Abstract
Mastitis is a common condition that predominates during the puerperium. Breast abscesses are less common, however when they do develop, delays in specialist referral may occur due to lack of clear protocols. In secondary care abscesses can be diagnosed by ultrasound scan and in the past the management has been dependent on the receiving surgeon. Management options include aspiration under local anesthetic or more invasive incision and drainage (I&D). Over recent years the availability of bedside/clinic based ultrasound scan has made diagnosis easier and minimally invasive procedures have become the cornerstone of breast abscess management. We review the diagnosis and management of breast infection in the primary and secondary care setting, highlighting the importance of early referral for severe infection/breast abscesses. As a clear guideline on the management of breast infection is lacking, this review provides useful guidance for those who rarely see breast infection to help avoid long-term morbidity.
Keywords: Mastitis, abscess, infection, lactation
Introduction
Mastitis is a relatively common breast condition; it can affect patients at any time but predominates in women during the breast-feeding period (1). It is defined as inflammation of the breast with or without infection. Mastitis with infection may be lactational (puerperal) or non-lactational (e.g., duct ectasia). Causes of non-infectious mastitis include idiopathic granulomatous inflammation and other inflammatory conditions (e.g., foreign body reaction). Timely management of mastitis with antibiotics can help avoid complications.
A breast abscess is a localized collection of purulent material within the breast (2), which can be a complication of mastitis. Breast abscesses most commonly affect women aged between 18 and 50 years. In women of reproductive age these are predominantly lactational but non-lactational abscesses are also seen in premenopausal older woman. Breast abscesses are occasionally noted in neonates (3). Non-lactational abscesses are more common in obese patients and smokers than in the general population (4). In the United Kingdom (UK) these patients may be reviewed in a variety of healthcare settings including general practice, Accident and Emergency (A&E) or in surgical clinics. Early referrals are essential to prevent evolution into severe infection and even sepsis. There has been a lack of consensus in the past regarding appropriate management pathways and delays in treatment has resulted in worse outcomes (5). Treatment regimens generally include antibiotics; and for breast abscesses - percutaneous drainage and/or surgical I&D. As effective ultrasound-guided drainage becomes more commonplace, this has begun to circumvent the need for invasive I&D, even for large abscesses. The predominance of Staphylococcus (S) aureus allows a rational choice of antibiotic without having to wait for the results of bacteriological culture (5). Relatively few randomized-control-trials have been carried out to evaluate the treatment rationale; hence, management and guideline design requires a review of available evidence.
Clinical and research implications
Epidemiology
Breast infections are the most common benign breast problem during pregnancy and the puerperium (6). The global prevalence of mastitis in lactating women ranges from 1–10% (7–11). However, a recent Cochrane review suggested that the incidence of mastitis could be as high as 33% (12). The incidence is highest in the first few weeks postpartum, decreasing gradually after that (5). Duct ectasia (peri-ductal mastitis or dilated ducts associated with inflammation) occurs in 5–9% of non-lactating women (13). Tubercular mastitis is rare, even in tuberculosis (TB)-endemic countries, with a reported incidence between 0.1–3% (14). Granulomatous mastitis is rare chronic inflammatory condition of the breast that can mimic inflammatory breast cancer and periductal mastitis (15). Mammary fistulae, which can be a complication of breast infection, occurs in 1–2% of women with mastitis (16).
Breast abscesses as a complication, develop in 3 to 11% of women with mastitis, with a reported incidence of 0.1–3% in breastfeeding women (5, 9, 17). Approximately 50% of infants with neonatal mastitis will develop a breast abscess (3). Breast abscesses in lactating and non-lactating women are two distinct clinical entities, each with a discrete pathogenesis. Lactational breast abscesses remain more common although the incidence has been decreasing in recent years (18). About 90% of non-lactational breast abscesses are sub-areolar (19). The remaining non-lactational breast abscesses are caused by rare granulomatous, bacterial or fungal etiologies (13, 15). Non-lactational, sub-areolar abscesses tend to occur in women toward the end of their reproductive years (13).
Etiology
Mastitis may occur with or without infection. Infectious mastitis and breast abscesses are predominantly caused by bacteria that colonize the skin. S. aureus is the most common causative agent, followed by coagulase-negative Staphylococci. The majority of S. aureus isolated are now methicillin-resistant S. aureus (MRSA) (20, 21).
