Abstract
Objective:
Response to menopausal hormone therapy (MHT) shows individual variation. SLCO1B1 encodes the OATP1B1 transporter expressed in the liver that transports many endogenous substances, including estrone sulfate, from the blood into hepatocytes. This study evaluated the relationship between genetic variation in SLCO1B1 and response to MHT in women enrolled in the Kronos Early Estrogen Prevention Study (KEEPS) at Mayo Clinic, Rochester, MN.
Methods:
KEEPS participants were randomized to oral conjugated equine estrogen (n = 33, oCEE), transdermal 17β-estradiol (n = 33, tE2), or placebo (n = 34) for 48 months. Menopausal symptoms (hot flashes, night sweats, insomnia, palpitations) were self-reported before treatment and at 48 months. Estrone (E1), E2, and sulfated conjugates (E1S, E2S) were measured using high-performance liquid chromatography-tandem mass spectrometry. SLCO1B1 rs4149056 (c.521T>C, p.Val174Ala) was genotyped using a TaqMan assay.
Results:
After adjusting for treatment, there was a significant association between the SLCO1B1 rs4149056 TT genotype (encoding normal function transporter) and lower E1S, E1S/E1, and E2S (P = 0.032, 0.010, and 0.008, respectively) compared with women who were heterozygous (TC) or homozygous (CC) for the reduced function allele. The interactions between genotype, treatment, and E2S concentration were stronger in women assigned to tE2 (P = 0.013) than the women taking oCEE (P = 0.056). Among women assigned to active treatment, women with the CT genotype showed a significantly greater decrease in night sweats (P = 0.041) than those with the TT genotype.
Conclusions:
Individual variation in sulfated estrogens is explained, in part, by genetic variation in SLCO1B1. Bioavailability of sulfated estrogens may contribute to relief of night sweats.
Keywords: 17β-estradiol, conjugated equine estrogens, Kronos Early Estrogen Prevention Study, OATP1B1, personalized therapy
Perimenopausal and postmenopausal women commonly experience significant neurovascular dysregulation including hot flashes and night sweats, insomnia, and palpitations that impact their quality of life, and in some women last for a decade or longer.1,2 Although there have been many advances in recent years in pharmacogenomics and personalized medicine,3,4 dosing of menopausal hormone therapy (MHT) to relieve menopausal symptoms currently relies on a trial-and-error approach, with an overarching goal to use the “lowest most effective dose.”5
Circulating and local tissue estrogen levels may be regulated by several enzymes. For example, estrone (E1) can be converted to estradiol (E2) by 17-beta-hydroxysteroid dehydrogenase (17-β-HSD). Both E1 and E2 can be converted into more hydrophilic sulfated forms by sulfotransferases. While this process was originally thought to be a step toward elimination, the sulfated forms are now thought to serve as a storage pool for estrogens. Steroid sulfatases can remove the sulfate group to convert the estrogens back from the storage pool to their active forms.6 Local tissue expression of estrogens can vary,7 and may, in part, be due to activity of the sulfotransferases and steroid sulfatases.6 In addition, transporters may also regulate estrogen concentration and distribution.
SLCO1B1 encodes the membrane-bound, sodium-independent organic anion transporter protein (OATP1B1) that is primarily expressed in the liver. OATP1B1 transports many endogenous substances, including estrone sulfate and bilirubin, from the blood into hepatocytes.8,9 The single-nucleotide polymorphism (SNP) that results in the c.521T>C (p.Val174Ala, rs4149056) variant has been the focus of many prior studies, most notably for its association with statin-induced myopathy.10 The c.521T>C variant, present in the ∗5 and ∗15 haplotypes, decreases transport of substrates, including estrone-3-sulfate and estradiol 17ß-D-glucuronide, potentially due to decreased membrane expression of the transporter.9,11-15 Furthermore, in a cohort of 424 individuals in the Rotterdam Scan Study, a population-based study of older individuals in the Netherlands, carriers of the variant 174Ala allele had higher concentrations of estrone sulfate than noncarriers.9 In the California Teachers Study cohort, another variant in SLCO1B1, rs4149013, was associated with an increased risk of breast cancer among postmenopausal women who were using combined estrogen and progestin therapy at the time of study enrollment.16 Of note, the rs4149056 SNP was not included in that study. In a recent genome-wide association study performed in a cohort of 774 postmenopausal women with resected early-stage ER+ breast cancer, several SNPs in SLCO1B1 were significantly associated with higher levels of estrone conjugates and an increased ratio of estrone conjugates to estrone.17 Of those, the SNP with the lowest P value (P = 3.74 × 10−11) was rs4149056.
