Abstract
Background
Advanced non-small-cell lung cancer (nsclc) represents a major health issue globally. Systemic treatment decisions are informed by clinical trials, which, over years, have improved the survival of patients with advanced nsclc. The applicability of clinical trial results to the broad lung cancer population is unclear because strict eligibility criteria in trials generally select for optimal patients.
Methods
We performed a retrospective chart review of all consecutive patients with advanced nsclc seen in outpatient consultation at our academic institution between September 2009 and September 2012, collecting data about patient demographics and cancer characteristics, treatment, and survival from hospital and pharmacy records. Two sets of arbitrary trial eligibility criteria were applied to the cohort. Scenario A stipulated Eastern Cooperative Oncology Group performance status (ecog ps) 0–1, no brain metastasis, creatinine less than 120 μmol/L, and no second malignancy. Less-strict scenario B stipulated ecog ps 0–2 and creatinine less than 120 μmol/L. We then used the two scenarios to analyze treatment and survival of patients by trial eligibility status.
Results
The 528 included patients had a median age of 67 years, with 55% being men and 58% having adenocarcinoma. Of those 528 patients, 291 received at least 1 line of palliative systemic therapy. Using the scenario A eligibility criteria, 73% were trial-ineligible. However, 46% of “ineligible” patients actually received therapy and experienced survival similar to that of the “eligible” treated patients (10.2 months vs. 11.6 months, p = 0.10). Using the scenario B criteria, only 35% were ineligible, but again, the survival of treated patients was similar in the ineligible and eligible groups (10.1 months vs. 10.9 months, p = 0.57).
Conclusions
Current trial eligibility criteria are often strict and limit the enrolment of patients in clinical trials. Our results suggest that, depending on the chosen drug, its toxicities and tolerability, eligibility criteria could be carefully reviewed and relaxed.
Keywords: Non-small-cell lung cancer, nsclc, clinical trial eligibility
INTRODUCTION
Lung cancer is the most common cancer in the world and the leading cause of cancer death worldwide1,2. Although 5-year survival in lung cancer has slowly improved to approximately 18% in 2011 from 12% in the 1970s1, the disease remains lethal for most1,3.
Clinical trials have undoubtedly improved the outcomes of nsclc treatment in both early- and late-stage disease4–11. However, fewer than 5% of all cancer patients participate in clinical trials5,12–14. Lung cancer patients represent only about 12.5% of all cancer clinical trial participants14, being 3rd in participation after breast and colorectal cancer patients, which demonstrates a true underrepresentation of lung cancer despite its remarkable epidemiology and lethality15,16.
One important obstacle to participation is the high selectivity of lung cancer clinical trials, which often have very restrictive eligibility criteria17–19. In fact, studies show that eligibility for a trial might require meeting as many as 44 criteria19–21. Restrictive eligibility not only constitutes a barrier to clinical trial enrolment, but also creates other problems, including difficulty in generalizing results to the broader patient population22–28.
In the present study, we took an existing dataset of patients with advanced nsclc that had previously been reported29. We then used hypothetical clinical trial eligibility criteria to explore how many patients might be trial-eligible. We further assessed how outcomes varied between patient groups based on their trial eligibility and treatments actually received.
METHODS
Patient Data
After ethics approval, we performed a chart review of all patients with de novo advanced nsclc (stage iiib palliative and all stage iv) seen in the outpatient department at The Ottawa Hospital Cancer Centre between September 2009 and September 2012. The Ottawa Hospital Cancer Centre is an academic centre that is the sole provider of medical and radiation oncology services to a population of approximately 1.5 million in Eastern Ontario.
Data collected from hospital and pharmacy records included patient demographics, cancer characteristics, treatment details, and survival information. The primary analysis has previously been reported29.
Clinical Trial Criteria
We designed two clinical trial eligibility scenarios and then assessed how many patients in the cohort would have been “trial eligible” based on the inclusion criteria in each scenario. Subsequently, for each scenario, we compared the trial-eligible and -ineligible patients, the proportion of each group that received systemic therapy, and survival in the two groups.
Scenario A had more-strict eligibility criteria. Patients had to have an Eastern Cooperative Oncology Group performance status (ps) of 0 or 1, absence of brain metastasis, creatinine less than 120 μmol/L (approximately 1.5 times the upper limit normal), and absence of a second malignancy.
