Abstract
Background
Despite advances in systemic therapy choices for patients with early-stage breast cancer, optimal practices for intravenous (IV) access remain unknown. That lack of knowledge holds particularly true for the use of central venous access devices (cvads) such as peripherally inserted central catheters (piccs) and implanted vascular access devices (ports).
Methods
Using a survey of Canadian oncologists and oncology nurses responsible for the care of breast cancer patients, we evaluated current access practices, perceptions of complications, and perceptions of risk, and we estimated complication rates and evaluated perceived risk factors for lymphedema.
Results
Survey responses were received from 25 physicians and 57 oncology nurses. Administration of trastuzumab or an anthracycline was associated with a higher likelihood of a cvad being recommended. Other factors associated with recommendation of a cvad included prior difficult IV access and a recommendation from the chemotherapy nurse. Although the complication rates perceived to be associated with the use of piccs and ports remained high, respondents felt that cvads might improve patient quality of life. Risk factors perceived to be associated with the risk of lymphedema were axillary lymph node dissection, radiation to the axilla, and line-associated infection. Factors known to be unrelated to lymphedema risk (specifically, blood draws and blood pressure measurement) continue to be perceived as posing a higher risk.
Conclusions
Despite widespread use of chemotherapy for patients with breast cancer, the type of venous access used for treatment varies significantly, as do perceptions about the risks of cvad use and the risk for lymphedema development. Further prospective studies are needed to identify best-practice strategies.
Keywords: Early-stage breast cancer, physician surveys, nurse surveys, vascular access
BACKGROUND
Systemic chemotherapy for early-stage breast cancer is in widespread use. Yet despite changes in its nature (that is, less anthracycline use) and duration (for example, trastuzumab administration every 3 weeks for 1 year), optimal practices for intravenous (IV) access remain unknown1,2.
Broadly, IV therapies can be administered through a peripheral IV access inserted into a vein in the arm during each visit to the chemotherapy unit and removed before the patient returns home, or through central venous access devices (cvads) such as peripherally inserted central catheters (piccs) and implanted vascular access devices (“ports”). A picc is a percutaneous central line that is inserted into the upper arm and that stays in the arm for the entire duration of the chemotherapy. It is easily removed after chemotherapy treatment is complete. A port is a reservoir that is surgically placed under the skin in the chest. Its removal after systemic therapy is finished is more complex3. These intravenous access techniques differ significantly in terms of morbidity and cost to the health care system. The lack of knowledge about optimal access is reflected in surveys of patients4 and in an ongoing systematic review, which shows that breast cancer–specific data are minimal and prospective clinical trial data directly comparing the various forms of vascular access for chemotherapy in breast cancer are absent (Robinson A. Personal communication).
Each of the IV administration routes has its own merits and complications that have to be weighed for each individual patient and systemic regimen being delivered. Thus, although peripheral IV access means that minimal follow-up care is required, risks for peripheral phlebitis and chemotherapy extravasation (that is, accidental leakage into surrounding tissues rather than containment in the blood vessel) are increased. On the other hand, the use of a picc or port is thought to reduce the risk of extravasation, to ensure reliable access for infusion, to improve patient satisfaction, and to eliminate the long-term effects on peripheral veins that can be damaged by the administration of vesicant drugs5. However, cvads are also associated with an increased risk of thrombotic and infectious complications6–9.
Given uncertainty about best practices within the national and international oncology community3,10–15, we surveyed oncology nurses and physicians with the aim of evaluating how medical professionals decide on venous access methods. The survey was also designed to assess practice variability with respect to the modern systemic therapy regimens and perceived rates of complications for the various access techniques. In addition, given the continued presence of various “urban legends” reported by breast cancer patients about the risk for lymphedema being higher with the use of the surgical arm for blood draws, chemotherapy administration, and blood pressure measurement4, our survey also contained questions about risk factors for the development of lymphedema16. The findings emerging from the survey will be used to inform knowledge users about potential future prospective randomized trials aiming to optimize vascular access strategies in patients with early-stage breast cancer.
METHODS
Questionnaire Design and Distribution
The survey was developed by clinicians and researchers with expertise in medical oncology, epidemiology, knowledge translation, and survey design. To reflect the different and common roles that medical oncologists and oncology nurses have in patient management, separate surveys were designed for those two groups. Recruitment for both surveys ran from March to June 2016. All surveys and related documentation were reviewed and approved by the Ottawa Health Science Network Research Ethics Board. Participant consent was implied upon completion of the survey. The primary purpose of the study was to identify, for both nurses and physicians, current practices and perceived challenges and risks associated with the use of peripheral- and central-line access in early-stage breast cancer patients. A secondary objective was to determine the perceived risk factors for lymphedema.
