cell expression of 50% or greater, no EGFR mutation or ALK translocation, and a life expectancy of more than 3 months. Based on a preplanned interim analysis, the trial was stopped early because of pembrolizumab superiority. The 10.3-month median pfs in the pembrolizumab group significantly exceeded the pfs in the platinum-based chemotherapy group. The estimated 80.2% 6-month survival and 44.8% response rate were also higher in the pembrolizumab group.
The heterogeneity in results between keynote-024 and CheckMate 026 might be attributable to differences in study design and inclusion criteria, including previous treatments, the PD-L1 biomarker test and expression level cut-off used, and intrinsic differences in biochemical efficacy between the PD-1 inhibitors76.
Atezolizumab
Atezolizumab, a monoclonal antibody to PD-L1, has been tested in multiple clinical trials that have led to its approval by hc as a second-line therapy for advanced nsclc. The phase ii poplar trial48 compared atezolizumab with docetaxel in the second-line setting. The trial enrolled patients with nsclc who had progressed after platinum-based chemotherapy. In the atezolizumab group, os was significantly improved (hr: 0.69; p = 0.01), with an orr of 15%. No difference in median pfs was observed. Those results led to the associated phase iii oak trial72. The oak trial made the same treatment comparison for patients with previously treated advanced nsclc. The os was significantly greater in the atezolizumab group than in the docetaxel group, although no difference in pfs was observed. The atezolizumab group experienced a 13.6% orr, a 2.8-month median pfs, and a 12.6-month median os. In subgroup analyses, even patients with less than 1% PD-L1 expression in tumour cells experienced better os (hr: 0.75; p = 0.02). The overall quality of the evidence in the trials was considered moderate75.
The only trial to study atezolizumab as first-line therapy was the phase ii birch trial73. It enrolled 142 patients with previously untreated advanced nsclc and tumour (or til) PD-L1 expression of 5% or greater. Results included a 25% orr, a 7.3-month median pfs, and a 23.5-month median os.
Durvalumab
Durvalumab is another monoclonal antibody to PD-L1 that has been studied in clinical trials, but that is not currently under hc review for use in Canada. A phase i clinical trial77 evaluating durvalumab in 59 patients with treatment-naïve advanced nsclc showed a 25% orr that was not associated with histologic subtype. No pfs or os values were reported. The phase iii pacific trial74 compared durvalumab as consolidation therapy with placebo in 709 patients with stage iii nsclc who had no disease progression after initial chemoradiotherapy. Patients treated with durvalumab consolidation therapy had a significantly greater orr, pfs, and time until death or distant metastasis (28.4%, 16.8 months, and 23.2 months respectively). Development of new lesions and brain metastases was also significantly less frequent in the durvalumab group than in the placebo group. Within the durvalumab group, no difference in pfs was evident based on PD-L1 tumour cell expression with a threshold of 25%. The os data for the study have yet to be reported.
THE CHALLENGE OF ACQUIRED RESISTANCE
Despite an objective clinical response to PD-1 axis blockade being obtained in approximately 20% of nsclc patients, most patients will ultimately acquire drug resistance and subsequently experience disease progression53. However, the mechanisms that underpin acquired resistance to PD-1/PD-L1 inhibitors are poorly understood. Two proposed mechanisms that could contribute to the phenomenon are the evolution of the landscape of tumour neoantigens78 and the upregulation of other immune checkpoint proteins that are independent of the PD-1/PD-L1 axis79.