Scenario B had less-strict eligibility criteria: Eastern Cooperative Oncology Group ps 0–2 and creatinine less than 120 μmol/L. If data relating to the eligibility criteria were missing, the patient was excluded from the analysis. Eastern Cooperative Oncology Group ps was missing for 8% of patients, and baseline creatinine, for 2%.
Statistical Methods
For this retrospective analysis, the chi-square test was applied. The survival analysis used the Kaplan–Meier method. All analyses were conducted using the SAS software application (version 9.3: SAS Institute, Cary, NC, U.S.A.).
RESULTS
The full descriptive analysis for this cohort of patients was reported in a previous publication29. In brief, 528 patients were included in the study (Table i). Median age in the cohort was 67.5 years; 55% of all patients were men; 43% were current smokers.
TABLE I.
Demographic data for the study cohort
| Variable | Value |
|---|---|
| Patients (n) | 528 |
| Age at diagnosis (years) | |
| Median | 67.5 |
| Range | 34.9–89.7 |
| Sex [n (%)] | |
| Men | 292 (55) |
| Women | 236 (45) |
| ECOG PS [n (%)] | |
| 0 | 46 (9) |
| 1 | 220 (42) |
| 2 | 111 (21) |
| 3 | 92 (17) |
| 4 | 19 (4) |
| Unknown | 40 (8) |
| Smoking status [n (%)] | |
| Current smoker | 228 (43) |
| Ex-smoker | 257 (49) |
| Never-smoker | 37 (7) |
| Unknown | 6 (1) |
| Weight loss [n (%)] | |
| <5% | 235 (45) |
| >5% | 255 (48) |
| Unknown | 38 (7) |
| Histologic subtype [n (%)] | |
| Adenocarcinoma | 308 (58) |
| Large-cell | 27 (5) |
| Mixed | 1 (0.2) |
| Other NSCLC | 29 (6) |
| Squamous cell | 118 (22) |
| Unknown | 45 (9) |
| Stage [n (%)] | |
| IIIB | 35 (7) |
| IV | 493 (93) |
| Reason for no CTx (if stated) [n (%)] | |
| Poor performance status | 158 (67) |
| Age | 3 (1) |
| Comorbidities | 5 (2) |
| Patient choice | 49 (23) |
| Others | 22 (9) |
ECOG PS = Eastern Cooperative Oncology Group performance status; NSCLC = non-small-cell lung cancer; CTx = chemotherapy.
Of all nsclcs, 58% were adenocarcinomas; only 22% were squamous cell carcinomas. Patients with stage iv disease represented 93% of the population; the remaining 7% had stage iiib disease and were treated with palliative intent. Half the patients had a ps of 0 or 1.
Nearly half the patients (n = 237, 45%) did not receive any systemic therapy. Treated patients were younger (median age: 64.8 years vs. 71 years for untreated patients, p < 0.0001). A platinum doublet was the most common first-line therapy (88%); pemetrexed–docetaxel was the most common therapy in the second-line setting.
Only 5% of the patients participated in a clinical trial for any given line of therapy. In 89 patients (17%), a second malignancy had been diagnosed. In about 40 patients, creatinine was elevated above 120 μmol/L, and 16 of them were still treated with chemotherapy.
Survival Analysis
Scenario A
Table ii presents the patient demographic data for scenario A by trial eligibility. Using scenario A (strict criteria), only 27% of the patients (n = 144) would have been trial-eligible. Of those 144 patients, 113 (78%) were treated with at least 1 line of systemic therapy. Of the 384 patients (73%) who were not eligible, 178 (46%) were still treated with systemic therapy (Table iii).
TABLE II.
