Abstract
Purpose:
To evaluate the validity and reliability of a novel handheld osmolarity system (I-PEN Osmolarity System; I-MED Pharma Inc, Dollard-des-Ormeaux, Quebec, Canada) for measurement of the osmolarity of a National Institute of Standards and Technology (NIST) traceable solution at a variety of ambient temperatures.
Methods:
A total of 65 measurements of an NIST solution with a verified osmolarity of 290 ± 2 mOsmol/L were taken using 3 separate handheld osmolarity systems, 65 unique single-use sensors (SUSs) from 3 different lots, and 2 adaptors. Mean values were calculated using the device, SUS, and adaptor. Measurements were taken using a handheld osmolarity system, an adaptor, and 56 individual SUSs at 6 different ambient temperatures ranging from 17.7 to 26.5°C.
Results:
Overall, the mean osmolarity measured was 294.06 mOsmol/L (SD ±2.29; percent coefficient of variation 0.78), ranging from 286.60 to 298.18 mOsmol/L. This fell within a prespecified acceptable variability of ±4 mOsmol/L (SD ±7). Mean values did not vary across devices, adaptors, or single-use sensors used. Mean osmolarity measurements increased with rising ambient temperatures, with an R2 = 0.88. The temperature correction factor was calculated to be 2.01 mOsmol/L per °C.
Conclusions:
The osmolarity system reliably and accurately measured the osmolarity of an NIST solution in a laboratory setting, using an adaptor to correct for differences in resistance between a laboratory NIST solution measurement and direct measurements on the palpebral conjunctiva of the eyelid. The handheld osmolarity system represents a rapid and accurate instrument for measurement of tear osmolarity in a simulated testing setting.
Key Words: dry eye disease, tear osmolarity, osmometer
Dry eye disease (DED) is a multifactorial condition that frequently presents with symptoms of eye discomfort, visual disturbance, and tear film instability. There may also be damage to the ocular surface.1
DED presents a diagnostic challenge for clinicians because of its multifactorial nature. It is associated with multiple underlying causative factors and nonspecific symptoms. Some patients may even be asymptomatic. Recognition and management are important regardless of symptomatology, however, because if left untreated, the condition can result in damage to the ocular surface, loss of goblet cells, and disturbances in mucin expression, which can eventually result in the release of inflammatory mediators into tears.1
Tear osmolarity and tear instability are the recognized primary mechanisms of DED. In particular, a tear osmolarity level above the homeostatic range is considered to be the causative factor driving the immunopathological cascade.
Measuring tear osmolarity has emerged as an important clinical tool for objectively and quantitatively identifying DED and monitoring its progression and response to therapy. In recent years, 2 devices using novel technology have come onto the market that simplify in-office measurement of tear osmolarity. These are replacing vapor pressure osmometers, which, while providing accurate, specific, and sensitive measurements, also require a substantial amount of time and several steps to obtain readings. A multistep process increases the likelihood of tear evaporation, which can alter results and is of particular concern among patients who already have low tear volumes, in whom the tear fluid available for analysis may fall below 10 μL.2,3
One of these new tear osmometers is the TearLab Osmolarity System (TearLab Corp, San Diego, CA), which works, according to the manufacturer's instruction manual,4 by measuring the electrical impedance of a 50-nL sample of the tear film. The TearLab device has been shown to provide highly accurate results in a point-of-care setting,2 but a recent study revealed that at least 3 consecutive measurements were required to provide clinically reliable and accurate osmolarity readings.5 The same study also showed that outlying readings frequently occur, so using the maximum osmolarity value obtained may lead to a false-positive diagnosis of DED.5
The novel handheld osmolarity system, the I-PEN Osmolarity System (I-MED Pharma Inc, Dollard-des-Ormeaux, Quebec, Canada), tested in this study has become commercially available and has been approved for sale in Canada, Europe, Australia, and South Korea and more recently submitted for approval by the US FDA.
Similar to the TearLab, the I-PEN Osmolarity System measures tear osmolarity by using electrical impedance measurements. According to the manufacturer's instructions,6 an I-PEN Osmolarity Single Use Sensor (single-use sensor) must first be inserted into the device. Next, patients are asked to gently squeeze their eyelids closed for 30 to 60 seconds. Patients then open their eyes, and the tip of the single-use sensor is placed at a 30- to 45-degree angle directly onto the palpebral conjunctiva on the inside of the lower eyelid, with both gold nodes from the single-use sensor in good contact with the palpebral conjunctiva. After several seconds in this position, the handheld osmolarity system makes an audible beep and displays the osmolarity reading on its liquid crystal display (LCD) screen (Fig. 1A).