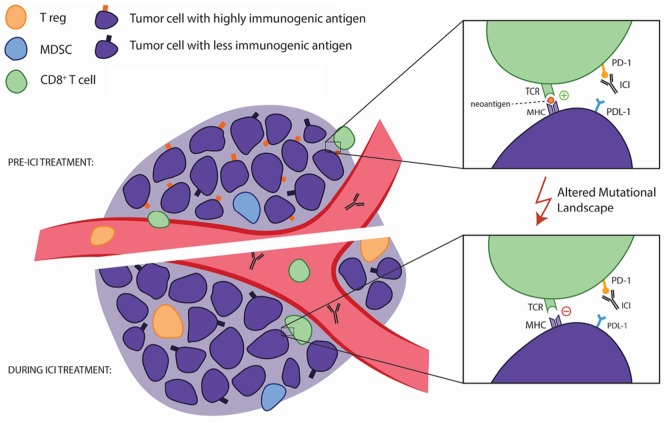
A recent study by Anagnostou et al.78 examined the evolving landscape of tumour neoantigens during the emergence of acquired resistance in patients with nsclc after initial response to immune checkpoint blockade with anti–PD-1 antibodies. They analyzed matched pre-treatment and resistant tumours, and identified genomic changes resulting in the loss of 7–18 putative mutation-associated neoantigens in resistant clones. They then generated peptides from the eliminated neoantigens that could elicit clonal T cell expansion in autologous T cell cultures—suggesting that those neoantigens could generate a functional immune response. Furthermore, they demonstrated that neoantigen loss occurred through the elimination of tumour subclones or through deletion of chromosomal regions with truncal alterations. Taken together, those data demonstrated for the first time that acquired resistance to PD-1 blockade could be a result of an evolving landscape of tumour mutations, some of which encode immunogenic neoantigens (Figure 1). The authors suggested that the putative neoantigens identified before and at the emergence of resistance could be leveraged to develop patient-specific vaccines and adoptive T cell transfer with engineered T cell receptor T cells.
FIGURE 1.
A possible mechanism for acquired resistance to PD-1 inhibition. Anagnostou and colleagues76 discovered that acquired resistance to blockade of the PD-1/PD-L1 axis can potentially be explained by a shifting mutational landscape, which might cause the loss of neoantigens. That loss abrogates the ability of T cells in the tumour microenvironment to recognize the malignant cells as foreign, which renders therapy with PD-1 axis blockade ineffective. CD8+ T cells, regulatory T cells (T regs) and myeloid-derived suppressor cells (MDSCs) are all pictured here as part of the tumour microenvironment. ICI = immune checkpoint inhibitor; TCR = T cell receptor; MHC = major histocompatibility complex.
In addition to the shifting landscape of neoantigens, upregulation of other immune checkpoint proteins can contribute to acquired resistance to PD-1 axis inhibition. Using two fully immunocompetent murine models of lung adenocarcinoma, Koyama et al.79 observed the upregulation of tim-3 in PD-1 antibody-bound T cells. Further, they showed that mice treated with a tim-3–blocking antibody after failure of PD-1 blockade was associated with a survival advantage. To confirm that principle, they also analyzed tumour specimens from human patients, which showed similar tim-3 upregulation in blocking antibody-bound T cells at the time of anti–PD-1 treatment failure. Hypothetically, the measurement of multiple immune checkpoints at time of biopsy could inform specific and personalized immunotherapeutic strategies. Ultimately, to develop beneficial therapeutic approaches, preclinical studies with larger sample sizes will be needed to better understand the dynamics and diversity of alternative immune checkpoint expression.
SUMMARY AND FUTURE DIRECTIONS
The last decade has uncovered the importance of targeting the PD-1/PD-L1 axis for the treatment of nsclc. With hc approval now in place for the anti–PD-1 antibodies nivolumab, pembrolizumab, and atezolizumab, the clinical significance of blocking this immunosuppressive axis is clear. However, as things now stand, patient response to these therapeutics has substantial heterogeneity. No single biomarker has shown perfect reliability to date, and future studies will have to elucidate how administration of these agents can be optimized for patients. It remains to be seen whether future recipients will be selected based on a combination of currently studied clinicopathologic biomarkers or whether more predictive biomarkers will be found.
Furthermore, the scope of use of the PD-1/PD-L1 inhibitors will continue to evolve in coming years. The interim findings of the aforementioned pacific trial74 are certainly promising, and the hope is that its final results will lead to durvalumab being established as a safe and efficacious option for patients with nonresectable nsclc after standard chemoradiotherapy. In addition, the ongoing phase iii br.31 trial (see NCT02273375 at http://ClinicalTrials.gov) led by the Canadian Cancer Trials Group, which is assessing the role of adjuvant immunotherapy in the postsurgical setting, has the potential to be practice-changing.
Although the present review has focused on the current landscape of PD-1/PD-L1 axis inhibitors as monotherapy, numerous trials are evaluating the use of those agents in a wide variety of combinations. Table iii summarizes a selection of currently completed early-phase studies evaluating PD-1/PD-L1 axis blockade in combination with chemotherapy, radiotherapy, or other immune checkpoint inhibitors such as ctla-4.
TABLE III.