Demographic data, scenario A
| Variable | Patient group | |||
|---|---|---|---|---|
|
| ||||
| Trial eligible | Trial ineligible | |||
|
| ||||
| Treated | Untreated | Treated | Untreated | |
| Patients (n) | 113 | 31 | 178 | 206 |
| Age (years) | ||||
| Mean | 64.6±9.7 | 71.2±11.3 | 64.4±8.5 | 70.8±9.8 |
| Median | 64.8 | 71.1 | 64.7 | 70.9 |
| Range | 34.9–83.8 | 43.6–87.5 | 43.0–86.7 | 46.2–89.7 |
| Sex [n (%)] | ||||
| Men | 61 (54) | 19 (61) | 99 (55.62) | 113 (54.85) |
| Women | 52 (46) | 12 (39) | 79 (44.38) | 93 (45.15) |
| Weight loss >5% [n (%)] | 45 (40.9) | 16 (53.33) | 75 (46.01) | 119 (63.64) |
| Histology [n (%)] | ||||
| Adenocarcinoma | 77 (71.96) | 18 (58.06) | 106 (66.25) | 107 (57.84) |
| Squamous cell | 20 (18.69) | 12(38.71) | 35 (21.88) | 51 (27.57) |
| Large-cell | 6 (5.61) | 0 | 11 (6.88) | 10 (5.41) |
| Other NSCLC | 4 (3.74) | 1 (3.23) | 8 (5.0) | 16 (8.64) |
| Mixed | 0 | 0 | 0 | 1 (0.54) |
| ECOG PS [n (%)] | ||||
| 0 | 18 | 5 | 18 (11.54) | 5 (2.66) |
| 1 | 95 | 26 | 70 (44.87) | 29 (15.43) |
| 2 | 0 | 0 | 54 (34.62) | 57 (30.32) |
| 3 | 0 | 0 | 13 (8.33) | 79 (42.02) |
| 4 | 0 | 0 | 1 (0.64) | 18 (9.57) |
| Stage [n (%)] | ||||
| IIIB | 10 (8.85) | 5 (16.13) | 3 (1.69) | 17 (8.25) |
| IV | 103 (91.15) | 26 (83.87) | 175 (98.31) | 189 (91.75) |
NSCLC = non-small-cell lung cancer; ECOG PS = Eastern Cooperative Oncology Group performance status.
TABLE III.
Proportion of treated ineligible patients in scenarios A and B
| Trial eligibility | Scenario A patients [n (%)] | Scenario B patients [n (%)] | ||
|---|---|---|---|---|
|
|
|
|||
| Total | Treated | Total | Treated | |
| Eligible | 144 (27) | 113 (78.5) | 343 (65) | 240 (70) |
| Ineligible | 384 (73) | 178 (46) | 185 (35) | 51 (28) |
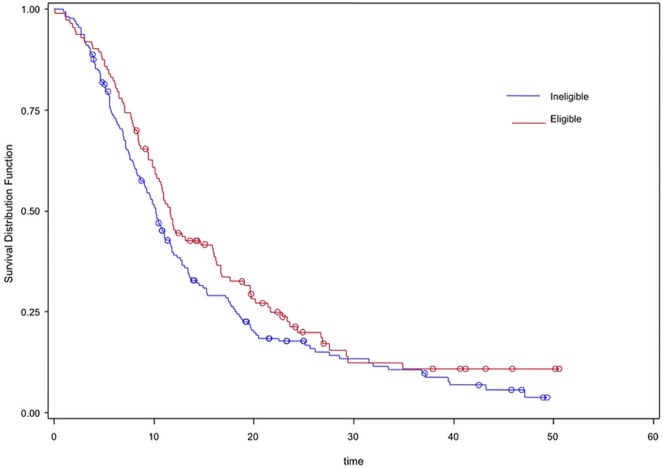
The patients who were treated experienced similar median overall survival (os) regardless of whether they were trial-eligible or -ineligible (11.6 months vs. 10.2 months, p = 0.1). However, compared with ineligible untreated patients, the eligible untreated patients experienced significantly superior survival (8.1 vs. 3.8 months, p = 0.003, Table iv, Figure 1).
TABLE IV.
Overall survival in scenarios A and B, in months
| Trial eligibility | Scenario A | Scenario B | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Treated | Untreated | Treated | Untreated | |||||
|
|
|
|
|
|||||
| Median | 95% CI | Median | 95% CI | Median | 95% CI | Median | 95% CI | |
| Eligible | 11.6 | 10.1 to 15.9 | 8.1 | 3.4 to 12.9 | 10.9 | 9.9 to 11.8 | 4.9 | 3.6 to 6.5 |
| Ineligible | 10.2 | 8.7 to 11.5 | 3.8 | 3.2 to 4.2 | 10.1 | 6.3 to 13.4 | 3.5 | 3.1 to 4.0 |
| p=0.1 | p=0.003 | p=0.57 | p<0.001 | |||||
FIGURE 1.
Kaplan–Meier survival curves for trial eligibility scenario A, reflecting treated eligible (red) and treated ineligible (blue) patients.