Some breast infections (and up to 40% of breast abscesses) may be polymicrobial, with isolation of aerobes (Staphylococcus, Streptococcus, Enterobacteriaceae, Corynebacterium, Escherichia coli, and Pseudomonas) as well as anaerobes (Peptostreptococcus, Propionibacterium, Bacteroides, Lactobacillus, Eubacterium, Clostridium, Fusobacterium, and Veillonella) (3, 20, 22, 23). Anaerobes are sometimes isolated in abscesses and in chronic recurrent cases. A study of primary and recurrent breast abscesses showed that smokers were more likely to have anaerobes recovered (isolated in 15% of patients) (24).
More unusual pathogens may include Bartonella henselae (the agent of cat-scratch disease), mycobacteria (TB and atypical mycobacteria), Actinomyces, Brucella, fungi (Candida and Cryptococcus), parasites, and maggot infestation. Unusual breast infections may be the initial presentation of HIV infection (25). Typhoid is a well-recognized cause of breast abscesses in countries where this disease is prevalent (26, 27).
Non-infectious mastitis may result from underlying duct ectasia (peri-ductal mastitis or plasma cell mastitis) and infrequently from foreign material (e.g., nipple piercing, breast implant, or silicone) (28, 29). Granulomatous (lobular) mastitis is a benign disease once considered idiopathic, however there is growing evidence of an association with corynebacteria infection (30).
Pathophysiology
In lactational mastitis, it is likely that bacteria (often originating from the mouth of the infant) gain entry via cracks or fissures in the nipple surface. Once the primary defenses are breached, organisms have an ideal culture environment in nutrient rich maternal milk leading to rapid replication. This can be augmented by milk stasis and overproduction leading to mastitis (6, 11). In neonates, transient breast enlargement secondary to maternal hormones can make them vulnerable to mastitis.
In duct ectasia, the mammary duct-associated inflammatory disease sequence involves squamous metaplasia of lactiferous ducts, causing blockage (obstructive mastopathy) with peri-ductal inflammation and possible duct rupture (16). Inflamed ducts are prone to bacterial infection (31, 32).
In tubercular mastitis, mycobacterium tuberculosis can enter the breast from a direct inoculation (via a nipple abrasion) or more commonly from secondary spread from a distal source such as lymphatic spread, miliary dissemination, or contiguous spread (e.g., empyema necessitans). Clinical presentation is usually of a solitary, ill-defined, unilateral hard lump situated in the upper outer quadrant of the breast. Primary TB of the breast is rare. Necrotizing granulomas are the histopathological hallmark of TB infection.
In granulomatous mastitis, granulomas are usually non-necrotizing, inflammation is focused around breast lobules that clinically may present as a painless mass (15).
Left untreated, mastitis may cause tissue destruction resulting in an abscess. Lactational abscesses tend to be located in the peripheral breast and are often a progression of mastitis or lactational breast inflammation (6). Occasionally spread is hematogenous from an infection elsewhere. Risk factors for lactational breast abscess formation include the first pregnancy at maternal age over 30 years, pregnancy more than 41 weeks of gestation, and mastitis (4, 33). Early infection is usually localized to a single segment within the breast, extension to another segment is a late sign. Lactose-rich milk provides an ideal growth environment, so bacterial dispersion in the vascular and distended segment is easy. The pathological process is similar to any acute inflammatory event, although the nature of the lactating breast architecture; with its loose parenchyma and stagnation of milk in an engorged segment may allow the infection to spread quickly both within the stroma and through the milk ducts (18).
Non-lactational breast abscesses are often sub-areolar and were first described as fistulas of lactiferous ducts by Zuska et al. (34). It was noted that this results in chronically draining sinuses and abscess formation near the areola (34). This form has a known association with squamous metaplasia of the lactiferous duct epithelium, duct obstruction and subareolar duct dilation or duct ectasia (19, 35, 36). This is proceeded by inflammation of the surrounding duct, infection of these terminal lactiferous ducts, duct rupture and subsequent peri-areolar fistula and sub-areolar breast abscess formation (11, 19, 36). These abscesses have a chronic course, often with recurrent obstruction of the ducts with keratin plugs and have a tendency to form extensive fistulas (19, 36). Central (peri-areolar) non-lactational abscesses are usually due to periductal mastitis (2). Smoking and Diabetes mellitus are significant risk factors for periductal mastitis and non-lactational abscesses (2, 11).