Selected clinical trials of PD-1/PD-L1 inhibitors as combination therapy non-small-cell lung cancer (NSCLC)
| Study name | Drug | Phase | Treatment | Population | Survival (months) | Response rate (%) | |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Progression-free | Overall | ||||||
| CheckMate 01280 | Nivolumab 10 mg/kg with gemcitabine–cisplatin | I | First line | 56 Advanced NSCLC randomized based on histologic type | 24-Week rate: 51% | 2-year rate: 25% | 33 |
| Nivolumab 10 mg/kg with pemetrexed–cisplatin | 24-Week rate: 71% | 2-year rate: 33% | 47 | ||||
| Nivolumab 10 mg/kg with paclitaxel–carboplatin | 24-Week rate: 38% | 2-year rate: 27% | 47 | ||||
| Nivolumab 5 mg/kg with paclitaxel–carboplatin | 24-Week rate: 51% | 2-year rate: 62% | 43 | ||||
| CheckMate 01281 | Nivolumab 3 mg/kg every 2 weeks with ipilimumab 1 mg/kg every 12 weeks | I | First line | 77 Advanced NSCLC | 8.1 | Not reached | 38 |
| Nivolumab 3 mg/kg every 2 weeks with ipilimumab 1 mg/kg every 6 weeks | 3.9 | Not reached | 47 | ||||
| KEYNOTE 02182 | Paclitaxel–carboplatin | I/II | First line | 123 Advanced NSCLC, no EGFR or ALK mutation | 8.9 | 20.9 | 32 |
| Pembrolizumab with paclitaxel–carboplatin | 19.0 (HR: 0.54; p=0.0067) | Not reached (HR: 0.59; p=0.0344) | 57 | ||||
| KEYNOTE 02183 | Pembrolizumab with pemetrexed–carboplatin | II | First line | 123 Advanced nonsquamous NSCLC, no EGFR or ALK mutation | 13.0 | 13 | 55 |
| Pemetrexed–carboplatin | 8.9 (HR: 0.53; p=0.01) | 14 (HR: 0.90; p=0.39) | 29 | ||||
| KEYNOTE 18984 | Pembrolizumab with pemetrexed–platinum agent | III | First line | 616 Advanced nonsquamous NSCLC, no EGFR or ALK mutations | 8.8 | Not reached | 48 |
| Pemetrexed-platinum agent | 4.9 (HR: 0.52; p<0.001) | 11.3 (HR: 0.49; p<0.001) | 19 | ||||
To summarize, despite room for optimization in the administration of PD-1/PD-L1 inhibitors and for combinatorial regimens to demonstrate superiority compared with monotherapy, it is important to recognize that, arguably, no bigger advance than the use of these inhibitors has yet occurred in the treatment of nsclc. PD-1/PD-L1 inhibitors are extending the survival of patients with nsclc and are doing so with less patient morbidity. We therefore look forward to the future and, hopefully, to continued improvement in nsclc outcomes.
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare that we have none.
REFERENCES
- 1.Canadian Cancer Society’s Advisory Committee on Cancer Statistics . Canadian Cancer Statistics 2017. Toronto, ON: Canadian Cancer Society; 2017. [Google Scholar]
- 2.Novello S, Barlesi F, Califano R, et al. on behalf of the esmo Guidelines Committee Metastatic non-small-cell lung cancer: esmo clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27(suppl 5):v1–27. doi: 10.1093/annonc/mdw326. [DOI] [PubMed] [Google Scholar]
- 3.Midha A, Dearden S, McCormack R. EGFR mutation incidence in non-small-cell lung cancer of adenocarcinoma histology: a systematic review and global map by ethnicity (mutmapii) Am J Cancer Res. 2015;5:2892–911. [PMC free article] [PubMed] [Google Scholar]
- 4.Dearden S, Stevens J, Wu YL, Blowers D. Mutation incidence and coincidence in non small–cell lung cancer: meta-analyses by ethnicity and histology (mutmap) Ann Oncol. 2013;24:2371–6. doi: 10.1093/annonc/mdt205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 6.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 7.Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320–30. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 8.Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373:1803–13. doi: 10.1056/NEJMoa1510665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferris RL, Blumenschein G, Fayette J, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;375:1856–67. doi: 10.1056/NEJMoa1602252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373:123–35. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1–positive non-small-cell lung cancer. N Engl J Med. 2016;375:1823–33. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 12.Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1–positive, advanced non-small-cell lung cancer (keynote-010): a randomised controlled trial. Lancet. 2016;387:1540–50. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 13.Liang SC, Latchman YE, Buhlmann JE, et al. Regulation of PD-1, PD-L1, and PD-L2 expression during normal and autoimmune responses. Eur J Immunol. 2003;33:2706–16. doi: 10.1002/eji.200324228. [DOI] [PubMed] [Google Scholar]
