Abstract
Sarcoidosis is a multisystemic granulomatous disease of unknown causes, and cutaneous sarcoidosis (CS) is an early manifestation of the disease. Dermoscopy has gained increasing interest in the past few years as an aid in the clinical diagnosis of inflammatory and infectious skin manifestations. We present a case report about a single, erythematous, and asymptomatic plaque on the face with unexpected dermoscopy characteristics of CS.
Learning points:
CS on the face of a therapy-resistant actinic keratosis should be considered a differential diagnosis.
Dermoscopy can change the diagnosis and lead to the correct management.
Keywords: plaque, cutaneous sarcoidosis, dermoscopy, inflammatory disease, face, granulomatous disease
Case Presentation
A 43-year-old woman was referred to our skin cancer unit with a history of therapy-resistant actinic keratosis (AK), which had been treated by a practice-based dermatologist with topical isotretinoin and 3% diclofenac in 2.5% hyaluronic acid without improvement. Clinically, the lesion appeared as a reddish, slightly scaling plaque 6 mm in diameter on the right temple (Figures 1 and 2). The patient recalled the presence of the lesion for the past several months but denied any symptoms or history of trauma.
Figure 1.
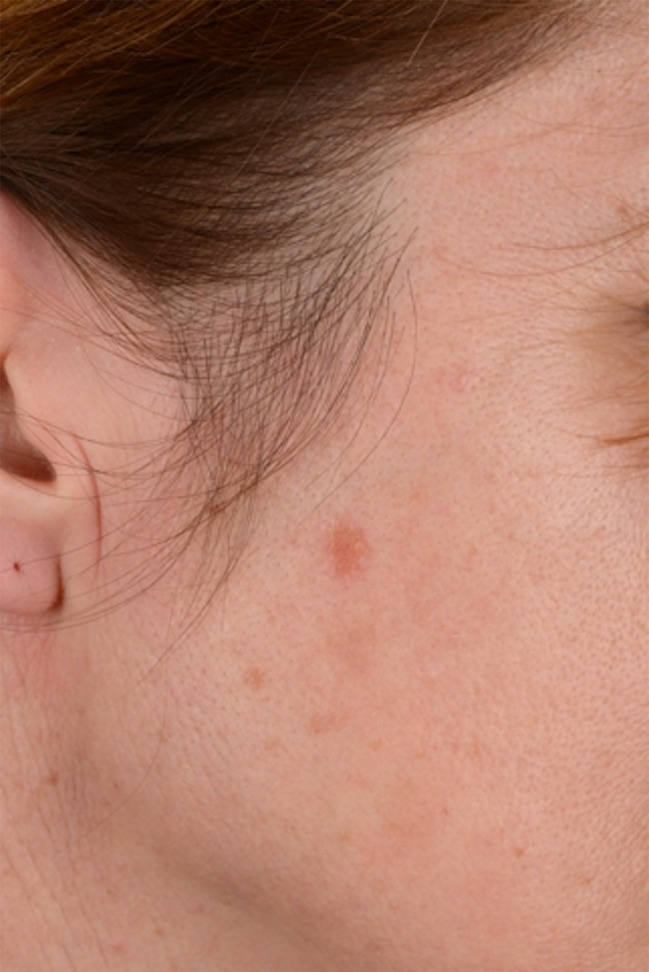
Clinical image of a single erythematous plaque on temple area. [Copyright: ©2018 Conforti et al.]
Figure 2.
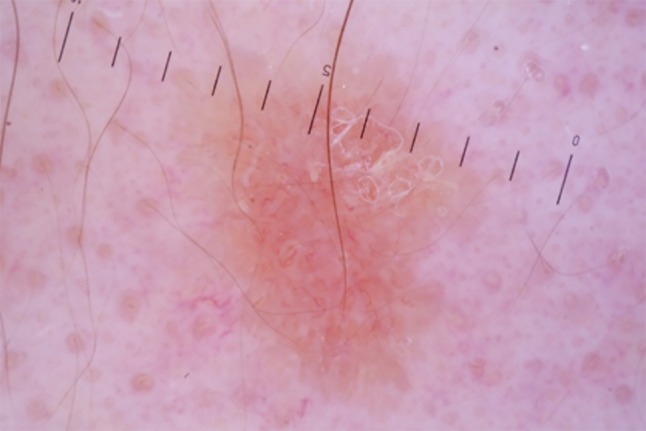
Dermoscopy (polarized ×20) exhibits whitish-yellow scales and whitish structureless areas (red arrow) on a yellow- orange background (black arrow), with diffuse linear irregular vessels. [Copyright: ©2018 Conforti et al.]
The digital epiluminescence microscope system with polarized light (DermLite, 3Gen, ×20) with immersion oil revealed whitish-yellow scales and whitish structureless areas on a yellow-orange background, with diffuse linear irregular vessels (Figure 2). Due to the yellow-to-orange color, which is typically seen in granulomatous diseases, the differential diagnosis included CS, lupus vulgaris (LV), and cutaneous leishmaniasis. A biopsy was performed. Subsequent histopathology confirmed the suspected dermoscopic diagnosis of CS, showing noncaseating epithelioid granulomas (Figure 3).
