Abstract
The treatment of hormone-positive breast cancer (bca) is a rapidly evolving field. Improvement in the understanding of the mechanisms of action and resistance to anti-hormonal therapy has translated, in the past decade, into multiple practice-changing clinical trials, with the end result of increased survivorship for patients with all stages of hormone-positive cancer. The primary care physician will thus play an increasing role in the routine care, surveillance, and treatment of issues associated with anti-hormonal therapy. The aim of the present review was to provide a focused description of the issues relevant to primary care, while briefly highlighting recent advances in the field of anti-hormonal therapy.
Key Points
■ Hormone-positive bca is the most prevalent form of bca and, compared with the other subtypes, is usually associated with better survival.
■ Survivorship has significantly increased for all stages of hormone-positive bca, making the primary care physician a key player in the care of affected patients.
■ The two most common classes of anti-hormonal agents used in these patients are selective estrogen receptor modulators and aromatase inhibitors. Each class of medication is associated with signature side effects.
■ Within the past decade, multiple novel estrogen receptor blockers (for example, fulvestrant) and agents aimed at circumventing resistance to endocrine therapy [inhibitors of cyclin-dependent kinase 4/6 and of mtor (the mechanistic target of rapamycin)] have gained clinical ground. Understanding their side effects will be increasingly relevant to primary care physicians.
■ Multidisciplinary care is always encouraged in the care of cancer patients receiving anti-hormonal therapy.
Keywords: Breast cancer, endocrine therapy, primary care, survivorship
INTRODUCTION
Breast cancer (bca) affects 1 in 8 women during their lifetime1. Approximately 3 in 4 of those cancers are positive for either the estrogen or the progesterone receptor, where estrogen and progesterone are the key drivers of carcinogenesis2. Endocrine therapy, which lowers estrogen levels and inhibits the growth of the cancer, remains the mainstay systemic treatment for hormone receptor–positive bca in the adjuvant, metastatic, and (occasionally) neoadjuvant settings. Given increased survivorship and the long duration of treatment exposure in these patients (often 5–10 years), there is an increased need for primary care involvement and collaboration between general practitioners and oncologists3. In the present review, we summarize the latest evidence for the use of endocrine therapy in the adjuvant and metastatic settings, and we provide practical tips for managing the adverse effects of that therapy.
ADJUVANT ENDOCRINE THERAPY FOR BCa
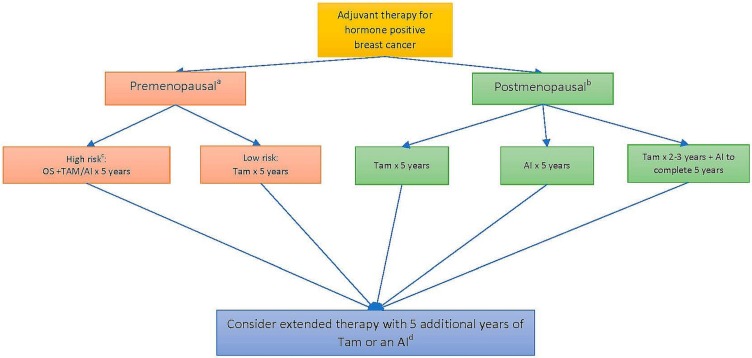
Adjuvant therapy is often decided by the oncologist based on clinical, pathologic, and genetic scoring parameters. Several meta-analyses have demonstrated a consistent benefit for a patient’s survival with the addition of endocrine therapy in hormone receptor–positive nonmetastatic bca. The three classes of agents used are the selective estrogen receptor modulators (such as tamoxifen), the aromatase inhibitors (ais), and ovarian suppression. Figure 1 summarizes the sequencing strategies for those agents, and notes key definitions (for example, the definition of menopause, and low-risk vs. high-risk patients).
FIGURE 1.