Although there is emerging information on genetic variants that may impact estrogen metabolism, most studies have been performed in the context of cancer. It remains unclear whether these variants contribute to interindividual differences in estrogen requirement for relief of menopausal symptoms in postmenopausal women. Therefore, this study aimed to: replicate the prior findings of a relationship between rs4149056 and estrogen concentrations among naturally menopausal healthy women; explore whether this SNP impacts estrogen, estrone, and their sulfated conjugates among women taking two different formulations of estrogen in a prospective randomized double blind clinical trial; and explore the relationship between this SNP and menopausal symptoms in these women.
METHODS
Participants
Women (n = 100) enrolled in the Kronos Early Estrogen Prevention Study (KEEPS) from the Mayo Clinic, Rochester, MN site, were included in this study. Participants were randomized for 48 months to one of the following three regimens: oral conjugated equine estrogens (oCEE; 0.45 mg/d, n = 33) with micronized progesterone (200 mg/d for the first 12 days of the month), transdermal 17β-estradiol (tE2; 50 μg/d, n = 33) with micronized progesterone (200 mg/d for the first 12 days of the month), or placebo pills and patch (n = 34). Demographic and phenotypic characteristics of KEEPS participants did not differ across treatment assignments.18,19 Women were excluded from KEEPS if they had a hysterectomy, low-density lipoprotein cholesterol (LDL-C) >190 mg/dL, body mass index (BMI) >35 kg/m2, fasting glucose >126 mg/dL, uncontrolled hypertension (systolic blood pressure >150 mm Hg or diastolic blood pressure >95 mm Hg), history of smoking >10 cigarettes/d, or pre-existing coronary artery calcification (score >50 AU). At the time of enrollment, women averaged 52.7 years of age (range 42-58) and were 1.4 years (range 0.5-3.0) past menopause. Other baseline characteristics of this group of participants have been described previously.19 All were of European ancestry (white), based on ancestry-informative markers from a prior genotyping study.20 All participants provided written, informed consent, and the study was approved by the Mayo Clinic Institutional Review Board.
Genotyping
The SLCO1B1 rs4149056 SNP (c.521T>C, p.Val174Ala) was genotyped using a TaqMan assay in the Mayo Clinic Medical Genome Facility Genotyping Center. This SNP is present in the ∗5, ∗15, and ∗17 SLCO1B1 haplotypes.
Measurement of serum hormones
Estrone, estrone sulfate (E1S), 17β-estradiol (E2), and estradiol sulfate (E2S) were measured in fasting serum samples by liquid chromatography-tandem mass spectrometry (LC-MS/MS) as described previously.19 Blood was collected before randomization to treatment and at the final study visit without regard to the timing of the last application or dosing of the active treatments.
Menopausal symptoms
All participants completed a menopausal symptom checklist before randomization and again at 6, 12, 24, 36, and 48 months.21 The self-reported menopausal symptoms included in the present analysis were: hot flashes, night sweats, insomnia, and palpitations. Symptoms were scored on a 5-point ordinal scale: 0 (no symptoms) to 5 (severe symptoms).
Statistical analyses
Statistical analyses were performed using R software version 3.0.1 or JMP version 10.22,23 E1 and E2 concentrations and ratios of sulfated to nonsulfated estrogens that were not normally distributed were log-transformed before analysis. Linear regression models were used to test for associations between SLCO1B1 rs4149056 genotype and hormone levels, hormone ratios, and symptoms after correcting for treatment assignment. Symptoms were scored on a scale of 0 to 5, with 5 being the most severe, but in our analysis, symptoms were dichotomized to mild (scores 0-2) or severe (scores 3-5). Fisher's exact test was used to evaluate relationships between symptoms and genotype (comparing T/T with T/C and C/C) before treatment.
