Abstract
To explore alterations in γ-aminobutyric acid (GABA) levels in response to levetiracetam (LEV) treatment in patients with migraine. Patients with migraine (N=14) were treated with LEV for 12 weeks. The levels of GABA+ in the anterior cingulate cortex/medial prefrontal cortex (ACC/mPFC) and the posterior cingulate cortex (PCC) were examined by proton magnetic resonance spectroscopy before (baseline) and after treatment. LEV showed good efficacy in the reduction of headache frequency and intensity in patients with migraine. Among the 14 patients, good-quality spectral data of GABA+ in the PCC region were obtained in 11 patients. There was a significant decrease in GABA+ levels in the PCC region after LEV treatment. ACC/mPFC GABA+ was assessed by proton magnetic resonance spectroscopy in eight patients with migraine. LEV had no significant effect on GABA+ levels in the ACC/mPFC region. The decreased GABA+ levels after LEV treatment in patients with migraine suggest that GABA is a migraine biomarker.
Keywords: γ-aminobutyric acid, levetiracetam, magnetic resonance spectroscopy, migraine
Introduction
Migraine is one of the most prevalent and disabling chronic conditions globally, which exerts significant burdens on individuals and the society 1. However, the underlying pathological mechanisms remain poorly understood. It is thus very important to investigate the clinical characteristics of migraine and to identify potential biomarkers, which will benefit the diagnosis and treatment of migraine.
γ-Aminobutyric acid (GABA) is a predominant inhibitory neurotransmitter in the central nervous system 2 and may serve as a possible biomarker for migraine. It is distributed widely in the brain and has been implicated in the temporal modulation of neuronal excitability and neuronal disorders, such as pain 3. As an important regulator of excitation and inhibition 4, the change in brain GABA levels could result in pathophysiological events leading to migraine 5. Therefore, it is important to study the GABA levels in migraine to gain an understanding of the pathogenesis of migraine.
Levetiracetam (LEV) is a new antiepileptic drug (AED). It can inhibit high-voltage-gated N-type Ca2+ channel current, Zn2+-associated negative modulation of GABA, and GABA release 6. In addition to epilepsy, LEV may also be effective in treating migraine according to some small open-label trials 7–9. However, to the best of our knowledge, there has been no report on the alterations in intracerebral GABA+ levels in patients with migraine after LEV treatment.
Patients and methods
Patients
Patients who were diagnosed with migraine by the Headache Specific Outpatients of Shandong Provincial Hospital were followed up between March 2016 and September 2016. This research was approved by the Human Research Ethics Committee of Shandong Provincial Hospital Affiliated to Shandong University (no. 2017-213). All participants provided written informed consent.
The patients fulfilled the International Classification of Headache Disorders-III criteria for migraine and had at least one migraine attack per month identified by the attending neurologist/physician. Exclusion criteria included nonmigrainous headaches according to International Classification of Headache Disorders-III criteria, a history of neck injury, claustrophobia or severe depression (i.e. Depression Anxiety Stress Scales-21 score of >21), pregnancy, presence of conditions that would compromise spectroscopy data (e.g. implants, tattoo, dental braces), or use of medications known to alter GABA levels.
Methods
Participants with migraine provided information on headache characteristics including history of migraine, headache frequency (attacks per month), typical duration of each migraine episode, and headache intensity in the past month using the visual analogue scale (with anchors at 0 and 10: 0=no pain, 10=worst pain possible). After participants underwent initial proton magnetic resonance spectroscopy (1H-MRS), LEV was initiated at 250 mg/day for the first week and incremented to a total dose of 500 mg/day (250 mg, twice daily) after 1 week. Following 12 weeks of treatment, 1H-MRS was repeated. During LEV therapy, patients stopped using other medications, except ibuprofen. Headache frequency and intensity were recorded at baseline and after 12 weeks of treatment.
Proton magnetic resonance spectroscopy
Scans were performed on an Achieva 3.0-T TX scanner equipped with an eight-channel phased-array head coil (Philips Healthcare, Best, The Netherlands). Before examination, T1-weighted 3D turbo field echo images were used for localization and the scanning parameters were set as follows: TR=8.1 ms; TE=3.7 ms; slice thickness=1 mm; matrix: 256×256; field of view=24×24 cm2; and flip angle=8°. The MRS voxels (volume size 3×3×3 cm3 and 3×2×3 cm3) were set on the anterior cingulate cortex/medial prefrontal cortex (ACC/mPFC) and the posterior cingulate cortex (PCC) (Fig. 1). The median sagittal plane was selected as a reference slice for voxel localization. The ACC/mPFC is the frontal part of the cingulate cortex that resembles a ‘collar’ surrounding the frontal part of the corpus callosum. The PCC is the caudal part of the cingulate cortex, located posterior to the ACC. All of the MRS voxels were positioned in such a manner as to avoid the lateral ventricle and skull.
