Abstract
Patients with multiple atypical nevi are at higher risk of developing melanoma. Among different techniques, sequential digital dermatoscopic imaging (SDDI) is a state-of-the art method to enhance diagnostic accuracy in evaluating pigmented skin lesions. It relies on analyzing digital dermatoscopic images of a lesion over time to find specific dynamic criteria inferring biologic behavior. SDDI can reduce the number of necessary excisions and finds melanomas in an early—and potentially curable—stage, but precautions in selecting patients and lesions have to be met to reach those goals.
Keywords: dermatoscopy, monitoring, melanoma, nevi, digital, screening
Introduction
Dermatoscopy has progressed to a state-of-the art technique not only to distinguish melanoma from nevi [1,2], but also to diagnose all kinds of pigmented and nonpigmented skin tumors [3]. This is due to its proven increase in diagnostic accuracy compared to the unaided eye [4], an improvement that recently has also been shown to be present in nonpigmented lesions that are inherently more difficult to diagnose [5].
But there is a specific aspect of pigmented and nonpigmented skin lesion diagnosis with dermatoscopy that stands apart, namely, screening high-risk patients. Why is this different? Not only are these patients much more likely to be diagnosed with melanoma [6,7], they are also more difficult to diagnose [8]. This is partially because early melanoma can be featureless, but also because nevi on those patients can have a worrisome morphology. Some approaches have been proposed to tackle these problems.
The morphologic differentiability can be overcome partly by comparing nevi clusters of the same pattern in a patient [9,10], which has become well known as the comparative approach set forth by Argenziano [11]. This comparative approach has its limitations though; for example, in an experimental setting, dermatologists were not able to distinguish melanomas and nevi well in lesions of high-risk patients [8,12].
Total-body imaging is widely used for screening high-risk patients, but because pigmented skin lesions can change or occur, especially in young patients [13–15], it is most commonly not applied solely but in combination with other diagnostic methods [16,17].
A German group presented a rather innovative method in which they removed the skin of the entire back of a patient to reduce his melanoma risk [18]. Though seemingly promising, this approach may not be a solution for usual high-risk patients: The removed nevi are most likely not the precursors of a potential melanoma [19], and possible melanoma risks due to germline mutations [20] would still be present. Finally, such an overwhelming surgical procedure defeats the purpose of a screening method, namely reducing invasive procedures. Rather, a noninvasive and more specific method has to be chosen for that purpose. One technique that fulfills those requirements, and overcomes some drawbacks mentioned previously, is digital dermatoscopic follow-up, or sequential digital dermatoscopic imaging (SDDI) [21,22].
By comparing 2 images of a lesion taken at different time points, additional information about the dynamics, and thus biologic behavior, can be obtained. This additional information has gained interest when being added as an additional “E” criterion to the classic “ABCDs” [23]. In a study of patients with a high risk of melanoma [24], about 20% to 50% of melanomas could only be detected with the help of digital follow-up, but not with a single dermatoscopic examination. In addition to monitoring multiple nevi, digital dermatoscopy is also used to enhance specificity on individual suspicious lesions. Here, a shorter interval (2–3 months [25]; short-term follow-up) is usually chosen [26] for single lesions and even small dermatoscopic changes are regarded as suspicious, whereas in the screening of patients with many nevi an interval of 6–12 months (long-term follow-up) is more common. In the following sections, general rules for practical application of SDDI are discussed.
Selection
Risk Factors
The first consideration in applying digital dermatoscopic monitoring is the patient collective, as it has to meet certain criteria [27]. A previous report [24] has shown that digital dermatoscopy is particularly useful for patients with a familial atypical mole and multiple melanoma (FAMMM) syndrome and an atypical mole syndrome (AMS; >50 nevi and >3 atypical nevi) in a strict sense. Conversely, conventional dermatoscopy was sufficient for the detection of melanomas in patients with solely a large number of (inconspicuous) nevi: in this patient group, more than 80% of melanomas were diagnosed over a period of 10 years by means of a single dermatoscopic examination or other clinical information. In 2 additional studies with a shorter period of time, no melanoma was found in patients with low risk among the dermatoscopically monitored lesions [28,29]. Thus, there is no compelling evidence for applying digital dermatoscopic monitoring to low-risk patient groups. They most likely will not benefit from it at all; instead, it may even do harm, as recent literature shows a positive association of false positive findings with a number of monitored lesions in a patient [30].
Compliance
An often-underestimated drawback is lack of patient compliance [29,31]; that is, patients do not show up for the follow-up appointments. The reason this is an issue is changed sensitivity at the baseline visit [32]. One basic mechanism of the increased diagnostic accuracy of digital dermatoscopic monitoring is that one increases specificity by leaving a lesion untouched in good faith, the lesion—on the patient—will come back after a specified interval. This increased specificity comes at the price of lower sensitivity, which can only be overcome by finding missed melanomas at a second examination. Thus, the physician has to ensure the patient returns to the office. While the lack of compliance is not without dispute [33], an Italian group [28] found compliance was higher for shorter intervals and that long-term monitoring may be started with shorter periods.