FIGURE 1.
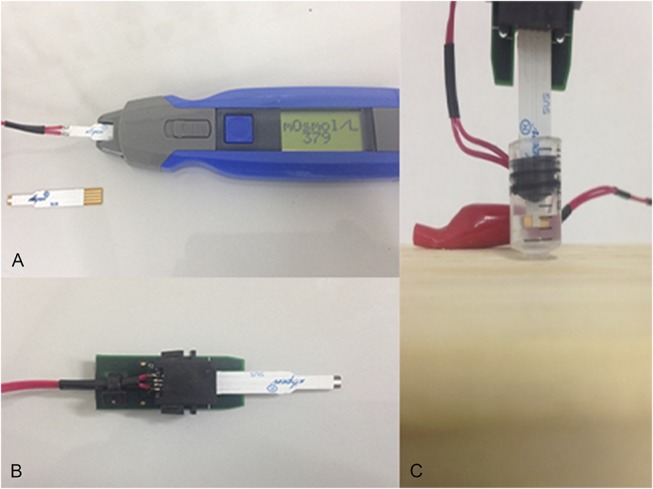
A, Handheld osmolarity system and single-use sensor. B, Adaptor and single-use sensor. C, Single-use sensor immersed in an NIST traceable solution.
Precision and accuracy remain a hallmark of good tear osmolarity measurement because differences of as little as 11 mOsmol/L can be clinically significant.7 Expected difference in tear osmolarity between mean values of patients with and without DED can be as little as 20 mOsmol/L,8 and intereye differences of ≥8 mOsmol/L are considered diagnostic of tear film instability.9
The present study was designed to test the accuracy and reliability of this novel handheld osmolarity system for measurement of osmolarity of a National Institute of Standards and Technology (NIST) traceable solution with a verified osmolarity of 290 ± 2 mOsmol/L (at 22°C referenced temperature) in a laboratory setting. Because this handheld osmolarity system is designed to test tear osmolarity in an in vivo setting by taking measurements directly on the extraocular tissues of the eye, an adaptor was required to allow for accurate measurements of a solution in an in vitro laboratory setting. The manufacturer of this handheld osmolarity system states that the adaptor simply provides an additional resistor, which mimics the resistance normally offered by the palpebral conjunctiva and allows the handheld osmolarity system to record measurements within its normal range of resistances when the single-use sensor is touched to the palpebral conjunctiva.
Previous studies have demonstrated that measurement of tear osmolarity by electrical impedance, when performed in vitro, can be affected by temperature variation.10 This handheld osmolarity system is normally used only in vivo in clinical practice, in which the effect of temperature is a negligible factor because the palpebral conjunctiva has been shown to remain at a steady temperature of 36.2 ± 0.6°C.11 Because this study examines the use of the handheld osmolarity system in vitro, additional testing of the handheld osmolarity system was undertaken at a range of temperatures to determine the effect of ambient temperature on measurement of osmolarity of the NIST traceable solution.
MATERIALS AND METHODS
This two-center study was conducted in 2 parts. The first part of the study involved repeating the same measurements at various ambient temperatures to determine the effect of ambient temperature on the accuracy and repeatability of the handheld osmolarity system measurements when used in vitro. The second part of the study involved testing the accuracy and repeatability of the handheld osmolarity system for measurement of an NIST traceable solution with a verified osmolarity of 290 ± 2 mOsmol/L in 2 separate centers on 2 separate days by separate investigators.
Part 1
For this study, the materials were the following: 3 handheld osmolarity systems (I-PEN Osmolarity System, I-MED Pharma Inc), 2 adapters (I-MED Pharma Inc), 1 single-use sensor mounted onto the adaptor, 65 single-use sensors (SUSs) packaged in the normal commercially available individual wrapped SUSs in a box of 50 units (I-MED Pharma Inc), a traceable NIST solution of 290 ± 2 mOsmol/L (Advanced Instruments, Norwood, MA), a mini digital LCD thermometer (Eccobox, KY), a syringe that was adapted to receive the 0.5 mL of NIST solution, a wooden base to hold the syringe and NIST solution, and a pipette used to deliver the 0.5-mL volume of NIST solution (Fig. 1C).