RESULTS
Genotyping
In this cohort of 100 women, 75 women were homozygous TT (normal function) genotype, 24 were heterozygous CT, and only 1 was homozygous CC (reduced function) for the SLCO1B1 rs4149056 variant (Table 1). The minor allele frequency (C allele) was 13.0%. Women with TT or CT SLCO1B1 rs4149056 genotypes were evenly distributed among the three treatment groups; the single participant with the CC genotype was in the placebo group.
TABLE 1.
Hormone levels by SLCO1B1 rs4149056 genotype and treatmenta
| Placebo | oCEE | tE2 | |||||||
| CC | CT | TT | CC | CT | TT | CC | CT | TT | |
| (n = 1) | (n = 13) | (n = 20) | (n = 0) | (n = 7) | (n = 26) | (n = 0) | (n = 4) | (n = 29) | |
| E1 | 14 | 19.23 (6.80) | 19.43 (10.46) | — | 69.71 (34.42) | 59.95 (39.49) | — | 42.00 (10.03) | 34.58 (14.64) |
| E1S | 385 | 373.62 (255.06) | 298.80 (314.05) | — | 3491 (2884.26) | 1810.58 (1348.51) | — | 1586.00 (1108.82) | 904.74 (784.91) |
| E1S/E1 | 27.5 | 18.17 (10.89) | 13.57 (7.26) | — | 50.03 (38.58) | 29.18 (15.95) | — | 35.15 (14.97) | 22.65 (12.67) |
| E2 | 3.6 | 6.37 (2.13) | 5.58 (2.61) | — | 13.76 (6.06) | 11.87 (6.85) | — | 40.25 (12.28) | 31.23 (29.09) |
| E2S | 4.9 | 7.92 (4.06) | 7.16 (4.39) | — | 43.46 (28.88) | 21.28 (16.44) | — | 56.48 (38.29) | 19.54 (13.58) |
| E2S/E2 | 1.38 | 1.26 (0.53) | 1.29 (0.32) | — | 3.40 (2.53) | 1.76 (0.85) | — | 1.64 (1.43) | 0.92 (0.51) |
CC, homozygous alleles for reduced function of enzyme; CT, heterozygous alleles; E1, serum estrone; E1S, serum estrone sulfate; E1S/E1, ratio of estrone sulfate to estrone in serum; E2, serum 17β-estradiol; E2S, serum estradiol sulfate; E2S/E2, ratio of estradiol sulfate to 17β-estradiol in serum; oCEE, oral conjugated equine estrogen; tE2, transdermal 17β-estradiol; TT, homozygous alleles for common function of enzyme.
aData are shown as mean (standard deviation).
Association of SLCO1B1 genotype and serum hormone levels
Serum hormone levels among the three groups was previously published.19 In general, E1 and E1S levels and the E1S/E1 ratio were highest among women randomized to oCEE, followed by tE2, and lowest among women on placebo. E2 was highest among women taking tE2, followed by those taking oCEE, and lowest among those on placebo. E2S levels were similar among women on active treatment (oCEE or tE2), but significantly higher than women randomized to placebo. The E2S/E2 ratio was highest among those taking oCEE, with those on tE2 or placebo being similar.
After adjusting for treatment, there was a significant association (P = 0.032) between SLCO1B1 rs4149056 SNP genotype and E1S, with women homozygous for the T (normal function) allele having lower E1S serum concentration than those who were heterozygotes (TC) (Fig. 1, Table 1). Similarly, after adjustment for treatment, the T allele was associated with a lower E1S/E1 ratio (P = 0.010). There was no interaction between SNP genotype and treatment in either analysis, indicating that the SNP genotype did not have a greater impact within a particular treatment group. There was no association between E1 concentration and rs4149056 genotype (P = 0.380).
FIG. 1.