Lesions
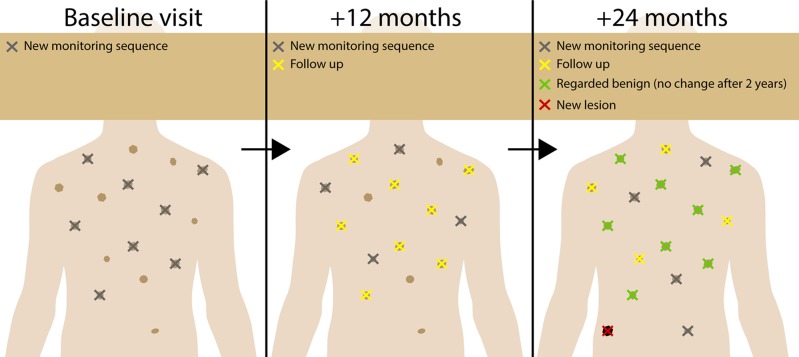
Previous studies have shown that in high-risk patients one cannot estimate at baseline which of the lesions on the patient is more prone to become a melanoma [8,12]. While other authors argue that only lesions with some sign of atypia should be followed over time [34], those results suggest a possible benefit in integrating inconspicuous lesions. One should not follow, though, that all lesions on a patient have to be monitored at every visit. Taking photographs of all lesions at every visit is not only impossible to do in a reasonable amount of time, but it may also decrease diagnostic accuracy as more monitored lesions per patient are positively correlated with false positive findings [30]. A survey showed that the majority of experts in the field in fact do not perform dermatoscopic monitoring of every single lesion on a patient [35], and indirect evidence indicates this is truly not necessary. In a retrospective analysis of our own high-risk center, where only a random subset of lesions is monitored at every visit with monitoring being stopped after no change has been seen for 2 (or 3) years, almost half of melanomas were in situ and mean invasion depth was well below 1 mm for 10 years [30]. Therefore, because we cannot estimate which pigmented skin lesion turns out to be a melanoma, selection of lesion monitoring has to be random and can be incremental to save resources (incremental SDDI, Figure 1).
Figure 1.
Incremental SDDI. Because with current methods it is not feasible to image every lesion at every visit, we selected a random sample of new lesions at every visit (gray), which were imaged in subsequent visits (yellow), but were discarded from follow-up after showing no change for 2 years (green). With this method, we were able to map all lesions eventually, to suggest if one lesion has occurred in the last interval even without TBP (red). [Copyright: ©2018 Tschandl.]
One cannot choose which lesions specifically should be monitored, but there are rules as to which lesions should not be. Important exclusion criteria are: (1) nodular (black, brown, gray, red or blue) lesions, as thick melanomas would progress to higher invasion depths more quickly [36,37]; (2) blue lesions [38], as monitoring cannot reliably evaluate changes in the dermis; (3) regressive lesions, as a potential melanoma may be completely regressed at follow-up; (4) lesions with a dermatoscopic clod pattern, as they show a faster growth [39]; and (5) spitzoid lesions [40], not including Reed nevi [41], as the latter can show fairly symmetric growth and stabilization. Lesions with these characteristics should be removed immediately, unless they are clearly benign at the baseline visit.
Finally, what should lesions selected for SDDI look like? Ideally, they are medium-sized, flat, and show a dermatoscopic reticular pattern. But, as with any recommendation, the preceding recommendations are due to change with new findings, specifically in the advent of automated full-body imaging, where monitoring of every single lesion of a patient seems feasible in the future. Until then, it is justifiable to discard monitoring a single lesion if no change has occurred for 2–3 years because a diagnosed single melanocytic nevus is at very low risk of transforming into a melanoma at 0.0005% to 0.003% per year [42].
Evaluation
Melanocytic nevi generally grow symmetrically and follow 1 of 3 variants: a reticular pattern (slow growth), a surrounding rim of clods (moderate to fast growth), or peripheral pseudopodia and radial lines (fast growth) [43]. The following changes (summarized in Table 1 and Figures 2–4 and adapted from Kittler et al [44]) have been associated with melanoma in previous studies [45,46] and should lead to the removal of a lesion: (1) changed architecture; (2) asymmetric increase in size; (3) new colors, depigmentation, and focal color change; and (4) the appearance of melanoma criteria such as black dots or regression.
TABLE 1.