Scenario B
Table v presents the patient demographic data for scenario B by trial eligibility. Using scenario B (relaxed criteria), more than half the patients (65%, n = 343) would have been trial-eligible. Of those 343 patients, 240 (70%) were treated (Table iii). Of the 185 patients (35%) who were not eligible, only 51 (28%) were still treated (Table iii).
TABLE V.
Demographic data, scenario B
| Variable | Patient group | |||
|---|---|---|---|---|
|
| ||||
| Trial eligible | Trial ineligible | |||
|
| ||||
| Treated | Untreated | Treated | Untreated | |
| Patients (n) | 240 | 103 | 51 | 134 |
| Age (years) | ||||
| Mean | 64.7±8.9 | 70.4±11.1 | 63.2±9.1 | 71.2±9.1 |
| Median | 65.0 | 70.2 | 62.5 | 71.8 |
| Range | 34.9–86.7 | 43.6–88.9 | 45.4–83.5 | 49.1–89.7 |
| Sex [n (%)] | ||||
| Men | 129 (53.75) | 57 (55.34) | 31 (60.78) | 75 (55.79) |
| Women | 111 (46.25) | 46 (44.66) | 20 (39.22) | 59 (44.03) |
| Weight loss >5% [n (%)] | 99 (43.23) | 52 (53.61) | 21 (47.73) | 83 (69.17) |
| Histology [n (%)] | ||||
| Adenocarcinoma | 152 (69.09) | 55 (58.51) | 31 (65.96) | 70 (57.38) |
| Squamous cell | 46 (20.91) | 29 (30.85) | 9 (19.15) | 34 (27.87) |
| Large-cell | 13 (5.91) | 4 (4.26) | 4 (8.51) | 6 (4.92) |
| Other NSCLC | 9 (4.09) | 6 (6.38) | 3 (6.38) | 11 (9.02) |
| Mixed | 0 | 0 | 0 | 1 (0.82) |
| ECOG PS [n (%)] | ||||
| 0 | 35 (14.58) | 9 (8.74) | 1 (3.45) | 1 (0.86) |
| 1 | 155 (64.58) | 46 (44.66) | 10 (34.48) | 9 (7.76) |
| 2 | 50 (20.83) | 48 (46.6) | 4 (13.79) | 9 (7.76) |
| 3 | 0 | 0 | 13 (44.83) | 79 (68.1) |
| 4 | 0 | 0 | 1 (3.45) | 18 (15.52) |
| Stage [n (%)] | ||||
| IIIB | 13 (5.42) | 9 (8.74) | 0 | 13 (9.7) |
| IV | 227 (94.58) | 94 (91.26) | 51 (100) | 121 (90.3) |
NSCLC = non-small-cell lung cancer; ECOG PS = Eastern Cooperative Oncology Group performance status.
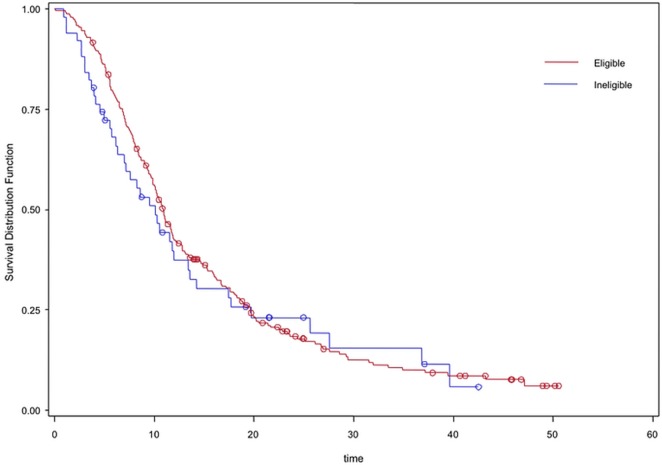
As in scenario A, survival for the patients who received systemic therapy was similar whether they were trial-eligible or -ineligible (10.9 months vs. 10.1 months, p = 0.57). However, compared with ineligible untreated patients, the eligible untreated patients experienced significantly better survival (4.9 months vs. 3.5 months, p < 0.001); however, the difference was less dramatic than in scenario A (Table iv, Figure 2).
FIGURE 2.
Kaplan–Meier survival curves for trial eligibility scenario B, reflecting treated eligible (red) and treated ineligible (blue) patients.
Importantly, despite using the relaxed criteria in scenario B, median os was superior in the eligible treated patients compared with the eligible untreated patients (10.9 months vs. 4.9 months, p < 0.0001).