For the first part of the study, a total of 3 different handheld osmolarity systems were outfitted with a single-use sensor and one of the 2 adaptors. The adaptor is a conversion tool specifically designed to allow the handheld osmolarity system to read measurements taken directly from a solution because the device is designed to read osmolarity in tears soaking the palpebral conjunctiva in vivo. Each handheld osmolarity system was used to take 20 to 25 measurements of a traceable NIST solution of 290 ± 2 mOsmol/L, using a new single-use sensor for each measurement, for a total of 65 measurements [20 measurements taken with I-PEN 1, 25 with I-PEN 2, and 20 with I-PEN 3 (Table 1)]. Two different adaptors and SUSs from 2 different production lots were used during the study. Serial numbers of each handheld osmolarity system and adaptor and lot numbers of NIST solutions and SUSs were recorded. The temperature was recorded using the mini digital LCD thermometer by placing the temperature probe in the area immediately adjacent to the small (0.5 mL) adapted syringe container in which the NIST traceable solutions were measured. The mini digital LCD thermometer used to perform these measurements was previously calibrated against an NIST traceable glass/mercury thermometer. Temperatures recorded in both test sites ranged between 20.8 and 25.5°C.
TABLE 1.
Variability of 3 Handheld Osmolarity Systems for Measurement of an NIST Traceable Solution Corrected Using the Temperature Correction Factor
A coefficient of correlation of R2 < 0.5 was considered to represent a low precision measurement, and a coefficient of correlation of R2 > 0.7 was considered to be sufficient for measurements in a therapeutic or clinical setting. Precision was calculated as percent coefficient of variation (%CV).
Part 2
For the second part of the study, materials were essentially the same as described in part 1: 1 handheld osmolarity system, 1 adapter, 1 single-use sensor, 56 SUSs, a traceable NIST solution of 290 ± 2 mOsmol/L, a syringe, a wooden base, a pipette, and a calibrated mini digital LCD thermometer.
To determine the validity and reliability of the handheld osmolarity system for measuring a verified, NIST traceable solution of 290 ± 2 mOsmol/L at different ambient temperatures, additional measurements were taken using the handheld osmolarity system, equipped with a single-use sensor and an adaptor, as shown in Figure 1B. A total of 56 measurements were taken of the NIST solution at 6 mean temperature points ranging from 19.2 to 26.5°C and individual temperature measurements ranging from 17.7 to 26.6°C. Again, a new single-use sensor was used for each reading. The ambient temperature of the air adjacent to the NIST solution container was adjusted and recorded using a digital thermometer, and the NIST solution was allowed to equilibrate with the ambient temperature for 3 minutes between each reading.
All handheld osmolarity system readings using the adaptor were undertaken using the same procedure. A rubber piston from a syringe plunger was mounted onto the single-use sensor. The syringe barrel was fixed in an upright position onto a wooden base. Using a pipette, 0.5 mL of NIST traceable solution was removed from its original ampoule and transferred to the syringe barrel, taking care not to introduce air bubbles. The single-use sensor with the mounted rubber piston was then inserted into the adaptor at one end and into the barrel of the syringe at the other, ensuring that the NIST traceable solution liquid covered both electrodes on the single-use sensor completely (Fig. 1C). The handheld osmolarity system was then turned on. According to the laboratory protocol, the handheld osmolarity system had to be primed by using a single-use sensor with a computer chip. This single-use sensor was then removed and immediately replaced with the single-use sensor permanently connected to the adaptor, which was immersed in the NIST traceable solution (Fig. 1C).
RESULTS
Before the study, significant variation was defined as the mean of each of 65 measurements >±7 mOsmol/L of the verified 290 ± 2 mOsmol/L osmolarity of the NIST traceable solution and an SD of >±4 from the mean.
Effects of Temperature on In Vitro Handheld Osmolarity System Measurements
A total of 56 measurements were taken using the handheld osmolarity system at mean ambient temperatures ranging from 19.2 to 26.6°C (Table 2). The correlation between increasing ambient temperature and increasing osmolarity readings yielded a coefficient of determination, R2 = 0.88. The temperature correction factor was calculated as 2.01 mOsmol/L per °C using the rise over run of the calculation of the graph slope in the graph, as presented in Figure 2.
TABLE 2.