Differentiation of Nevus and Melanoma with Follow-Up Images*
| Change | Nevus | Melanoma |
|---|---|---|
| Change in size | None or symmetrical growth | Asymmetrical growth |
| Change in color | No change or even lighter/darker brown or erythema | New colors, especially focally and depigmentation |
| Change in structure | No or subtle changes such as accentuation of existing structures | Architecture changes and the appearance of new structures including classical melanoma criteria and regression and signs of regression |
Adapted from Kittler et al [50].
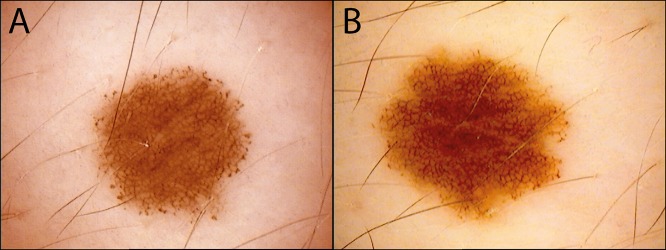
Figure 2.
A compound nevus (A) with peripheral clods showing (B) symmetrical growth after 1 year. Nevi with peripheral clods very commonly show symmetric enlargement over time [70]. [Copyright: ©2018 Tschandl.]
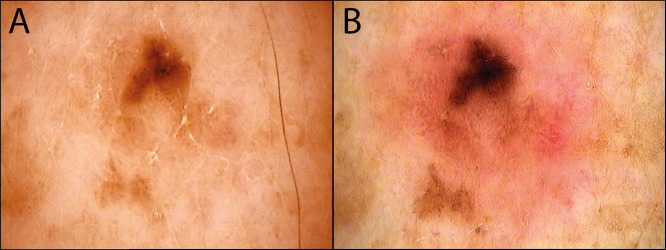
Figure 3.
This histopathologically verified lentigo maligna initially presented with only structureless brown areas (A) at the baseline visit. (B) After 14 months of follow-up, the pigment has become darker, grown asymmetrically, and an additional pink structureless area can be seen. [Copyright: ©2018 Tschandl.]
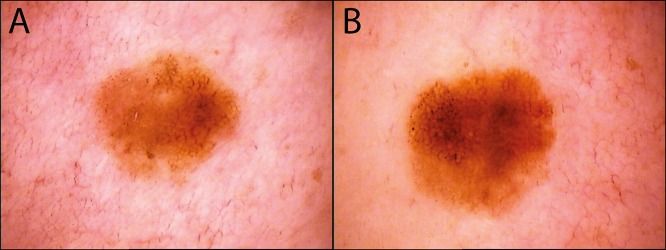
Figure 4.
While this lesion (A) initially appears inconspicuous, after (B) 6 months it shows additional black dots and asymmetric growth. Histopathological evaluation revealed a superficially spreading melanoma with an invasion depth of 0.4 mm. [Copyright: ©2018 Tschandl.]
Notably, all criteria rely heavily on asymmetry (chaos), which is one of the most (interrater) reliable features in dermatoscopy [47], but evaluation of the necessary extent of change still relies on the subjective judgment of the examining physician. Additionally, many additional factors have to be taken into account and these are (1) time, (2) medication, (3) anatomic location, and (4) age. First, with shorter intervals between 2 images, less change indicates a probable melanoma [26,48], whereas nevi generally change more slowly and in a limited fashion [39,49,50]. In contrast, recurrence of a benign nevus may occur earlier than recurring melanoma [51]. Second, new checkpoint inhibitors such as dabrafenib [52] or vemurafenib [53] may lead to drastic changes in nevi. Third, congenital nevi of the nail apparatus may show growth and involution [54]. Fourth, growing lesions raise more suspicion in older patients, as nevi are expected to change in younger patients to some extent [14,15].
General Considerations
Effectiveness
Regarding diagnostic accuracy, a meta-analysis has shown that by using SDDI, 54.6% of melanomas can be excised in situ. The number of lesions needed to monitor differs significantly between studies (31–1 008), most possibly reflecting different methods of executing SDDI. Undeniably, this number is the lowest for short-term SDDI [21,48] because it is mainly used for increasing specificity (ie, avoiding excision of single suspicious lesions rather than scanning all lesions on a patient). It therefore does not have the identical purpose as long-term SDDI and is commonly combined with other screening methods such as total-body photography, conventional skin examination, and dermatoscopy [16,17]. The number of needed excisions (NNE) to find a melanoma under long-term SDDI is low (1:12; melanoma: benign nevi as diagnosed by histopathology), but here also short-term SDDI is lower (1:5) [21], as it includes only suspicious lesions, decreasing the pretest probability of false positive findings. The low NNE for any kind of SDDI is thought to be one of the main reasons the NNE has decreased in recent years in specialized centers [55].