Survey of Medical Oncologists
Practicing Canadian oncologists who were known to treat breast cancer, whose e-mail addresses were publically available, and who had been involved in previous surveys of this type17,18, were sent electronic invitations through Fluid Surveys (http://www.fluidsurveys.com). Using a modified Dillman technique, reminders were sent by e-mail to non-responders at 2-week intervals for 4 mail-outs19. If physicians did not want to participate electronically, the option of a paper survey was given. No financial or other incentive was offered for completion of the survey. Only physicians whose self-reported practice involved the initiation of chemotherapy for breast cancer patients were considered. The oncologist survey consisted of a 10-item questionnaire, including questions about demographics and clinical experience (supplementary Appendix 1). The questions explored the factors that influence a recommendation for peripheral or central access, the percentage of patients for whom central access is recommended upfront, the perceived risks of complications, and the current wait times for insertion. The perceived risk factors for the development of postsurgical lymphedema were examined. Most questions were formulated to require a multiple-choice response or a number or degree of importance as a response.
Survey of Oncology Nurses
Chemotherapy, breast cancer clinic, and oncology ward nurses at 3 Canadian cancer centres (The Ottawa Hospital Cancer Center, the Irving Greenberg Family Cancer Centre, and the Cancer Centre of Southeastern Ontario) were approached by their respective nurse managers. Nurse managers were individually approached by members of the research team. Information sheets were handed out to interested nurses, who then contacted the research team to complete a 13-item paper-based questionnaire (supplementary Appendix 2). If nurses were not interested in completing the survey, they did not have to contact the research team. Nurses were asked questions about their demographics and clinical experience. Questions also solicited their most accurate description of current practice trends in the use of central venous access, perceived rates of complications, and risk factors for the development of lymphedema.
Statistical Analysis
Both surveys consisted of close-ended multiple-choice and hybrid questions (that is, rate the importance or provide numbers), which are summarized descriptively as proportions with their 95% confidence intervals. The analysis was conducted in Microsoft Excel 2010 (Microsoft Corporation, Redmond, WA, U.S.A.). No formal hypotheses were tested. Data from the physician and nurse surveys were analyzed separately. Our group has extensive experience in performing surveys of knowledge users, and therefore the study had no predefined sample size. The response rates and findings from the surveys were summarized individually in tables and narratively compared.
RESULTS
Respondent Demographics
The survey response rate for physicians was 93% (25 responses of 27 requests), with 20 responses (80%) coming from an academic cancer centre; 4 (16%), from a community cancer centre; and 1 (4%), from “other.” Respondents reported treating an average of 22 (range: 6–65) new breast cancer patients monthly (Table i).
TABLE I.
Respondent characteristics
| Characteristic | Value |
|---|---|
| Physicians (n) | 25 |
| Province, Ontario [n (%)] | 25 (100) |
| Centre [n (%)] | |
| Academic | 20 (80) |
| Community | 4 (16) |
| Other | 1 (4) |
| Chemotherapy initiation [n (%)] | 25 (100) |
| New breast cancer patients per month (n) | |
| Median | 22 |
| Range | 6–65 |
| Nurses | 57 |
| Province, Ontario [n (%)] | 57 (100) |
| Role [n (%)]a | |
| Chemotherapy nurse | 31 (54) |
| Oncology clinic nurse | 20 (35) |
| Oncology ward nurse | 10 (18) |
| Years of experience | |
| Median | 9 |
| Range | 2–28 |
| Breast cancer patients per week | |
| Median | 22 |
| Range | 1–28 |
Four respondents reported more than 1 nursing role.
The 57 surveys completed and returned by oncology nurses included 31 (54%) from chemotherapy nurses, 20 (35%) from oncology clinic nurses, and 10 (18%) from oncology ward nurses. More than one nursing role was reported by 4 nurses. Respondents had an average of 9 years of oncology experience (range: 2–28 years) and reported seeing an average of 22 breast cancer patients weekly (range: 1–28) during the course of their clinical responsibilities (Table i).
Who Decides the Type of Venous Access That Patients Receive?
