Abstract
Objective:
Seventy percent of postmenopausal women experience vasomotor symptoms, which can be highly disruptive and persist for years. Hormone therapy and other treatments have variable efficacy and/or side effects. Neurokinin B signaling increases in response to estrogen deficiency and has been implicated in hot flash (HF) etiology. We recently reported that a neurokinin 3 receptor (NK3R) antagonist reduces HF in postmenopausal women after 4 weeks of treatment. In this article we report novel data from that study, which shows the detailed time course of this effect.
Methods:
Randomized, double-blind, placebo-controlled, single-center, crossover trial of an oral NK3R antagonist (MLE4901) for vasomotor symptoms in women aged 40 to 62 years, experiencing ≥7 HF/24 hours some of which were reported as bothersome or severe (Clinicaltrials.gov NCT02668185). Thirty-seven women were randomized and included in an intention-to-treat analysis. To ascertain the therapeutic profile of MLE4901, a post hoc time course analysis was completed.
Results:
By day 3 of treatment with MLE4901, HF frequency reduced by 72% (95% CI, −81.3 to −63.3%) compared with baseline (51 percentage point reduction compared with placebo, P < 0.0001); this effect size persisted throughout the 4-week dosing period. HF severity reduced by 38% compared with baseline by day 3 (95% CI, −46.1 to −29.1%) (P < 0.0001 compared with placebo), bother by 39% (95% CI, −47.5 to −30.1%) (P < 0.0001 compared with placebo), and interference by 61% (95% CI, −79.1 to −43.0%) (P = 0.0006 compared with placebo); all continued to improve throughout the 4-week dosing period (to −44%, −50%, and −70%, respectively by day 28, all P < 0.0001 compared with placebo).
Conclusions:
NK3R antagonism rapidly relieves vasomotor symptoms without the need for estrogen exposure.
Keywords: Hot flashes, Neurokinin 3 receptor antagonist, NK3R, RCT, Sleep, Vasomotor symptoms
Seventy percent of postmenopausal women experience vasomotor symptoms, which can be highly disruptive and persist for years; 10% describe them as intolerable.1,2 For the majority of participants in the MsFLASH 02 study, the two most bothersome symptoms of menopause were vasomotor symptoms and sleep disturbance.3 Hormone therapy and other alternative treatments, including some antidepressants, gabapentin, cognitive behavioral therapy, and herbal remedies, have variable efficacy and/or limited availability, and/or significant adverse profiles with recommended contraindications for some women including those with a history of breast cancer for example.4-8 As such a novel therapeutic that safely and effectively treated hot flashes (HFs) could benefit millions of women worldwide.
Scientific research has changed our understanding of HF etiology over the last 20 years with two critical findings. The first was the role of specialized hypothalamic neurons that colocalize kisspeptin, neurokinin B (NKB), and dynorphin receptors (KNDy neurons) across the reproductive lifespan9; and the second was the work of Rance and colleagues who have elucidated the neurocircuitry of hypothalamic NKB signaling together with its receptor, the neurokinin 3 receptor (NK3R), in the thermoregulatory autonomic system in response to estrogen deficiency.10-15 Two recent publications further implicate NKB/NK3R signaling in menopausal flushing: (1) peripheral administration of NKB in premenopausal women resulted in HFs that were typical of those described by postmenopausal women,16 and (2) a population-based study suggested genetic variation in TACR3, the gene that encodes NK3R could be associated with the variability in vasomotor symptoms experienced by postmenopausal women.17 Collectively, the prior literature led us to hypothesize that NKB/NK3R signaling is critical in menopausal flushing. We therefore carried out a study to determine whether vasomotor symptoms in postmenopausal women could be attenuated by administration of an oral NK3R antagonist. This trial completed earlier this year and confirmed that an NK3R antagonist can reduce HFs in postmenopausal women after 4 weeks of treatment.18 In this article we report novel data from that study, which shows the detailed time course of this effect.
METHODS
Study design and participants
This randomized, double-blind, placebo-controlled, single-center, crossover study recruited women aged 40 to 62 years who were having at least seven flashes/24-h period (of which some were reported as being severe or bothersome), and who had not had a menstrual period for at least 12 months (Clinicaltrials.gov NCT02668185). Sixty-eight women were screened, of which 45 were confirmed eligible to enter the study which started with a 2-week baseline “run in” period to establish “steady state” and familiarity with recording symptoms.18 Thirty-seven participants were confirmed to be eligible to enter the active phase of the study, and so received 4 weeks of treatment with an oral selective NK3R antagonist twice daily (MLE4901; Millendo Therapeutics, Inc., Ann Arbor, MI) and 4 weeks of exact-match placebo twice daily in the order generated by central randomization separated by a 2-week washout period (Fig. 1).18 Participants were ambulatory during the study and no restriction was placed on lifestyle. Full details outlining inclusion and exclusion criteria and study design are as previously described.18 Approvals were granted by the West London Regional Ethics Committee (15/LO/1481), and the Medicine and Healthcare Products Regulatory Agency (EudraCT 2015-001553-32). The trial was registered in full at ClinicalTrials.gov before study start (NCT02668185), and performed in accordance with Good Clinical Practice Guidelines.
FIG. 1.