For every screening method, not only diagnostic accuracy, but also immediate and follow-up costs have to be taken into account. Literature suggesting that even skin cancer awareness interventions can increase costs alongside even lower quality-adjusted life years [56] show the importance of being careful and constantly critical of population-wide decisions about any kind of screening method [57]. When limiting interventions to high-risk patients, there is repeated evidence for cost-effectiveness of screening in general [58,59], and SDDI specifically [60].
Combinations
SDDI is never applied alone, but is at a minimum combined with a total-body exam from a physician with or without a handheld dermatoscope. Combinations with other examination techniques have been shown to be effective in skin cancer screening of high-risk patients.
Total-body photography (TBP): By comparing clinical images of 2 time points, TBP itself may reduce the number of excised lesions in pigmented lesion clinics [61,62] by detecting clinically new or changing lesions. Especially with the advent of high-resolution photography and automated detection of new lesions [63], TBP has the ability to become even more important in screening a large number of patients. The evidence and use of TBP for screening are promising, but an in-depth review is beyond the scope of this review. Regarding digital dermatoscopy, TBP performs very well when combined with SDDI in screening programs, as both possibly detect distinct subsets of melanoma [16,17].
Reflectance confocal microscopy (RCM): To further reduce the number of unnecessary excisions, RCM has been applied as a “second-level” exam for doubtful lesions found by digital dermatoscopy [64]. Though repeatedly found helpful in further studies [65–67], application is currently limited to highly specialized centers with access to this technique.
Limitations
At the time of publication, this review may be outdated. It gives a current review of state-of-the art knowledge about digital dermatoscopic monitoring, but screening and monitoring high-risk melanoma patients may change in the future. New methods such as automated skin lesion tracking [63,68] as well as classifications by artificial intelligence [69] will most likely fundamentally rearrange perspective in the next years.
Footnotes
Funding: None.
Competing interests: The authors have no conflicts of interest to disclose.
References
- 1.Clinical Practice Guidelines for the Management of Melanoma in Australia and New Zealand. Available: http://www.cancer.org.au/content/pdf/HealthProfessionals/ClinicalGuidelines/ClinicalPracticeGuidelines-ManagementofMelanoma.pdf.
- 2.S3-Leitlinie Melanom. Available: http://www.awmf.org/uploads/tx_szleitlinien/032-024OLl_S3_Melanom_2016-08.pdf.
- 3.Rosendahl C, Tschandl P, Cameron A, Kittler H. Diagnostic accuracy of dermatoscopy for melanocytic and nonmelanocytic pigmented lesions. J Am Acad Dermatol. 2011;64(6):1068–1073. doi: 10.1016/j.jaad.2010.03.039. [DOI] [PubMed] [Google Scholar]
- 4.Kittler H, Pehamberger H, Wolff K, Binder M. Diagnostic accuracy of dermoscopy. Lancet Oncol. 2002;3(3):159–165. doi: 10.1016/S1470-2045(02)00679-4. [DOI] [PubMed] [Google Scholar]
- 5.Sinz C, Tschandl P, Rosendahl C, et al. Accuracy of dermatoscopy for the diagnosis of nonpigmented cancers of the skin. J Am Acad Dermatol. 2017;77(6):1100–1109. doi: 10.1016/j.jaad.2017.07.022. [DOI] [PubMed] [Google Scholar]
- 6.Fusaro RM, Lynch HT, Kimberling WJ. Familial atypical multiple mole melanoma syndrome (FAMMM) Arch Dermatol. 1983;119(1):2–3. doi: 10.1001/archderm.1983.01650250006002. [DOI] [PubMed] [Google Scholar]
- 7.Clark WH, Jr, Reimer RR, Greene M, Ainsworth AM, Mastrangelo MJ. Origin of familial malignant melanomas from heritable melanocytic lesions. The B-K mole syndrome. Arch Dermatol. 1978;114(5):732–738. doi: 10.1001/archderm.1978.01640170032006. [DOI] [PubMed] [Google Scholar]
- 8.Tschandl P, Hofmann L, Fink C, Kittler H, Haenssle HA. Melanomas vs. nevi in high-risk patients under long-term monitoring with digital dermatoscopy: do melanomas and nevi already differ at baseline? J Eur Acad Dermatol Venereol. 2017;31(6):972–977. doi: 10.1111/jdv.14065. [DOI] [PubMed] [Google Scholar]