Algorithm for choice of endocrine therapy in the adjuvant setting. aMenopause: Defined as any patient less than 60 years of age who previously underwent bilateral oophorectomy or who has not had any menstrual periods for 12 months or more in the absence of tamoxifen, chemotherapy, or ovarian suppression, and whose serum estradiol is in the postmenopausal range or who is amenorrheic on tamoxifen, with follicle-stimulating hormone and serum estradiol in the postmenopausal range. bAny patient 60 years of age or older. cAny patient less than 35 years of age or any premenopausal patient who has received chemotherapy in the adjuvant setting. dAdditional treatments to be decided in conjunction with an oncologist, on a case-by-case basis. OS = ovarian suppression; Tam = tamoxifen; AI = aromatase inhibitor (letrozole, anastrozole, exemestane).
Premenopausal Patients
For premenopausal women at high risk of recurrence, the choice is between tamoxifen and an ai, plus ovarian suppression in high risk cases, based on a discussion with the patient about the benefits and risks.
In the high-risk subgroup (defined as <35 years of age at diagnosis or a need for adjuvant chemotherapy after surgery), the addition of ovarian suppression (whether chemical or surgical) to tamoxifen or exemestane is associated with a 4.5%–7.7% absolute reduction in bca recurrence at 5 years4. For all other premenopausal patients at standard risk, tamoxifen remains the treatment of choice. Tamoxifen is administered once daily as a 20 mg pill for a minimum of 5 years. Compared with placebo, its use is associated with a significant absolute reduction in bca mortality to 23.9% from 33.1% at 15 years (an improvement of 9.2% ± 1.0%), preventing 1 bca death for every 11 patients treated5,6. Notably, ais should not be used as monotherapy in premenopausal women, because they might induce ovarian reactivation and estrogen production, but they can be used in combination therapy with ovarian suppression in high-risk patients. Any patient on ai therapy who experiences a recurrence of their menstrual periods should be promptly evaluated, with measurement of serum estradiol and a referral back to the oncologist.
Postmenopausal Patients
In the postmenopausal setting, the 3 most commonly used ais (which are equally effective) are anastrozole (1 mg daily), letrozole (2.5 mg daily), and exemestane (25 mg daily) administered for 5 years. Compared with tamoxifen, the ais are associated with a reduction in bca recurrence, particularly during years 0–1 [relative risk (rr): 0.64; 95% confidence interval (ci): 0.52 to 0.78] and years 2–4 (rr: 0.80; 95% ci: 0.68 to 0.93)6. In a patient approaching, but not yet having reached a menopausal state, consideration might be given to starting tamoxifen for the first 2–3 years and switching to an ai afterward. Compared with a 5-year course of tamoxifen, the switch strategy is associated with a reduction in bca recurrence during years 2–4 (rr: 0.56; 95% ci: 0.46 to 0.67) and with fewer deaths from bca (rr: 0.84; 95% ci: 0.72 to 0.96). No further benefit for recurrence is observed beyond the treatment period7.
Duration of Therapy
After 5 years of tamoxifen, either continuation of tamoxifen or a switch to an ai for an additional 5 years is effective in reducing the odds of distant recurrence and of new primary bcas, with some evidence suggesting improved bca-free and overall survival8,9. Some, but not all, data suggest that, for women completing 5 years on an ai, an additional 5 years of ai also improves recurrence-free survival, without improvement in overall survival10,11. The absolute benefit of extended therapy is small and must be weighed against the potential side effects of venous thrombosis and endometrial cancer with tamoxifen and of osteoporotic fractures with the ais. A decision for extended treatment should be made in consultation with an experienced oncologist.
SIDE EFFECTS AND HEALTH-RELATED ISSUES WITH ENDOCRINE THERAPY
Each class of anti-endocrine agent has its signature-specific side effects (summarized in Table i).
TABLE I.
