Abstract
Background and Objectives
Percutaneous peripheral nerve stimulation (PNS) is an analgesic modality involving the insertion of a lead through an introducing needle followed by the delivery of electric current. This modality has been reported to treat chronic pain as well as postoperative pain the day following knee surgery. However, it remains unknown if this analgesic technique may be used in ambulatory subjects following foot procedures beginning within the recovery room immediately following surgery, and with only short series of patients reported to date, the only available data are derived from strictly observational studies. The purposes of this proof-of-concept study were to demonstrate the feasibility of using percutaneous sciatic nerve PNS to treat postoperative pain following ambulatory foot surgery in the immediate postoperative period and provide the first available data from a randomized controlled study design to provide evidence of analgesic effect.
Methods
Preoperatively, an electrical lead (SPRINT; SPR Therapeutics, Inc, Cleveland, Ohio) was percutaneously inserted posterior to the sciatic nerve between the subgluteal region and bifurcation with ultrasound guidance. Following hallux valgus osteotomy, subjects received 5 minutes of either stimulation or sham in a randomized, double-masked fashion followed by a 5-minute crossover period and then continuous stimulation until lead removal on postoperative days 14 to 28.
Results
During the initial 5-minute treatment period, subjects randomized to stimulation (n = 4) experienced a downward trajectory in their pain over the 5 minutes of treatment, whereas those receiving sham (n = 3) reported no such change until their subsequent 5-minute stimulation crossover. During the subsequent 30 minutes of stimulation, pain scores decreased to 52% of baseline (n = 7). Three subjects (43%) used a continuous popliteal nerve block for rescue analgesia during postoperative days 0 to 3. Overall, resting and dynamic pain scores averaged less than 1 on the numeric rating scale, and opioid use averaged less than 1 tablet daily with active stimulation. One lead dislodged, 2 fractured during use, and 1 fractured during intentional withdrawal.
Conclusions
This proof-of-concept study demonstrates that percutaneous sciatic nerve PNS is feasible for ambulatory foot surgery and suggests that this modality provides analgesia and decreases opioid requirements following hallux valgus procedures. However, lead dislodgement and fracture are concerns.
Clinical Trial Registration
This study was registered at Clinicaltrials.gov, identifier NCT02898103.
Ambulatory orthopedic surgery frequently results in pain that is difficult to control with current analgesic options. Neuromodulation is a technique that involves the application of electric current to relieve pain. Although the exact mechanism remains undetermined, the most commonly cited model involves “gate control” theory in which large-diameter afferent nerve fibers are stimulated, inhibiting the transfer of pain signals from small-diameter afferent fibers to the central nervous system at the level of the spinal cord.1 Although used extensively to relieve chronic pain,2 its application to acute pain has been essentially nonexistent because of the invasive nature of implanted systems: conventional units typically require an implantable pulse generator and multiple electrodes in close proximity to the peripheral nerve that require invasive and time-consuming surgery to both place and remove.3,4
In contrast, ultrasound-guided percutaneous peripheral nerve stimulation (PNS) involves the insertion of a lead through an introducing needle, obviating the requirement of a surgical incision and avoiding overstimulation of cutaneous nerve fibers.5 Removal is achieved with gentle traction, similar to a perineural catheter used for local anesthetic administration. Theoretical benefits over opioids include a lack of systemic adverse effects such as nausea, respiratory depression, and cognitive dysfunction, as well as potential for addiction, diversion, and abuse.6 Possible benefits over local anesthetic–based peripheral nerve blocks include a lack of induced motor, sensory, and proprioception deficits that possibly increase the risk of falling and decrease the ability to participate in physical therapy.7 In addition, although perineural catheters are frequently inserted immediately adjacent to and within the same fascial plane as the target nerve, these leads are optimally inserted 1 to 3 cm away from the nerve, theoretically decreasing the risk of needle-to-nerve contact and possible neurologic injury.8
Furthermore, helically coiled electrical leads have a dramatically lower risk of infection than perineural catheters—fewer than 1 per 32,000 indwelling days9,10—and, available pulse generators (“stimulators”) are now so small that they may be simply adhered to the patient's skin with no infusion pump or large local anesthetic reservoir to carry. Combined with a historically lower dislodgement rate than perineural catheters, helically coiled leads are often used to provide PNS for multiple months and even years compared with the far more-limited duration of continuous peripheral nerve blocks, which are typically utilized for only a few days.11
The recent US Food and Drug Administration clearance of a percutaneous lead (Fig. 1A) and wearable stimulator (Fig. 1B) to treat acute postoperative pain raises the possibility of providing a nonopioid analgesic that outlasts surgical procedure-related pain.12,13 A short series of patients using this system adjacent to the femoral and sciatic nerves beginning the day following inpatient knee arthroplasty is currently In Press.14 However, subjects initiated stimulation postoperative day (POD) 1 so that no efficacy data were available for the day of surgery; all subjects remained hospitalized for at least 3 days, and no control group was included.
FIGURE 1.
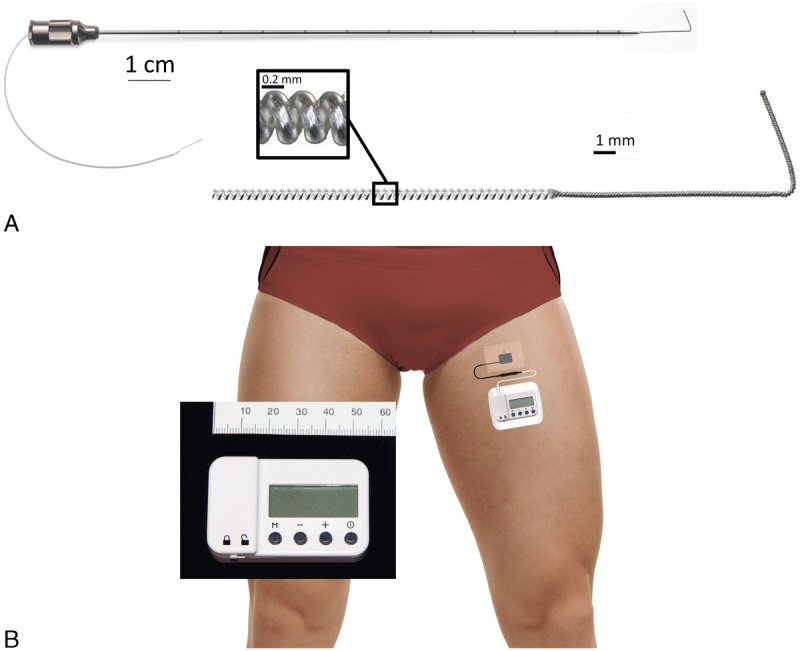
The PNS equipment used for this study: A 12.5-cm, 20-gauge needle with a preloaded helically coiled monopolar insulated electrical lead (A; MicroLead, SPR Therapeutics, Inc; illustration used with permission from B.M.I.) and a stimulator attached to the surface return electrode (B; SPR Therapeutics, Inc; illustration used with permission from B.M.I.). The power source (battery) for the pulse generator is integrated into the white surface return electrode pad.