Clinical features and diagnosis
The clinical diagnosis of mastitis or breast abscess is usually made based on the clinical presentation (Box 1) and by an individual’s history with a breast abscess tending to present with pain and/or a lump (Figure 1). Lactational breast abscesses tend to be found peripherally in the breast, and non-lactational abscesses are typically found in a peri-areolar or sub-areolar location (37).
Box 1. Signs and symptoms of breast infection.
Mastitis:
flu-like symptoms, malaise, and myalgia
fever
breast pain
decreased milk outflow
breast warmth
breast tenderness
breast firmness
breast swelling
breast erythema
enlargement of axillary lymph nodes
Breast abscess (in addition to the above):
Well-circumscribed fluctuant mass in the affected breast (although not always palpable if deep in breast tissue
Figure 1. a, b.
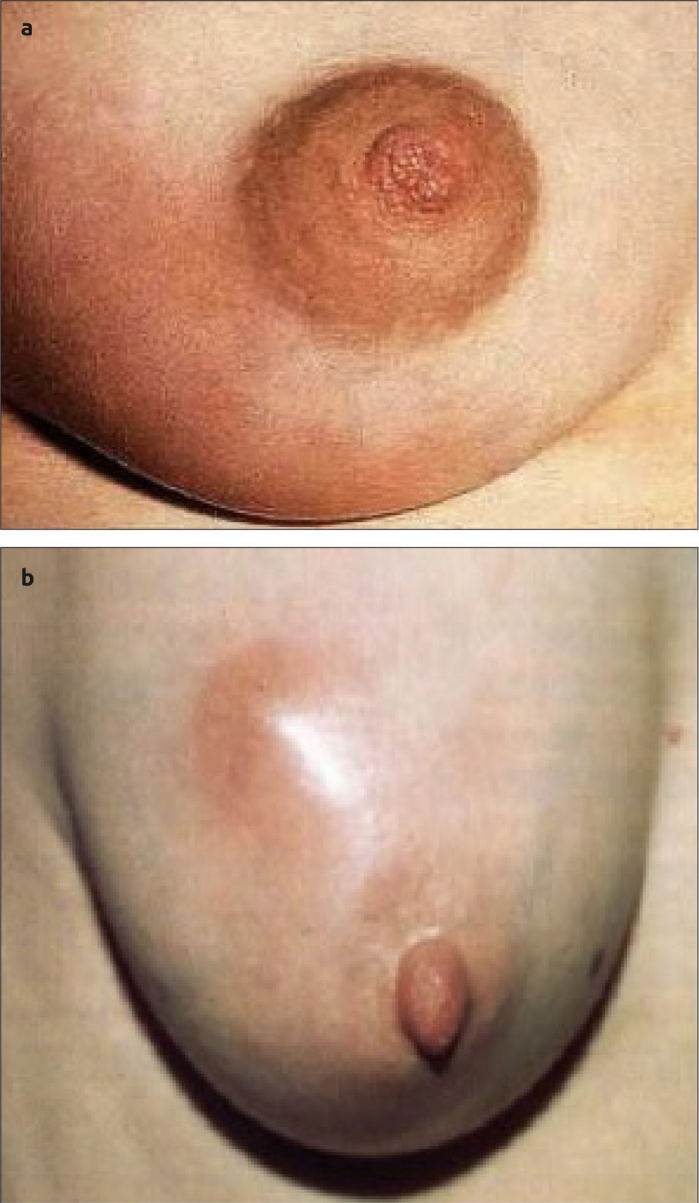
(a) Erythema associated with mastitis and (b) lactational breast abscess with visible swelling and erythema
Initial investigations
Ultrasound is a useful diagnostic tool in the initial workup; an abscess would be seen as a hypoechoic lesion, it may be well circumscribed, macrolobulated, irregular, or ill-defined with possible septa (Figure 2a). A hypoechoic rim may indicate the thick wall of a chronic abscess (Figure 2b). Ultrasound is the preferred imaging modality for all age groups with suspected breast infection (including neonates)(38). Fine needle aspiration can be used to drain a breast abscess for diagnostic and therapeutic purposes. Purulent fluid on diagnostic needle aspiration drainage indicates a breast abscess. This sample is often sent for cytology to rule out malignancy. Milk, aspirate, discharge, or biopsy tissue is sent for culture and sensitivity; a positive culture indicates infection and sensitivities should be used to guide antibiotic therapy. Consider performing a pregnancy test if occurrence of mastitis is unexpected (e.g. in an adolescent).