Mean Osmolarity Measurements of a Standard NIST Solution Taken at 6 Different Ambient Temperatures (°C)
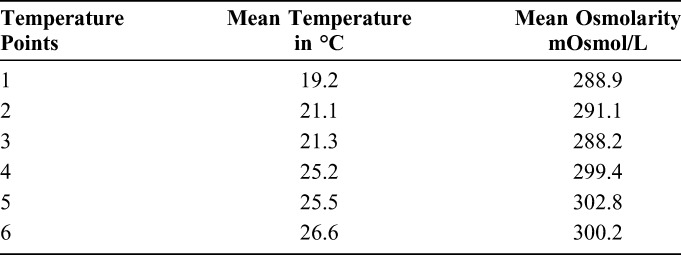
FIGURE 2.
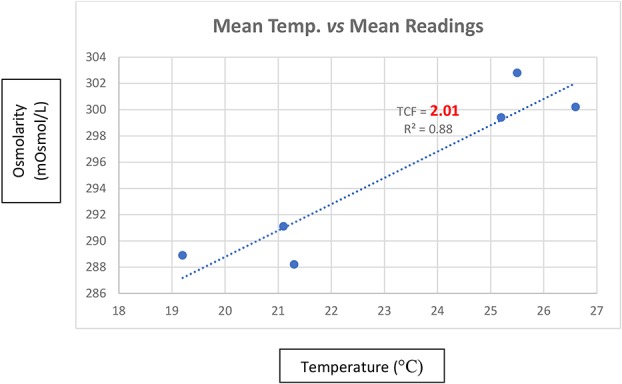
Mean osmolarity readings as a function of temperature (°C). TCF, temperature correction factor; R2, coefficient of determination.
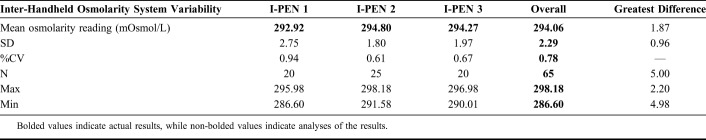
Inter-Handheld Osmolarity System Variability
The mean osmolarity measured was 294.06 mOsmol/L (SD ±2.29; %CV = 0.78), ranging from 298.18 to 286.60 mOsmol/L (Table 1).
Osmolarity measurement using the I-PEN 1 was 292.92 mOsmol/L (SD ±2.75; %CV = 0.94), with minimum and maximum values of 286.60 and 295.98 mOsmol/L, respectively. For I-PEN 2, the mean osmolarity was measured as 294.80 mOsmol/L (SD ±1.80; %CV = 0.61), with a maximum range of measurements from 291.58 to 298.18 mOsmol/L. The mean osmolarity measured by I-PEN 3 was 294.27 mOsmol/L (SD ±1.97; %CV = 0.67), ranging from 290.01 to 296.98 mOsmol/L. The greatest difference in the mean measurements with the 3 handheld osmolarity systems was 1.87 mOsmol/L (Table 1).
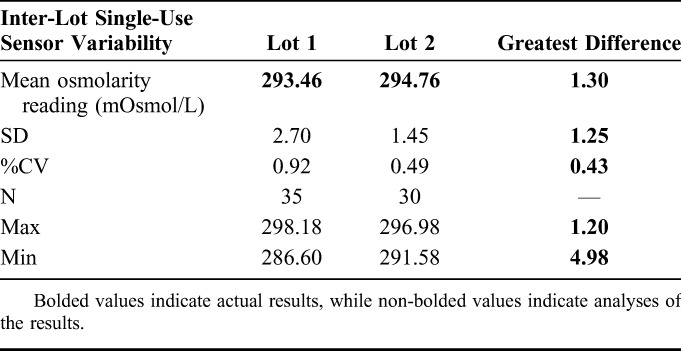
Inter-Lot Single-Use Sensor Variability
Osmolarity measurements were taken using SUSs from 2 different manufacturing lots. Measurements using Lot 1 produced a mean of 293.46 mOsmol/L (SD ±2.7; %CV = 0.92). For Lot 2, the mean measurement was 294.76 mOsmol/L (SD ±1.45; %CV = 0.49) (Table 3).
TABLE 3.