Endocrine therapy in the adjuvant setting: benefits and adverse effects and their management
| Therapeutic agent | Benefit | Adverse effects | ||
|---|---|---|---|---|
|
| ||||
| Type | Prevalence | Management | ||
| Tamoxifen | ||||
| When used for 5–10 years in the adjuvant setting, is associated with a 9.2%±1% absolute reduction in breast cancer mortality over 15 years | ||||
| Hot flashes | 40%–80% |
|
||
| Venous thromboembolism (VTE) | Relative increase in VTE by a factor of 2–3 Pulmonary embolism: 0.2% over 5 years |
|
||
| Endometrial cancer | Relative increase by a factor of 2.7 (1.2/1000 patient–years) |
|
||
| Ocular pathologies | Cataract: 3.7% of patients |
|
||
| Fatty liver disease | Up to 33% of patients |
|
||
| Aromatase inhibitors | ||||
| When used for 5–10 years in the adjuvant setting, are associated, compared with tamoxifen, with a reduction in the relative risk for breast cancer recurrence of 36%±13% in year 1 and 20%±12% in years 2–4 | ||||
| Osteoporosis and fractures | Relative increase in fractures of 47%±13%; absolute increase of 2% |
|
||
| Arthralgias, musculoskeletal symptoms | 45%–50% |
|
||
| Sexual dysfunction | Varies with symptoms but loss of libido, vaginal dryness, and dyspareunia each reported in the range of 10%–20% |
|
||
| Cardiovascular disease (CVD) | Similar rate of serious CVD; increased risk of hypertension and hypercholesterolemia |
|
||
SSRI = selective serotonin reuptake inhibitor; SNRI = serotonin and norepinephrine reuptake inhibitor; NSAIDs = nonsteroidal anti-inflammatory drugs.
Up to 94% of patients experience side effects while taking endocrine therapy, and as many as 18% discontinue treatment12. Although most side effects will be managed by the oncologist, an increasing number of patients will present to their primary care physician with the initial complaint. It’s therefore crucial for the general practitioner to be able to identify, target, and institute the multidisciplinary interventions appropriate for the successful treatment of the side effects. Successful treatment will, in turn, maintain patient adherence with their therapy and prevent rare life-threatening complications.
Managing the Side Effects of AIs
AI-Induced Bone Loss
Estrogen deficiency has long been recognized as a risk factor for osteoporosis by increasing bone resorption through osteoclastogenesis. In a meta-analysis of seven trials comparing ais with tamoxifen in postmenopausal women with early-stage bca, use of ais significantly increased the risk of bone fractures (odds ratio: 1.47; 95% ci: 1.34 to 1.61)13. The U.S. National Comprehensive Cancer Network recommends an evaluation with baseline bone mineral density testing and follow-up every 2 years for women with bca undergoing therapy that lowers sex steroids14. Given osteoclastogenesis from estrogen deficiency, the use of osteoclast inhibitors such as bisphosphonates not only prevents bone loss, but, in postmenopausal women, is associated with a reduction in bca recurrence (rr: 0.86; 95% ci: 0.78 to 0.94) and bca mortality (rr: 0.82; 95% ci: 0.73 to 0.93)15. The latest guidelines from Cancer Care Ontario and the American Society of Clinical Oncology therefore indicate that postmenopausal women and patients on ovarian suppression receiving adjuvant systemic treatment should be considered for intravenous zoledronic acid (4 mg every 6 months) or oral clodronate (1600 mg daily) for 3–5 years16. Supplementation with 1200 mg elemental calcium (total diet plus supplement) and 800 IU vitamin D daily is recommended for all patients14.
AI-Induced Musculoskeletal Symptoms
The use of ais has been associated with an increased risk of arthralgias, significantly increased tendon thickness, and carpal tunnel syndrome17–19. Most of the carpal tunnel syndrome cases were mild-to-moderate in severity and did not require treatment.
The arthralgia or musculoskeletal syndrome induced by ais is variously defined in the literature, but is usually symmetrical, affecting hands and wrists with morning stiffness20. Given the lack of a standard definition, prevalence varies in the clinical trials, but is estimated to be 45%–50%. Severity also varies, with the intensity of the symptoms attenuating after prolonged exposure to ais21. However, the symptoms can at times also be debilitating, and this side effect is one of the most common causes of therapy discontinuation. No clear treatment has yet been established, but exercise, massage therapy, acupuncture, and nonsteroidal anti-inflammatory drugs have all been shown to lessen the burden of symptoms to varying degrees22. Switching the ai can result in better tolerance and compliance, with 72% of patients continuing letrozole at 6 months after anastrozole was stopped because of musculoskeletal symptoms23.