- 9.Grob JJ, Bonerandi JJ. The “ugly duckling” sign: identification of the common characteristics of nevi in an individual as a basis for melanoma screening. Arch Dermatol. 1998;134(1):103–104. doi: 10.1001/archderm.134.1.103-a. [DOI] [PubMed] [Google Scholar]
- 10.Wazaefi Y, Gaudy-Marqueste C, Avril M-F, et al. Evidence of a limited intra-individual diversity of nevi: intuitive perception of dominant clusters is a crucial step in the analysis of nevi by dermatologists. J Invest Dermatol. 2013;133(10):2355–2361. doi: 10.1038/jid.2013.183. [DOI] [PubMed] [Google Scholar]
- 11.Argenziano G, Catricalà C, Ardigo M, et al. Dermoscopy of patients with multiple nevi: improved management recommendations using a comparative diagnostic approach. Arch Dermatol. 2011;147(1):46–49. doi: 10.1001/archdermatol.2010.389. [DOI] [PubMed] [Google Scholar]
- 12.Skvara H, Teban L, Fiebiger M, Binder M, Kittler H. Limitations of dermoscopy in the recognition of melanoma. Arch Dermatol. 2005;141(2):155–160. doi: 10.1001/archderm.141.2.155. [DOI] [PubMed] [Google Scholar]
- 13.Banky JP, Kelly JW, English DR, Yeatman JM, Dowling JP. Incidence of new and changed nevi and melanomas detected using baseline images and dermoscopy in patients at high risk for melanoma. Arch Dermatol. 2005;141(8):998–1006. doi: 10.1001/archderm.141.8.998. [DOI] [PubMed] [Google Scholar]
- 14.Zalaudek I, Schmid K, Marghoob AA, et al. Frequency of dermoscopic nevus subtypes by age and body site: a cross-sectional study. Arch Dermatol. 2011;147(6):663–670. doi: 10.1001/archdermatol.2011.149. [DOI] [PubMed] [Google Scholar]
- 15.Scope A, Marchetti MA, Marghoob AA, et al. The study of nevi in children: principles learned and implications for melanoma diagnosis. J Am Acad Dermatol. 2016;75(4):813–823. doi: 10.1016/j.jaad.2016.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malvehy J, Puig S. Follow-up of melanocytic skin lesions with digital total-body photography and digital dermoscopy: a two-step method. Clin Dermatol. 2002;20(3):297–304. doi: 10.1016/S0738-081X(02)00220-1. [DOI] [PubMed] [Google Scholar]
- 17.Salerni G, Carrera C, Lovatto L, et al. Benefits of total body photography and digital dermatoscopy (“two-step method of digital follow-up”) in the early diagnosis of melanoma in patients at high risk for melanoma. J Am Acad Dermatol. 2012;67(1):e17–e27. doi: 10.1016/j.jaad.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brod C, Schippert W, Breuninger H. Dysplastic nevus syndrome with development of multiple melanomas. A surgical concept for prophylaxis. J Dtsch Dermatol Ges. 2009;7(9):773–775. doi: 10.1111/j.1610-0387.2009.07095.x. [DOI] [PubMed] [Google Scholar]
- 19.Pampena R, Kyrgidis A, Lallas A, Moscarella E, Argenziano G, Longo C. A meta-analysis of nevus-associated melanoma: prevalence and practical implications. J Am Acad Dermatol. 2017;77(5):938–945.e4. doi: 10.1016/j.jaad.2017.06.149. [DOI] [PubMed] [Google Scholar]
- 20.Kefford RF, Newton Bishop JA, Bergman W, Tucker MA. Counseling and DNA testing for individuals perceived to be genetically predisposed to melanoma: A consensus statement of the Melanoma Genetics Consortium. J Clin Oncol. 1999;17(10):3245–3251. doi: 10.1200/JCO.1999.17.10.3245. [DOI] [PubMed] [Google Scholar]
- 21.Salerni G, Terán T, Puig S, et al. Meta-analysis of digital dermoscopy follow-up of melanocytic skin lesions: a study on behalf of the International Dermoscopy Society. J Eur Acad Dermatol Venereol. 2013;27(7):805–814. doi: 10.1111/jdv.12032. [DOI] [PubMed] [Google Scholar]
- 22.Watts CG, Dieng M, Morton RL, Mann GJ, Menzies SW, Cust AE. Clinical practice guidelines for identification, screening and follow-up of individuals at high risk of primary cutaneous melanoma: a systematic review. Br J Dermatol. 2015;172(1):33–47. doi: 10.1111/bjd.13403. [DOI] [PubMed] [Google Scholar]
- 23.Thomas L, Tranchand P, Berard F, Secchi T, Colin C, Moulin G. Semiological value of ABCDE criteria in the diagnosis of cutaneous pigmented tumors. Dermatology. 1998;197(1):11–17. doi: 10.1159/000017969. [DOI] [PubMed] [Google Scholar]