Variability of SUSs From 2 Different Manufacturers' Lots for Measurement of an NIST Traceable Solution Corrected Using the Temperature Correction Factor
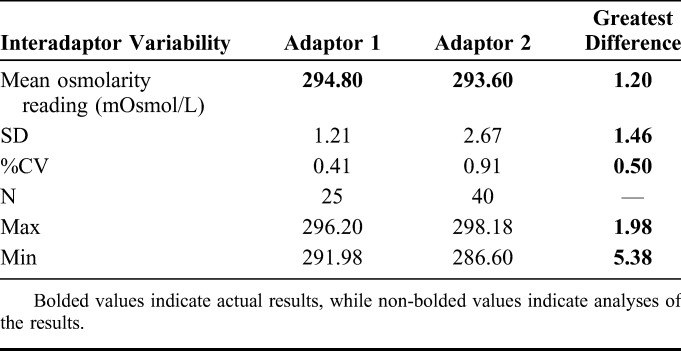
Interadaptor Variability
Osmolarity measurements were taken using 2 different adaptors attached to the handheld osmolarity system. Measurements taken using Adaptor 1 resulted in a mean of 294.8 mOsmol/L (SD ±1.21; %CV = 0.41). The mean value taken using Adaptor 2 was 293.6 mOsmol/L (SD ±2.67; %CV = 0.91) (Table 4).
TABLE 4.
Variability of 2 Different Adaptors Attached to a Handheld Osmolarity System for Measurement of an NIST Traceable Solution Corrected Using the Temperature Correction Factor
DISCUSSION
DED is a common clinical problem that requires prompt treatment to prevent serious sequelae, such as damage to the ocular surface. Symptomatology alone is not adequate for detection and diagnosis of DED.1 Therefore, objective measurements of tear osmolarity remain an important clinical tool.12 In the past several years, 2 devices that allow point-of-care testing of tear osmolarity by measuring tear electrical impedance have become available in the marketplace. These allow clinicians to rapidly and efficiently test for DED without the need to send samples for laboratory analysis.
The current investigation was undertaken to confirm that the novel handheld osmolarity system provides valid, reliable, and repeatable measurements of 1 NIST solution in the physiological range (290 mOsmol/L) in an in vitro setting. Three handheld osmolarity systems were tested using a verified NIST traceable solution with a confirmed osmolarity of 290 ± 2 mOsmol/L. Additional testing was conducted to determine whether ambient temperatures have an effect on results when measured in vitro.
The mean osmolarity of the NIST traceable solution, which has a verified osmolarity of 290 ± 2 mOsmol/L, was measured by the handheld osmolarity systems as 294.06 mOsmol/L. This falls below the prespecified definition of significant variation of >±7 mOsmol/L. It is also well within the intereye differential of 8 mOsmol/L needed to diagnose tear film instability9 and the 11 mOsmol/L differentials previously identified as clinically significant.7 Importantly, there was little variation from one measurement to the next, as demonstrated by the overall SD of ±2.29 and %CV of 0.78. This falls well below the prespecified definition of significant variation of an SD of >±4 mOsmol/L.
Testing conducted at various temperatures demonstrated that, as with any testing device that uses electrical impedance to measure tear osmolarity, the handheld osmolarity system measurements, when performed in vitro, were sensitive to changes in temperature, with a correction factor of 2.01 mOsmol/L required per °C above or below 22°C. The 22°C is confirmed by the manufacturer of the NIST traceable solution as the temperature at which the NIST solution was calibrated. This correction factor was applied to all the readings performed with the handheld osmolarity system, and the corrected readings are reported in Tables 1, 3, and 4.
The results of the handheld osmolarity system compared with the NIST standard solution of 290 ± 2 mOsmol/L are consistently higher by approximately 4 mOsmol/L. The variability in the measurements using the handheld osmolarity system, however, is remarkably small, with low SDs and %CVs regardless of the unit used, the manufacturing lot of the single-use sensor, or the adaptor used. This would indicate that the handheld osmolarity system provides accurate and consistently reproducible results when used to perform in vitro measurements.
The slight upward shift of the handheld osmolarity system readings compared with the NIST traceable solution may result from various factors, such as variability within the NIST traceable solution itself, which is rated at ±2 mOsmol/L, inability to perform direct measurements of the temperature of the NIST solution, the variability of the mini digital LCD thermometer, and the variability introduced by the use of an adaptor to allow in vitro measurements.