We therefore conducted a proof-of-concept study to evaluate the feasibility of providing percutaneous sciatic nerve PNS following ambulatory hallux valgus osteotomy. A brief, randomized, double-masked, sham-controlled, partial-crossover study was performed within the recovery room, providing the first available efficacy data for an acute pain indication both with a control group and in the immediate postoperative period. Stimulation was subsequently provided to all subjects for 14 to 28 days on an outpatient basis.
METHODS
This study adhered to Good Clinical Practice quality standards and ethical guidelines defined by the Declaration of Helsinki. Study protocol approval, as well as data and safety oversight, was conducted by the University of California San Diego Institutional Review Board (IRB no. 151094; San Diego, California). Written, informed consent was obtained from all subjects participating in the trial. The trial was prospectively registered at Clinicaltrials.gov (NCT02898103, principal investigator: B.M.I., date of registration: September 13, 2016) prior to initiation of enrollment.
Enrollment was offered to adults at least 18 years old scheduled for primary, unilateral hallux valgus osteotomy. Exclusion criteria were a postoperative analgesic plan that included a single-injection peripheral nerve block in the surgical extremity; chronic high-dose opioid use (daily use within the 2 weeks prior to surgery and duration of use >4 weeks); neuromuscular deficit within the sciatic nerve distribution; anticipated magnetic resonance imaging within the following 2 weeks; compromised immune system based on medical history or other conditions that increase the risk of infection; implanted spinal cord stimulator, cardiac pacemaker/defibrillator, deep brain stimulator, or other implantable neurostimulator; history of bleeding disorder; antiplatelet or anticoagulation therapies other than aspirin; allergy to local anesthetics, occlusive dressings, tape, or bandages; incarceration; or pregnancy.
Leads were inserted within 1 week prior to surgery. Subjects were positioned prone and had their ipsilateral limb prepared with chlorhexidine gluconate/isopropyl alcohol solution and sterile drapes. The insertion point was between the sciatic bifurcation and subgluteal region, depending on operator preference after ultrasound viewing of the anatomy and experience with prior subjects' responses as the pilot study progressed. Immediately prior to lead insertion, muscle strength was evaluated with an isometric force electromechanical dynamometer (MicroFET2; Lafayette Instrument Company, Lafayette, Indiana) to measure the force produced during a maximum voluntary isometric contraction during plantar flexion. The dynamometer was placed against the bed's foot board (immobile), and the subject was asked to take 2 seconds to come to maximum effort plantar flexing, maintain this effort for 5 seconds, and then relax.
Lead Placement Technique
A portable ultrasound (M-Turbo; SonoSite, Bothell, Washington) and linear array transducer (HFL38x; SonoSite) within a sterile sleeve were utilized for lead insertion. The sciatic nerve was imaged in a transverse cross-sectional (short-axis) view. A local anesthetic skin wheal was raised lateral to the ultrasound transducer. A 12.5-cm, 20-gauge needle (Fig. 1A) with a preloaded, helically coiled, insulated lead (MicroLead; SPR Therapeutics, Inc, Cleveland, Ohio) was inserted through the skin wheal and advanced toward a point approximately 1 cm posterior to the midpoint of the sciatic nerve. When the needle tip was immediately posterior to the lateral border of the sciatic nerve, the lead was subsequently attached to an external pulse generator or “stimulator” (SPRINT; SPR Therapeutics, Inc), and a surface return electrode was placed on the ipsilateral limb (Fig. 1B).
Stimulation was delivered at 100 Hz, and amplitude (range, 0.2–20 mA) and pulse duration (range, 15–200 microseconds) were adjusted until the subject reported sensory changes in the ipsilateral leg or until muscle contractions occurred, while keeping pulse duration as low as possible.15 The optimal sensory changes targeted the toes, and if changes occurred cephalad to the foot or muscle contractions occurred, the current was decreased to the minimum settings, stimulator was switched off, and needle was advanced.
This process was repeated until sensory changes (often described as a “pleasant massage”) were reported in the toes, or the needle tip had reached the medial border of the sciatic nerve (whichever came first). If the latter, an additional pass with a new lead at a different level or slightly different trajectory was attempted until the optimal sensory changes with stimulation were achieved. The preloaded lead has a 1.5-cm anchor at its tip and is deployed by withdrawing the needle over the lead. After needle removal, the lead was again connected to the stimulator to ensure lead dislodgement did not occur during needle withdrawal (if so, a new lead was inserted). Wound closure adhesive (2-octyl 2-cyanoacrylate) was applied to the exit point (for the final 4 subjects), a connector block attached to the lead approximately 2 cm from the skin entry point, the excess lead removed, and the lead entry site was covered with a sterile dressing.
The lead was again connected to the stimulator, and settings were recorded. During stimulation, maximum voluntary isometric contraction during plantar flexion was again assessed using the same technique described for the prestimulation measurement. The stimulator was removed, and the subject returned home with the only limitation being a prohibition on submerging the lead entry site in water (eg, swimming or taking a bath). Throughout the study, subjects were asked to rate using the numeric rating scale (NRS, 0–10) both the worst and “average” pain they experienced within the specified time period.