- 24.Haenssle HA, Korpas B, Hansen-Hagge C, et al. Selection of patients for long-term surveillance with digital dermoscopy by assessment of melanoma risk factors. Arch Dermatol. 2010;146(3):257–264. doi: 10.1001/archdermatol.2009.370. [DOI] [PubMed] [Google Scholar]
- 25.Altamura D, Avramidis M, Menzies SW. Assessment of the optimal interval for and sensitivity of short-term sequential digital dermoscopy monitoring for the diagnosis of melanoma. Arch Dermatol. 2008;144(4):502–506. doi: 10.1001/archderm.144.4.502. [DOI] [PubMed] [Google Scholar]
- 26.Moscarella E, Tion I, Zalaudek I, et al. Both short-term and long-term dermoscopy monitoring is useful in detecting melanoma in patients with multiple atypical nevi. J Eur Acad Dermatol Venereol. 2017;31(2):247–251. doi: 10.1111/jdv.13840. [DOI] [PubMed] [Google Scholar]
- 27.Moloney FJ, Guitera P, Coates E, et al. Detection of primary melanoma in individuals at extreme high risk: a prospective 5-year follow-up study. JAMA Dermatol. 2014;150(8):819–827. doi: 10.1001/jamadermatol.2014.514. [DOI] [PubMed] [Google Scholar]
- 28.Argenziano G, Mordente I, Ferrara G, Sgambato A, Annese P, Zalaudek I. Dermoscopic monitoring of melanocytic skin lesions: clinical outcome and patient compliance vary according to follow-up protocols. Br J Dermatol. 2008;159(2):331–336. doi: 10.1111/j.1365-2133.2008.08649.x. [DOI] [PubMed] [Google Scholar]
- 29.Schiffner R, Schiffner-Rohe J, Landthaler M, Stolz W. Long-term dermoscopic follow-up of melanocytic naevi: clinical outcome and patient compliance. Br J Dermatol. 2003;149(1):79–86. doi: 10.1046/j.1365-2133.2003.05409.x. [DOI] [PubMed] [Google Scholar]
- 30.Rinner C, Tschandl P, Sinz C, Kittler H. Long-term evaluation of the efficacy of digital dermatoscopy monitoring at a tertiary referral center. J Dtsch Dermatol Ges. 2017;15(5):517–522. doi: 10.1111/ddg.13237. [DOI] [PubMed] [Google Scholar]
- 31.Gadens GA. Lack of compliance: a challenge for digital dermoscopy follow-up. An Bras Dermatol. 2014;89(2):242–244. doi: 10.1590/abd1806-4841.20142547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kittler H, Binder M. Risks and benefits of sequential imaging of melanocytic skin lesions in patients with multiple atypical nevi. Arch Dermatol. 2001;137(12):1590–1595. doi: 10.1001/archderm.137.12.1590. [DOI] [PubMed] [Google Scholar]
- 33.Madigan LM, Treyger G, Kohen LL. Compliance with serial dermoscopic monitoring: An academic perspective. J Am Acad Dermatol. Elsevier. 2016;75:1171–1175. doi: 10.1016/j.jaad.2016.07.012. [DOI] [PubMed] [Google Scholar]
- 34.Thomas L, Puig S. Dermoscopy, Digital dermoscopy and other diagnostic tools in the early detection of melanoma and follow-up of high-risk skin cancer patients. Acta Derm Venereol. 2017;9(218) doi: 10.2340/00015555-2719. [DOI] [PubMed] [Google Scholar]
- 35.Moscarella E, Pampena R, Kyrgidis A, et al. Digital dermoscopy monitoring in patients with multiple nevi: how many lesions should we monitor per patient? J Am Acad Dermatol. 2015;73(1):168–170. doi: 10.1016/j.jaad.2015.03.033. [DOI] [PubMed] [Google Scholar]
- 36.Lin MJ, Mar V, McLean C, Kelly JW. An objective measure of growth rate using partial biopsy specimens of melanomas that were initially misdiagnosed. J Am Acad Dermatol. 2014;71(4):691–697. doi: 10.1016/j.jaad.2014.04.068. [DOI] [PubMed] [Google Scholar]
- 37.Betti R, Agape E, Vergani R, Moneghini L, Cerri A. An observational study regarding the rate of growth in vertical and radial growth phase superficial spreading melanomas. Oncol Lett. 2016;12(3):2099–2102. doi: 10.3892/ol.2016.4813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Argenziano G, Longo C, Cameron A, et al. Blue-black rule: a simple dermoscopic clue to recognize pigmented nodular melanoma. Br J Dermatol. 2011;165(6):1251–1255. doi: 10.1111/j.1365-2133.2011.10621.x. [DOI] [PubMed] [Google Scholar]