- 14.Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027–34. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Latchman Y, Wood CR, Chernova T, et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol. 2001;2:261–8. doi: 10.1038/85330. [DOI] [PubMed] [Google Scholar]
- 16.Tseng SY, Otsuji M, Gorski K, et al. B7-DC, a new dendritic cell molecule with potent costimulatory properties for T cells. J Exp Med. 2001;193:839–46. doi: 10.1084/jem.193.7.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keir ME, Liang SC, Guleria I, et al. Tissue expression of PD-L1 mediates peripheral T cell tolerance. J Exp Med. 2006;203:883–95. doi: 10.1084/jem.20051776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He J, Hu Y, Hu M, Li B. Development of PD-1/PD-L1 pathway in tumor immune microenvironment and treatment for non–small cell lung cancer. Sci Rep. 2015;5:13110. doi: 10.1038/srep13110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Charlton JJ, Chatzidakis I, Tsoukatou D, Boumpas DT, Garinis GA, Mamalaki C. Programmed death-1 shapes memory phenotype CD8 T cell subsets in a cell-intrinsic manner. J Immunol. 2013;190:6104–14. doi: 10.4049/jimmunol.1201617. [DOI] [PubMed] [Google Scholar]
- 20.Montler R, Bell RB, Thalhofer C, et al. ox40, PD-1 and ctla-4 are selectively expressed on tumor-infiltrating T cells in head and neck cancer. Clin Transl Immunology. 2016;5:e70. doi: 10.1038/cti.2016.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahmadzadeh M, Johnson LA, Heemskerk B, et al. Tumor antigen–specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 2009;114:1537–44. doi: 10.1182/blood-2008-12-195792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Konishi J, Yamazaki K, Azuma M, Kinoshita I, Dosaka-Akita H, Nishimura M. B7-H1 expression on non–small cell lung cancer cells and its relationship with tumor-infiltrating lymphocytes and their PD-1 expression. Clin Cancer Res. 2004;10:5094–500. doi: 10.1158/1078-0432.CCR-04-0428. [DOI] [PubMed] [Google Scholar]
- 23.Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 24.Kondo A, Yamashita T, Tamura H, et al. Interferon-gamma and tumor necrosis factor-alpha induce an immunoinhibitory molecule, B7-H1, via nuclear factor-κB activation in blasts in myelodysplastic syndromes. Blood. 2010;116:1124–31. doi: 10.1182/blood-2009-12-255125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taube JM, Anders RA, Young GD, et al. Colocalization of inflammatory response with B7-H1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med. 2012;4:127ra37. doi: 10.1126/scitranslmed.3003689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marzec M, Zhang Q, Goradia A, et al. Oncogenic kinase npm/alk induces through stat3 expression of immunosuppressive protein CD274 (PD-L1, B7-H1) Proc Natl Acad Sci U S A. 2008;105:20852–7. doi: 10.1073/pnas.0810958105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ota K, Azuma K, Kawahara A, et al. Induction of PD-L1 expression by the eml4-alk oncoprotein and downstream signaling pathways in non-small cell lung cancer. Clin Cancer Res. 2015;21:4014–21. doi: 10.1158/1078-0432.CCR-15-0016. [DOI] [PubMed] [Google Scholar]
- 28.Chen N, Fang W, Zhan J, et al. Upregulation of PD-L1 by egfr activation mediates the immune escape in egfr-driven nsclc: implication for optional immune targeted therapy for nsclc patients with EGFR mutation. J Thorac Oncol. 2015;10:910–23. doi: 10.1097/JTO.0000000000000500. [DOI] [PubMed] [Google Scholar]
- 29.Azuma K, Ota K, Kawahara A, et al. Association of PD-L1 overexpression with activating EGFR mutations in surgically resected nonsmall-cell lung cancer. Ann Oncol. 2014;25:1935–40. doi: 10.1093/annonc/mdu242. [DOI] [PubMed] [Google Scholar]