Day of Surgery
On POD 0 prior to osteotomy, the lead was again attached to a stimulator, and the current was increased with the revised settings recorded. The stimulator allowed minimum, intermediate, and maximum pulse duration to be set by the health care provider that was subsequently controlled by subjects. The stimulator was removed, and the lead connecting wire was covered with gauze and an occlusive dressing for the surgery. Preoperatively, an ultrasound-guided perineural catheter was inserted adjacent to the sciatic nerve bifurcation using exclusively normal saline via the needle to be used as a rescue analgesia, as described previously (a minimum of 2 cm caudad to the lead).16 For surgical anesthesia, subjects received a general anesthetic with inhaled volatile anesthetic in nitrous oxide and oxygen. Intravenous fentanyl, hydromorphone, and/or morphine were administered intraoperatively, as needed.
Randomization
Within the recovery room, baseline measurements were recorded, including a pain score at the surgical site using the NRS, sensory deficits on the ipsilateral great toe (binary end point measured with an alcohol pad and von Frey filament, compared with the contralateral limb, with any decrease considered a positive finding), and the ability to move the ipsilateral great toe (binary end point with any amount of decrease considered a positive finding). Subjects were randomized to 1 of 2 groups using computer-generated lists and opaque, sealed envelopes: an initial 5 minutes of either electrical stimulation or sham, followed by 5 additional minutes of the opposite treatment. Two separate stimulators were programmed with the intermediate preoperative settings, one set to deliver active stimulation and the other set to sham (the sham mode is available for this stimulator model and is identical in appearance to the setting that delivers the current). The investigator recording the outcome measures and remaining masked to treatment group was provided the initial “Stimulator A” by an assistant, attached it to the lead, and initiated the stimulator. All investigators, clinical health care providers, and the subjects were masked to treatment group with the exception of the single assistant who opened the sealed envelope. Outcome measures were recorded every minute for 5 minutes, at which time the alternative “Stimulator B” was attached to the lead and initiated. Outcome measures were again recorded every minute for 5 minutes, after which a “Stimulator C” programmed to deliver actual current for all subjects was initiated, and end points were measured after 5 and 30 minutes.
Thirty minutes following Stimulator C initiation, a portable infusion pump (ambIT Preset; Summit Medical, Salt Lake City, Utah) and 500 mL reservoir of ropivacaine 0.2% were attached to the perineural catheter (basal 6 mL/h, bolus 4 mL, 30-minute lockout) in the off setting. From this point forward, subjects could receive intravenous fentanyl or hydromorphone prior to discharge and initiate their perineural local anesthetic infusion at any time until the catheters were removed (PODs 1–3). Subjects and their caretakers were provided verbal and written instructions on stimulator/pump and lead/catheter care and management. The contact information of an investigator was provided (available at all times during the treatment period). Subjects were discharged home with a prescription for oxycodone 5-mg tablets, replacement lead dressings, enough stimulator batteries for the duration of treatment, and their lead/catheter in situ. To increase analgesia, subjects were instructed to first increase the stimulation level on their pulse generators, then take oral opioids, and use their perineural infusion as a last resort. They were free to leave the infusion running continuously or trigger the infusion pump for any duration of their choosing.
Subjects were contacted by telephone daily for data collection on PODs 1 to 14, 30, and 90. Information included pain level at the surgical site, opioid consumption, perceived sensory deficits (cold and light touch) in the ipsilateral toes, perceived muscle strength decrease, and whether the perineural infusion had been triggered in the previous 24 hours. Perineural catheters were removed at home by the subjects or their caretakers on PODs 1 to 3, determined by subject preference. Subjects returned to the orthopedic clinic for lead withdrawal, which entailed an investigator removing the occlusive dressing and continuous, gentle traction on the lead, similar to a perineural catheter extraction.
Statistical Analysis
This was a proof-of-concept study to demonstrate feasibility and generate data to help design and power a subsequent clinical trial. Therefore, a convenience sample of 7 subjects was enrolled, and statistics were not applied to the data because of the small sample size.
RESULTS
Seven subjects enrolled, and all had a lead inserted successfully without sedation and reporting minimal pain (Tables 1 and 2). Plantar flexion maximum voluntary isometric contraction remained essentially unchanged during stimulation compared with baseline values (Table 1). Within the recovery room following osteotomy, subjects who were randomized initially to stimulation (n = 4) experienced a downward trend in their surgical pain over the 5 minutes of treatment, whereas those randomized to sham (n = 3) reported no change in their level of pain during the same period (Fig. 2). The subjects initially receiving sham treatment experienced a similar downward trend in their surgical pain over the second 5-minute crossover period of stimulation (Fig. 2). Pain levels for both groups continued to decrease to a mean of 52% of baseline (n = 7) during the subsequent 30 minutes with stimulation. No sensory deficits (light touch or cold) or motor block was detected in any subject at any time point within the first 40 minutes following baseline. Following this time point, 2 subjects requested supplemental opioids and one subsequently initiated the continuous popliteal-sciatic nerve block (50 minutes after baseline). A third subject initiated the continuous block 120 minutes after baseline to prophylactically minimize pain while returning home.
TABLE 1.
Anthropomorphic and Preoperative Lead/Stimulator Characteristics (n = 7)
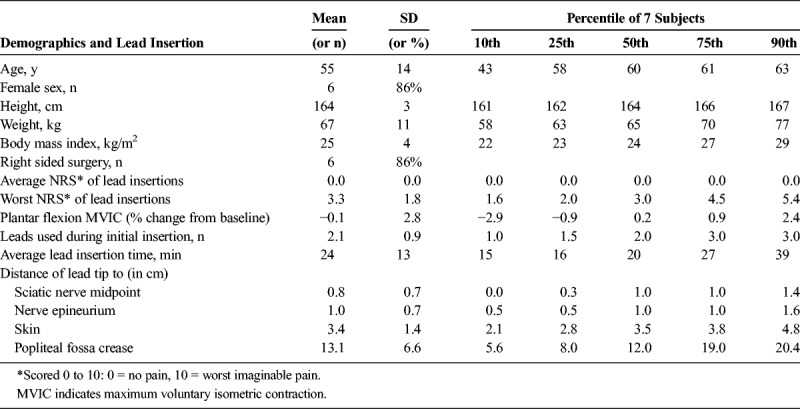
TABLE 2.