Fig. 1.
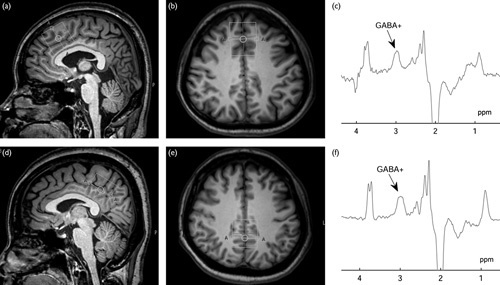
Magnetic resonance spectroscopy voxel placement and resulting spectra. T1-weighted turbo field echo (TFE) images show single-voxel placements centered on the anterior cingulated cortex/median prefrontal cortex (ACC/mPFC) in the sagittal (a), axial (b) projections and on the posterior cingulated cortex (PCC) in the sagittal (d), axial (e) projections. Representative spectra were obtained from the ACC/mPFC (c) and the PCC (f) using the MEGA-PRESS sequence. γ-Aminobutyric acid (GABA) was resolved at 3.02 ppm.
We quantified GABA using the MEGA-PRESS method 10 with the following parameters: TR=2000 ms; TE=68 ms; 320 signal averages; acquisition bandwidth=1000 Hz; and scan duration 8 min 48 s. J-evolution for GABA was refocused during odd-numbered acquisitions (ON), but not during even numbered acquisitions (OFF) by applying a Gaussian inversion pulse to the 3CH2 resonance of GABA at 1.9 ppm (ON) and at 7.5 ppm symmetrically about the water peak (OFF), respectively. Water suppression was carried out using chemical shift-selective pulses after automatic optimization. FASTMAP shimming of the volume-of-interest was performed automatically before each acquisition. Eight averages of the unsuppressed water signal were obtained for quantification. The difference between ‘ON’ and ‘OFF’ spectra provided an edited spectrum of GABA. As the measured GABA signal at 3.02 ppm is also likely to contain a contribution from co-edited macromolecules and homocarnosine resonances 11, therefore, we will refer to the GABA signals as GABA+.
Statistical analysis
The baseline GABA+ levels and clinical data were compared with comparative data after 12-week LEV monotherapy using a paired-samples t-test. Statistical significance was defined as P value of less than 0.05. Statistical analysis was carried out using the SPSS 19.0 software package (IBM Corp., Armonk, New York, USA).
T1-weighted turbo field echo images show single-voxel placements centered on the ACC/mPFC in the sagittal (a), axial (b) projections and on the PCC in the sagittal (d), axial (e) projections. Representative spectra were obtained from the ACC/mPFC (c) and the PCC (f) using the MEGA-PRESS sequence.GABA is resolved at 3.02 ppm.
Results
Clinical results
Twenty-five patients were recruited into the study. Eleven patients dropped out, either lost to follow-up, withdrew from the study, or did not adhere to medication. Finally, 14 patients with migraine were analyzed in this study. These migraine patients were between 15 and 60 years of age (mean, 40.19 years) and 12 patients were females. Patients had experienced migraine for a duration of between 3 and 30 years (mean, 12.21 years). There was one migraine patient with aura and 13 migraine patients without aura in the research. Patients treated with LEV showed a significant decrease in headache frequency and intensity compared with pretreatment (Table 1).
Table 1.
Headache of migraine patients before and after levetiracetam treatment
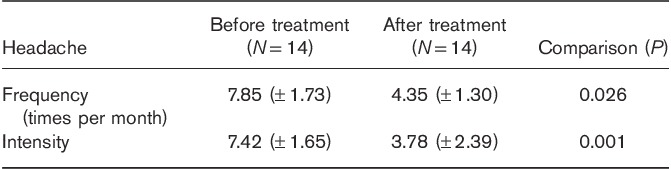
Results of proton magnetic resonance spectroscopy
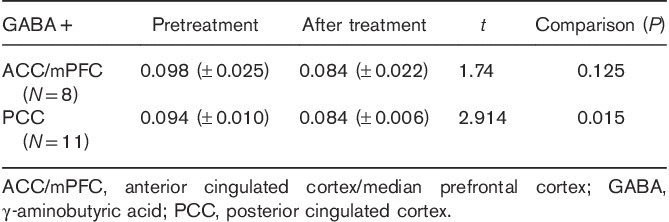
There were 14 patients who underwent MRS re-examination after 12 weeks of LEV therapy. For eleven of these patients, good-quality spectral data of GABA+ in PCC were obtained. Eight of these patients showed good-quality spectral results of GABA+ in ACC/mPFC. Neurotransmitter results from 1H-MRS are shown in Table 2.