AI-Induced Sexual Dysfunction
The diminishment of estrogen synthesis during ai therapy is similar to that seen with aging. The consequences often include vaginal dryness and atrophy, which can in turn result in cystitis, vaginitis, painful intercourse (dyspareunia), and decreased libido24. Because those effects are often underreported, and because they result in significant physical and emotional distress for patients and their partners alike, sexual dysfunction should be actively discussed with patients25. The complexity of female sexual dysfunction necessitates a biopsychosocial approach to assessment and management. Potential interventions range from education and lifestyle changes to sexual counselling, sexual aids (for example, lubricants and lidocaine preparations), medications (non-hormonal), and dietary supplements. All of the foregoing approaches have been shown to be helpful to varying degrees26. For refractory cases, low-dose vaginal estrogen could be considered in collaboration with an oncologist25.
AI-Induced Cardiovascular Disease
Estrogen has a cardioprotective role in women, and compared with tamoxifen therapy, ai therapy has been associated with higher rates of hyperlipidemia and hypertension27. However, rates of severe cardiovascular disease such as myocardial infarction and stroke in patients treated with ais are similar to those in the non-cancer population28. Patients taking ais should be routinely screened for hypertension, hyperlipidemia, and metabolic syndrome.
Managing the Side Effects of Tamoxifen
Hot Flashes
Hot flashes are one of the most common and bothersome side effects of tamoxifen, being reported in up to 80% of patients undergoing therapy29. They also occur to a much lesser extent in patients taking ais. Drugs that inhibit the activity of CYP2D6, such as the selective serotonin reuptake inhibitors, reduce the occurrence of tamoxifen-related hot flashes by decreasing the conversion of tamoxifen to its most active metabolite, endoxifen30. However, strong CYP2D6 inhibitors could adversely affect drug efficacy. Therefore, moderate CYP2D6 inhibitors (such as sertraline and duloxetine) are preferred over strong inhibitors (such as paroxetine and fluoxetine) for the treatment of hot flashes.
Venous Thromboembolism
The relative risk of venous thromboembolism is increased by a factor of 2–3 in older women receiving tamoxifen31,32. The risk seems to be further pronounced when therapy is extended to 10 years from 5 in the adjuvant setting8. Risk factors for tamoxifen-induced venous thromboembolism include prior surgery, fracture, immobilization, and heterozygous factor v Leiden carrier status33. However, the risk of fatal pulmonary embolism does not seem to increase with tamoxifen use extended to 10 years (0.2%), especially for women less than 54 years of age6,8.
Endometrial Cancer
Tamoxifen has been associated with a risk for both endometrial cancer and uterine sarcoma that is increased by a factor of 2.7; however, the absolute annual risk of endometrial cancer remains low at 1.2 per 1000 patient–years34,35. The elevated risk of cancer persists as long as the patient takes tamoxifen and declines after treatment discontinuation36. The following recommendations are in place for surveillance of uterine cancer in women taking tamoxifen37:
■ In premenopausal women, any irregular bleeding should be investigated by hysteroscopy or endometrial biopsy (or both). The same recommendation applies to postmenopausal women experiencing new-onset vaginal bleeding.
■ In postmenopausal women without vaginal bleeding, routine age-appropriate screening is recommended.
Tamoxifen-Induced Ocular Pathologies
Exposure to tamoxifen is associated with an increased risk of cataracts (3.7%) and, less commonly, with reversible corneal pigmentation and irreversible retinal deposits in association with macular edema and vision loss38. Although these issues are less common, a routine annual eye exam is recommended for all patients.
Tamoxifen-Induced Fatty Liver Disease
Overall, tamoxifen has a favourable effect on lipid profile39. However, it has recently been reported that, based on ultrasonography, fatty liver is incidentally found in one third of patients taking tamoxifen40. Clinically relevant steatohepatitis is nevertheless very uncommon, and no routine screening is therefore required. Tamoxifen can be continued unless liver function tests reach twice the upper limit of normal. For patients with documented fatty liver disease, liver function tests every 3–6 months are recommended.