Scenario B included patients with brain metastasis (n = 96), 60 of whom were treated with systemic therapy, and 36 of whom were not. The survival analysis showed superior os in treated compared with untreated patients with brain metastasis (10.0 months vs. 5.0 months, p = 0.001).
Scenario Comparison
Statistically, the median os for ineligible patients treated in scenarios A and B did not differ (10.2 months vs. 10.1 months respectively, p = 0.83). A detailed multivariate analysis of the overall cohort was previously published29. That analysis indicated that omission of chemotherapy, poor performance status, and weight loss greater than 5% are associated with poor os.
DISCUSSION
Patients with nsclc represent about 87% of all patients diagnosed with lung cancer30, and about 40% of that group present with stage iv disease31. Our results demonstrate that, whether trial-eligible or not, if patients are considered by their treating physicians to be fit for systemic therapy, they experience similar os. That finding has not been well described before. Using a simple yet logical concept, we were able to identify important clinical findings. Our study shows clearly that even trial-ineligible patients derive clinical benefit from chemotherapy. That observation highlights questions about the usefulness of strict eligibility criteria in clinical trials. Given similar survival in treated patients, whether trial-eligible or not, it could be argued that the physician’s judgment is as effective as trial eligibility criteria for anticipating benefit from therapy, and therefore trial eligibility criteria could be relaxed.
The stricter of our trial eligibility scenarios (scenario A) had only 4 criteria, but they were enough to exclude 73% of patients. We were limited by the data points collected, but presumably, if more extensive criteria had been applied to the dataset, more and more patients would have been excluded. That 73% is close to what has been reported previously32,33. In fact, a study showed that the average number of trial eligibility criteria was about 2319, making it even more difficult to find eligible participants to enrol in clinical trials. Surprisingly, two studies showed that half of all exclusion criteria in clinical trials might not be backed by strong clinical evidence19,34,35.
Eligibility criteria are commonly used to achieve more homogenous populations and to minimize the chance that confounding factors will affect trial results. However, some of the exclusions are seemingly unwarranted, and variety in the enrolled patients might not significantly affect results. There is increasing evidence that patients with a ps of 2, although having a poorer prognosis than those with a ps of 0–1, can still derive a significant survival advantage from systemic therapy36–38. In addition, diagnosis of a prior malignancy in advanced lung cancer patients might not be relevant, with one manuscript failing to report worse survival for such patients compared with their counterparts not having a prior diagnosis22. Furthermore, the presence of brain metastasis remains an exclusion criterion in many ongoing clinical trials—or in others, at least requires that central nervous system–directed therapy be given39. That criterion has clinical implications when patients present with asymptomatic millimetric central nervous system disease that might have little immediate clinical relevance, but that would require time-consuming brain radiotherapy (with its associated risks and short-term toxicities)—and a mandated radiation washout period—before the patient could subsequently enrol in a trial.
Although the present manuscript concentrates on the effect of inclusion and exclusion criteria, there are, of course, other major factors that limit clinical trial enrolment. Those factors include patient participation factors (for example, worry about uncertainty), physician participation factors (for example, problems complying with the protocol), and other factors such as the cost of clinical trials, legislation, and public health policies40. A comprehensive effort to increase trial enrolment would address all those factors.
The limitations of our study include its single-centre nature and its retrospective design, which meant that the data available for collection were limited to what had been recorded in the patient chart during management. The limited data led to the small number of eligibility criteria used for the study scenarios, unlike the case of a real clinical trial. Given its retrospective nature, our study could not provide prospective data about quality of life and treatment-related toxicity; however, for this same cohort of patients, we were able to show that scores from the Edmonton Symptom Assessment System were able to predict survival, as published in a separate paper41. Furthermore, our cohort did not include hospitalized patients, and it largely included patients managed before reflexive molecular profiling for EGFR mutations and ALK translocations became a standard of care.
In the last century, strong initiatives set out to have what is called “proportionality” in clinical trials. “Proportionality” meant enrolling participants of different races and ages to mirror the general distribution of the cancer patient population42,43. We would argue that ongoing initiatives are needed to further that process by reviewing trial eligibility criteria. Seeking to include patients with poorer performance status, brain metastasis, prior malignancy, or significant organ impairment should help not only to increase trial accrual, but also to make results more applicable to a general lung cancer population. The U.S. Food and Drug Administration, the American Society of Clinical Oncology, and the Friends of Cancer Research have launched an initiative to modernize clinical trial eligibility. The initiative is “designed to identify opportunities where eligibility criteria could be broadened, and ultimately influence investigators and sponsors to adjust these criteria where clinically appropriate”44,45.