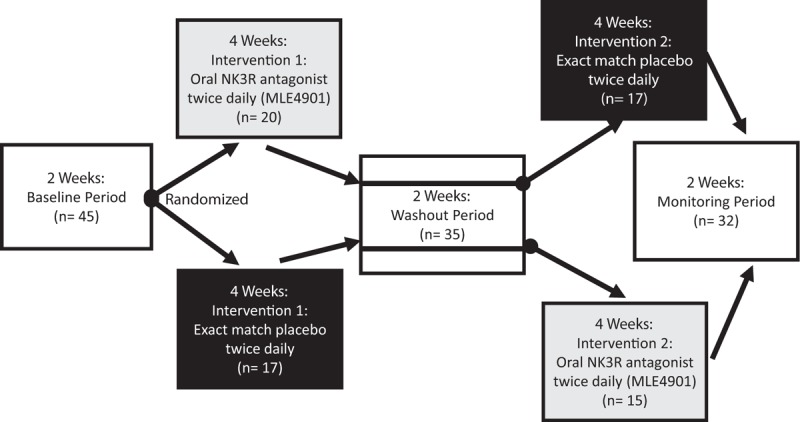
Summary of protocol: baseline period: participants underwent a 2-week period to gather baseline data on hot flush (HF) frequency, HF severity, HF bother, and perceived HF interference (Hot Flash-Related Daily Interference Scale). If the inclusion criteria regarding HF frequency and severity were met at the end of this period, then they were assigned to the active phase of the study after randomization (black circle). Intervention 1 (double-blind): all participants randomized to either 4 weeks of treatment with oral, 40 mg twice daily MLE4901 or exact-match placebo. Washout period: all participants underwent a 2-week washout period after intervention 1 (half-life of MLE4901 is 8.5 h). Intervention 2 (double-blind): all participants then switched to receive either four weeks treatment with oral twice daily exact-match placebo or oral 40 mg twice daily MLE4901 depending on which intervention they received first. Monitoring period: a subsequent 2-week period to complete safety monitoring. Figure available under the terms of the CC BY licence from http://dx.doi.org/10.1016/S0140-6736(17)30823-1 (Prague et al, Lancet, 201718).
Outcomes
The primary outcome was total number of HFs during the fourth week of treatment with MLE4901 and placebo. Secondary outcomes included HF severity, bother, interference, reproductive hormone concentrations, Menopause-Specific Quality of Life (MENQOL) domain scores, and objective measurement of HFs using a skin conductance monitor (Bahr monitor). HF frequency, severity, and bother data were collated twice daily to capture symptoms that occurred during the daytime and those that occurred during the nighttime separately. For all outcomes, outlined a priori in our protocol, comparison was made between the average daily value during the fourth week of treatment with MLE4901 and placebo, and also between the average daily value during the fourth week of both treatment periods and the second week of the baseline period. Full details outlining study design methodology are as previously described.18 Post hoc time course analysis was subsequently conducted to ascertain the therapeutic profile of MLE4901 by comparing mean daily total at day 3, and mean weekly total after week 1, week 2, week 3, and week 4 of both treatment periods, and also compared with the second week of the baseline period. To assess the impact on sleep, post hoc analyses were completed on daytime and nighttime vasomotor symptoms separately, and a selection of individual MENQOL and Hot Flash Related Daily Interference Scale (HFRDIS) items (MENQOL: “difficulty sleeping,” “lethargy,” “tiredness,” “stamina,” “muscle ache,” “physical strength”; HFRDIS: “sleep,” “concentration”). All post hoc analyses are reported in this article.
Statistical analysis
Our a priori statistical plan was strictly followed as previously described18; in summary, analyses were completed for the intention-to-treat (ITT; n = 37) and per-protocol (n = 28) data sets using generalized linear mixed models and standard crossover analysis to estimate the adjusted (least squares) means, and differences between treatment means, together with associated 95% CIs and P value. A similar approach was used for our post hoc analyses in our modified ITT cohort using only observed data rather than an imputation technique (therefore using a minimum of n = 33 and maximum n = 35 out of a total number of 37 participants, except for percentage change from baseline for the HFRDIS items “sleep” and “concentration” where the minimum was n = 27 due to 7 participants scoring 0 at baseline). Data were analyzed using generalized linear mixed models with an unstructured covariance matrix. For all models used, a standard crossover analysis was implemented with period, administration sequence, and treatment as fixed effects and subject as a random effect as previously described.18 In the a priori analyses, the final model only necessitated inclusion of the baseline value as a covariate.18 Similarly, our post hoc analyses only required the baseline value as a covariate as well. For each subject, the percentage change from baseline was calculated at each time point, with baseline defined from the data captured during the second week of the baseline period. The percentage change from baseline was then analyzed using the above-described generalized linear mixed model. From each model, as before, adjusted (least squares) means and differences between treatment means were estimated, together with associated 95% CIs, and a P value from a comparison of the mean values of the two treatments.18 Post hoc analyses of linear correlation calculated the Pearson correlation coefficient. A priori sample size and power calculation were performed using published data from studies with similar methodology; including an anticipated 25% improvement in symptoms with placebo19-23 as previously described.18
Funding
This was an academic investigator initiated and led study, which was funded by the UK Medical Research Council (grant reference MR/M024954/1) and an National Institute for Health Research Professorship to WSD (grant reference RP-2014-05-001).