Figure 2. a, b.
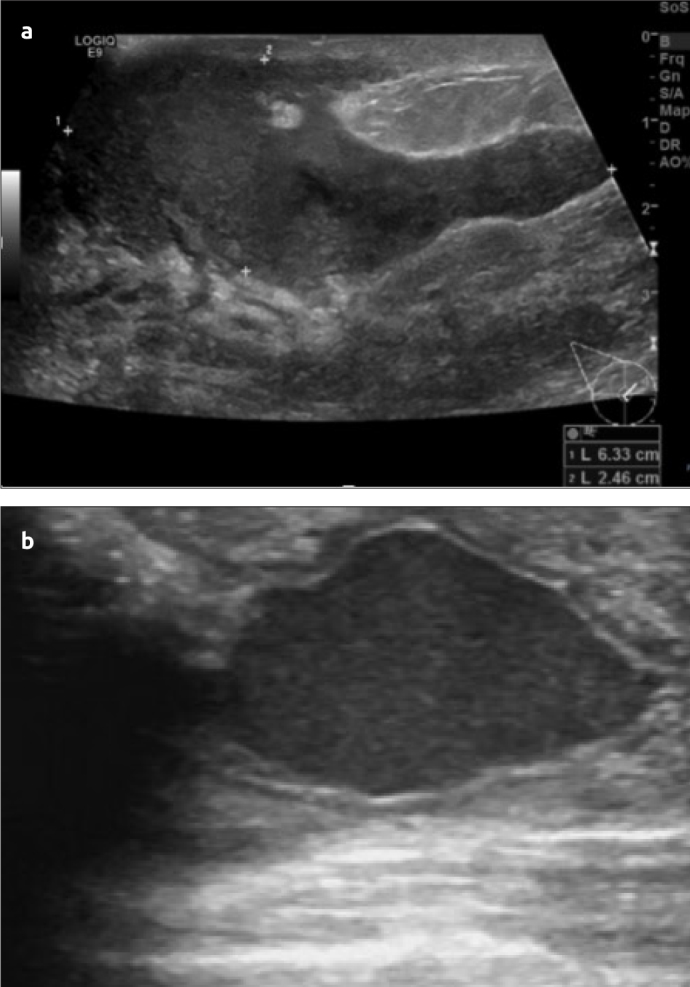
US scan (a) shows a well circumscribed hypoechoic lesion and US scan (b) shows a chronic abscess with a hypoechoic rim
Microbiology and pathology investigations
For routine cases of mastitis, a biopsy is not usually indicated. For all other cases, such as a suspected abscess, atypical presentation, uncertain diagnosis, or a potential complication (e.g., recurrent infection or treatment failure), a biopsy may be warranted. A biopsy includes fine-needle aspiration biopsy (which can be performed with/without ultrasound guidance) or tissue biopsy (which may be an excisional or incisional biopsy, a core-needle biopsy, or other vacuum-assisted biopsy). Tissue biopsy permits examination of involved tissue for infection, granulomatous inflammation, and malignancy. Excised tissue should be sent for histopathological evaluation (cytology) for a possible malignancy and infection (e.g., fungal stains and acid-fast bacilli for TB), especially in refractory and recurrent cases. Skin-punch biopsy can be undertaken to diagnose inflammatory breast carcinoma.
Milk, nipple discharge, aspirated material, or excised tissue should be sent for Gram-stain, culture (aerobic and anaerobic) with sensitivity, and fungal and mycobacterial studies.
Culture may be performed in all patients or only in select cases such as:
Hospital-acquired infection
Severe or unusual cases
Failure to respond to antibiotics within 2 days
Recurrent mastitis (7).