Stimulation Parameters
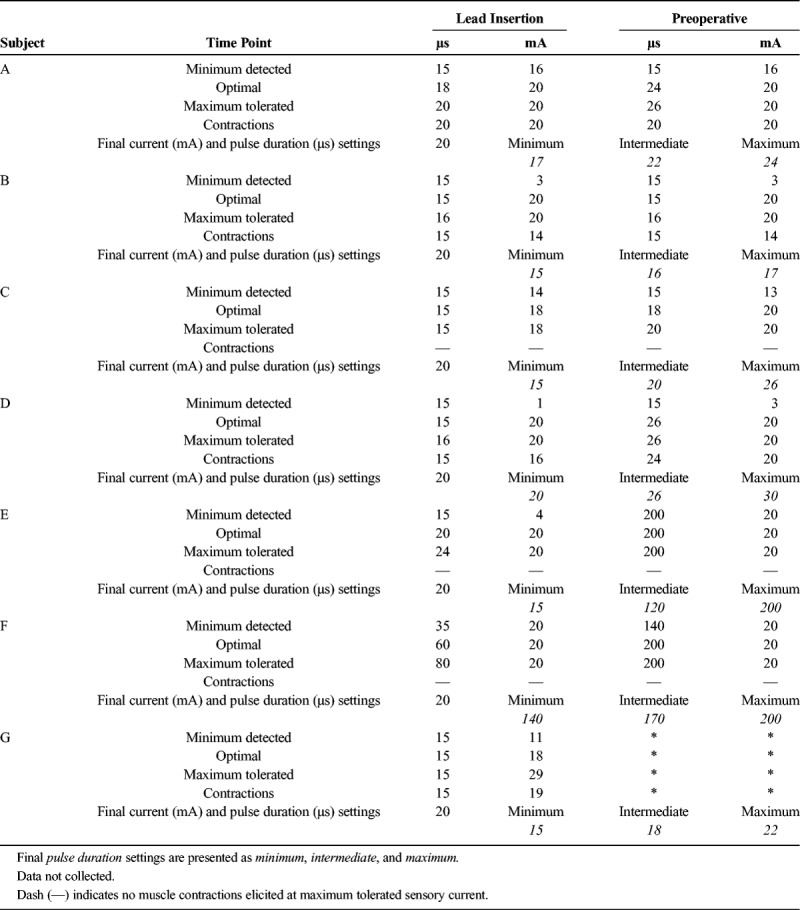
FIGURE 2.
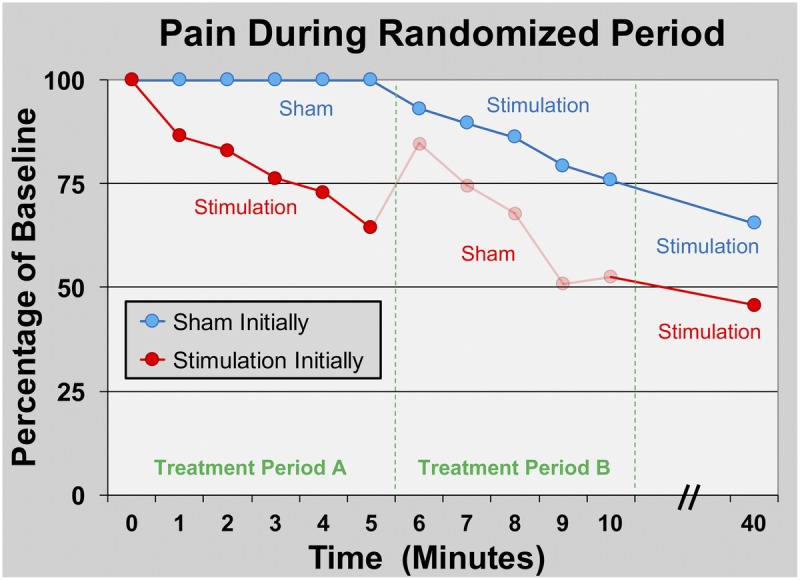
Effects of PNS of the sciatic nerve on surgical pain within the recovery room immediately following hallux valgus osteotomy. Subjects were randomized to receive 5 minutes of either electric current (“stimulation”; n = 4) or sham (n = 3) in a double-masked fashion (Treatment Period A) followed by a 5-minute crossover period (Treatment Period B). Stimulation was subsequently delivered to all subjects (n = 7) for 30 additional minutes. Data are presented as means at each time point with the original pain scores measured using the NRS. Given the relatively small sample size, statistics were not applied to the data. The group who received stimulation during the initial treatment has data shown in ghost during the subsequent period because peripheral nerve stimulation has a “carryover” effect, and these data points are therefore difficult to interpret.
No sensory deficits (light touch or cold) or motor block was detected in any subject at any time point during the follow-up period with the exception of during continuous popliteal-sciatic nerve block use. During the first 2 PODs, 3 subjects triggered their perineural infusions for at least 10 minutes each day, falling to 2 subjects on POD 3 when the catheters were discontinued. Overall, resting and dynamic pain scores as well as opioid requirements were very low (Figs. 3 and 4), with the average resting pain less than 1 on the NRS and 1 or fewer opioid tablets consumed daily with the exception of POD 2 (Fig. 5). Leads were removed on PODs 14 to 22 with 2 exceptions: one subject withdrew from the study the morning of POD 1 (details below), and another had a lead fracture on POD 7 and the lead removed 3 days later.
FIGURE 3.