METASTATIC BCa
Inhibitors of Cyclin-Dependent Kinase 4/6
Endocrine therapy remains the mainstay treatment for patients with metastatic hormone receptor–positive, her2-negative bca not presenting in visceral crisis. However, the advent of inhibitors of cyclin-dependent kinase 4/6 (cdk4/6), such as palbociclib, ribociclib, and abemaciclib, in combination with endocrine therapy to overcome endocrine resistance, has been a breakthrough for the first-line treatment of postmenopausal women with hormone-positive metastatic disease. The addition of a cdk4/6 inhibitor to letrozole or anastrozole has been shown to increase progression-free survival to 24–25 months compared with 14–15 months with letrozole or anastrozole alone, with overall survival data currently being immature41–43. These newer agents are associated with significant rates of neutropenia and lymphopenia (affecting up to 92% of patients on therapy) and, less commonly, with anemia and thrombocytopenia41–43.
Unlike traditional chemotherapy, which causes neutropenia through bone marrow cell apoptosis, cdk4/6 inhibitors cause cell-cycle arrest without depleting the bone marrow of precursor white cells. As a result, the occurrence of febrile neutropenia is rare (1%–2%), and neutropenia is rapidly reversed within 48 hours of therapy cessation, without the need for stimulating factors. It is therefore very common for patients followed in primary care to have their blood results flagged for abnormal counts while receiving these therapies. Inhibitors of cdk4/6 should be discontinued only if the patient has clinical signs of an infection. Dose adjustments for severe cases will be performed by the oncologist who actively follows the patient.
In summary, the cdk4/6 inhibitors require monitoring for the initial 2 weeks and then with monthly clinic visits, a complete blood count, and liver enzymes41–43. For ribociclib, baseline electrocardiography should be obtained, with a follow-up in 2 weeks, and then monthly monitoring for prolongation of QTc.
Fulvestrant
Given intramuscularly, fulvestrant is another novel endocrine agent that works by selectively degrading the estrogen receptor. Currently, fulvestrant is used only in the metastatic setting, either in the first line for patients presenting with de novo metastatic hormone-positive disease, or in later lines in combination with the cdk4/6 inhibitor palbociclib44,45. Like the ais, fulvestrant is associated with arthralgias and hot flashes, but it is associated with higher rates of liver enzyme elevation and local injection site reactions44,45.
Everolimus
Everolimus, an inhibitor of mtor (the mechanistic target of rapamycin), has been added to endocrine therapy with exemestane to overcome resistance to endocrine therapy. Currently, the use of everolimus in combination with exemestane is limited to postmenopausal patients who have progressed on prior endocrine therapy. The addition of everolimus increases progression-free survival by 4.1 months, but is also associated with increased risks for stomatitis, pneumonitis (3%), anemia, hyperglycemia, and fatigue46.
SUMMARY
The treatment of hormone receptor–positive bca is a constantly evolving field. Newer agents that aim to circumvent resistance, combination therapies, and the duration of therapy are often the subjects of large practice-changing clinical trials. As therapies improve, so does the survivorship of affected patients with cancer at all stages. As in many other chronic diseases, such as hypertension and diabetes, the primary care physician will have to be aware of the specific issues affecting the increasingly prevalent bca population and of the screening and interventions required to adequately manage the consequences of anti-hormonal therapy. A multi-disciplinary approach and consultation with oncology specialists are always recommended.
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare the following interests: AA has received advisory fees from Novartis for their drug ribociclib. KE has no conflicts of interest to declare.