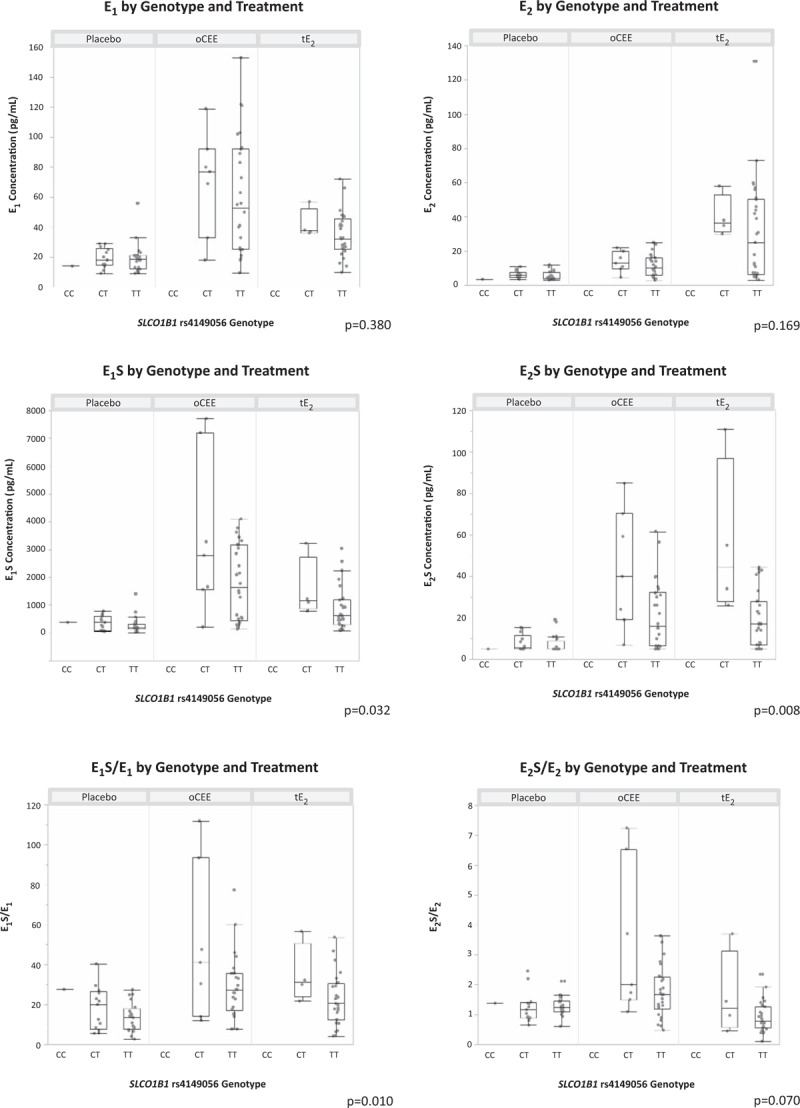
Association between hormone levels and SLCO1B1 rs4149056 genotype by treatment group. Quantile box plots overlay individual data points. P-values provided are for the association of hormone concentration or ratio with SLCO1B1 rs4149056 genotype after adjustment for treatment. E1, serum estrone; E1S, serum estrone sulfate; E1S/E1, ratio of estrone sulfate to estrone in serum; E2, serum 17β-estradiol, E2S, serum estradiol sulfate, E2S/E2 = ratio of estradiol sulfate to 17β-estradiol in serum.
The serum concentration of E2S also was associated with SLCO1B1 rs4149056 SNP genotype after adjustment for treatment (P = 0.008). The TT genotype was associated with a lower serum E2S concentration than the CT or CC genotypes (Fig. 1, Table 1). Further analysis for interactions between genotype, treatment, and E2S concentration revealed that the strength of the association between rs4149056 SNP genotype and E2S concentration was stronger in the group of women assigned to tE2 than the women taking oCEE (P value for the interaction between genotype and treatment arm in the model was 0.056 for the SNP × oCEE treatment group and 0.013 for the SNP × tE2 treatment group). The ratio of E2S/E2 trended toward a lower ratio with increasing T alleles, but was not significant (P = 0.070). The interaction term for SNP × oCEE treatment in the model was significant (P = 0.043), indicating that the ratio of E2S/E2 is significantly associated with rs4149056 genotype in this treatment group. There was no association between E2 concentration and rs4149056 before or after adjusting for treatment group (P = 0.493 and 0.169, respectively).
Association of SLCO1B1 genotype and menopausal symptoms
Based on prior studies, genetic variation in SLCO1B1 may be associated with endogenous hormone levels, and also with levels of exogenous hormones as observed in this study; therefore, we evaluated menopausal symptoms before randomization and after 48 months of treatment. No relationship was observed with SLCO1B1 rs4149056 genotype and hot flashes, night sweats, insomnia, or total symptoms before treatment (P = 1.00, 1.00, 0.22, 1.00, respectively). No women had severe palpitations at baseline.