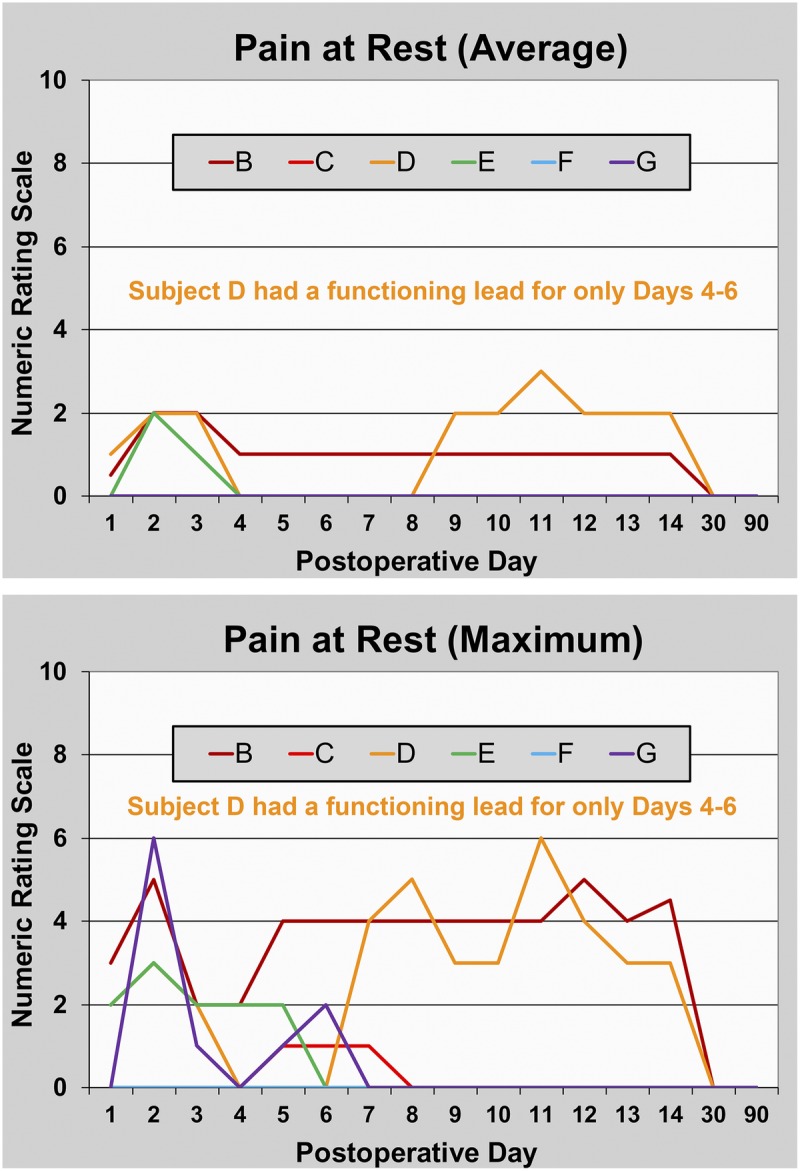
Pain at rest during PNS of the sciatic nerve following hallux valgus osteotomy. Data are presented for each subject (subject A withdrew prior to any data collection). Subject D had a functioning lead for only PODs 4 to 6. This subject and subjects B and E triggered their perineural infusions for at least 10 minutes each of the first 2 PODs, falling to 2 subjects (B and E) on POD 3.
FIGURE 4.
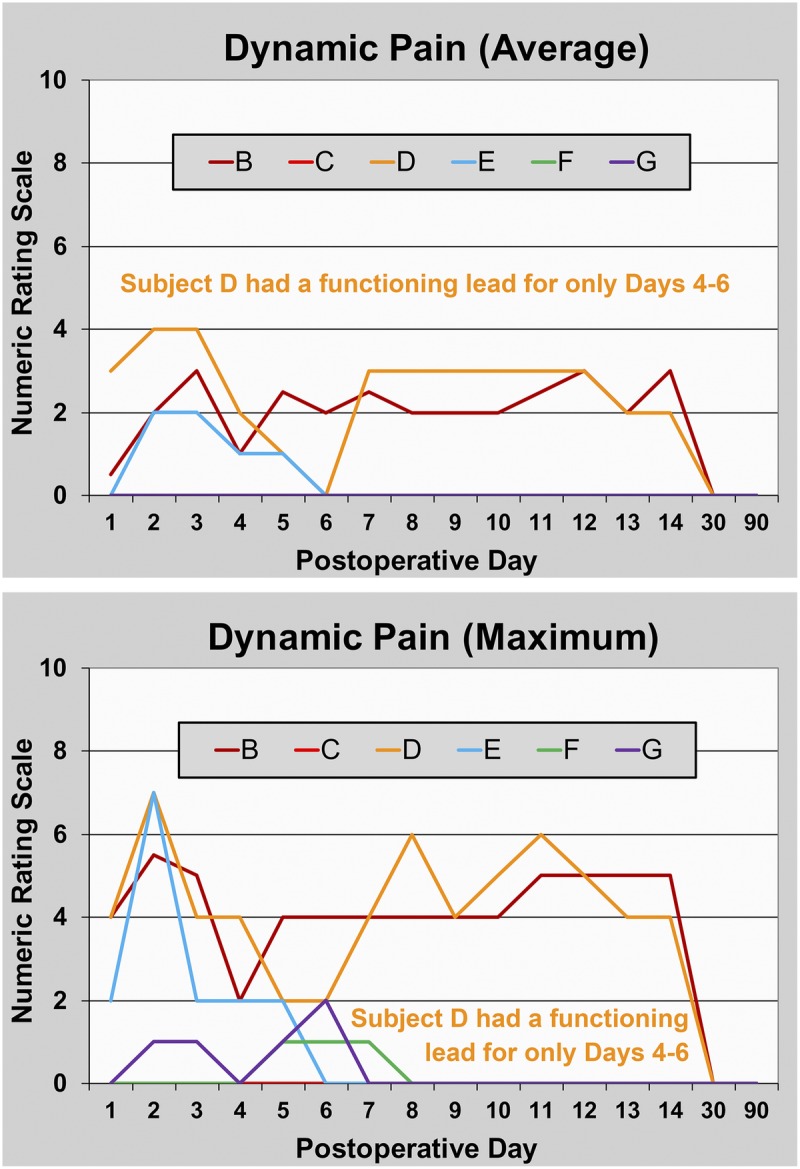
Pain with movement during PNS of the sciatic nerve following hallux valgus osteotomy. Data are presented for each subject (subject A withdrew prior to any data collection). Subject D had a functioning lead for only PODs 4 to 6. This subject and subjects B and E triggered their perineural infusions for at least 10 minutes each of the first 2 PODs, falling to 2 subjects (B and E) on POD 3.
FIGURE 5.