This study confirms that this novel handheld osmolarity system, when used with an adaptor for in vitro measurements, provides accurate, precise, and reproducible results using 3 different devices and different single-use sensor lots and adaptors. The handheld osmolarity system allows the clinician to perform osmolarity measurements in a rapid manner, with readings obtained in less than 4 seconds. There is no requirement for calibration because the device is autocalibrated with each use, and there is no requirement for sample transport, thus avoiding the inherent temperature effects associated with the other device on the market, which works as an in vitro measurement system. In addition, the measurements performed by the handheld osmolarity system are not affected by tear film volume variations, which may occur in patients with very severe dry eye. Because the measurement with this device is conducted in vivo, by simply touching the palpebral conjunctiva with the single-use sensor for a very brief time, the variability introduced for a precise tear film sample is eliminated.
This study demonstrates that this novel handheld osmolarity system, although it is not designed to perform direct in vitro measurements of a solution on its own,13 can perform accurate and reproducible readings of an NIST traceable solution when used with an adaptor in an in vitro setting for testing a solution with a known osmolarity. This study allows for the conclusion that when the I-PEN Osmolarity System is used in vivo in accordance with the manufacturer's instructions, it should provide precise and reproducible results.
Footnotes
I. Hofmann is an employee and Chief Scientific Officer of I-MED Pharma Inc. that distributes the device under study. G. Rocha is a member of a speakers' bureau of, serves as a consultant to, and has received research funds from Valeant; is a member of a speakers' bureau of, serves as a consultant to, and serves in the advisory board of Shire; is a member of a speakers' bureau of Abbott; and has received research funds from TearLab. C. C. Chan has received previous honoraria from Alcon Labs Inc, Allergan, Bausch & Lomb, Shire, Santen, and TearLab. A. Borovik and E. Gulliver have no industry support to acknowledge.
Trademarks: TearLab® is a Registered Trademark of TearLab Corporation. I-PEN® Osmolarity System is a Registered Trademark of I-MED Pharma Inc.
REFERENCES
- 1.Craig J, Nelson JD, Azar DT, et al. TFOS DEWS II report executive summary. Ocul Surf. 2017;15:802–812. [DOI] [PubMed] [Google Scholar]
- 2.Tomlinson A, McCann LC, Pearce EI. Comparison of human tear film osmolarity measured by electrical impedance and freezing point depression techniques. Cornea. 2010;29:1036–1041. [DOI] [PubMed] [Google Scholar]
- 3.Gokhale M, Stahl U, Jalbert I. In situ osmometry: validation and effect of sample collection technique. Optom Vis Sci. 2013;90:359–365. [DOI] [PubMed] [Google Scholar]
- 4.TearLab Osmolarity Test Cards Instructions for Use, 930088 Rev G. San Diego, CA: TearLab Corporation; 2015. [Google Scholar]
- 5.Szczesna-Iskander D. Measurement variability of the TearLab osmolarity system. Cont Lens Anterior Eye. 2016;39:353–358. [DOI] [PubMed] [Google Scholar]
- 6.I-Pen Osmolarity System User Manual. Dollard Des Ormeaux, Quebec: I-MED Pharma Inc; 2017. [Google Scholar]
- 7.Fortes MB, Diment BC, Di Felice U, et al. Tear fluid osmolarity as a potential marker of hydration status. Med Sci Sports Exerc. 2011;43:1590–1597. [DOI] [PubMed] [Google Scholar]
- 8.Jacobi C, Jacobi A, Kruse FE, et al. Tear film osmolarity measurements in dry eye disease using electrical impedance technology. Cornea. 2011;30:1289–1292. [DOI] [PubMed] [Google Scholar]
- 9.Lemp MA, Bron AJ, Baudouin C, et al. Tear osmolarity in the diagnosis and management of dry eye disease. Am J Ophthalmol. 2011;151:792–798. [DOI] [PubMed] [Google Scholar]
- 10.Malmberg CG. Electrical conductivity of dilute solutions of “sea water” from 5 to 120°C. J Res Natl Stand Sec A. 1965;69A:39–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holden BA, Sweeney DF. The oxygen tension and temperature of the superior palpebral conjunctiva. Acta Ophthalmol (Copenh). 1985;63:100–103. [DOI] [PubMed] [Google Scholar]
- 12.Craig JP, Nichols KK, Akpek EK, et al. TFOS DEWS II definition and classification report. Ocul Surf. 2017;15:539–574. [DOI] [PubMed] [Google Scholar]
- 13.Rocha G, Gulliver E, Borovik A, et al. Randomized, masked, in vitro comparison of three commercially available tear film osmometers. Clin Ophthalmol. 2017;27:243–248. [DOI] [PMC free article] [PubMed] [Google Scholar]