After, 48 months of treatment, no relationship was observed with SLCO1B1 rs4149056 genotype and scores for hot flashes, insomnia, or total symptoms (P = 0.29, 0.13, and 0.91) after adjustment for treatment. No women had severe total symptoms, severe night sweats, or severe heart palpitations after 48 months of placebo or treatment. Change in symptom score from baseline (before treatment) to after 48 months of treatment was also evaluated by treatment group. Among women assigned to active treatment (oral or transdermal estrogen), women with the CT genotype showed a significantly greater reduction in night sweats (P = 0.041) than those with the TT genotype. There were no other significant findings.
DISCUSSION
In this cohort of postmenopausal women assigned to 48 months of placebo, oCEE, or tE2, a significant association was identified between the presence of the SLCO1B1 rs4149056 T allele (normal function) and lower serum concentration of E1S and E1S/E1, after adjusting for treatment. While these findings are consistent with prior studies performed to evaluate endogenous hormone levels in postmenopausal women,9,16,17 to our knowledge, this is the first study evaluating the influence of this SNP on serum hormone levels in healthy, naturally menopausal women taking MHT.
In addition, there was a significant association between the presence of the SLCO1B1 rs4149056 T allele and lower serum concentration of E2S, and a nonsignificant trend toward lower serum E2S/E2. While the OATP1B1 transporter encoded by SLCO1B1 is known to specifically transport E1S, the sulfated and desulfated forms of estrogens—including E1, E1S, E2, and E2S—exist in an equilibrium regulated by sulfatases, sulfotransferases, and membrane transport of sulfated steroids.24
Interestingly, we also identified an interaction between SNP genotype and type of treatment for both E2 and E2S/E2. We hypothesize that the effect of the SLCO1B1 rs4149056 SNP genotype differing by treatment may reflect important differences between the two treatment formulations. The primary component of tE2 is E2 and high levels of steroid sulfatases expressed in the dermis may be responsible for maintaining the E2 in the desulfated form as it reaches the circulation. In contrast, the primary components of oCEE are estrone and estrone sulphate, and due to the oral route of administration, the medication would be subject to the high levels of sulfotransferases in the gastrointestinal tract, and also first pass hepatic metabolism where sulfotransferases, sulfatases, and OATP1B1 are all highly expressed.
The rs4149056 genetic variation in SLCO1B1 was not associated with menopausal symptom severity before treatment, which might reflect differences in the timing of onset of symptoms relative to decreases in endogenous hormone levels. However, the greater reduction in night sweats among the women with the TC genotype than the TT genotype (P = 0.041) during treatment may be due to higher circulating levels of E1S and E2S, presumably due to decreased transporter activity resulting in less E1S and E2S uptake into the liver. The lower uptake may then allow higher total levels of E2 due to sulfatase activity at the local sites of neuronal and peripheral pathways involved with neurovascular thermoregulation, given that the sulfated form of estrogen is a storage pool in equilibrium with unconjugated estrogen.25 In an evaluation of sleep using the Pittsburgh Sleep Quality Index, sleep disturbances were alleviated to a greater extent with tE2 than oCEE in women of KEEPS.26 Together with the current findings, these data suggest that women with sleep disturbances, including night sweats, may respond better to tE2, particularly if they harbor a variant SLCO1B1 rs4149056 allele. Further studies are required to fully establish the impact of genetic variation in SLCO1B1 on levels of circulating E2 and E2S.