Physicians and nurses were asked to select all parties involved in the selection of a vascular access method. Shared decision-making was acknowledged in the design of the survey by allowing respondents to select all responses that applied. Oncologists most often reported themselves as the person responsible for making a decision about the type of venous access (22/25, 88%), but also listed chemotherapy nurses (19/25, 76%), patients (16/25, 64%), and clinic nurses (14/25, 56%) as being involved in a shared decision-making process. Similarly, nurses reported that the person most likely to make the decision was the medical oncologist (46/57, 81%), but they also listed patient preference (35/57, 61%), clinic nurses (33/57, 58%), and chemotherapy nurses (28/57, 49%) as other parties involved in the decision-making process.
What Effect Does the Chemotherapy Regimen Have on the Choice of Venous Access?
Oncologists were asked to specify the percentage of patients receiving a range of commonly used chemotherapy regimens for whom they would recommend either peripheral access, a picc, or a port. In parallel, nurses were asked to report real-time observations of access methods used (Figure 1). A second question then asked nurses to select their personal preference for vascular access based on the same chemotherapy regimens.
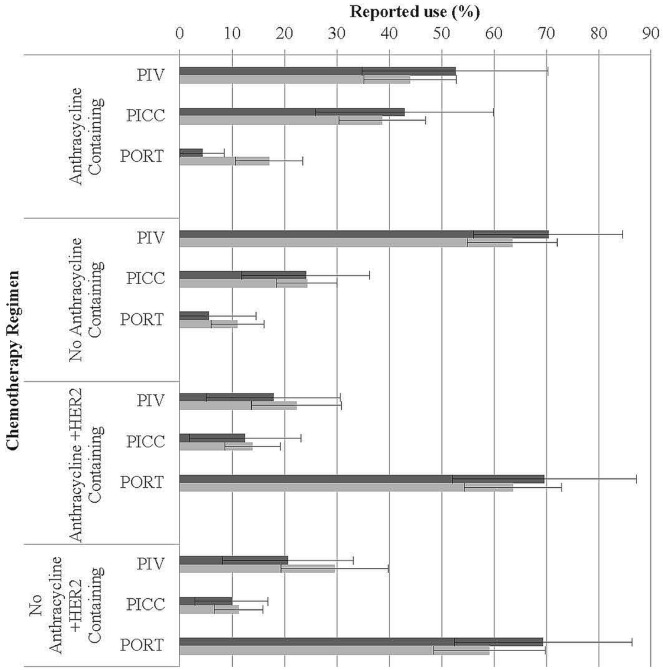
FIGURE 1.
Effect of chemotherapy regimen on method of vascular access selected by physicians and rates observed by nurses (bars represent the 95% confidence interval). PIV = peripheral intravenous; PICC = peripherally inserted central catheter; PORT = surgically implanted central catheter.
Choice of Access for Non-Trastuzumab-Containing Regimens
For non-trastuzumab-containing regimens that included both an anthracycline and a taxane (for example, doxorubicin–cyclophosphamide–paclitaxel, fluorouracil–epirubicin–cyclophosphamide followed by docetaxel), physicians selected peripheral IV in 53% of patients, a picc in 43%, and a port in 4%. For the same patients, the nurse respondents reported the use of a peripheral IV, a picc, and a port in 44%, 39%, and 16% of patients respectively. For non-anthracycline regimens such as docetaxel–cyclophosphamide and cyclophosphamide–methotrexate–fluorouracil, physicians selected a peripheral IV in 70%, a picc in 24%, and a port in 6% of cases. Nurses reported observed rates of 64% for peripheral IVs, 24% for piccs, and 11% for ports (Figure 1).
When nurses were asked for their opinion about the type of access that they would select for all non-trastuzumab-containing regimens, 19% (11/57) selected a peripheral IV; 47% (27/57), a picc; and 32% (18/57), a port. The reasons elicited for their choice related to improved quality of life (27/57, 47%) and fewer attempts at venipuncture (22/57, 39%) with cvads.
Choice of Access for Trastuzumab-Containing Regimens
When trastuzumab was added to the regimens already described, a port was selected by 70% of physicians for anthracycline-containing regimens and by 69% for non-anthracycline-containing regimens. A port was observed by nurses in 64% of anthracycline-containing regimens and in 59% of non-anthracycline regimens (Figure 1).