- 30.Li J, Chen Y, Shi X, et al. A systematic and genome-wide correlation meta-analysis of PD-L1 expression and targetable nsclc driver genes. J Thorac Dis. 2017;9:2560–71. doi: 10.21037/jtd.2017.07.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gainor JF, Shaw AT, Sequist LV, et al. EGFR mutations and ALK rearrangements are associated with low response rates to PD-1 pathway blockade in non–small cell lung cancer: a retrospective analysis. Clin Cancer Res. 2016;22:4585–93. doi: 10.1158/1078-0432.CCR-15-3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sato Y, Mukai K, Watanabe S, et al. Lymphocyte subsets in pulmonary venous and arterial blood of lung cancer patients. Jpn J Clin Oncol. 1989;19:229–36. [PubMed] [Google Scholar]
- 33.Woo EY, Yeh H, Chu CS, et al. Cutting edge: regulatory T cells from lung cancer patients directly inhibit autologous T cell proliferation. J Immunol. 2002;168:4272–6. doi: 10.4049/jimmunol.168.9.4272. [DOI] [PubMed] [Google Scholar]
- 34.Zhuang X, Xia X, Wang C, et al. A high number of CD8+ T cells infiltrated in nsclc tissues is associated with a favorable prognosis. Appl Immunohistochem Mol Morphol. 2010;18:24–8. doi: 10.1097/PAI.0b013e3181b6a741. [DOI] [PubMed] [Google Scholar]
- 35.Horne ZD, Jack R, Gray ZT, et al. Increased levels of tumor-infiltrating lymphocytes are associated with improved recurrence-free survival in stage 1A non-small-cell lung cancer. J Surg Res. 2011;171:1–5. doi: 10.1016/j.jss.2011.03.068. [DOI] [PubMed] [Google Scholar]
- 36.Tao H, Mimura Y, Aoe K, et al. Prognostic potential of foxp3 expression in non–small cell lung cancer cells combined with tumor-infiltrating regulatory T cells. Lung Cancer. 2012;75:95–101. doi: 10.1016/j.lungcan.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 37.Petersen RP, Campa MJ, Sperlazza J, et al. Tumor infiltrating foxp3+ regulatory T-cells are associated with recurrence in pathologic stage i nsclc patients. Cancer. 2006;107:2866–72. doi: 10.1002/cncr.22282. [DOI] [PubMed] [Google Scholar]
- 38.Mu CY, Huang JA, Chen Y, Chen C, Zhang XG. High expression of PD-L1 in lung cancer may contribute to poor prognosis and tumor cells immune escape through suppressing tumor infiltrating dendritic cells maturation. Med Oncol. 2011;28:682–8. doi: 10.1007/s12032-010-9515-2. [DOI] [PubMed] [Google Scholar]
- 39.Zhang Y, Wang L, Li Y, et al. Protein expression of programmed death 1 ligand 1 and ligand 2 independently predict poor prognosis in surgically resected lung adenocarcinoma. Onco Targets Ther. 2014;7:567–73. doi: 10.2147/OTT.S59959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tsao MS, Le Teuff G, Shepherd FA, et al. PD-L1 protein expression assessed by immunohistochemistry is neither prognostic nor predictive of benefit from adjuvant chemotherapy in resected non–small cell lung cancer. Ann Oncol. 2017;28:882–9. doi: 10.1093/annonc/mdx003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang CY, Lin MW, Chang YL, Wu CT, Yang PC. Programmed cell death–ligand 1 expression in surgically resected stage i pulmonary adenocarcinoma and its correlation with driver mutations and clinical outcomes. Eur J Cancer. 2014;50:1361–9. doi: 10.1016/j.ejca.2014.01.018. [DOI] [PubMed] [Google Scholar]
- 42.Wang A, Wang HY, Liu Y, et al. The prognostic value of PD-L1 expression for non–small cell lung cancer patients: a meta-analysis. Eur J Surg Oncol. 2015;41:450–6. doi: 10.1016/j.ejso.2015.01.020. [DOI] [PubMed] [Google Scholar]
- 43.Zhang M, Li G, Wang Y, et al. PD-L1 expression in lung cancer and its correlation with driver mutations: a meta-analysis. Sci Rep. 2017;7:10255. doi: 10.1038/s41598-017-10925-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin–paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–57. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 45.Michot JM, Bigenwald C, Champiat S, et al. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer. 2016;54:139–48. doi: 10.1016/j.ejca.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 46.Tartari F, Santoni M, Burattini L, Mazzanti P, Onofri A, Berardi R. Economic sustainability of anti–PD-1 agents nivolumab and pembrolizumab in cancer patients: recent insights and future challenges. Cancer Treat Rev. 2016;48:20–4. doi: 10.1016/j.ctrv.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 47.Ayers M, Lunceford J, Nebozhyn M, et al. ifn-γ–related mrna profile predicts clinical response to PD-1 blockade. J Clin Invest. 2017;127:2930–40. doi: 10.1172/JCI91190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fehrenbacher L, Spira A, Ballinger M, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (poplar): a multicentre, open-label, phase 2 randomised controlled trial. Lancet. 2016;387:1837–46. doi: 10.1016/S0140-6736(16)00587-0. [DOI] [PubMed] [Google Scholar]