CONCLUSIONS
The generalizability of clinical trial results can be questioned because of the high selectivity that results from restrictive eligibility criteria. Our research raises questions about whether simple clinical judgment and limited criteria could be as effective, but lead to improvements in clinical trial access and broad application of the results. We advocate a consideration of relaxed eligibility criteria to better represent the wider lung cancer patient community. Another option is to use the concept of “large simple trials.”
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare that we have none.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Stewart BW, Wild CW, editors. World Cancer Report 2014. Lyon, France: International Agency for Research on Cancer; 2015. [Google Scholar]
- 3.Allemani C, Weir HK, Carreira H, et al. Global surveillance of cancer survival 1995–2009: analysis of individual data for 25,676,887 patients from 279 population-based registries in 67 countries (concord-2) Lancet. 2015;385:977–1010. doi: 10.1016/S0140-6736(14)62038-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paskett ED, Cooper MR, Stark N, et al. Clinical trial enrollment of rural patients with cancer. Cancer Pract. 2002;10:28–35. doi: 10.1046/j.1523-5394.2002.101006.x. [DOI] [PubMed] [Google Scholar]
- 5.Lara PN, Jr, Higdon R, Lim N, et al. Prospective evaluation of cancer clinical trial accrual patterns: identifying potential barriers to enrollment. J Clin Oncol. 2001;19:1728–33. doi: 10.1200/JCO.2001.19.6.1728. [DOI] [PubMed] [Google Scholar]
- 6.Fenton L, Rigney M, Herbst RS. Clinical trial awareness, attitudes, and participation among patients with cancer and oncologists. Community Oncol. 2009;6:207–28. doi: 10.1016/S1548-5315(11)70546-0. [DOI] [Google Scholar]
- 7.Douillard JY, Rosell R, De Lena M, et al. Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage ib–iiia non-small-cell lung cancer (Adjuvant Navelbine International Trialist Association [anita]): a randomised controlled trial. Lancet Oncol. 2006;7:719–27. doi: 10.1016/S1470-2045(06)70804-X. [DOI] [PubMed] [Google Scholar]
- 8.Pignon JP, Tribodet H, Scagliotti GV, et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the lace collaborative group. J Clin Oncol. 2008;26:3552–9. doi: 10.1200/JCO.2007.13.9030. [DOI] [PubMed] [Google Scholar]
- 9.Butts CA, Ding K, Seymour L, et al. Randomized phase iii trial of vinorelbine plus cisplatin compared with observation in completely resected stage ib and ii non-small-cell lung cancer: updated survival analysis of jbr-10. J Clin Oncol. 2010;28:29–34. doi: 10.1200/JCO.2009.24.0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shepherd FA, Dancey J, Ramlau R, et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol. 2000;18:2095–103. doi: 10.1200/JCO.2000.18.10.2095. [DOI] [PubMed] [Google Scholar]
- 11.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373:123–35. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Howerton MW, Gibbons MC, Baffi CR, et al. Provider roles in the recruitment of underrepresented populations to cancer clinical trials. Cancer. 2007;109:465–76. doi: 10.1002/cncr.22436. [DOI] [PubMed] [Google Scholar]
- 13.Friedman MA, Cain DF. National Cancer Institute sponsored cooperative clinical trials. Cancer. 1990;65(suppl):2376–82. doi: 10.1002/1097-0142(19900515)65:10+<2376::AID-CNCR2820651504>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 14.Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: race-, sex-, and age-based disparities. JAMA. 2004;291:2720–6. doi: 10.1001/jama.291.22.2720. [DOI] [PubMed] [Google Scholar]
- 15.Cottin V, Arpin D, Lasset C, et al. Small-cell lung cancer: patients included in clinical trials are not representative of the patient population as a whole. Ann Oncol. 1999;10:809–15. doi: 10.1023/A:1008399831512. [DOI] [PubMed] [Google Scholar]