RESULTS
Full results of the a priori outcomes (mean HF frequency, severity, bother, interference, MENQOL domains, and sweat monitor data during the final week of the 4-wk treatment period with MLE4901 and placebo), luteinizing hormone pulsatility, and safety data are as previously reported.18
Post hoc analysis of questionnaire data (minimum n = 33 participants, maximum n = 35 participants) demonstrated that by day 3 of treatment with MLE4901, HF frequency reduced by 72% compared with baseline (95% CI, −81.3 to −63.3%; 51 percentage point decrease compared with placebo, P < 0.0001) and this effect size persisted throughout the 4-week dosing period. HF severity, bother, and interference, however, continued to improve throughout dosing. At day 3 HF severity reduced by 38% compared with baseline (95% CI, −46.1 to −29.1%; 31 percentage point reduction compared with placebo, P < 0.0001), which then reduced further to −43% by day 14 and −44% by day 28 (39 percentage point reduction compared with placebo); bother reduced by 39% (95% CI, −47.5 to −30.1; 34 percentage point reduction compared with placebo, P < 0.0001), which then reduced further to −45% by day 14 and −50% by day 28 (46 percentage point reduction compared with placebo), and interference reduced by 61% (95% CI, −79.1 to −43.0%; 37 percentage point reduction compared with placebo, P = 0.0006), which then reduced further to −64% by day 14 and −70% by day 28 (40 percentage point reduction compared with placebo) (for full time course data, see Fig. 2; day 28 data as previously reported [ITT: n = 37]18). Continued improvement in HF symptoms over the 4-week period of treatment was not seen with placebo (Fig. 2). HF frequency, severity, and bother were all positively correlated (r = 0.76-0.93, P < 0.0001). HF interference was also positively correlated with frequency, severity, and bother, but the strength of association was weaker (r = 0.62-0.65, P < 0.0001). Post hoc analysis also demonstrated that a similar improvement in HF symptoms was achieved during the daytime as during the nighttime after treatment with MLE4901, and again the improvement was rapid (Table 1).
FIG. 2.
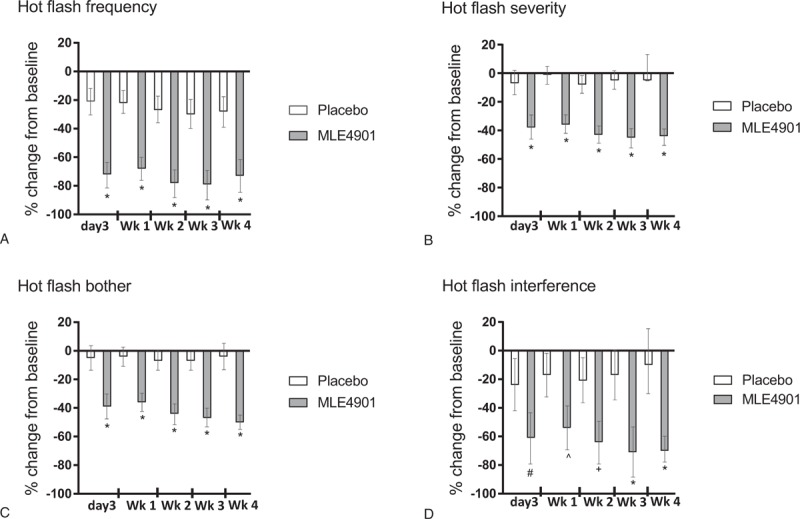
Hot flash frequency (A), severity (B), bother (C), and interference (D) outcomes: results are presented as percentage change with 95% CIs from baseline at each time point during the treatment period (ie, on day 3 of treatment, and then weekly mean total for each week (wk) of the 4-week treatment period for both placebo (white) and MLE4901 (gray). Minimum n = 33; maximum n = 37. ∗P < 0.0001, #P = 0.0006, ^P = 0.0011, +P = 0.0001. Week 4 data adapted from Prague et al, Lancet, 201718.
TABLE 1.
Hot flash frequency, severity, and bother during day time and night time
| Daytime vasomotor symptoms | Night vasomotor symptoms | |||||||||||
| n | Placebo (PBO) | n | MLE4901 (MLE) | Percentage point difference (MLE-PBO) | P | n | Placebo (PBO) | n | MLE4901 (MLE) | Percentage point difference (MLE-PBO) | P | |
| Frequency | ||||||||||||
| Day 3 | 34 | −25% (−35 to −16%) | 34 | −75% (−85 to −66%) | −50 (−62 to −38) | <0.0001 | 34 | −16% (−29 to −4%) | 34 | −70% (−83 to −57%) | −54 (−70 to −37) | <0.0001 |
| Week 1 | 34 | −24% (−33 to −15%) | 35 | −72% (−80 to −63%) | −48 (−59 to −37) | <0.0001 | 34 | −18% (−29 to −4%) | 35 | −63% (−72 to −54%) | −45 (−57 to −33) | <0.0001 |
| Week 2 | 34 | −29% (−38 to −19%) | 34 | −81% (−91 to −71%) | −52 (−66 to −39) | <0.0001 | 34 | −22% (−33 to −11%) | 34 | −75% (−86 to 63%) | −52 (−69 to −36) | <0.0001 |