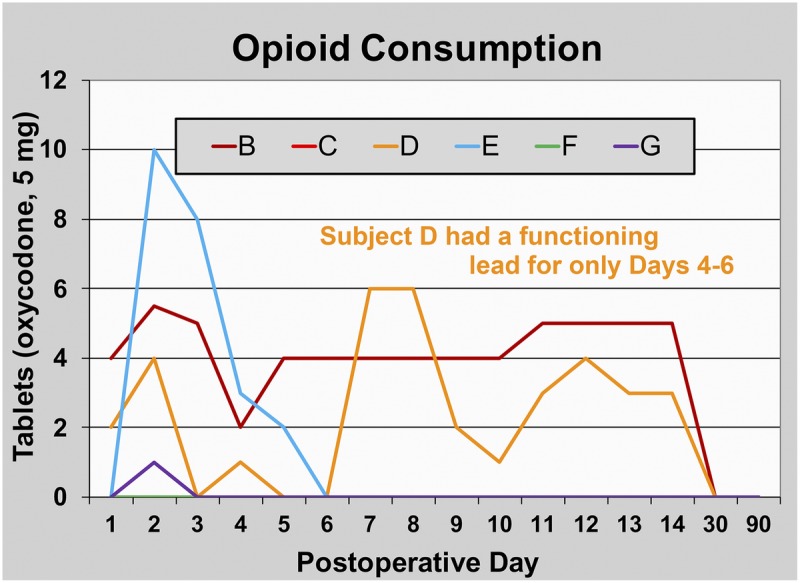
Opioid consumption during PNS of the sciatic nerve following hallux valgus osteotomy. Data are presented for each subject (subject A withdrew prior to any data collection). Subject D had a functioning lead for only PODs 4 to 6. This subject and subjects B and E triggered their perineural infusions for at least 10 minutes each of the first 2 PODs, falling to 2 subjects (B and E) on POD 3.
Adverse Events and Protocol Deviations
The first subject (A) had her lead inserted just superior to the sciatic bifurcation and reported excellent analgesia with stimulation following surgery, requesting neither supplemental opioids nor rescue perineural infusion. However, upon attempting to stand upon discharge, she experienced painful cramping within her operative foot that resolved when the electrical current was discontinued. She repeated this cycle of triggering the stimulator but again experienced the foot cramping and withdrew from the study the following morning without a recurrence of the cramping. She subsequently fell on POD 2 and fractured her radius, although this adverse event was determined to be unrelated to either the stimulator or continuous popliteal-sciatic nerve block as neither had been active for more than 24 hours. Her lead was removed on POD 8.
The fourth subject (D) had 3 leads inserted in the subgluteal area, but sensory changes were experienced only as caudad as midcalf. Therefore, on the day of surgery, her lead was removed and replaced just cephalad to the sciatic bifurcation with sensory changes in the arch of the foot. She reported excellent analgesia with stimulation following surgery, requesting neither supplemental opioids nor rescue perineural infusion. However, her intact lead was discovered dislodged the following morning and replaced on POD 3 within 2 cm of the original level. The stimulator reported a lead issue on POD 7, and her pain levels and opioid requirements dramatically increased after approximately 3 hours. She returned to clinic on POD 10, where it was discovered that her lead had fractured approximately 3.5 cm beneath the skin.
The fifth subject (E) had a very muscular thigh, and the insertion needle could not reach the sciatic nerve more cephalad than 12 cm proximal to the popliteal crease. He therefore had the lead inserted within the popliteal fossa and experienced cramping in his foot with high current following surgery. He was discharged with his stimulator set at a current below the threshold associated with the cramping, with excellent analgesia experienced until the lead was removed on POD 16.
The seventh subject had a lead inserted 5 cm proximal to the sciatic bifurcation that was discovered fractured 1.5 cm from the distal tip 1 week later on the day of surgery. It was replaced with a lead immediately cephalad to the sciatic bifurcation with sensory changes in the toes. The subject experienced almost no pain whatsoever without any opioid requirements until her stimulator reported a lead issue on POD 21. She returned to clinic the following day, and during removal, the lead fractured at the skin after approximately 2.5 cm had been extracted.
All lead remnants were left in situ. No lead infections, nerve injuries, or lead fracture remnant sequelae were identified during the final 2 data-collection phone calls on POD 30 or 90.
DISCUSSION
This proof-of-concept study demonstrates that percutaneous PNS is feasible for ambulatory foot surgery and suggests that this modality provides analgesia and decreases opioid requirements following hallux valgus procedures. To our knowledge, it is the first report of (1) using percutaneous PNS to treat postoperative pain following surgical procedures of the foot, (2) using PNS for ambulatory surgery, (3) inserting a lead in the region of the popliteal fossa for foot surgery, and (4) initiating PNS in the immediate postoperative period within the recovery room. Most importantly, this study (5) provides the first available efficacy data using a randomized, double-masked, controlled study design for an acute pain indication. Lastly, it (6) provides the first data derived from objective measurements that PNS does not induce muscle weakness, demonstrating a mean (range) plantar flexion change from baseline strength of −0.1% (−4.5% to 3.9%).
Lead Insertion Location
Because percutaneous PNS has not previously been reported for foot surgery, the optimal lead location is unknown. We therefore inserted the lead for our first subject just proximal to the sciatic bifurcation, based on experience with perineural catheters in this location for painful foot surgery.17 Although she (first subject) reported excellent analgesia, foot cramping led her to withdraw from the study. Four subsequent subjects had leads inserted in the popliteal region because of either difficulty eliciting sensory changes in the toes with a subgluteal lead (n = 1) or an inferior visualization of the sciatic nerve in the subgluteal region (n = 3). Of these 5, 3 patients (60%) reported cramping in their foot with stimulation. Of more than 50 percutaneous sciatic leads inserted at or proximal to the subgluteal region for this and previous investigations, none (0%) has induced foot cramping with stimulation.12–14 Whether this is a spurious finding, a true association, or direct causation remains to be determined, but certainly deserves further examination. Considering that the relative intraneural fascicular orientation greatly impacts the functional results of stimulation,3 the fascicular organization in the subgluteal regional may be preferred to the popliteal region when the stimulation of sensory fibers is desired over motor and mixed fascicles.