When nurses were asked about the type of access that they would select for trastuzumab-containing regimens, 81% (46/57) selected a port, with 42% (24/57) reporting that they chose it for its long-term nature, and 39% (22/57), for the freedom to perform activities of daily living without limitation.
What Factors Affect the Physician Choice of Venous Access?
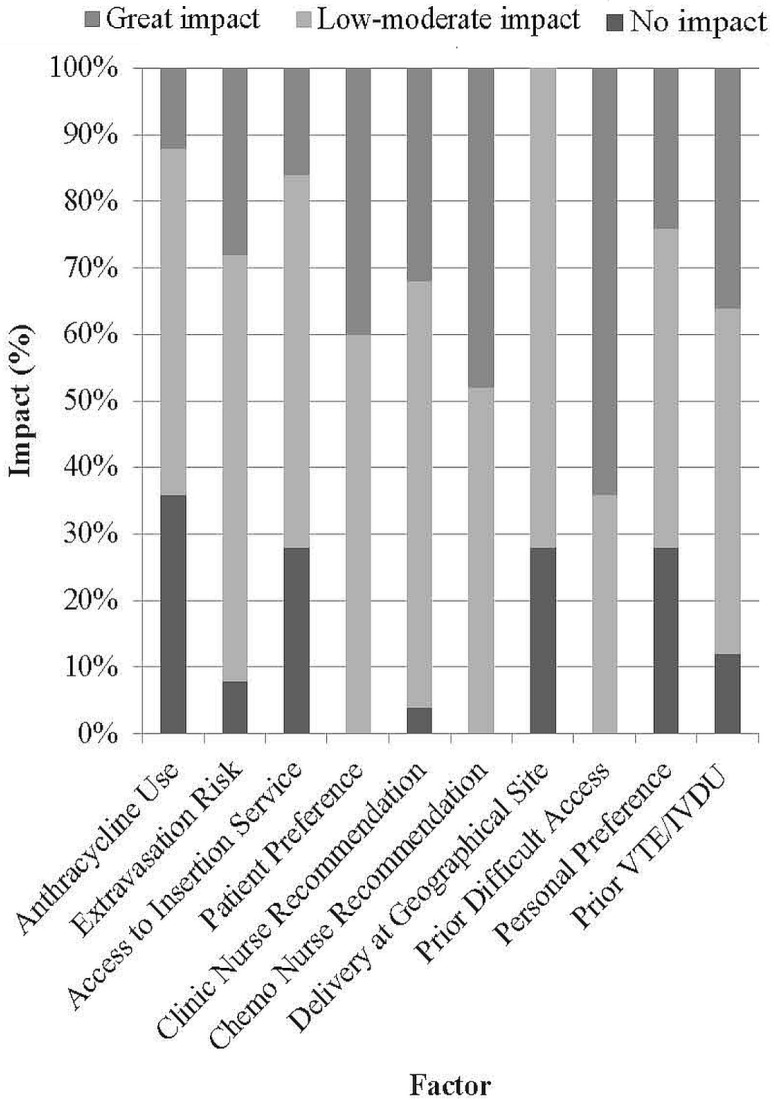
Oncologists were asked to rate the impact that a range of factors had on their decision to request a cvad (Figure 2). Factors most often reported as having a great impact were prior difficult IV access (16/25, 64%) and recommendation from the chemotherapy nurse if access was deemed problematic (12/25, 48%). Factors most often reported to have a moderate impact included the geographic site of chemotherapy delivery (18/25, 72%), clinic nurse recommendation (16/25, 64%), risk of extravasation with a chemotherapy regimen (16/25, 64%), patient preference (15/25, 60%), access to central line insertion services (14/25, 56%), anthracycline administration (13/25, 52%), patient factors such as prior thromboembolic disease or history of IV drug use (13/25, 52%), and personal preference (12/25, 48%). None of the factors in the survey were frequently selected as having no impact.
FIGURE 2.
Physician-reported factors influencing the venous access method. VTE = venous thromboembolism; IVDU = intravenous drug use.
What Is the Self-Reported Proficiency of Nurses for Peripheral IV Insertion?
Of the 57 nurse respondents, 53 indicated that they insert peripheral IVs for chemotherapy administration. With respect to proficiency, none of them self-rated as a novice, with 5% (3/57) self-rating as an advanced beginner; 21% (12/57), as competent; 23% (13/57), as proficient; and 44% (25/57), as expert. Nurses were also asked about the percentage of patients that they felt had “bad veins” and reported that an average of 33% of patients seen before chemotherapy had “bad veins.”