- 16.Sateren WB, Trimble EL, Abrams J, et al. How sociodemographics, presence of oncology specialists, and hospital cancer programs affect accrual to cancer treatment trials. J Clin Oncol. 2002;20:2109–17. doi: 10.1200/JCO.2002.08.056. [DOI] [PubMed] [Google Scholar]
- 17.Vardy J, Tannock IF. Quality of cancer care. Ann Oncol. 2004;15:1001–6. doi: 10.1093/annonc/mdh275. [DOI] [PubMed] [Google Scholar]
- 18.Somer RA, Sherman E, Langer CJ. Restrictive eligibility limits access to newer therapies in non-small-cell lung cancer: the implications of Eastern Cooperative Oncology Group 4599. Clin Lung Cancer. 2008;9:102–5. doi: 10.3816/CLC.2008.n.015. [DOI] [PubMed] [Google Scholar]
- 19.Begg CB, Engstrom PF. Eligibility and extrapolation in cancer clinical trials. J Clin Oncol. 1987;5:962–8. doi: 10.1200/JCO.1987.5.6.962. [DOI] [PubMed] [Google Scholar]
- 20.Garcia S, Bisen A, Yan J, et al. Thoracic oncology clinical trial eligibility criteria and requirements continue to increase in number and complexity. J Thorac Oncol. 2017;12:1489–95. doi: 10.1016/j.jtho.2017.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fuks A, Weijer C, Freedman B, Shapiro S, Skrutkowska M, Riaz A. A study in contrasts: eligibility criteria in a twenty-year sample of nsabp and pog clinical trials. National Surgical Adjuvant Breast and Bowel Program. Pediatric Oncology Group. J Clin Epidemiol. 1998;51:69–79. doi: 10.1016/S0895-4356(97)00240-0. [DOI] [PubMed] [Google Scholar]
- 22.Laccetti AL, Pruitt SL, Xuan L, Halm EA, Gerber DE. Effect of prior cancer on outcomes in advanced lung cancer: implications for clinical trial eligibility and accrual. J Natl Cancer Inst. 2015;107:pii. doi: 10.1093/jnci/djv002. djv002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gerber DE, Laccetti AL, Xuan L, Halm EA, Pruitt SL. Impact of prior cancer on eligibility for lung cancer clinical trials. J Natl Cancer Inst. 2014;106:pii. doi: 10.1093/jnci/dju302. dju302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCusker J, Wax A, Bennett JM. Cancer patient accessions into clinical trials: a pilot investigation into some patient and physician determinants of entry. Am J Clin Oncol. 1982;5:227–36. doi: 10.1097/00000421-198204000-00072. [DOI] [PubMed] [Google Scholar]
- 25.Gonzalez LE, Sutton SK, Pratt C, Gilbertson M, Antonia S, Quinn GP. The bottleneck effect in lung cancer clinical trials. J Cancer Educ. 2013;28:488–93. doi: 10.1007/s13187-013-0491-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kotwall CA, Mahoney LJ, Myers RE, DeCoste L. Reasons for non-entry in randomized clinical trials for breast cancer: a single institutional study. J Surg Oncol. 1992;50:125–9. doi: 10.1002/jso.2930500215. [DOI] [PubMed] [Google Scholar]
- 27.Lee JY, Breaux SR. Accrual of radiotherapy patients to clinical trials. Cancer. 1983;52:1014–16. doi: 10.1002/1097-0142(19830915)52:6<1014::AID-CNCR2820520614>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 28.Simon MS, Du W, Flaherty L, et al. Factors associated with breast cancer clinical trials participation and enrollment at a large academic medical center. J Clin Oncol. 2004;22:2046–52. doi: 10.1200/JCO.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 29.Brule SY, Al-Baimani K, Jonker H, et al. Palliative systemic therapy for advanced non–small cell lung cancer: investigating disparities between patients who are treated versus those who are not. Lung Cancer. 2016;97:15–21. doi: 10.1016/j.lungcan.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 30.Govindan R, Page N, Morgensztern D, et al. Changing epidemiology of small–cell lung cancer in the United States over the last 30 years: analysis of the Surveillance, Epidemiologic, and End Results database. J Clin Oncol. 2006;24:4539–44. doi: 10.1200/JCO.2005.04.4859. [DOI] [PubMed] [Google Scholar]