- 39.Beer J, Xu L, Tschandl P, Kittler H. Growth rate of melanoma in vivo and correlation with dermatoscopic and dermatopathologic findings. Dermatol Pract Concept. 2011;1(1):59–67. doi: 10.5826/dpc.dp0101a13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lallas A, Apalla Z, Ioannides D, et al. International Dermoscopy Society. Update on dermoscopy of Spitz/Reed naevi and management guidelines by the International Dermoscopy Society. Br J Dermatol. 2017;177(3):645–655. doi: 10.1111/bjd.15339. [DOI] [PubMed] [Google Scholar]
- 41.Bär M, Tschandl P, Kittler H. Differentiation of pigmented Spitz nevi and Reed nevi by integration of dermatopathologic and dermatoscopic findings. Dermatol Pract Concept. 2012;2(1):13–24. doi: 10.5826/dpc.0201a03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tsao H, Bevona C, Goggins W, Quinn T. The transformation rate of moles (melanocytic nevi) into cutaneous melanoma: a population-based estimate. Arch Dermatol. 2003;139(3):282–288. doi: 10.1001/archderm.139.3.282. [DOI] [PubMed] [Google Scholar]
- 43.Fonseca M, Marchetti MA, Chung E, et al. Cross-sectional analysis of the dermoscopic patterns and structures of melanocytic naevi on the back and legs of adolescents. Br J Dermatol. 2015;173(6):1486–1493. doi: 10.1111/bjd.14035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kittler H, Rosendahl C, Cameron A, Tschandl P. Dermatoscopy - An Algorithmic Method Based on Pattern Analysis. Vienna: Facultas.wuv; 2016. [Google Scholar]
- 45.Kittler H, Guitera P, Riedl E, et al. Identification of clinically featureless incipient melanoma using sequential dermoscopy imaging. Arch Dermatol. 2006;142(9):1113–1119. doi: 10.1001/archderm.142.9.1113. [DOI] [PubMed] [Google Scholar]
- 46.Salerni G, Carrera C, Lovatto L, et al. Characterization of 1152 lesions excised over 10 years using total-body photography and digital dermatoscopy in the surveillance of patients at high risk for melanoma. J Am Acad Dermatol. 2012;67(5):836–845. doi: 10.1016/j.jaad.2012.01.028. [DOI] [PubMed] [Google Scholar]
- 47.Carrera C, Marchetti MA, Dusza SW, et al. Validity and reliability of dermoscopic criteria used to differentiate nevi from melanoma: a web-based international dermoscopy society study. JAMA Dermatol. 2016;152(7):798–806. doi: 10.1001/jamadermatol.2016.0624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Menzies SW, Gutenev A, Avramidis M, Batrac A, McCarthy WH. Short-term digital surface microscopic monitoring of atypical or changing melanocytic lesions. Arch Dermatol. 2001;137(12):1583–1589. doi: 10.1001/archderm.137.12.1583. [DOI] [PubMed] [Google Scholar]
- 49.Braun RP, Lemonnier E, Guillod J, Skaria A, Salomon D, Saurat JH. Two types of pattern modification detected on the follow-up of benign melanocytic skin lesions by digitized epiluminescence microscopy. Melanoma Res. 1998;8(5):431–437. doi: 10.1097/00008390-199810000-00008. [DOI] [PubMed] [Google Scholar]
- 50.Kittler H, Pehamberger H, Wolff K, Binder M. Follow-up of melanocytic skin lesions with digital epiluminescence microscopy: patterns of modifications observed in early melanoma, atypical nevi, and common nevi. J Am Acad Dermatol. 2000;43(3):467–476. doi: 10.1067/mjd.2000.107504. [DOI] [PubMed] [Google Scholar]
- 51.Blum A, Hofmann-Wellenhof R, Marghoob AA, et al. Recurrent melanocytic nevi and melanomas in dermoscopy: results of a multicenter study of the International Dermoscopy Society. JAMA Dermatol. 2014;150(2):138–145. doi: 10.1001/jamadermatol.2013.6908. [DOI] [PubMed] [Google Scholar]
- 52.McClenahan P, Lin LL, Tan J-M, et al. BRAFV600E mutation status of involuting and stable nevi in dabrafenib therapy with or without trametinib. JAMA Dermatol. 2014;150(10):1079–1082. doi: 10.1001/jamadermatol.2014.436. [DOI] [PubMed] [Google Scholar]
- 53.Giurcaneanu C, Nitipir C, Popa LG, Forsea AM, Popescu I, Bumbacea RS. Evolution of melanocytic nevi under vemurafenib, followed by combination therapy with dabrafenib and trametinib for metastatic melanoma. Acta Dermatovenerol Croat. 2015;23(2):114–121. [PubMed] [Google Scholar]
- 54.Sawada M, Ishizaki S, Kobayashi K, Dekio I, Tanaka M. Long-term digital monitoring in the diagnosis and management of congenital nevi of the nail apparatus showing pseudo-Hutchinson’s sign. Dermatol Pract Concept. 2014;4(2):37–40. doi: 10.5826/dpc.0402a07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Argenziano G, Cerroni L, Zalaudek I, et al. Accuracy in melanoma detection: a 10-year multicenter survey. J Am Acad Dermatol. 2012;67(1):54–59. doi: 10.1016/j.jaad.2011.07.019. [DOI] [PubMed] [Google Scholar]