Relatedly, of the leads inserted in the popliteal fossa region, there was 1 dislodgement, 2 fractures below the skin during use, and 1 fracture during removal. In contrast, none of these adverse events occurred in subgluteal leads. Again, whether this is a spurious finding or true association/causation remains unknown. It is possible that leads inserted in the popliteal fossa region have a greater degree of applied tension due to repeated flexion and extension of the surrounding musculature relative to the subgluteal region, and this led to the increased incidence of dislodgement and fractures. Following the dislodgement in the fourth subject, we began applying wound closure adhesive at the lead entry site to aid fixation as is common with perineural catheters,18 and we have not observed a dislodgement since implementing this change (unpublished data).
Previous investigations involving the same helically coiled lead used in the current study have reported no spontaneous lead fractures during use and a 7.5% average incidence of fracture during removal.12–15,19–28 All previous fractured remnants have been left in situ with no negative sequelae reported in up to a 1-year period of assessment. Importantly, magnetic resonance imaging may be performed safely in patients with retained lead fragments at 1.5 T.29 Finally, most previously reported fractures occurred at or near the tip of the lead, leaving a relatively short remnant of less than 1.6 cm.29
Lead Design
The mean (range) insertion time for individual leads was 24 minutes (14–50 minutes), which included equipment setup for any second or third lead attempt. However, because multiple subjects had 2 (n = 2) or 3 (n = 3) lead insertions, the mean (range) overall treatment time for each subject was 55 minutes (14–99 minutes). Because these were the first leads ever used for foot surgery, we often attempted additional insertions in an effort to improve the location of induced sensory changes to/toward the toes, and many repeated insertions ultimately proved unnecessary. In addition, the number of insertion attempts and required duration of each considerably decreased with cumulative experience. One of the limitations of the current lead design is that the needle cannot be withdrawn without deploying the lead. Therefore, instead of withdrawing and repositioning the needle/lead combination if a first attempt passed the sciatic nerve without the desired response, an entirely new lead had to be inserted at a different level. This obviously added greatly to both the required insertion attempts and overall duration of treatment because multiple insertion kits and leads had to be prepared.
Limitations
Prior experience with percutaneous PNS in postoperative subjects 6 to 97 days following knee arthroplasty suggested that analgesia onset and peak were nearly instantaneous following the introduction of electrical current.12,13 We therefore designed the current randomized, sham-controlled, crossover portion of this study with only 5-minute treatment periods so that subjects randomized to sham initially would have a minimal duration without supplemental analgesia. However, our results suggest that for acute pain in the immediate postoperative period maximum PNS-induced analgesia requires far longer than 5 minutes: pain scores continued to decrease even as subjects emerged from general anesthesia through the 40-minute time point (Fig. 2). Unfortunately, no subsequent pain data were collected until the following day, so the duration for maximum analgesic effect remains to be determined.
In contrast, we were aware of a “carryover” effect following PNS so that subjects continue to receive a variable duration and degree of analgesia following electrical current discontinuation, possibly due to sustained modification of supraspinal pain processing.30 We knew that this carryover effect would make the data of the 5-minute sham period for the group who initially received active current difficult or impossible to interpret. However, to keep the double-masked study design, we had no choice but to collect the measurements from this 5-minute period. We therefore included the collected data but present it in ghost to indicate the uncertainty of its interpretation (Fig. 2).
CONCLUSIONS
This small pilot study demonstrates that percutaneous sciatic nerve PNS is feasible for ambulatory foot surgery and suggests that this modality provides analgesia and decreases opioid requirements following hallux valgus osteotomy procedures. The results of this pilot study indicate that a subsequent clinical trial is warranted.
ACKNOWLEDGMENTS
The authors thank Madelyn Bernard, RN (Hillcrest Hospital, San Diego, California), and Baharin Abdullah (Clinical Translational Research Center, University of California San Diego, La Jolla, California), for their invaluable assistance.
Footnotes
Conflict of interest: The institution of Drs. Ilfeld, Gabriel, Said, Sztain, Abramson, Khatibi, and Finneran–the University California San Diego (San Diego, CA)–has received funding and/or product for other research studies from SPR Therapeutics (Cleveland, OH).
Funding for this project provided by the University California Academic Senate (San Diego, CA) and the Department of Anesthesiology, University of California San Diego (San Diego, CA). SPR Therapeutics, Inc (Cleveland, OH), also provided the stimulators and leads used in this investigation. This company was given the opportunity to review the protocol and initial manuscript (minor revisions were suggested for each), but the investigators retained full control of the investigation, including study design, protocol implementation, data analysis, results interpretation, and manuscript preparation. The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the funding entities. None of the authors has a personal financial interest in this research.
This work was presented, in part, as a scientific abstract for the Annual Meeting of the American Society of Regional Anesthesia in New York, NY, April 19 to 21, 2018.