| Week 3 | 34 | −30% (−40 to −19%) | 34 | −82% (−93 to −71%) | −52 (−67 to −37) | <0.0001 | 34 | −28% (−38 to −17%) | 34 | −76% (−86 to −65%) | −48 (−63 to −33) | <0.0001 |
| Week 4 | 34 | −29% (−40 to −18%) | 34 | −82% (−93 to −72%) | −53 (−68 to −38) | <0.0001 | 34 | −22% (−35 to −10%) | 34 | −78% (−90 to −65%) | −55 (−72 to −39) | <0.0001 |
| Severity | ||||||||||||
| Day 3 | 33 | −4% (−13 to 5%) | 34 | −40% (−48 to −31%) | −36 (−47 to −24) | <0.0001 | 33 | −6% (−16 to 4%) | 35 | −37% (−47 to −27%) | −31 (−44 to −18) | <0.0001 |
| Week 1 | 34 | −4% (−10 to 3%) | 35 | −38% (−44 to −32%) | −34 (−42 to −26) | <0.0001 | 34 | −1% (−8 to 6%) | 35 | −35% (−42 to −28%) | −34 (−43 to −25) | <0.0001 |
| Week 2 | 34 | −8% (−15 to −0.4%) | 34 | −43% (−50 to −36%) | −36 (−46 to −26) | <0.0001 | 34 | −4% (−12 to 4%) | 34 | −43% (−51 to −36%) | −40 (−50 to −29) | <0.0001 |
| Week 3 | 34 | −7% (−14 to −0.4%) | 34 | −47% (−54 to −41%) | −40 (−49 to −31) | <0.0001 | 34 | −4% (−12 to 3%) | 34 | −46% (−53 to −38%) | −41 (−51 to −32) | <0.0001 |
| Week 4 | 34 | −8% (−15 to −1%) | 34 | −45% (−52 to −38%) | −37 (−46 to −29) | <0.0001 | 34 | −3% (−11 to 5%) | 34 | −47% (−55 to −39%) | −44 (−54 to −34) | <0.0001 |
| Bother | ||||||||||||
| Day 3 | 34 | −7% (−16 to 1%) | 33 | −42% (−51 to −33%) | −35 (−47 to −23) | <0.0001 | 33 | −6% (−16 to 4%) | 35 | −37% (−47 to −27%) | −31 (−45 to −17) | 0.0001 |
| Week 1 | 34 | −7% (−14 to −1%) | 35 | −40% (−47 to −34%) | −33 (−41 to − 24) | <0.0001 | 34 | −2% (−9 to 5%) | 35 | −36% (−43 to −29%) | −33 (−43 to −24) | <0.0001 |
| Week 2 | 34 | −9% (−17 to −2%) | 34 | −47% (−55 to −40%) | −38 (−48 to −28) | <0.0001 | 34 | −5% (−13 to 3%) | 34 | −44% (−52 to −36%) | −40 (−51 to −28) | <0.0001 |
| Week 3 | 34 | −10% (−17 to −4%) | 34 | −50% (−57 to −44%) | −40 (−49 to −31) | <0.0001 | 34 | −7% (−14 to 1%) | 34 | −46% (−54 to −39%) | −40 (−50 to −29) | <0.0001 |
| Week 4 | 34 | −9% (−15 to −3%) | 34 | −51% (−58 to −45%) | −42 (−51 to −33) | <0.0001 | 34 | −5% (−13 to 3%) | 34 | −48% (−56 to −40%) | −43 (−54 to −32) | <0.0001 |
Results are presented as percentage change with 95% CIs from baseline on day 3 of treatment and mean weekly total for each week (week 1, week 2, week 3, and week 4) for both placebo and MLE4901 and treatment periods. Daytime symptoms: all symptoms from the time of getting up to going to bed; nighttime symptoms: all symptoms from going to bed to getting up the following morning.
The psychosocial and physical domains of the MENQOL questionnaires significantly improved as a result of treatment with MLE4901.18 Post hoc analysis suggested that this was due to improved sleep as items less likely to be related to this such as “muscle ache” and “physical strength” were not significantly different (P = 0.3685 and P = 0.7808, respectively) after treatment with MLE4901, whereas those more likely to be related to improved sleep such as “difficulty sleeping,” “tiredness,” and “lethargy” were (P < 0.0001, P = 0.0019, and P = 0.0175, respectively) (Table 2). Improvements in sleeping, tiredness, and lethargy were significant by day 3 of treatment with MLE4901. Similar results were seen in post hoc analysis of two of the individual items of the HF-related daily interference score (HFRDIS): both “sleep” and “concentration” (n = 27-29 as 7 participants scored 0 at baseline) significantly improved with treatment with MLE4901, and again as early as day 3 ( Table 3). There was a linear concordance between the two sleep items in the two questionnaire measures “difficulty sleeping” in MENQOL and “sleep” in HFRDIS (r = 0.70, P < 0.0001).
TABLE 2.