Expressed milk or a midstream milk sample can be sent for leukocyte counts and microbiology studies, including bacteria quantification (39). Endogenous breast flora is similar with the one present on the skin. Although the presence of pathogenic bacteria and/or high bacterial counts (>10^3/mL of milk) indicates mastitis, the predictive value is low. Therefore, the presence of bacteria in milk does not necessarily indicate infection so must be interpreted in the clinical context (7). Moreover, many lactating women who have potentially pathogenic bacteria on their skin or in their milk will not develop mastitis (7) and many women who do develop mastitis may not have pathogenic organisms in their milk(7). Blood cultures should be obtained in patients who appear septic and in neonates before initiation of antibiotic therapy. In neonates, additional samples (e.g., cerebrospinal fluid, urine) should be submitted for microscopy and culture.
Mammography
Mammography has limited value in the acute assessment of mastitis and breast abscesses. It may be too painful to perform on a breast with an abscess and mammographic findings of breast infection and abscess are non-specific (40–43), these include:
No abnormality
Architectural distortion
A spiculated mass
Skin thickening or retraction
Micro-calcification
Focal/diffusely increased density
Mammographic findings often mimic cancer. Therefore, it is most useful after the acute phase has resolved and underlying breast lesions can be identified. All woman above the age of 40 years and those with complicated or atypical presentations, or where malignancy is suspected should have mammography on resolution of the acute phase (44).
Additional investigations
A full blood count (FBC) with a differential and blood cultures are indicated in patients with suspected systemic infection, abscess, recurrent infection, or treatment failure. Tests to diagnose possible TB include a tuberculin skin test (often positive in patients with active disease), microbiology studies, and/or biopsy. When lactational mastitis is suspected, examination of the neonate should be considered, specifically with regards the oral cavity, skin, and nappy area. For recurrent cases of lactational mastitis, cultures from the infant’s and mother’s oral cavity and nasopharynx should be submitted to determine their staphylococcal carrier status.
Medical Management
It is paramount that breast infection is diagnosed and treated early to prevent complications. Antibiotics should be given promptly when infection is suspected and early appropriate referral to secondary care will improve long-term outcomes. Indications for immediate admission/referral can be found in Box 2.
Box 2. Indications for immediate admission or referral (1).
Hospital admission required if:
There are signs of sepsis (such as tachycardia, fever, and chills).
The infection progresses rapidly.
The woman is haemodynamically unstable or immunocompromised.
The infant should be admitted with her to allow continuation of breastfeeding.
Arrange an urgent 2-week wait referral if there is an underlying mass or breast cancer is suspected.
Refer urgently to a general surgeon if a breast abscess is suspected.
Management of Mastitis
Lactational mastitis
All patients should receive supportive care (analgesia +/− warm compress (7)) and effective milk removal from the affected breast. If symptoms are not severe or prolonged and there are no systemic signs of infection (and/or negative culture) patients do not need further treatment. If symptoms are severe, prolonged or there are signs of systemic disease; patients should be treated with antibiotics in accordance with culture results and sensitivities. If MRSA has been excluded by culture or is not prevalent in the local area and there is no penicillin allergy, patients should be treated first line with an oral anti-staphylococcal penicillin (e.g. flucloxacillin (Bristol Laboratories Ltd, Berkhamsted, Hertfordshire, UK): 250–500 mg orally four times daily). Erythromycin (Bristol Laboratories Ltd, Berkhamsted, Hertfordshire, UK) (250–500 mg orally four times daily) or clarithromycin (Helm AG, Hamburg, Germany) (500 mg orally twice daily) can be used if the patient is penicillin allergic. If MRSA has been confirmed by culture or prevalent in the area, a non-beta-lactam antibiotic should be given (e.g. Co-amoxiclav (Bristol Laboratories Ltd, Berkhamsted, Hertfordshire, UK) 625 mg orally three times daily or clindamycin (Pfizer, New York, United States of America): 150–300 mg orally four times daily). If there is no improvement with oral therapy the patient should be reassessed and vancomycin (Pfizer, New York, United States of America) (15 mg/kg intravenously every 12 hours, maximum 4 g/day) or another antibiotic with activity against MRSA should be initiated. If indicated patients may also require antifungal therapy (mother and infant) for nipple candidiasis. Tetracycline, ciprofloxacin, and chloramphenicol are not suitable to be used to treat lactating breast infection as these drugs can enter the breast milk and be harmful to the baby.