REFERENCES
- 1.Melzack R, Wall PD. Pain mechanisms: a new theory. Science. 1965;150:971–979. [DOI] [PubMed] [Google Scholar]
- 2.Deer TR, Mekhail N, Provenzano D, et al. The appropriate use of neurostimulation of the spinal cord and peripheral nervous system for the treatment of chronic pain and ischemic diseases: the Neuromodulation Appropriateness Consensus Committee. Neuromodulation. 2014;17:515–550. [DOI] [PubMed] [Google Scholar]
- 3.Nashold BS, Jr, Goldner JL, Mullen JB, Bright DS. Long-term pain control by direct peripheral-nerve stimulation. J Bone Joint Surg Am. 1982;64:1–10. [PubMed] [Google Scholar]
- 4.Picaza JA, Hunter SE, Cannon BW. Pain suppression by peripheral nerve stimulation. Chronic effects of implanted devices. Appl Neurophysiol. 1977;40:223–234. [DOI] [PubMed] [Google Scholar]
- 5.Huntoon MA, Burgher AH. Ultrasound-guided permanent implantation of peripheral nerve stimulation (PNS) system for neuropathic pain of the extremities: original cases and outcomes. Pain Med. 2009;10:1369–1377. [DOI] [PubMed] [Google Scholar]
- 6.Kharasch ED, Brunt LM. Perioperative opioids and public health. Anesthesiology. 2016;124:960–965. [DOI] [PubMed] [Google Scholar]
- 7.Ilfeld BM, Duke KB, Donohue MC. The association between lower extremity continuous peripheral nerve blocks and patient falls after knee and hip arthroplasty. Anesth Analg. 2010;111:1552–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ilfeld BM, Grant SA. Ultrasound-guided percutaneous peripheral nerve stimulation for postoperative analgesia: could neurostimulation replace continuous peripheral nerve blocks? Reg Anesth Pain Med. 2016;41:720–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ilfeld BM, Gabriel RA, Saulino MF, et al. Infection rates of electrical leads used for percutaneous neurostimulation of the peripheral nervous system. Pain Pract. 2017;17:753–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Capdevila X, Bringuier S, Borgeat A. Infectious risk of continuous peripheral nerve blocks. Anesthesiology. 2009;110:182–188. [DOI] [PubMed] [Google Scholar]
- 11.Shimada Y, Matsunaga T, Misawa A, Ando S, Itoi E, Konishi N. Clinical application of peroneal nerve stimulator system using percutaneous intramuscular electrodes for correction of foot drop in hemiplegic patients. Neuromodulation. 2006;9:320–327. [DOI] [PubMed] [Google Scholar]
- 12.Ilfeld BM, Gilmore CA, Grant SA, et al. Ultrasound-guided percutaneous peripheral nerve stimulation for analgesia following total knee arthroplasty: a prospective feasibility study. J Orthop Surg Res. 2017;12:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ilfeld BM, Grant SA, Gilmore CA, et al. Neurostimulation for postsurgical analgesia: a novel system enabling ultrasound-guided percutaneous peripheral nerve stimulation. Pain Pract. 2017;17:892–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ilfeld BM, Ball ST, Gabriel RA, et al. A feasibility study of percutaneous peripheral nerve stimulation for the treatment of postoperative pain following total knee arthroplasty: A case series. Neuromodulation. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rauck RL, Cohen SP, Gilmore CA, et al. Treatment of post-amputation pain with peripheral nerve stimulation. Neuromodulation. 2014;17:188–197. [DOI] [PubMed] [Google Scholar]
- 16.Ilfeld BM, Sandhu NS, Loland VJ, et al. Ultrasound-guided (needle-in-plane) perineural catheter insertion: the effect of catheter-insertion distance on postoperative analgesia. Reg Anesth Pain Med. 2011;36:261–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ilfeld BM, Morey TE, Wang RD, Enneking FK. Continuous popliteal sciatic nerve block for postoperative pain control at home: a randomized, double-blinded, placebo-controlled study. Anesthesiology. 2002;97:959–965. [DOI] [PubMed] [Google Scholar]
- 18.Klein SM, Nielsen KC, Buckenmaier CC, III, Kamal AS, Rubin Y, Steele SM. 2-Octyl cyanoacrylate glue for the fixation of continuous peripheral nerve catheters. Anesthesiology. 2003;98:590–591. [DOI] [PubMed] [Google Scholar]
- 19.Chae J, Harley MY, Hisel TZ, et al. Intramuscular electrical stimulation for upper limb recovery in chronic hemiparesis: an exploratory randomized clinical trial. Neurorehabil Neural Repair. 2009;23:569–578. [DOI] [PubMed] [Google Scholar]
- 20.Chae J, Wilson RD, Bennett ME, Lechman TE, Stager KW. Single-lead percutaneous peripheral nerve stimulation for the treatment of hemiplegic shoulder pain: a case series. Pain Pract. 2013;13:59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chae J, Yu DT, Walker ME, et al. Intramuscular electrical stimulation for hemiplegic shoulder pain: a 12-month follow-up of a multiple-center, randomized clinical trial. Am J Phys Med Rehabil. 2005;84:832–842. [DOI] [PubMed] [Google Scholar]
- 22.Yu DT, Chae J, Walker ME, Fang ZP. Percutaneous intramuscular neuromuscular electric stimulation for the treatment of shoulder subluxation and pain in patients with chronic hemiplegia: a pilot study. Arch Phys Med Rehabil. 2001;82:20–25. [DOI] [PubMed] [Google Scholar]
- 23.Yu DT, Chae J, Walker ME, Hart RL, Petroski GF. Comparing stimulation-induced pain during percutaneous (intramuscular) and transcutaneous neuromuscular electric stimulation for treating shoulder subluxation in hemiplegia. Arch Phys Med Rehabil. 2001;82:756–660. [DOI] [PubMed] [Google Scholar]
- 24.Yu DT, Chae J, Walker ME, et al. Intramuscular neuromuscular electric stimulation for poststroke shoulder pain: a multicenter randomized clinical trial. Arch Phys Med Rehabil. 2004;85:695–704. [DOI] [PubMed] [Google Scholar]
- 25.Renzenbrink GJ, IJzerman MJ. Percutaneous neuromuscular electrical stimulation (P-NMES) for treating shoulder pain in chronic hemiplegia. Effects on shoulder pain and quality of life. Clin Rehabil. 2004;18:359–365. [DOI] [PubMed] [Google Scholar]
- 26.Wilson RD, Bennett ME, Lechman TE, Stager KW, Chae J. Single-lead percutaneous peripheral nerve stimulation for the treatment of hemiplegic shoulder pain: a case report. Arch Phys Med Rehabil. 2011;92:837–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilson RD, Harris MA, Gunzler DD, Bennett ME, Chae J. Percutaneous peripheral nerve stimulation for chronic pain in subacromial impingement syndrome: a case series. Neuromodulation. 2014;17:771–776; discussion 776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilson RD, Gunzler DD, Bennett ME, Chae J. Peripheral nerve stimulation compared with usual care for pain relief of hemiplegic shoulder pain: a randomized controlled trial. Am J Phys Med Rehabil. 2014;93:17–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shellock FG, Zare A, Ilfeld BM, Chae J, Strother RB. In vitro magnetic resonance imaging evaluation of fragmented, open-coil, percutaneous peripheral nerve stimulation leads. Neuromodulation. 2018;21:276–283. [DOI] [PubMed] [Google Scholar]
- 30.Ristic D, Spangenberg P, Ellrich J. Analgesic and antinociceptive effects of peripheral nerve neurostimulation in an advanced human experimental model. Eur J Pain. 2008;12:480–490. [DOI] [PubMed] [Google Scholar]