Questionnaire items from MENQOL which either are or are not likely related to improved sleep
| MENQOL item | n | Placebo (PBO) | n | MLE4901 (MLE) | Percentage point difference (MLE-PBO) | P |
| Difficulty sleeping | ||||||
| Day 3 | 34 | 14% (−23 to 50%) | 34 | −16% (−52 to 21%) | −29 (−66 to 7) | 0.1111a |
| Week 1 | 34 | 16% (−10 to 42%) | 35 | −13% (−39 to −13%) | −29 (−57 to −1) | 0.0463a |
| Week 2 | 34 | 12% (−6 to −30%) | 34 | −30% (−48 to −12%) | −41 (−63 to −20) | 0.0006a |
| Week 3 | 34 | 2% (−14 to −19%) | 34 | −34% (−51 to −18%) | −37 (−57 to −16) | 0.0012a |
| Week 4 | 34 | 14% (−4 to 32%) | 34 | −42% (−60 to −24%) | −56 (−80 to −32) | <0.0001a |
| Lethargy | ||||||
| Day 3 | 34 | 3% (−11 to 18%) | 35 | −15% (−29 to −1%) | −18 (−37 to 0) | 0.0474 |
| Week 1 | 34 | −2% (−13 to 9%) | 35 | −13% (−24 to −2%) | −11 (−22 to 1) | 0.0608 |
| Week 2 | 34 | 1% (−9 to 10%) | 34 | −13% (−23 to −3%) | −14 (−25 to −2) | 0.0233 |
| Week 3 | 34 | 1% (−13 to 15%) | 34 | −15% (−28 to −1%) | −16 (−33 to 1) | 0.0657 |
| Week 4 | 34 | 6% (−9 to 20%) | 34 | −17% (−32 to −3%) | −23 (−41 to −5) | 0.0128 |
| Tiredness | ||||||
| Day 3 | 34 | −6% (−19 to 8%) | 35 | −18% (−31 to −5%) | −13 (−22 to −3) | 0.0132 |
| Week 1 | 34 | −0.1% (−11 to 11%) | 35 | −16% (−27 to −5%) | −16 (−26 to −5) | 0.0042 |
| Week 2 | 34 | 0.4% (−11 to 12%) | 34 | −15% (−27 to −3%) | −15 (−28 to −3) | 0.0210 |
| Week 3 | 34 | −0.4% (−12 to 12%) | 34 | −23% (−36 to 13%) | −23 (−37 to −9) | 0.0023 |
| Week 4 | 34 | 1% (−10 to −12%) | 34 | −24% (−36 to −12%) | −25 (−37 to −13) | 0.0002 |
| Stamina | ||||||
| Day 3 | 34 | 2% (−18 to 22%) | 35 | −10% (−30 to 11%) | −12 (−38 to 15) | 0.3730 |
| Week 1 | 34 | 4% (−8 to 16%) | 35 | −6% (−18 to 7%) | −9 (−23 to 5) | 0.1790 |
| Week 2 | 34 | 9% (−3 to 21%) | 34 | −2% (−14 to 10%) | −11 (−22 to 1) | 0.0693 |
| Week 3 | 34 | 9% (−6 to 24%) | 34 | −6% (−20 to 10%) | −15 (−33 to 4) | 0.1177 |
| Week 4 | 34 | 5% (−10 to 19%) | 34 | −7% (−21 to 7%) | −12 (−26 to 3) | 0.1044 |
| Muscle ache | ||||||
| Day 3 | 34 | 4% (−17 to 25%) | 34 | −1% (−22 to 20%) | −5 (−33 to 24) | 0.7278 |
| Week 1 | 34 | 12% (−10% to 33%) | 35 | 13% (−8 to 35%) | 2 (−19 to 22) | 0.8680 |
| Week 2 | 34 | 15% (−10 to 40%) | 34 | 25% (−1 to 50%) | 10 (−9 to 28) | 0.2938 |
| Week 3 | 34 | 10% (−17 to 38%) | 34 | 16% (−12 to 43%) | 5 (−14 to 25) | 0.5813 |
| Week 4 | 34 | 15% (−14 to 43%) | 34 | 22% (−7 to −50%) | 7 (−9 to 23) | 0.3685 |
| Physical strength | ||||||
| Day 3 | 34 | −1% (−11 to 9%) | 35 | −1% (−12 to 9%) | 0 (−6 to 6) | 0.9346 |
| Week 1 | 34 | 2% (−7 to 11%) | 35 | 0.2% (−9 to 9%) | −2 (−10 to 6) | 0.5934 |
| Week 2 | 34 | 5% (−6 to 15%) | 34 | −2% (−13 to 8%) | −7 (−18 to 5) | 0.1810 |
| Week 3 | 34 | 12% (−6 to 29%) | 34 | −2% (−20 to 16%) | −13 (−37 to 10) | 0.2507 |
| Week 4 | 34 | −2% (−14 to 10%) | 34 | −4% (−16 to 9%) | −2 (−13 to 10) | 0.7808 |
Results are presented as percentage change with 95% CIs from baseline on day 3 of treatment and mean weekly total for week 1, week 2, week 3, and week 4 of the treatment periods for both placebo and MLE4901.
aSkewed data. Italics—significant P value.
MENQOL, Menopause-Specific Quality of Life.
TABLE 3.
Questionnaire items from HFRDIS which are likely related to improved sleep
| HFRDIS item | n | Placebo (PBO) | n | MLE4901 (MLE) | Percentage point difference (MLE-PBO) | P |
| Sleep | ||||||
| Day 3 | 28 | −16% (−45 to 13%) | 28 | −72% (−101 to −43%) | −56 (−97 to −15) | 0.0010a |
| Week 1 | 28 | −16% (−41 to 8%) | 28 | −61% (−86 to −36%) | −46 (−80 to −10) | 0.0148a |
| Week 2 | 28 | −11% (−34 to 13%) | 27 | −70% (−94% to −47%) | −60 (−93 to −27) | 0.0011a |
| Week 3 | 28 | −14% (−38 to 11%) | 27 | −67% (−93 to −42%) | −54 (−85 to −22) | 0.0017a |
| Week 4 | 28 | −20% (−42 to 1%) | 27 | −82% (−104 to −60%) | −62 (−93 to −32) | 0.0003a |
| Concentration | ||||||
| Day 3 | 29 | −25% (−46 to −4%) | 29 | −67% (−88 to −46%) | −42 (−72 to −12) | 0.0075a |
| Week 1 | 29 | −20% (−39 to −1%) | 29 | −55% (−74 to −36%) | −35 (−62 to −9) | 0.0118a |
| Week 2 | 29 | −13% (−37 to 11%) | 29 | −59% (−83 to −35%) | −46 (−81 to −12) | 0.0099a |
| Week 3 | 29 | −4% (−35 to 28%) | 29 | −56% (−87 to −24%) | −52 (−80 to −25) | 0.0007a |
| Week 4 | 29 | −14% (−32 to 4%) | 29 | −77% (−95 to −58%) | −62 (−88 to −37) | <0.0001a |
Results are presented as percentage change with 95% CIs from baseline on day 3 of treatment and mean weekly total for week 1, week 2, week 3, and week 4 of the treatment periods for both placebo and MLE4901.
aSkewed data. Italics—significant P value.