REFERENCES
- 1.Canadian Cancer Society’s Advisory Committee on Cancer Statistics . Canadian Cancer Statistics 2017. Toronto, ON: Canadian Cancer Society; 2017. [Google Scholar]
- 2.Howlader N, Altekruse SF, Li CI, et al. US incidence of breast cancer subtypes defined by joint hormone receptor and her2 status. J Natl Cancer Inst. 2014;106:pii. doi: 10.1093/jnci/dju055. dju055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rubin G, Berendsen A, Crawford SM, et al. The expanding role of primary care in cancer control. Lancet Oncol. 2015;16:1231–72. doi: 10.1016/S1470-2045(15)00205-3. [DOI] [PubMed] [Google Scholar]
- 4.Francis PA, Regan MM, Fleming GF, et al. on behalf of the soft investigators and the International Breast Cancer Study Group Adjuvant ovarian suppression in premenopausal breast cancer. N Engl J Med. 2015;372:436–46. doi: 10.1056/NEJMoa1412379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Early Breast Cancer Trialists’ Collaborative Group Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 6.Early Breast Cancer Trialists’ Collaborative Group Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet. 2015;386:1341–52. doi: 10.1016/S0140-6736(15)61074-1. [DOI] [PubMed] [Google Scholar]
- 7.Regan MM, Neven P, Giobbie-Hurder A, et al. Assessment of letrozole and tamoxifen alone and in sequence for postmenopausal women with steroid hormone receptor–positive breast cancer: the big 1-98 randomised clinical trial at 8.1 years median follow-up. Lancet Oncol. 2011;12:1101–8. doi: 10.1016/S1470-2045(11)70270-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davies C, Pan H, Godwin J, et al. on behalf of the atlas Collaborative Group Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor–positive breast cancer: atlas, a randomised trial. Lancet. 2013;381:805–16. doi: 10.1016/S0140-6736(12)61963-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goss PE, Ingle JN, Martino S, et al. A randomized trial of letrozole in postmenopausal women after five years of tamoxifen therapy for early-stage breast cancer. N Engl J Med. 2003;349:1793–802. doi: 10.1056/NEJMoa032312. [DOI] [PubMed] [Google Scholar]
- 10.Goss PE, Ingle JN, Pritchard KI, et al. Extending aromatase-inhibitor adjuvant therapy to 10 years. N Engl J Med. 2016;375:209–19. doi: 10.1056/NEJMoa1604700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tjan-Heijnen VCG, van Hellemond IEG, Peer PGM, et al. on behalf of the Dutch Breast Cancer Research Group for the data investigators Extended adjuvant aromatase inhibition after sequential endocrine therapy (data): a randomised, phase 3 trial. Lancet Oncol. 2017;18:1502–11. doi: 10.1016/S1470-2045(17)30600-9. [DOI] [PubMed] [Google Scholar]
- 12.Aiello Bowles EJ, Boudreau DM, Chubak J, et al. Patient-reported discontinuation of endocrine therapy and related adverse effects among women with early-stage breast cancer. J Oncol Pract. 2012;8:e149–57. doi: 10.1200/JOP.2012.000543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amir E, Seruga B, Niraula S, Carlsson L, Ocana A. Toxicity of adjuvant endocrine therapy in postmenopausal breast cancer patients: a systematic review and meta-analysis. J Natl Cancer Inst. 2011;103:1299–309. doi: 10.1093/jnci/djr242. [DOI] [PubMed] [Google Scholar]
- 14.Gralow JR, Biermann JS, Farooki A, et al. nccn Task Force report: bone health in cancer care. J Natl Compr Canc Netw. 2013;11(suppl 3):S1–50. doi: 10.6004/jnccn.2013.0215. [DOI] [PubMed] [Google Scholar]
- 15.Early Breast Cancer Trialists’ Collaborative Group Adjuvant bisphosphonate treatment in early breast cancer: meta-analyses of individual patient data from randomised trials. Lancet. 2015;386:1353–61. doi: 10.1016/S0140-6736(15)60908-4. [Erratum in: Lancet 2016;387:30] [DOI] [PubMed] [Google Scholar]