- 56.Gordon LG, Brynes J, Baade PD, et al. Cost-effectiveness analysis of a skin awareness intervention for early detection of skin cancer targeting men older than 50 years. Value Health. 2017;20(4):593–601. doi: 10.1016/j.jval.2016.12.017. [DOI] [PubMed] [Google Scholar]
- 57.Gilmore S. Melanoma screening: informing public health policy with quantitative modelling. PLoS One. 2017;12(9):e0182349. doi: 10.1371/journal.pone.0182349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tromme I, Legrand C, Devleesschauwer B, et al. Cost-effectiveness analysis in melanoma detection: a transition model applied to dermoscopy. Eur J Cancer. 2016;67:38–45. doi: 10.1016/j.ejca.2016.07.020. [DOI] [PubMed] [Google Scholar]
- 59.Watts CG, Cust AE, Menzies SW, Mann GJ, Morton RL. Cost-effectiveness of skin surveillance through a specialized clinic for patients at high risk of melanoma. J Clin Oncol. 2017;35(1):63–71. doi: 10.1200/JCO.2016.68.4308. [DOI] [PubMed] [Google Scholar]
- 60.Tromme I, Devleesschauwer B, Beutels P, et al. Selective use of sequential digital dermoscopy imaging allows a cost reduction in the melanoma detection process: a Belgian study of patients with a single or a small number of atypical nevi. PLoS One. 2014;9(10):e109339. doi: 10.1371/journal.pone.0109339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Goodson AG, Florell SR, Hyde M, Bowen GM, Grossman D. Comparative analysis of total body and dermatoscopic photographic monitoring of nevi in similar patient populations at risk for cutaneous melanoma. Dermatol Surg. 2010;36(7):1087–1098. doi: 10.1111/j.1524-4725.2010.01589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Truong A, Strazzulla L, March J, et al. Reduction in nevus biopsies in patients monitored by total body photography. J Am Acad Dermatol. 2016;75(1):135–143.e5. doi: 10.1016/j.jaad.2016.02.1152. [DOI] [PubMed] [Google Scholar]
- 63.Korotkov K, Quintana J, Puig S, Malvehy J, Garcia R. A new total body scanning system for automatic change detection in multiple pigmented skin lesions. IEEE Trans Med Imaging. 2015;34(1):317–338. doi: 10.1109/TMI.2014.2357715. [DOI] [PubMed] [Google Scholar]
- 64.Pellacani G, Pepe P, Casari A, Longo C. Reflectance confocal microscopy as a second-level examination in skin oncology improves diagnostic accuracy and saves unnecessary excisions: a longitudinal prospective study. Br J Dermatol. 2014;171(5):1044–1051. doi: 10.1111/bjd.13148. [DOI] [PubMed] [Google Scholar]
- 65.Alarcon I, Carrera C, Palou J, Alós L, Malvehy J, Puig S. Impact of in vivo reflectance confocal microscopy on the number needed to treat melanoma in doubtful lesions. Br J Dermatol. 2014;170(4):802–808. doi: 10.1111/bjd.12678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lovatto L, Carrera C, Salerni G, Alós L, Malvehy J, Puig S. In vivo reflectance confocal microscopy of equivocal melanocytic lesions detected by digital dermoscopy follow-up. J Eur Acad Dermatol Venereol. 2015;29(10):1918–1925. doi: 10.1111/jdv.13067. [DOI] [PubMed] [Google Scholar]
- 67.Stanganelli I, Longo C, Mazzoni L, et al. Integration of reflectance confocal microscopy in sequential dermoscopy follow-up improves melanoma detection accuracy. Br J Dermatol. 2015;172(2):365–371. doi: 10.1111/bjd.13373. [DOI] [PubMed] [Google Scholar]
- 68.Bogo F, Romero J, Peserico E, Black MJ. Automated detection of new or evolving melanocytic lesions using a 3D body model. Med Image Comput Comput Assist Interv. 2014;17:593–600. doi: 10.1007/978-3-319-10404-1_74. [DOI] [PubMed] [Google Scholar]
- 69.Menegola A, Tavares J, Fornaciali M, Li LT, Avila S, Valle E. RECOD Titans at ISIC Challenge 2017 [Internet] 2017. arXiv [cs.CV] Available: http://arxiv.org/abs/1703.04819.
- 70.Bajaj S, Dusza SW, Marchetti MA, et al. Growth-curve modeling of nevi with a peripheral globular pattern. JAMA Dermatol. 2015;151(12):1338–1345. doi: 10.1001/jamadermatol.2015.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]