HFRDIS, Hot Flash Related Daily Interference Scale.
DISCUSSION
In this post hoc analysis we have demonstrated that an oral NK3R antagonist (MLE4901) rapidly, and effectively, reduced frequency, severity, bother, and interference of vasomotor symptoms. Furthermore, similar improvements were seen in daytime and nighttime symptoms, and participants also experienced significant improvement in sleep. Considering that in the MsFLASH 02 study vasomotor symptoms and sleep were the two foremost symptom priorities for participants, these findings are particularly important3, and further advance the understanding of the specific therapeutic profile of NK3R antagonists both on symptomatology and speed of onset. Importantly, treatment was also well tolerated.18
It is difficult to compare the onset of action with other currently available treatments for vasomotor symptoms as the preexisting trials have only reported “end of study” data. For example, the reported data for hormone therapy in trials range from 3 months to 3 years,24 for paroxetine is after 6 weeks of treatment,25 and for gabapentin is after 12 weeks of treatment.26 Mean weekly total for week 1 was slightly worse than the total for day 3 in all outcomes after treatment with MLE4901 and this is likely because the weekly total was an average that included days 1 and 2 of treatment. Interestingly, participants anecdotally reported a noticeable change in their symptoms after approximately 48 hours of starting treatment with MLE4901, and also reported a similar time to offset on cessation.
It is also difficult to conclude to what extent the improvement in sleep and concentration were a result of less disruption through the night as flashes were less frequent and/or less severe/bothersome, so overall sleep quality was improved, or as a result of a direct effect on neuronal pathways involved in sleep by MLE4901. It is plausible that both explanations are contributory to the improvement in symptoms; especially as prior research has shown that melanin-concentrating hormone neurons, which are involved in the sleep–wake cycle, express NK3R.27,28 Furthermore, NK3R has also been shown to be present in the prefrontal cortex, which is an important brain area for concentration,29 and a prior meta-analysis suggested that hormone therapy may improve cognitive function in young women,30 though this was disputed in the WHI Memory Study31 but methodological differences may explain this disparity in findings. Further study in larger clinical trials of NK3R antagonists, as well as preclinical studies, may help to provide mechanistic and symptomatic detail.
As per previous studies the placebo effect was sizeable (28% reduction in HF frequency, which is similar to the reported rate in the literature of 25%), and this is why it is critical for trials investigating new treatments for vasomotor symptoms to be placebo controlled. The treatment effect size of MLE4901 above that achieved by placebo (percentage point reduction compared with placebo) was, however, highly significant for all outcomes. Although direct comparison with other available treatments is problematic as outlined above, our data suggest that the treatment effect of MLE4901 is similar to that of hormone therapy, and superior to that achieved by standard prescription doses of paroxetine or gabapentin,24-26 and thus is likely to be clinically meaningful.
Our results fit entirely with the preexisting data that have implicated NKB/NK3R signaling as a critical mediator of menopausal vasomotor symptoms. From the early work by Rance et al in postmortem brain specimens that demonstrated the marked hypertrophy and increased activity of hypothalamic neurons with upregulated NKB gene expression,15 to the more recent first report in a clinical trial of inducing typical flashes in premenopausal women by infusing NKB peripherally.16 Mechanistically, it seems clear that it is the subsequent increased activation of/input to the thermoregulatory autonomic pathway via increased NKB/NK3R signaling through the median preoptic nucleus in response to estrogen withdrawal that is critical.10-14 This heightened signaling pathway can seemingly now be silenced by pharmacological blockade with an oral NK3R antagonist, and thus vasomotor symptoms can be attenuated to the significant benefit of otherwise deeply affected women. Moreover, this can be achieved rapidly, and without the need for estrogen exposure making it a more attractive, or even clinically possible, option for many women than conventional hormone therapy. Furthermore, there may be additional health benefits of treatment with a NK3R antagonist for postmenopausal women. Cardiovascular disease for example is increased in women after estrogen levels decline, and there is some evidence that administering an NK3R antagonist in rats reverses spontaneous hypertension and lowers heart rate,32 and that this effect is achieved by reducing midbrain dopaminergic signaling in the ventral tegmental area that highly expresses NK3R.33 The NK3R is also present on vasopressin neurons,34 and neurokinin B activity has been shown to be potentiated by thromboxane A2.35 This hypothesis would need to be tested in very large clinical trials that were adequately powered for cardiovascular endpoints but if possible they could be highly informative, and offer a novel treatment strategy for a leading cause of mortality and morbidity.36
CONCLUSIONS
The novel data that we report in this manuscript, which details the time course of the effect of an NK3R antagonist to relieve menopausal symptoms and the impact on sleep, fit entirely with the preexisting literature and are timely as there is significant interest in the NK3R antagonist class as a future therapeutic for vasomotor symptoms.37 Larger scale studies assessing efficacy, safety, and optimal dosing strategy are already underway. If these studies are also positive and provide good long-term safety data, then this novel approach of using NK3R antagonism to treat menopausal flushing will be practice changing.