What Is the Perceived Incidence of Complications?
Medical oncologists and oncology nurses were both asked about the perceived incidence of complications with the various types of venous access (Table ii). Of the listed complications, sclerosis of the veins (31%) and risk of extravasation (7%–17%) were reported for peripheral IVs; no rates were reported for piccs and ports. The rate of delay in commencing chemotherapy was reported as 10% by physicians and 7% by nurses for a picc and as 26% and 8% respectively for a port. On average, physicians reported a wait time of 7 days for a picc and 17 days for a port. Access-related infections were reported by 7% of physicians and 13% of nurses for piccs, and by 6% of both groups for ports. Finally, catheter-associated thrombosis rates were reported as 9% by oncologists and 18% by nurses for piccs, and as 6% and 10% respectively for ports.
TABLE II.
Perceived complications with various methods of venous access
| Complication | Respondents (n) | Perceived to affect patients with ... | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| PIV | PICC | PORT | |||||
|
|
|
|
|||||
| (%) | (SE) | (%) | (SE) | (%) | (SE) | ||
| Delay initiation of chemotherapy | |||||||
| Physicians | 25 | 5 | 3 | 10 | 3 | 26 | 13 |
| Nurses | 48 | 8 | 5 | 7 | 3 | 8 | 4 |
| Extravasation | |||||||
| Physicians | 25 | 7 | 2 | <1 | 1 | <1 | 1 |
| Nurses | 48 | 17 | 8 | 1 | 1 | 1 | 1 |
| Access device–related infection (skin or line infection) | |||||||
| Physicians | 25 | 3 | 2 | 7 | 2 | 6 | 1 |
| Nurses | 46 | 7 | 4 | 13 | 4 | 6 | 2 |
| Catheter-associated thrombosis | |||||||
| Physicians | 25 | <1 | 1 | 9 | 3 | 6 | 3 |
| Nurses | 46 | 3 | 1 | 18 | 5 | 10 | 3 |
| Sclerosis of veins for future use | |||||||
| Physicians | 25 | 31 | 8 | 4 | 2 | 3 | 2 |
| Nurses | 0 | NA | NA | NA | NA | NA | NA |
| Need for ongoing maintenance (for example, flushing) | |||||||
| Physicians | 25 | <1 | 1 | 86 | 14 | 86 | 14 |
| Nurses | 0 | NA | NA | NA | NA | NA | NA |
| Complex insertion or removal process | |||||||
| Physicians | 25 | 3 | 2 | 18 | 13 | 46 | 17 |
| Nurses | 0 | NA | NA | NA | NA | NA | NA |
PIV = peripheral intravenous line; PICC = peripherally inserted central catheter; PORT = surgically implanted central catheter; SE = standard error; NA = not applicable.
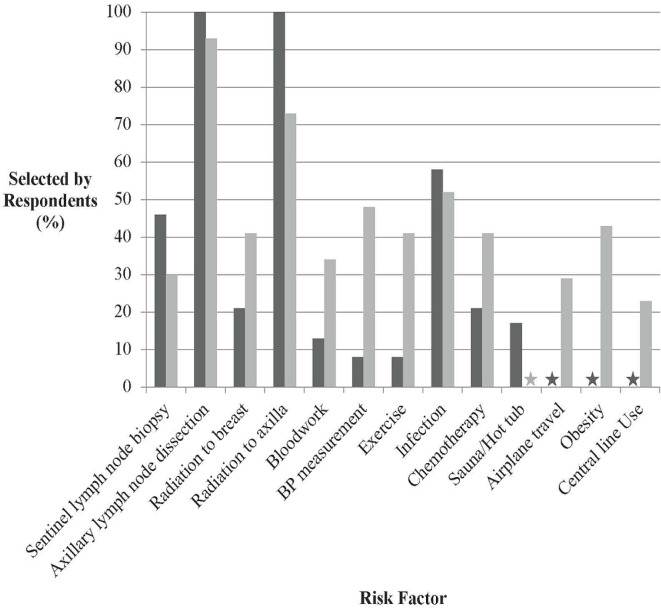
What Are the Risk Factors Associated with Lymphedema?