- 31.Morgensztern D, Ng SH, Gao F, Govindan R. Trends in stage distribution for patients with non–small cell lung cancer: a National Cancer Database survey. J Thorac Oncol. 2010;5:29–33. doi: 10.1097/JTO.0b013e3181c5920c. [DOI] [PubMed] [Google Scholar]
- 32.Horn L, Keedy VL, Campbell N, et al. Identifying barriers associated with enrollment of patients with lung cancer into clinical trials. Clin Lung Cancer. 2013;14:14–18. doi: 10.1016/j.cllc.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 33.Vardy J, Dadasovich R, Beale P, Boyer M, Clarke SJ. Eligibility of patients with advanced non–small cell lung cancer for phase iii chemotherapy trials. BMC Cancer. 2009;9:130. doi: 10.1186/1471-2407-9-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van Spall HG, Toren A, Kiss A, Fowler RA. Eligibility criteria of randomized controlled trials published in high-impact general medical journals: a systematic sampling review. JAMA. 2007;297:1233–40. doi: 10.1001/jama.297.11.1233. [DOI] [PubMed] [Google Scholar]
- 35.Gerber DE, Pruitt SL, Halm EA. Should criteria for inclusion in cancer clinical trials be expanded? J Comp Eff Res. 2015;4:289–91. doi: 10.2217/cer.15.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gajra A, Marr AS, Ganti AK. Management of patients with lung cancer and poor performance status. J Natl Compr Canc Netw. 2014;12:1015–25. doi: 10.6004/jnccn.2014.0098. [DOI] [PubMed] [Google Scholar]
- 37.Schluckebier L, Garay OU, Zukin M, Ferreira CG. Carboplatin plus pemetrexed offers superior cost-effectiveness compared to pemetrexed in patients with advanced non–small cell lung cancer and performance status 2. Lung Cancer. 2015;89:274–9. doi: 10.1016/j.lungcan.2015.06.015. [DOI] [PubMed] [Google Scholar]
- 38.Zukin M, Barrios CH, Pereira JR, et al. Randomized phase iii trial of single-agent pemetrexed versus carboplatin and pemetrexed in patients with advanced non-small-cell lung cancer and Eastern Cooperative Oncology Group performance status of 2. J Clin Oncol. 2013;31:2849–53. doi: 10.1200/JCO.2012.48.1911. [DOI] [PubMed] [Google Scholar]
- 39.McCoach CE, Berge EM, Lu X, Baron AE, Camidge DR. A brief report of the status of central nervous system metastasis enrollment criteria for advanced non–small cell lung cancer clinical trials: a review of the ClinicalTrials.gov trial registry. J Thorac Oncol. 2016;11:407–13. doi: 10.1016/j.jtho.2015.10.024. [DOI] [PubMed] [Google Scholar]
- 40.Tournoux C, Katsahian S, Chevret S, Levy V. Factors influencing inclusion of patients with malignancies in clinical trials. Cancer. 2006;106:258–70. doi: 10.1002/cncr.21613. [DOI] [PubMed] [Google Scholar]
- 41.McGee SF, Zhang T, Jonker H, et al. The impact of baseline Edmonton Symptom Assessment Scale Scores on treatment and survival in patients with advanced non-small-cell lung cancer. Clin Lung Cancer. 2018;19:e91–9. doi: 10.1016/j.cllc.2017.05.018. [DOI] [PubMed] [Google Scholar]
- 42.Tejeda HA, Green SB, Trimble EL, et al. Representation of African-Americans, Hispanics, and whites in National Cancer Institute cancer treatment trials. J Natl Cancer Inst. 1996;88:812–16. doi: 10.1093/jnci/88.12.812. [DOI] [PubMed] [Google Scholar]
- 43.Harris DJ, Douglas PS. Enrollment of women in cardiovascular clinical trials funded by the National Heart, Lung, and Blood Institute. N Engl J Med. 2000;343:475–80. doi: 10.1056/NEJM200008173430706. [DOI] [PubMed] [Google Scholar]
- 44.Kim ES, Bernstein D, Hilsenbeck SG, et al. Modernizing eligibility criteria for molecularly driven trials. J Clin Oncol. 2015;33:2815–20. doi: 10.1200/JCO.2015.62.1854. [DOI] [PubMed] [Google Scholar]
- 45.American Society of Clinical Oncology (asco) Initiative to modernize eligibility criteria for clinical trials launched [Web page] Alexandria, VA: ASCO; 2016. [Available at: https://www.asco.org/advocacy-policy/asco-in-action/initiative-modernize-eligibility-criteria-clinical-trials-launched; 17 May 2016] [Google Scholar]