Table 2.
GABA+ levels in migraine patients before and after levetiracetam treatment
Discussion
Here, we found a significant decrease in PCC GABA+ levels in patients with migraine responding to LEV monotherapy (P=0.015). This finding suggests an indirect influence of LEV on the GABAergic system and a potential biomarker role of GABA in migraine treatment.
The underlying pathophysiology of migraine remains incompletely defined, but it most likely involves complex mechanisms of the peripheral nervous system and the central nervous system. To date, no robust biomarkers have been identified for migraine, but many studies have suggested cerebral dysfunction in migraine patients, most notably including altered excitability of the brain, as well as abnormal levels of the neurotransmitters glutamate and GABA in the occipital cortex and the cingulate cortex, which are involved in pain processing 12–16.
GABA is one of the possible biomarkers for migraine. Given the inhibitory function of GABA in most brain synapses, including its involvement in vasodilatation, GABA has been implicated in the pathophysiology of migraine 17–20. It has been reported that patients had higher GABA levels in the cerebrospinal fluid during a migraine attack than those during the headache-free period 21. GABA levels were also found to be higher in the blood platelet of patients with tension headache 22. Recently, GABA was shown to cause vasodilation during the interaction with neurons, blood vessels, and astrocytes during cortical spreading depression and the trigeminovascular system activation 23–25.
LEV is a new AED, and its mechanism of action is not fully understood. Synaptic vesicle protein 2A has been identified as the binding site of LEV in the brain, suggesting a novel mechanism of action distinct from that of other AEDs. LEV can inhibit high-voltage-gated N-type Ca2+ channel current, Zn2+-associated negative modulation of GABA, and GABA release 6. GABA-mediated inhibition is involved in the pathophysiological events underlying migraine and may represent a target for AEDs in migraine treatment. Our results showed a good efficacy of LEV – a novel AED, in the reduction of migraine frequency and intensity in patients with migraine. We successfully obtained the PCC GABA+ levels by 1H-MRS in 11 patients with migraine. Accordingly, a significant decrease in GABA+ levels was found in the PCC region of these patients. Indeed, increased GABA concentration in the human cerebral cortex has been noted during painful stimulation 3. In addition, PCC GABA+ levels were elevated in migraine during the interictal period 15. Thus, the association between the decreased GABA+ levels and the clinical benefits of LEV treatment indicates a change in brain chemistry specific to migraine. These results together support a putative role for GABA in migraine pathophysiology. There might exist an indirect influence of LEV on the GABAergic system in patients with migraine, which warrants further studies to validate GABA as a migraine biomarker. It is speculated that the brain GABA levels decrease with the alleviation of migraine pain.
There are some limitations of the present study that should be noted. First, GABA+ was measured in single-voxel areas of the ACC/mPFC and PCC regions. It was not clear whether the changes in GABA+ levels were different in other regions. Second, spectroscopy was performed only during the interictal period. Additional spectroscopic studies during the ictal period are needed. Nevertheless, our current data have shown the decrease in GABA+ levels associated with effective treatment, shedding light on novel treatment strategies and clinical outcome.
Conclusion
LEV showed good efficacy in the reduction of headache frequency and intensity in patients with migraine and was associated with a significant decrease in GABA+ levels in the PCC region. Further study is warranted to validate GABA as a migraine biomarker.
Acknowledgements
Conflicts of interest
There are no conflicts of interest.