- 49.Johnson DB, Estrada MV, Salgado R, et al. Melanoma-specific mhc-ii expression represents a tumour-autonomous phenotype and predicts response to anti–PD-1/PD-L1 therapy. Nat Commun. 2016;7:10582. doi: 10.1038/ncomms10582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Routy B, Le Chatelier E, Derosa L, et al. Gut microbiome influences efficacy of PD-1–based immunotherapy against epithelial tumors. Science. 2018;359:91–7. doi: 10.1126/science.aan3706. [DOI] [PubMed] [Google Scholar]
- 51.Gopalakrishnan V, Spencer CN, Nezi L, et al. Gut microbiome modulates response to anti–PD-1 immunotherapy in melanoma patients. Science. 2018;359:97–103. doi: 10.1126/science.aan4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Herbst RS, Soria JC, Kowanetz M, et al. Predictive correlates of response to the anti–PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563–7. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gettinger SN, Horn L, Gandhi L, et al. Overall survival and long-term safety of nivolumab (anti-programmed death 1 antibody, BMS-936558, ONO-4538) in patients with previously treated advanced non-small-cell lung cancer. J Clin Oncol. 2015;33:2004–12. doi: 10.1200/JCO.2014.58.3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372:2018–28. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 55.Rizvi N, Balmanoukian A, Goldberg SB, et al. Phase 1b study of the safety and antitumour activity of durvalumab (MEDI4736) + tremelimumab in advanced nsclc. Ann Oncol. 2015;26:ix126. doi: 10.1093/annonc/mdv532.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373:1627–39. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rizvi NA, Mazières J, Planchard D, et al. Activity and safety of nivolumab, an anti–PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol. 2015;16:257–65. doi: 10.1016/S1470-2045(15)70054-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Passiglia F, Bronte G, Bazan V, et al. PD-L1 expression as predictive biomarker in patients with nsclc: a pooled analysis. Oncotarget. 2016;7:19738–47. doi: 10.18632/oncotarget.7582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Abdel-Rahman O. Correlation between PD-L1 expression and outcome of nsclc patients treated with anti-PD-1/PD-L1 agents: a meta-analysis. Crit Rev Oncol Hematol. 2016;101:75–85. doi: 10.1016/j.critrevonc.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 60.Hirsch FR, McElhinny A, Stanforth D, et al. PD-L1 immunohistochemistry assays for lung cancer: results from phase 1 of the Blueprint PD-L1 IHC Assay Comparison Project. J Thorac Oncol. 2017;12:208–22. doi: 10.1016/j.jtho.2016.11.2228. [DOI] [PubMed] [Google Scholar]
- 61.Han JJ, Kim DW, Koh J, et al. Change in PD-L1 expression after acquiring resistance to gefitinib in EGFR-mutant non-small-cell lung cancer. Clin Lung Cancer. 2016;17:263–70.e2. doi: 10.1016/j.cllc.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 62.Grigg C, Rizvi NA. PD-L1 biomarker testing for non–small cell lung cancer: truth or fiction? J Immunother Cancer. 2016;4:48. doi: 10.1186/s40425-016-0153-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti–PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti–PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–65. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kelderman S, Schumacher TN, Kvistborg P. Mismatch repair–deficient cancers are targets for anti–PD-1 therapy. Cancer Cell. 2015;28:11–13. doi: 10.1016/j.ccell.2015.06.012. [DOI] [PubMed] [Google Scholar]