Although the questions asked in the two groups overlapped considerably, some questions were asked of only one group (Figure 3). The factors selected by physicians and nurses as being most associated with the risk of lymphedema were axillary lymph node dissection (96% physicians, 91% nurses), radiation to the axilla (96% physicians, 72% nurses), and line-associated infections (56% physicians, 51% nurses). Other factors such as blood draws (12% physicians, 33% nurses) and blood pressure measurements (8% physicians, 47% nurses) were also reported as risk factors for lymphedema.
FIGURE 3.
Risk factors perceived to be associated with lymphedema by physicians and nurses. BP = blood pressure.
DISCUSSION
In recent years, major changes have occurred in the types of chemotherapy that breast cancer patients receive20. Those changes have been accompanied by significant equipoise with respect to the chemotherapy regimens and the type of vascular access used to administer them; furthermore, no national or international guidelines guiding decisions about vascular access have been published5,21,22.
The current equipoise is reflected in surveys of patients4 and the absence of prospective clinical trial data directly comparing various forms of vascular access for chemotherapy administration in breast cancer patients. The resulting variability is of great importance, given that cvads are not only expensive, but that all types of vascular access are associated with complications and affect the patient’s quality of life. However, few studies have looked at the optimal type of venous access to use in daily oncology practice. We identified a systematic review evaluating the risks of central lines with respect to infection9 and another evaluating the risks of central lines with respect to venous thromboembolism8. Existing retrospective literature demonstrates that the risk of thrombotic and infectious complications might be increased with central access devices6–8, but those data are prone to many biases and confounders. The rates of complications for those cvads expected by health care professionals (including physicians and chemotherapy nurses) and by the patients themselves also vary because of the lack of prospective data.
Our study suggests that oncologists usually make the final decision about the type of vascular access used to administer chemotherapy. The factors listed as most important include prior difficult IV access and a recommendation from the chemotherapy nurse. Study findings also suggest considerable clinical equipoise with respect to the use of vascular access methods, not only with different chemotherapy regimens, but also with the same chemotherapy regimen. However, trends can be observed: anthracycline- and trastuzumab-containing regimens are preferably administered using a cvad, as selected by physicians and corroborated by nurse observation. Interestingly, when nurses were asked for their opinions about the best type of vascular access, most selected cvads regardless of the use of anthracyclines or trastuzumab, because they believed that those access devices increase quality of life and preserve veins.
Complication rates associated with cvads were appreciated by both oncologists and nurses, although higher rates were estimated by nurses than by physicians. Specifically, the incidence of line infections was estimated as 6% by oncologists and 13% by nurses, when the actual rate is 1%–5%9. The rate of thrombosis associated with cvads was estimated as 6% by oncologists and 18% by nurses, when the actual rate is 7%8. For peripheral IVs, health care professionals estimated the rate of vein sclerosis as 31% (actual incidence unknown)23 and extravasation as 7%–17% (actual incidence: 1%–7%)24. Despite the recognition of those risks, cvads continue to be the preferred route of vascular access for regimens containing trastuzumab and are more frequently chosen for regimens containing an anthracycline. Moreover, our results show that medical oncologists are the main decision-makers when vascular access for chemotherapy administration is being chosen and that the choice is made with little evidence to guide best practice. Further research is needed to guide those decisions.
Finally, lymphedema remains an important side effect associated with treatment for breast cancer. Although a number of studies have confirmed the risk factors for lymphedema, we are not aware of any other surveys that have tried to evaluate ongoing “urban myths” propagated about the risk factors for development of lymphedema. Results of our survey suggest that most oncologists and oncology nurses recognize axillary lymph node dissection, radiation to the axilla, and line-associated infection as risk factors for the development of lymphedema. Obesity was frequently not recognized as a risk factor despite having one of the most significant associations with the development of lymphedema25. More importantly, factors such as blood draws, blood pressure measurements, and air travel, which have been shown not to affect the risk of lymphedema, continue to be perceived as risk factors. Those perceptions lead to patients receiving misleading information and make it clear that further research and education are needed26–28.
Study Limitations
Although the present study targeted oncologists and oncology nurses across Canada, all nurse respondents were health care professionals within a single province, which might limit the generalizability of the results. Furthermore, the survey questions included risk factors identified by a team of experts in the topic; however, the provision of a selected list of expected complications without allowing respondents to elaborate has inherent bias. Finally, although the questions asked of the two groups overlapped considerably, some factors relating to complications from vascular access (sclerosis, need for frequent flushing, complex removal) and to risk factors for lymphedema (obesity, plane travel, high temperatures) were asked of only one group and therefore could not be compared between groups. Ultimately, however, those answers generate more questions for future studies that could evaluate in greater detail the differences in risk perception between oncology physicians and nurses.