- 16.Dhesy-Thind S, Fletcher GG, Blanchette PS, et al. Use of adjuvant bisphosphonates and other bone-modifying agents in breast cancer: a Cancer Care Ontario and American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2017;35:2062–81. doi: 10.1200/JCO.2016.70.7257. [DOI] [PubMed] [Google Scholar]
- 17.Nishihori T, Choi J, DiGiovanna MP, et al. Carpal tunnel syndrome associated with the use of aromatase inhibitors in breast cancer. Clin Breast Cancer. 2008;8:362–5. doi: 10.3816/CBC.2008.n.043. [DOI] [PubMed] [Google Scholar]
- 18.Dizdar O, Ozcakar L, Malas FU, et al. Sonographic and electrodiagnostic evaluations in patients with aromatase inhibitor–related arthralgia. J Clin Oncol. 2009;27:4955–60. doi: 10.1200/JCO.2008.20.5435. [DOI] [PubMed] [Google Scholar]
- 19.Hershman DL, Loprinzi C, Schneider BP. Symptoms: aromatase inhibitor induced arthralgias. Adv Exp Med Biol. 2015;862:89–100. doi: 10.1007/978-3-319-16366-6_7. [DOI] [PubMed] [Google Scholar]
- 20.Niravath P. Aromatase inhibitor–induced arthralgia: a review. Ann Oncol. 2013;24:1443–9. doi: 10.1093/annonc/mdt037. [DOI] [PubMed] [Google Scholar]
- 21.Beckwee D, Leysen L, Meuwis K, Adriaenssens N. Prevalence of aromatase inhibitor–induced arthralgia in breast cancer: a systematic review and meta-analysis. Support Care Cancer. 2017;25:1673–86. doi: 10.1007/s00520-017-3613-z. [DOI] [PubMed] [Google Scholar]
- 22.Younus J, Kligman L. Management of aromatase inhibitor-induced arthralgia. Curr Oncol. 2010;17:87–90. doi: 10.3747/co.v17i1.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Briot K, Tubiana-Hulin M, Bastit L, Kloos I, Roux C. Effect of a switch of aromatase inhibitors on musculoskeletal symptoms in postmenopausal women with hormone-receptor-positive breast cancer: the atoll (Articular Tolerance of Letrozole) study. Breast Cancer Res Treat. 2010;120:127–34. doi: 10.1007/s10549-009-0692-7. [DOI] [PubMed] [Google Scholar]
- 24.Kwan KW, Chlebowski RT. Sexual dysfunction and aromatase inhibitor use in survivors of breast cancer. Clin Breast Cancer. 2009;9:219–24. doi: 10.3816/CBC.2009.n.037. [DOI] [PubMed] [Google Scholar]
- 25.Carter J, Lacchetti C, Andersen BL, et al. Interventions to address sexual problems in people with cancer: American Society of Clinical Oncology clinical practice guideline adaptation of Cancer Care Ontario guideline. J Clin Oncol. 2018;36:492–511. doi: 10.1200/JCO.2017.75.8995. [DOI] [PubMed] [Google Scholar]
- 26.Derzko C, Elliott S, Lam W. Management of sexual dysfunction in postmenopausal breast cancer patients taking adjuvant aromatase inhibitor therapy. Curr Oncol. 2007;14(suppl 1):S20–40. doi: 10.3747/co.2007.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Foglietta J, Inno A, de Iuliis F, et al. Cardiotoxicity of aromatase inhibitors in breast cancer patients. Clin Breast Cancer. 2017;17:11–17. doi: 10.1016/j.clbc.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 28.Ligibel JA, James O’Malley A, Fisher M, Daniel GW, Winer EP, Keating NL. Risk of myocardial infarction, stroke, and fracture in a cohort of community-based breast cancer patients. Breast Cancer Res Treat. 2012;131:589–97. doi: 10.1007/s10549-011-1754-1. [DOI] [PubMed] [Google Scholar]
- 29.Day R. Quality of life and tamoxifen in a breast cancer prevention trial: a summary of findings from the nsabp P-1 study. National Surgical Adjuvant Breast and Bowel Project. Ann N Y Acad Sci. 2001;949:143–50. doi: 10.1111/j.1749-6632.2001.tb04012.x. [DOI] [PubMed] [Google Scholar]
- 30.Goetz MP, Rae JM, Suman VJ, et al. Pharmacogenetics of tamoxifen biotransformation is associated with clinical outcomes of efficacy and hot flashes. J Clin Oncol. 2005;23:9312–18. doi: 10.1200/JCO.2005.03.3266. [DOI] [PubMed] [Google Scholar]
- 31.Davies C, Godwin J, Gray R, et al. on behalf of the Early Breast Cancer Trialists’ Collaborative Group Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011;378:771–84. doi: 10.1016/S0140-6736(11)60993-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cuzick J, Forbes J, Edwards R, et al. on behalf of the ibis investigators First results from the International Breast Cancer Intervention Study (ibis-I): a randomised prevention trial. Lancet. 2002;360:817–24. doi: 10.1016/S0140-6736(02)09962-2. [DOI] [PubMed] [Google Scholar]