Acknowledgments
We also thank Tricia Tan (Imperial College London), Niamh Martin (Imperial College London), and Vincenzo Libri (Director of the NIHR UCLH Clinical Research Facility and Head of the Leonard Wolfson Experimental Neurology Centre at UCL—Institute of Neurology) for their time and expertise in monitoring the safety of the trial. The views expressed are those of the authors and not necessarily those of the above-mentioned funders, the NHS, the NIHR, or the Department of Health.
Footnotes
Funding/support: The study was funded by the UK Medical Research Council as part of their Developmental Pathway Funding Scheme (grant reference MR/M024954/1) and by the award of a Research Professorship to WSD by the National Institute for Health Research (grant reference RP-2014-05-001). The Section of Investigative Medicine is funded by grants from the MRC, BBSRC and is supported by the NIHR Imperial Biomedical Research Centre Funding Scheme. The research study was supported by the NIHR/Wellcome Trust CRF at Imperial College Healthcare NHS Trust. JKP is funded by the MRC. RER is funded by an NIHR Academic Foundation Programme award. ANC is funded by the NHS and BRC. SC is funded by NIHR. WSD is funded by an NIHR Professorship (grant reference RP-2014-05-001).
Financial disclosure/conflicts of interest: JKP is funded by the UK MRC. RER and SC are funded by the NIHR. CNJ is an inventor on a patent application 14762086.8-1453, which is registered to Imperial Innovations. PM and VHL are employees of Millendo Therapeutics. NP has lectured for and acted in an advisory capacity for Abbott, Bayer, Besins, Consilient, Meda, MSD, Mylan, Novo Nordisk, Pfizer, and Shionogi. LCW is an employee of AstraZeneca UK. AstraZeneca licensed the compound and associated patents to Millendo Therapeutics (WO2014170648A1, pending; US9475773 B2, granted). WSD is funded by the NIHR, is an inventor on a patent application 14762086.8-1453, which is registered to Imperial Innovations, and was previously an investigator for a separate study of MLE4901 in polycystic ovarian syndrome, for which a consultancy fee was paid. Millendo Therapeutics provided some financial support for an administrative assistant for the study of MLE4901 in menopausal flushing. TPS worked as a statistical consultant for Millendo Therapeutics. ANC and MSH declare no competing interests.
REFERENCES
- 1.Stearns V, Ullmer L, Lopez JF, Smith Y, Isaacs C, Hayes D. Hot flushes. Lancet 2002; 360:1851–1861. [DOI] [PubMed] [Google Scholar]
- 2.Avis NE, Crawford SL, Greendale G, et al. Duration of menopausal vasomotor symptoms over the menopause transition. JAMA Intern Med 2015; 175:531–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carpenter JS, Woods NF, Otte JL, et al. MsFLASH participants’ priorities for alleviating menopausal symptoms. Climacteric 2015; 18:859–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marjoribanks J, Farquhar C, Roberts H, Lethaby A, Lee J. Long-term hormone therapy for perimenopausal and postmenopausal women. Cochrane Database Syst Rev 2017; 1:CD004143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Institute for Health and Care Excellence. Menopause: Diagnosis and Management; 2015. Available at: https://www.nice.org.uk/guidance/ng23/chapter/recommendations Accessed August 7, 2017. [PubMed] [Google Scholar]
- 6.Drewe J, Bucher KA, Zahner C. A systematic review of non-hormonal treatments of vasomotor symptoms in climacteric and cancer patients. Springerplus 2015; 4:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ayers B, Smith M, Hellier J, Mann E, Hunter MS. Effectiveness of group and self-help cognitive behavior therapy in reducing problematic menopausal hot flushes and night sweats (MENOS 2): a randomized controlled trial. Menopause 2012; 19:749–759. [DOI] [PubMed] [Google Scholar]
- 8.Franco OH, Chowdhury R, Troup J, et al. Use of plant-based therapies and menopausal symptoms: a systematic review and meta-analysis. JAMA 2016; 315:2554–2563. [DOI] [PubMed] [Google Scholar]
- 9.Navarro VM, Gottsch ML, Chavkin C, Okamura H, Clifton DK, Steiner RA. Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J Neurosci 2009; 29:11859–11866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mittelman-Smith MA, Williams H, Krajewski-Hall SJ, McMullen NT, Rance NE. Role for kisspeptin/neurokinin B/dynorphin (KNDy) neurons in cutaneous vasodilatation and the estrogen modulation of body temperature. Proc Natl Acad Sci USA 2012; 109:19846–19851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakamura K, Morrison SF. A thermosensory pathway mediating heat-defense responses. Proc Natl Acad Sci USA 2010; 107:8848–8853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dacks PA, Krajewski SJ, Rance NE. Activation of neurokinin 3 receptors in the median preoptic nucleus decreases core temperature in the rat. Endocrinology 2011; 152:4894–4905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mittelman-Smith MA, Krajewski-Hall SJ, McMullen NT, Rance NE. Neurokinin 3 receptor-expressing neurons in the median preoptic nucleus modulate heat-dissipation effectors in the female rat. Endocrinology 2015; 156:2552–2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rometo AM, Krajewski SJ, Voytko ML, Rance NE. Hypertrophy and increased kisspeptin gene expression in the hypothalamic infundibular nucleus of postmenopausal women and ovariectomized monkeys. J Clin Endocrinol Metab 2007; 92:2744–2750. [DOI] [PubMed] [Google Scholar]