It is important to recognize that the clinical response to estrogen therapy in menopausal women is determined by an interplay of multiple factors. The pathways of estrogen metabolism and action are complex, and are affected by interindividual differences in binding proteins, receptor binding/action, plasma membrane transporters, and drug metabolizing enzymes. Moreover, while there is clear evidence that estrogen therapy reduces vasomotor symptoms,27-30 there is no association between the presence and severity of vasomotor symptoms and circulating estrogen levels,31 suggesting that local estrogen levels in the brain may differ from those in the periphery. In addition, there are significant ethnic and racial variations in vasomotor symptom reporting, with additional contributions from lifestyle and environmental factors.32 In particular, being overweight, a history of premenstrual syndrome, smoking, or being of African descent are risk factors for vasomotor symptoms.1,32,33 Thus, the exact mechanism by which estrogen contributes to autonomic thermoregulatory instability has yet to be elucidated. It is unlikely that interindividual differences in a single gene or metabolic pathway will have a significant clinical impact on response to hormone therapy. However, identifying genetic variants impacting estrogen metabolism contributes to our understanding of this complex pathway and may ultimately allow more individualized treatment of women with menopausal symptoms.
This study was limited by small sample size. This weakness is balanced by the strength that the KEEPS participants represent a well-characterized cohort of healthy women, thus allowing exploration of these genetic variants in the absence of comorbidities. Metabolism of estrogen is complex involving multiple enzymatic pathways each of which may have variants that could impact local and serum levels of the metabolites. In addition, it is unclear how environmental variables such as interaction with other drugs or the microbiome may influence estrogen metabolism.6,24,34,35 Therefore, the response to MHT is multifactorial and may ultimately require a complex algorithm for individualizing MHT in terms of the dose, formulation, and route of administration that may be appropriate for each woman. For example, there are several relationships between SULT1A1 genotype, estrogen levels, and onset of menopause.19SULT1A1 and SULT1E1 encode enzymes that conjugate a sulfate moiety onto estrogens, whereas STS encodes an enzyme that removes the sulfate moiety.6,24 The current study involves SLCO1B1, which encodes the OATP1B1 transporter that can facilitate movement of sulfate-conjugated estrogens across membranes. To fully elucidate a complicated pathway such as this one, the use of many samples and the ability to evaluate multiple variables—such as SULT1A1, SULT1E1, STS, and SLCO1B1 genetic variants—simultaneously may be necessary. However, candidate gene studies, such as this one, are important in establishing which variables may be important for inclusion in future models and algorithms of genotypes to personalize therapy.
CONCLUSIONS
In this exploratory study, the common rs4149056 genetic variant in SLCO1B1 was associated with levels of sulfated estrogens in recently, naturally menopausal women randomized to either oCEE or tE2. The variant was also associated with the magnitude of reduction in night sweats in women randomized to active treatment, particularly those using tE2. Understanding genetic variants that may influence individual plasma concentrations of estrogens in postmenopausal women on MHT may allow individualized MHT, avoiding the lengthy trial-and-error process currently used to identify an effective dose, formulation, or route of administration for symptom relief. This may ultimately contribute to better management of menopausal symptoms, improved quality of life, and potential mitigation of cardiovascular risk in menopausal women.36-38
Acknowledgments
The authors thank the dedicated volunteers participating in this study and co-workers in the Women's Health Clinic.
Footnotes
Funding/support: This work was supported by grants from the Aurora Foundation to the Kronos Longevity Research Institute, U19 GM61388 (The Pharmacogenomics Research Network), NIH P50 AG044170, RO1 GM28157, UL1TR00013 (from the National Center for Research Resources (NCRR), a component of the National Institutes of Health [NIH], and the NIH Roadmap for Medical Research. Contents of this paper are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. Information on NCRR is available at http://www.ncrr.nih.gov/. Information on Reengineering the Clinical Research enterprise can be obtained from http://nihroadmap.nih.gov), American Heart Association-Scientist Development Grant, AHA 08-30503Z, American Heart Association, Grant-in-Aid, 12GRNT12050147, and the Mayo Foundation.
Financial disclosure/conflicts of interest: None declared.