- 33.Garber JE, Halabi S, Tolaney SM, et al. on behalf of the Cancer and Leukemia Group B Factor v Leiden mutation and thromboembolism risk in women receiving adjuvant tamoxifen for breast cancer. J Natl Cancer Inst. 2010;102:942–9. doi: 10.1093/jnci/djq211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Braithwaite RS, Chlebowski RT, Lau J, George S, Hess R, Col NF. Meta-analysis of vascular and neoplastic events associated with tamoxifen. J Gen Intern Med. 2003;18:937–47. doi: 10.1046/j.1525-1497.2003.20724.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fisher B, Costantino JP, Redmond CK, et al. Endometrial cancer in tamoxifen-treated breast-cancer patients—findings from the National Surgical Adjuvant Breast and Bowel Project (nsabp) B-14. J Natl Cancer Inst. 1994;86:527–37. doi: 10.1093/jnci/86.7.527. [DOI] [PubMed] [Google Scholar]
- 36.Cuzick J, Forbes JF, Sestak I, et al. on behalf of the International Breast Cancer Intervention Study i investigators Long-term results of tamoxifen prophylaxis for breast cancer— 96-month follow-up of the randomized ibis-i trial. J Natl Cancer Inst. 2007;99:272–82. doi: 10.1093/jnci/djk049. [DOI] [PubMed] [Google Scholar]
- 37.Committee on Practice Bulletins–Gynecology acog practice bulletin no. 126: management of gynecologic issues in women with breast cancer. Obstet Gynecol. 2012;119:666–82. doi: 10.1097/AOG.0b013e31824e12ce. [DOI] [PubMed] [Google Scholar]
- 38.Paganini-Hill A, Clark LJ. Eye problems in breast cancer patients treated with tamoxifen. Breast Cancer Res Treat. 2000;60:167–72. doi: 10.1023/A:1006342300291. [DOI] [PubMed] [Google Scholar]
- 39.Hayes DF, Skaar TC, Rae JM, et al. Estrogen receptor genotypes, menopausal status, and the effects of tamoxifen on lipid levels: revised and updated results. Clin Pharmacol Ther. 2010;88:626–9. doi: 10.1038/clpt.2010.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hong N, Yoon HG, Seo DH, et al. Different patterns in the risk of newly developed fatty liver and lipid changes with tamoxifen versus aromatase inhibitors in postmenopausal women with early breast cancer: a propensity score–matched cohort study. Eur J Cancer. 2017;82:103–14. doi: 10.1016/j.ejca.2017.05.002. [DOI] [PubMed] [Google Scholar]
- 41.Goetz MP, Toi M, Campone M, et al. monarch 3: abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol. 2017;35:3638–46. doi: 10.1200/JCO.2017.75.6155. [DOI] [PubMed] [Google Scholar]
- 42.Hortobagyi GN, Stemmer SM, Burris HA, et al. Ribociclib as first-line therapy for hr-positive, advanced breast cancer. N Eng J Med. 2016;375:1738–48. doi: 10.1056/NEJMoa1609709. [DOI] [PubMed] [Google Scholar]
- 43.Turner NC, Ro J, Andre F, et al. Palbociclib in hormone-receptor-positive advanced breast cancer. N Eng J Med. 2015;373:209–19. doi: 10.1056/NEJMoa1505270. [DOI] [PubMed] [Google Scholar]
- 44.Robertson JFR, Bondarenko IM, Trishkina E, et al. Fulvestrant 500 mg versus anastrozole 1 mg for hormone receptor–positive advanced breast cancer (falcon): an international, randomised, double-blind, phase 3 trial. Lancet. 2016;388:2997–3005. doi: 10.1016/S0140-6736(16)32389-3. [DOI] [PubMed] [Google Scholar]
- 45.Cristofanilli M, Turner NC, Bondarenko I. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, her2-negative metastatic breast cancer that progressed on previous endocrine therapy (paloma-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016;17:425–39. doi: 10.1016/S1470-2045(15)00613-0. [DOI] [PubMed] [Google Scholar]
- 46.Baselga J, Campone M, Piccart M, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Eng J Med. 2012;366:520–9. doi: 10.1056/NEJMoa1109653. [DOI] [PMC free article] [PubMed] [Google Scholar]