- 15.Rance NE, Young WS., 3rd Hypertrophy and increased gene expression of neurons containing neurokinin-B and substance-P messenger ribonucleic acids in the hypothalami of postmenopausal women. Endocrinology 1991; 128:2239–2247. [DOI] [PubMed] [Google Scholar]
- 16.Jayasena CN, Comninos AN, Stefanopoulou E, et al. Neurokinin B administration induces hot flushes in women. Sci Rep 2015; 5:8466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crandall CJ, Manson JAE, Hohensee C, et al. Association of genetic variation in the tachykinin receptor 3 locus with hot flashes and night sweats in the Women's Health Initiative Study. Menopause 2017; 24:252–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prague JK, Roberts RE, Comninos AN, et al. Neurokinin 3 receptor antagonism as a novel treatment for menopausal hot flushes: a phase 2, randomised, double-blind, placebo-controlled trial. Lancet 2017; 389:1809–1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sloan JA, Loprinzi CL, Novotny PJ, Barton DL, Lavasseur BI, Windschitl H. Methodologic lessons learned from hot flash studies. J Clin Oncol 2001; 19:4280–4290. [DOI] [PubMed] [Google Scholar]
- 20.Freeman EW, Guthrie KA, Caan B, et al. Efficacy of escitalopram for hot flashes in healthy menopausal women: a randomized controlled trial. JAMA 2011; 305:267–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Newton KM, Carpenter JS, Guthrie KA, et al. Methods for the design of vasomotor symptom trials: the menopausal strategies: finding lasting answers to symptoms and health network. Menopause 2014; 21:45–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gordon PR, Kerwin JP, Boesen KG, Senf J. Sertraline to treat hot flashes: a randomized controlled, double-blind, crossover trial in a general population. Menopause 2006; 13:568–575. [DOI] [PubMed] [Google Scholar]
- 23.Joffe H, Guthrie KA, LaCroix AZ, et al. Low-dose estradiol and the serotonin-norepinephrine reuptake inhibitor venlafaxine for vasomotor symptoms: a randomized clinical trial. JAMA Intern Med 2014; 174:1058–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maclennan AH, Broadbent JL, Lester S, Moore V. Oral oestrogen and combined oestrogen/progestogen therapy versus placebo for hot flushes. Cochrane Database Syst Rev 2004; 4:CD002978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stearns V, Beebe KL, Iyengar M, Dube E. Paroxetine controlled release in the treatment of menopausal hot flashes: a randomized controlled trial. JAMA 2003; 289:2827–2834. [DOI] [PubMed] [Google Scholar]
- 26.Guttuso T, Jr, Kurlan R, McDermott MP, Kieburtz K. Gabapentin's effects on hot flashes in postmenopausal women: a randomized controlled trial. Obstet Gynecol 2003; 101:337–345. [DOI] [PubMed] [Google Scholar]
- 27.Cvetkovic V, Poncet F, Fellmann D, Griffond B, Risold PY. Diencephalic neurons producing melanin-concentrating hormone are influenced by local and multiple extra-hypothalamic tachykininergic projections through the neurokinin 3 receptor. Neuroscience 2003; 119:1113–1145. [DOI] [PubMed] [Google Scholar]
- 28.Li N, Nattie E, Li A. The role of melanin concentrating hormone (MCH) in the central chemoreflex: a knockdown study by siRNA in the lateral hypothalamus in rats. PLoS One 2014; 9:e103585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chao OY, Huston JP, Nikolaus S, de Souza Silva MA. Concurrent assessment of memory for object and place: Evidence for different preferential importance of perirhinal cortex and hippocampus and for promnestic effect of a neurokinin-3 R agonist. Neurobiol Learn Mem 2016; 130:149–158. [DOI] [PubMed] [Google Scholar]
- 30.Hogervorst E, Yaffe K, Richards M, Huppert F. Hormone replacement therapy for cognitive function in postmenopausal women. Cochrane Database Syst Rev 2002; 3:CD003122. [DOI] [PubMed] [Google Scholar]
- 31.Resnick SM, Maki PM, Rapp SR, et al. Effects of combination estrogen plus progestin hormone treatment on cognition and affect. J Clin Endocrinol Metab 2006; 91:1802–1810. [DOI] [PubMed] [Google Scholar]
- 32.Lessard A, Campos MM, Neugebauer W, Couture R. Implication of nigral tachykinin NK3 receptors in the maintenance of hypertension in spontaneously hypertensive rats: a pharmacologic and autoradiographic study. Br J Pharmacol 2003; 138:554–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Brito Gariepy H, Couture R. Blockade of tachykinin NK3 receptor reverses hypertension through a dopaminergic mechanism in the ventral tegmental area of spontaneously hypertensive rats. Br J Pharmacol 2010; 161:1868–1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pineda R, Sabatier N, Ludwig M, Millar RP, Leng G. A direct neurokinin b projection from the arcuate nucleus regulates magnocellular vasopressin cells of the supraoptic nucleus. J Neuroendocrinol 2016; 28:10.1111/jne.12342. [DOI] [PubMed] [Google Scholar]
- 35.Pal S, Wu J, Murray JK, et al. An antiangiogenic neurokinin-B/thromboxane A2 regulatory axis. J Cell Biol 2006; 174:1047–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schooling CM. Tachykinin neurokinin 3 receptor antagonists: a new treatment for cardiovascular disease? Lancet 2017; 390:709–711. [DOI] [PubMed] [Google Scholar]
- 37.Cully M. Deal watch: neurokinin 3 receptor antagonist revival heats up with Astellas acquisition. Nat Rev Drug Discov 2017; 16:377. [DOI] [PubMed] [Google Scholar]