REFERENCES
- 1.Freeman EW, Sammel MD, Sanders RJ. Risk of long-term hot flashes after natural menopause: evidence from the Penn Ovarian Aging Study cohort. Menopause 2014; 21:924–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tepper PG, Brooks MM, Randolph JF, Jr, et al. Characterizing the trajectories of vasomotor symptoms across the menopausal transition. Menopause 2016; 23:1067–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collins SL, Carr DF, Pirmohamed M. Advances in the pharmacogenomics of adverse drug reactions. Drug Saf 2016; 39:15–27. [DOI] [PubMed] [Google Scholar]
- 4.Relling MV, Evans WE. Pharmacogenomics in the clinic. Nature 2015; 526:343–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Villiers TJ, Hall JE, Pinkerton JV, et al. Revised global consensus statement on menopausal hormone therapy. Climacteric 2016; 19:313–315. [DOI] [PubMed] [Google Scholar]
- 6.Reed MJ, Purohit A, Woo LW, Newman SP, Potter BV. Steroid sulfatase: molecular biology, regulation, and inhibition. Endocr Rev 2005; 26:171–202. [DOI] [PubMed] [Google Scholar]
- 7.Blankenstein MA, van de Ven J, Maitimu-Smeele I, et al. Intratumoral levels of estrogens in breast cancer. J Steroid Biochem Mol Biol 1999; 69:293–297. [DOI] [PubMed] [Google Scholar]
- 8.Abe T, Kakyo M, Tokui T, et al. Identification of a novel gene family encoding human liver-specific organic anion transporter LST-1. J Biol Chem 1999; 274:17159–17163. [DOI] [PubMed] [Google Scholar]
- 9.van der Deure WM, Friesema EC, de Jong FJ, et al. Organic anion transporter 1B1: an important factor in hepatic thyroid hormone and estrogen transport and metabolism. Endocrinology 2008; 149:4695–4701. [DOI] [PubMed] [Google Scholar]
- 10.Group SC, Link E, Parish S, et al. SLCO1B1 variants and statin-induced myopathy: a genomewide study. N Engl J Med 2008; 359:789–799. [DOI] [PubMed] [Google Scholar]
- 11.Kameyama Y, Yamashita K, Kobayashi K, Hosokawa M, Chiba K. Functional characterization of SLCO1B1 (OATP-C) variants, SLCO1B1∗5, SLCO1B1∗15 and SLCO1B1∗15+C1007G, by using transient expression systems of HeLa and HEK293 cells. Pharmacogenet Genomics 2005; 15:513–522. [DOI] [PubMed] [Google Scholar]
- 12.Tirona RG, Leake BF, Merino G, Kim RB. Polymorphisms in OATP-C: identification of multiple allelic variants associated with altered transport activity among European- and African-Americans. J Biol Chem 2001; 276:35669–35675. [DOI] [PubMed] [Google Scholar]
- 13.Nozawa T, Nakajima M, Tamai I, et al. Genetic polymorphisms of human organic anion transporters OATP-C (SLC21A6) and OATP-B (SLC21A9): allele frequencies in the Japanese population and functional analysis. J Pharmacol Exp Ther 2002; 302:804–813. [DOI] [PubMed] [Google Scholar]
- 14.Iwai M, Suzuki H, Ieiri I, Otsubo K, Sugiyama Y. Functional analysis of single nucleotide polymorphisms of hepatic organic anion transporter OATP1B1 (OATP-C). Pharmacogenetics 2004; 14:749–757. [DOI] [PubMed] [Google Scholar]
- 15.Michalski C, Cui Y, Nies AT, et al. A naturally occurring mutation in the SLC21A6 gene causing impaired membrane localization of the hepatocyte uptake transporter. J Biol Chem 2002; 277:43058–43063. [DOI] [PubMed] [Google Scholar]
- 16.Lee E, Schumacher F, Lewinger JP, et al. The association of polymorphisms in hormone metabolism pathway genes, menopausal hormone therapy, and breast cancer risk: a nested case-control study in the California Teachers Study cohort. Breast Cancer Res 2011; 13:R37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dudenkov TM, Ingle JN, Buzdar AU, et al. SLCO1B1 polymorphisms and plasma estrone conjugates in postmenopausal women with ER+ breast cancer: genome-wide association studies of the estrone pathway. Breast Cancer Res Treat 2017; 164:189–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harman SM, Black DM, Naftolin F, et al. Arterial imaging outcomes and cardiovascular risk factors in recently menopausal women: a randomized trial. Ann Intern Med 2014; 161:249–260. [DOI] [PubMed] [Google Scholar]