CONCLUSIONS
To date, no clear consensus about best practice for the use of vascular access routes has been reached. Very few trials have compared the various strategies—namely, the relative merits and complications of peripheral access, picc, and port; use of those methods is also associated with considerable clinical equipoise. The results of our survey also confirm that urban legends about risk factors for the development of lymphedema continue to be propagated. The results of the present survey and our parallel survey of patients receiving chemotherapy for breast cancer have been used to assist in the appropriate design of ongoing clinical trials (see NCT03132454, NCT02632435, and NCT02688998 at http://ClinicalTrials.gov) that will help to answer those important clinical questions.
Supplemental material available at http://www.current-oncology.com
ACKNOWLEDGMENTS
Funding for this project came from local sources. Funding for two salaries came from the Rethinking Clinical Trials Program through the Canadian Institutes of Health Research’s Strategy for Patient-Oriented Research. We are grateful to all the nurses and physicians who completed the survey.
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare the following interests: SMc holds an advisory role with AngioDynamics. All remaining authors declare that they have no conflicts to disclose.
REFERENCES
- 1.Freytes CO. Indications and complications of intravenous devices for chemotherapy. Curr Opin Oncol. 2000;12:303–7. doi: 10.1097/00001622-200007000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Chopra V, Flanders SA, Saint S, et al. on behalf of the Michigan Appropriateness Guide for Intravenous Catheters Panel The Michigan Appropriateness Guide for Intravenous Catheters (magic): results from a multispecialty panel using the rand/ucla appropriateness method. Ann Intern Med. 2015;163(suppl):S1–40. doi: 10.7326/M15-0744. [DOI] [PubMed] [Google Scholar]
- 3.Alexander M, Corrigan A, Gorski L, Hankins J, Perucca R. Infusion Nursing: An Evidence Based Approach. 3rd ed. St Louis, MO: Saunders–Elsevier; 2010. [Google Scholar]
- 4.LeVasseur N, Stober C, Ibrahim M, et al. Perceptions concerning vascular access for intravenous systemic therapy and risk factors for lymphedema in early-stage breast cancer—a patient survey. Curr Oncol. 2018;25:e305–e310. doi: 10.3747/co.25.3911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gallieni M, Pittiruti M, Biffi R. Vascular access in oncology patients. CA Cancer J Clin. 2008;58:323–46. doi: 10.3322/CA.2008.0015. [DOI] [PubMed] [Google Scholar]
- 6.Piran S, Ngo V, McDiarmid S, Le Gal G, Petrcich W, Carrier M. Incidence and risk factors of symptomatic venous thromboembolism related to implanted ports in cancer patients. Thromb Res. 2014;133:30–3. doi: 10.1016/j.thromres.2013.10.026. [DOI] [PubMed] [Google Scholar]
- 7.Aw A, Carrier M, Koczerginski J, McDiarmid S, Tay J. Incidence and predictive factors of symptomatic thrombosis related to peripherally inserted central catheters in chemotherapy patients. Thromb Res. 2012;130:323–6. doi: 10.1016/j.thromres.2012.02.048. [DOI] [PubMed] [Google Scholar]
- 8.Chopra V, Anand S, Hickner A, et al. Risk of venous thromboembolism associated with peripherally inserted central catheters: a systematic review and meta-analysis. Lancet. 2013;382:311–25. doi: 10.1016/S0140-6736(13)60592-9. [DOI] [PubMed] [Google Scholar]
- 9.Chopra V, O’Horo JC, Rogers MA, Maki DG, Safdar N. The risk of bloodstream infection associated with peripherally inserted central catheters compared with central venous catheters in adults: a systematic review and meta-analysis. Infect Control Hosp Epidemiol. 2013;34:908–18. doi: 10.1086/671737. [DOI] [PubMed] [Google Scholar]
- 10.Hill J, Whynot A, Broadhurst D, et al. Occlusion management guideline for central venous access devices (cvads) Vascular Access. 2013;7(suppl 1):1–34. [Google Scholar]
- 11.Infusion Nurses Society Infusion nursing standards of practice. J Infus Nurs. 2006;29(suppl):S1–92. doi: 10.1097/00129804-200601001-00001. [DOI] [PubMed] [Google Scholar]