References
- 1.Leonardi M, Raggi A. Burden of migraine: international perspectives. Neurol Sci 2013; 34 (Suppl 1):S117–S118. [DOI] [PubMed] [Google Scholar]
- 2.Enna SJ, McCarson KE. The role of GABA in the mediation and perception of pain. Adv Pharmacol 2006; 54:1–27. [DOI] [PubMed] [Google Scholar]
- 3.Kupers R, Danielsen ER, Kehlet H, Christensen R, Thomsen C. Painful tonic heat stimulation induces GABA accumulation in the prefrontal cortex in man. Pain 2009; 142:89–93. [DOI] [PubMed] [Google Scholar]
- 4.Mann EO, Paulsen O. Role of GABAergic inhibition in hippocampal network oscillations. Trends Neurosci 2007; 30:343–349. [DOI] [PubMed] [Google Scholar]
- 5.Vecchia D, Pietrobon D. Migraine: a disorder of brain excitatory-inhibitory balance? Trends Neurosci 2012; 35:507–520. [DOI] [PubMed] [Google Scholar]
- 6.Klitgaard H, Pitkanen A. Antiepileptogenesis, neuroprotection, and disease modification in the treatment of epilepsy: focus on levetiracetam. Epileptic Disord 2003; 5 (Suppl 1):S9–S16. [PubMed] [Google Scholar]
- 7.Miller GS. Efficacy and safety of levetiracetam in pediatric migraine. Headache 2004; 44:238–243. [DOI] [PubMed] [Google Scholar]
- 8.Verma A, Srivastava D, Kumar A, Singh V. Levetiracetam in migraine prophylaxis: a randomized placebo-controlled study in a rural medical institute in northern India. Clin Neuropharmacol 2013; 36:193–197. [DOI] [PubMed] [Google Scholar]
- 9.Sadeghian H, Motiei-Langroudi R. Comparison of levetiracetam and sodium valproate in migraine prophylaxis: a randomized placebo-controlled study. Ann Indian Acad Neurol 2015; 18:45–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mescher M, Merkle H, Kirsch J, Garwood M, Gruetter R. Simultaneous in vivo spectral editing and water suppression. NMR Biomed 1998; 11:266–272. [DOI] [PubMed] [Google Scholar]
- 11.Rothman DL, Behar KL, Prichard JW, Petroff OA. Homocarnosine and the measurement of neuronal pH in patients with epilepsy. Magn Reson Med 1997; 38:924–929. [DOI] [PubMed] [Google Scholar]
- 12.Bridge H, Stagg CJ, Near J, Lau CI, Zisner A, Cader MZ. Altered neurochemical coupling in the & occipital cortex in migraine with visual aura. Cephalalgia 2015; 35:1025–1030. [DOI] [PubMed] [Google Scholar]
- 13.González de la Aleja J, Ramos A, Mato-Abad V, Martínez-Salio A, Hernández-Tamames JA, Molina JA, et al. Higher glutamate to glutamine ratios in occipital regions in women with migraine during the interictal state. Headache 2013; 53:365–375. [DOI] [PubMed] [Google Scholar]
- 14.Siniatchkin M, Sendacki M, Moeller F, Wolff S, Jansen O, Siebner H, Stephani U. Abnormal changes of synaptic excitability in migraine with aura. Cereb Cortex 2012; 22:2207–2216. [DOI] [PubMed] [Google Scholar]
- 15.Aguila ME, Lagopoulos J, Leaver AM, Rebbeck T, Hübscher M, Brennan PC, Refshauge KM. Elevated levels of GABA+ in migraine detected using 1H-MRS. NMR Biomed 2015; 28:890–897. [DOI] [PubMed] [Google Scholar]
- 16.Bigal ME, Hetherington H, Pan J, Tsang A, Grosberg B, Avdievich N, et al. Occipital levels of GABA are related to severe headaches in migraine. Neurology 2008; 70:2078–2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anwar N, Mason PFJ. Two actions of gamma-aminobutyric acid (GABA) on the responses of isolated basilar artery from the rabbit. Br J Pharmacol 1982; 75:177–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alborch E, Torregrosa G, Terrasa JC, Estrada C. GABA receptors mediate cerebral vasodilation in the unanesthetized goat. Brain Res 1984; 321:103–110. [DOI] [PubMed] [Google Scholar]
- 19.Fergus A, Lee KS. GABAergic regulation of cerebral microvascular tone in the rat. J Cereb Blood Flow Metab 1997; 17:992–1003. [DOI] [PubMed] [Google Scholar]
- 20.Barbelivien A, Noel C, MacKenzie ET, Dauphin F. Cerebrovascular evidence for a GABAergic modulation of the cholinergic vasodilatatory basalocortical system in the rat. Brain Res 1999; 834:223–227. [DOI] [PubMed] [Google Scholar]
- 21.Welch KMA, Chabi E, Bartosh K, Achar VS, Meyer JS. Cerebrospinal fluid γ aminobutyric acid levels in migraine. Br Med J 1975; 3:516–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kowa H, Shimomura T, Takahashi K. Platelet gamma-aminobutyric acid levels in migraine and tension-type headache. Headache 1992; 32:229–232. [DOI] [PubMed] [Google Scholar]
- 23.Brennan KC, Charles A. An update on the blood vessel in migraine. Curr Opin Neurol 2010; 23:266–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hamel E. Perivascular nerves and the regulation of cerebrovascular tone. J Appl Physiol 2006; 100:1059–1064. [DOI] [PubMed] [Google Scholar]
- 25.Kocharyan A, Fernandes P, Tong XK, Vaucher E, Hamel E. Specific subtypes of cortical GABA interneurons contribute to the neurovascular coupling response to basal forebrain stimulation. J Cereb Blood Flow Metab 2008; 28:221–231. [DOI] [PubMed] [Google Scholar]