- 19.Moyer AM, de Andrade M, Weinshilboum RM, Miller VM. Influence of SULT1A1 genetic variation on age at menopause, estrogen levels, and response to hormone therapy in recently postmenopausal white women. Menopause 2016; 23:863–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller VM, Petterson TM, Jeavons EN, et al. Genetic polymorphisms associated with carotid artery intima-media thickness and coronary artery calcification in women of the Kronos Early Estrogen Prevention Study. Physiol Genomics 2013; 45:79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Santoro N, Allshouse A, Neal-Perry G, et al. Longitudinal changes in menopausal symptoms comparing women randomized to low-dose oral conjugated estrogens or transdermal estradiol plus micronized progesterone versus placebo: the Kronos Early Estrogen Prevention Study. Menopause 2017; 24:238–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.R Core Team. R: A language and environment for statistical computing. Available at: http://www.R-project.org Accessed March 22, 2018. [Google Scholar]
- 23.JMP [computer program]. Version 10. Cary, NC: SAS Institute Inc; 1989-2014. [Google Scholar]
- 24.Mueller JW, Gilligan LC, Idkowiak J, Arlt W, Foster PA. The regulation of steroid action by sulfation and desulfation. Endocr Rev 2015; 36:526–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ruder HJ, Loriaux L, Lipsett MB. Estrone sulfate: production rate and metabolism in man. J Clin Invest 1972; 51:1020–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cintron D, Lahr BD, Bailey KR, et al. Effects of oral versus transdermal menopausal hormone treatments on self-reported sleep domains and their association with vasomotor symptoms in recently menopausal women enrolled in the Kronos Early Estrogen Prevention Study (KEEPS). Menopause 2018; 25:145–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maclennan AH, Broadbent JL, Lester S, Moore V. Oral oestrogen and combined oestrogen/progestogen therapy versus placebo for hot flushes. Cochrane Database Syst Rev 2004; CD002978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCoy N, Culter W, Davidson JM. Relationships among sexual behavior, hot flashes, and hormone levels in perimenopausal women. Arch Sex Behav 1985; 14:385–394. [DOI] [PubMed] [Google Scholar]
- 29.Whiteman MK, Staropoli CA, Benedict JC, Borgeest C, Flaws JA. Risk factors for hot flashes in midlife women. J Womens Health (Larchmt) 2003; 12:459–472. [DOI] [PubMed] [Google Scholar]
- 30.Woods NF, Smith-Dijulio K, Percival DB, Tao EY, Taylor HJ, Mitchell ES. Symptoms during the menopausal transition and early postmenopause and their relation to endocrine levels over time: observations from the Seattle Midlife Women's Health Study. J Womens Health (Larchmt) 2007; 16:667–677. [DOI] [PubMed] [Google Scholar]
- 31.Sturdee DW, Hunter MS, Maki PM, et al. The menopausal hot flush: a review. Climacteric 2017; 20:296–305. [DOI] [PubMed] [Google Scholar]
- 32.Freeman EW, Sammel MD, Rinaudo PJ, Sheng L. Premenstrual syndrome as a predictor of menopausal symptoms. Obstet Gynecol 2004; 103 (5 Pt 1):960–966. [DOI] [PubMed] [Google Scholar]
- 33.Smith RL, Gallicchio LM, Flaws JA. Understanding the complex relationships underlying hot flashes: a Bayesian network approach. Menopause 2018; 25:182–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sier JH, Thumser AE, Plant NJ. Linking physiologically-based pharmacokinetic and genome-scale metabolic networks to understand estradiol biology. BMC Syst Biol 2017; 11:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vieira AT, Castelo PM, Ribeiro DA, Ferreira CM. Influence of oral and gut microbiota in the health of menopausal women. Front Microbiol 2017; 8:1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Silveira JS, Clapauch R, Souza M, Bouskela E. Hot flashes: emerging cardiovascular risk factors in recent and late postmenopause and their association with higher blood pressure. Menopause 2016; 23:846–855. [DOI] [PubMed] [Google Scholar]
- 37.Thurston RC, Chang Y, Barinas-Mitchell E, et al. Physiologically assessed hot flashes and endothelial function among midlife women. Menopause 2017; 24:886–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thurston RC, Johnson BD, Shufelt CL, et al. Menopausal symptoms and cardiovascular disease mortality in the Women's Ischemia Syndrome Evaluation (WISE). Menopause 2017; 24:126–132. [DOI] [PMC free article] [PubMed] [Google Scholar]