Figure 3.
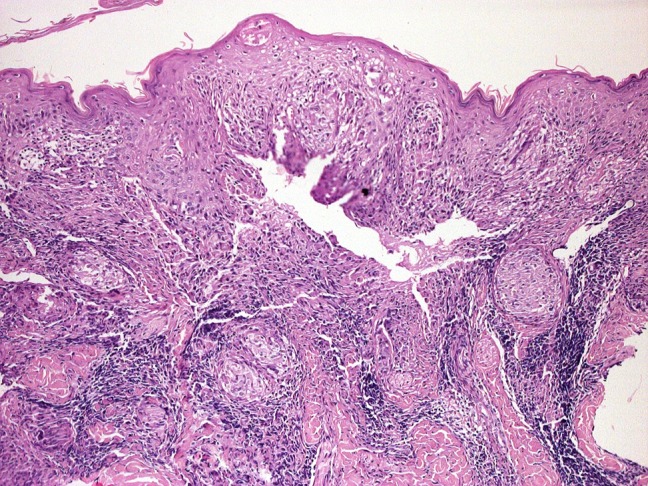
Histology (×40) shows noncaseating epithelioid granulomas. [Copyright: ©2018 Conforti et al.]
Blood tests showed a high level of angiotensin converting enzyme (ACE) and antinuclear antibody (ANA). The patient did not present with pulmonary involvement.
Discussion
Sarcoidosis is a multisystemic granulomatous disease of unknown causes. It may involve various tissues and organs, but the lungs, lymph nodes, eyes, and skin are commonly involved. CS is usually an early manifestation of the systemic disease. Specific cutaneous manifestations of sarcoidosis are highly polymorphous, including solitary or multiple, red, yellow-brown or purple macules or papules, plaques, infiltrations, annular lesions, and subcutaneous nodules [1–3].
In past years, specific dermoscopic patterns of a variety of inflammatory and infectious skin disorders have been described, including some commonly considered CS in differential diagnosis [4,5]. Pellicano et al [4] reported a retrospective dermoscopic evaluation of the 7 cases of histologically confirmed CS. Linear vessels overlying the translucent orange ovoid structures were the only vascular structures seen in all cases [4]. In line with this, Vázquez-López et al evaluated the dermoscopic patterns in a large series of different nontumoral dermatoses; their series included 3 cases of CS, of which 2 lesions exhibited monomorphous linear vessels [5]. We also found linear vessels as the only vascular pattern in our case.
Histologically, CS shows noncaseating epithelioid granulomas, with minimal or absent lymphocytes or plasma cells (naked granuloma) [6]. Inside giant cells, Schaumann bodies and asteroid bodies may be found, although they are not specific. Many of these features are not exclusively seen in CS and may also occur in infectious diseases, immunologic/foreign body granulomas, neoplasia, immunodeficiency disorders, and drug eruptions. For this reason, sarcoidosis is one of the “great imitators” clinically and histopathologically, though its diagnosis is facilitated by dermoscopy.
Conclusions
In our patient, clinical features were indeed consistent with the diagnosis of AK. However, dermoscopy exhibited whitish structureless areas on a yellow-orange background associated with linear vessels, compatible with granulomatous skin disease. The dermoscopy findings changed the diagnosis from tumoral to granulomatous disease and led to the correct management.
Footnotes
Funding: None.
Competing interests: The authors have no conflicts of interest to disclose.
References
- 1.Tchernev G. Cutaneous sarcoidosis: the “great imitator”: etiopathogenesis, morphology, differential diagnosis, and clinical management. Am J Clin Dermatol. 2006;7(6):375–382. doi: 10.2165/00128071-200607060-00006. [DOI] [PubMed] [Google Scholar]
- 2.Elgart ML. Cutaneous sarcoidosis: definitions and types of lesions. Clin Dermatol. 1986;4(4):35–45. doi: 10.1016/0738-081X(86)90032-5. [DOI] [PubMed] [Google Scholar]
- 3.Fernandez-Faith E, McDonnell J. Cutaneous sarcoidosis: differential diagnosis. Clin Dermatol. 2007;25(3):276–287. doi: 10.1016/j.clindermatol.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 4.Pellicano R, Tiodorovic-Zivkovic D, Gourhant JY, et al. Dermoscopy of cutaneous sarcoidosis. Dermatology. 2010;221(1):51–54. doi: 10.1159/000284584. [DOI] [PubMed] [Google Scholar]
- 5.Vázquez-López F, Kreusch J, Marghoob AA. Dermoscopic semiology: further insights into vascular features by screening a large spectrum of nontumoral skin lesions. Br J Dermatol. 2004;150(2):226–231. doi: 10.1111/j.1365-2133.2004.05753.x. [DOI] [PubMed] [Google Scholar]
- 6.Wilson NJ, King CM. Cutaneous sarcoidosis. Postgrad Med J. 1998;74(877):649–652. doi: 10.1136/pgmj.74.877.649. [DOI] [PMC free article] [PubMed] [Google Scholar]