- 12.Weinstein S, Hagle M, editors. Plumer’s Principles and Practice of Infusion Therapy. 9th ed. Philadelphia, PA: Wolters Kluwer Health, Lippincott Williams and Wilkins; 2014. [Google Scholar]
- 13.Vescia S, Baumgartner AK, Jacobs VR, et al. Management of venous port systems in oncology: a review of current evidence. Ann Oncol. 2008;19:9–15. doi: 10.1093/annonc/mdm272. [DOI] [PubMed] [Google Scholar]
- 14.Vardy J, Engelhardt K, Cox K, et al. Long-term outcome of radiological-guided insertion of implanted central venous access port devices (cvapd) for the delivery of chemotherapy in cancer patients: institutional experience and review of the literature. Br J Cancer. 2004;91:1045–9. doi: 10.1038/sj.bjc.6602082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fu MR, Deng J, Armer JM. Putting evidence into practice: cancerrelated lymphedema. Clin J Oncol Nurs. 2014;(suppl):68–79. doi: 10.1188/14.CJON.S3.68-79. [DOI] [PubMed] [Google Scholar]
- 16.Greene AK. Myths associated with lymphedema. In: Greene A, Slavin S, Brorson H, editors. Lymphedema. Cham, Switzerland: Springer International Publishing; 2015. [Google Scholar]
- 17.Jacobs C, Clemons M, Mazzarello S, et al. Enhancing accrual to chemotherapy trials for patients with early stage triple-negative breast cancer: a survey of physicians and patients. Support Care Cancer. 2017;25:1881–6. doi: 10.1007/s00520-017-3580-4. [DOI] [PubMed] [Google Scholar]
- 18.Jacobs C, Ibrahim MF, Clemons M, et al. Treatment choices for patients with invasive lobular breast cancer: a doctor survey. J Eval Clin Pract. 2015;21:740–8. doi: 10.1111/jep.12379. [DOI] [PubMed] [Google Scholar]
- 19.Hoddinott SN, Bass MJ. The Dillman total design survey method. Can Fam Physician. 1986;32:2366–8. [PMC free article] [PubMed] [Google Scholar]
- 20.Early Breast Cancer Trialists’ Collaborative Group Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 21.Schiffer CA, Mangu PB, Wade JC, et al. Central venous catheter care for the patient with cancer: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2013;31:1357–70. doi: 10.1200/JCO.2012.45.5733. [DOI] [PubMed] [Google Scholar]
- 22.Sousa B, Furlanetto J, Hutka M, et al. on behalf of the esmo Guidelines Committee Central venous access in oncology: esmo clinical practice guidelines. Ann Oncol. 2015;26(suppl 5):v152–68. doi: 10.1093/annonc/mdv296. [DOI] [PubMed] [Google Scholar]
- 23.Bolton-Maggs P, Flavin A. Epirubicin for breast cancer may cause considerable venous sclerosis. BMJ. 2005;331:816. doi: 10.1136/bmj.38574.659225.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perez Fidalgo JA, Garcia Fabregat L, Cervantes A, et al. on behalf of the esmo Guidelines Working Group Management of chemotherapy extravasation: esmo-eons clinical practice guidelines. Eur J Oncol Nurs. 2012;16:528–34. doi: 10.1016/j.ejon.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 25.Ridner SH, Dietrich MS, Stewart BR, Armer JM. Body mass index and breast cancer treatment–related lymphedema. Support Care Cancer. 2011;19:853–7. doi: 10.1007/s00520-011-1089-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferguson CM, Swaroop MN, Horick N, et al. Impact of ipsilateral blood draws, injections, blood pressure measurements, and air travel on the risk of lymphedema for patients treated for breast cancer. J Clin Oncol. 2016;34:691–8. doi: 10.1200/JCO.2015.61.5948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.United States, Department of Health and Human Services, National Institutes of Health, National Cancer Institute (nci) Lymphedema (PDQ)–Health Professional Version [Web page] Bethesda, MD: NCI; 2015. [Available at: https://www.cancer.gov/about-cancer/treatment/side-effects/lymphedema/lymphedema-hp-pdq; cited 27 June 2017] [Google Scholar]
- 28.Meneses KD, McNees MP. Upper extremity lymphedema after treatment for breast cancer: a review of the literature. Ostomy Wound Manage. 2007;53:16–29. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material available at http://www.current-oncology.com