- 66.Lawrence MS, Stojanov P, Polak P, et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature. 2013;499:214–18. doi: 10.1038/nature12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non–small cell lung cancer. Science. 2015;348:124–8. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McGranahan N, Furness AJS, Rosenthal R, et al. Clonal neo-antigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science. 2016;351:1463–9. doi: 10.1126/science.aaf1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gettinger S, Rizvi NA, Chow LQ, et al. Nivolumab monotherapy for first-line treatment of advanced non-small-cell lung cancer. J Clin Oncol. 2016;34:2980–7. doi: 10.1200/JCO.2016.66.9929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Carbone DP, Reck M, Paz-Ares L, et al. First-line nivolumab in stage iv or recurrent non-small-cell lung cancer. N Engl J Med. 2017;376:2415–26. doi: 10.1056/NEJMoa1613493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hui R, Garon EB, Goldman JW, et al. Pembrolizumab as first-line therapy for patients with PD-L1–positive advanced non–small cell lung cancer: a phase 1 trial. Ann Oncol. 2017;28:874–81. doi: 10.1093/annonc/mdx008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (oak): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389:255–65. doi: 10.1016/S0140-6736(16)32517-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Garassino M, Rizvi N, Besse B, et al. Atezolizumab as 1L therapy for advanced nsclc in PD-L1–selected patients: updated orr, pfs and os data from the birch study [abstract OA03.02] J Thorac Oncol. 2017;12:S251–2. doi: 10.1016/j.jtho.2016.11.239. [DOI] [Google Scholar]
- 74.Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after chemoradiotherapy in stage iii non-small-cell lung cancer. N Engl J Med. 2017;377:1919–29. doi: 10.1056/NEJMoa1709937. [DOI] [PubMed] [Google Scholar]
- 75.Ellis PM, Vella ET, Ung YC. Immune checkpoint inhibitors for patients with advanced non–small-cell lung cancer: a systematic review. Clin Lung Cancer. 2017;18:444–59.e1. doi: 10.1016/j.cllc.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 76.Remon J, Pardo N, Martinez-Martí A, et al. Immune-checkpoint inhibition in first-line treatment of advanced non–small cell lung cancer patients: current status and future approaches. Lung Cancer. 2017;106:70–5. doi: 10.1016/j.lungcan.2017.02.002. [Corrigendum in: Lung Cancer 2018;117:80] [DOI] [PubMed] [Google Scholar]
- 77.Antonia S, Rizvi N, Brahmer J, et al. Safety and clinical activity of durvalumab (MEDI4736), an anti–programmed cell death ligand-1 (PD-L1) antibody, in patients with non–small cell lung cancer (nsclc) [abstract A047] Cancer Immunol Res. 2016;4 doi: 10.1158/2326-6074.CRICIMTEATIAACR15-A047. [DOI] [Google Scholar]
- 78.Anagnostou V, Smith KN, Forde PM, J, et al. Evolution of neo-antigen landscape during immune checkpoint blockade in non–small cell lung cancer. Cancer Discov. 2017;7:264–76. doi: 10.1158/2159-8290.CD-16-0828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Koyama S, Akbay EA, Li YY, et al. Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nat Commun. 2016;7:10501. doi: 10.1038/ncomms10501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rizvi NA, Hellmann MD, Brahmer JR, et al. Nivolumab in combination with platinum-based doublet chemotherapy for first-line treatment of advanced non-small-cell lung cancer. J Clin Oncol. 2016;34:2969–79. doi: 10.1200/JCO.2016.66.9861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hellmann MD, Rizvi NA, Goldman JW, et al. Nivolumab plus ipilimumab as first-line treatment for advanced non-small-cell lung cancer (CheckMate 012): results of an open-label, phase 1, multicohort study. Lancet Oncol. 2017;18:31–41. doi: 10.1016/S1470-2045(16)30624-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Borghaei H, Langer CJ, Gadgeel S, et al. Updated results from keynote-021 cohort G: a randomized, phase 2 study of pemetrexed and carboplatin (pc) with or without pembrolizumab (pembro) as first-line therapy for advanced nonsquamous nsclc [abstract LBA49] Ann Oncol. 2017;28(suppl 5) [Google Scholar]
- 83.Langer CJ, Gadgeel SM, Borghaei H, et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label keynote-021 study. Lancet Oncol. 2016;17:1497–508. doi: 10.1016/S1470-2045(16)30498-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. on behalf of the keynote-189 investigators Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378:2078–92. doi: 10.1056/NEJMoa1801005. [DOI] [PubMed] [Google Scholar]