Non-lactational mastitis in adults and adolescents
All patients should receive supportive care. In adults with low suspicion of MRSA and no penicillin allergy the first line therapy should be with an oral anti-staphylococcal penicillin (e.g. flucloxacillin: 250–500 mg orally four times daily) or topical therapy (e.g. mupirocin topical (GlaxoSmithKline, Brentford, Middlesex, UK): (2%) apply to the affected area(s) two to three times daily). If MRSA is suspected, then the patient should be started on a non-beta-lactam antibiotic (e.g. co-amoxiclav 625 mg orally three times daily) or if patient is allergic to penicillin; clindamycin: 150–300 mg orally four times daily. If a second line therapy is required, the patient should be reassessed and then started on vancomycin or other antibiotic with activity against MRSA. There should be a switch to appropriate therapy for underlying cause if needed.
Mastitis in neonates, infants and children (<12 years of age) should be treated with supportive care and if MRSA is excluded by culture or not prevalent in area (and no penicillin allergy) then they should receive intravenous anti-staphylococcal penicillin (e.g. flucloxacillin: infants and children: 25–50 mg/kg intravenously every 4–6 hours; consult specialist for guidance on neonatal doses) or first-generation cephalosporin (e.g. cefazolin (MIP Pharma GmbH, Blieskastel, Germany): infants and children: 25–100 mg/kg/day intravenously given in divided doses every 6–8 hours, maximum 6 g/day; consult specialist for guidance on neonatal doses) as first line therapy, otherwise they can be treated with non-beta-lactam antibiotics (e.g. trimethoprim/sulfamethoxazole (Roche, Basal, Switzerland): children >2 months of age: 8–10 mg/kg/day intravenously/orally given in divided doses every 12 hours). If the patient does not improve the diagnosis should be reassessed.
As periductal mastitis is almost exclusively associated with tobacco abuse, smoking cessation advice should be given to these patients (32).
Granulomatous mastitis
Medical treatment with corticosteroids provides significant regression of the inflammatory disease, allowing more conservative surgery (45).
Management of Breast Abscesses
Breast abscesses rarely resolve with antibiotics alone. Abscesses generally require drainage in conjunction with antibiotics.
Non-MRSA breast abscesses
In adults, if MRSA has not been isolated or infection occurring in an area where MRSA is not prevalent, then intravenous (IV) or oral antibiotics with activity against methicillin-sensitive S. aureus (MSSA) (e.g. flucloxacillin: 250–500 mg orally four times daily or 0.5 to 2 g intravenously every 6 hours) should be started alongside supportive care. Supportive measures include analgesia if required. Antibiotic duration should be 7–10 days. The choice to start IV or oral antibiotics should be guided by the severity of the condition and the clinical judgement of the treating clinician.
In infant’s children and neonates, if MRSA can be excluded, a breast abscess can be treated with an intravenous antibiotic that is active against MSSA (e.g. flucloxacillin: children: 12.5 to 25 mg/kg orally four times daily; consult specialist for guidance on neonatal doses). Duration of treatment will be guided by the clinical response but is generally 7 to 10 days. Doxycycline (Chanelle Medical, County Galway, Ireland) is not appropriate for those less than 8 years of age. Supportive measures including analgesia should also be administered as appropriate.
MSRA breast abscesses
If MRSA is isolated or suspected a non-beta lactam antibiotic should be selected in addition to supportive care. If Community-acquired MRSA (CA-MRSA) is suspected or confirmed, or in a patient with a penicillin allergy, trimethoprim/sulfamethoxazole (160/800 mg orally twice daily), doxycycline (100 mg orally twice daily), or clindamycin (150–300 mg orally four times daily) can be used. Mothers should not continue to breastfeed on trimethoprim/sulfamethoxazole if the infant is younger than 2 months of age. Mothers should not breastfeed at all if on doxycycline. Vancomycin (15 mg/kg intravenously every 12 hours) can be used in more severe cases and in hospitalized patients where hospital-acquired MRSA is suspected. Alternatives, especially for patients exhibiting signs of systemic illness, include linezolid, tigecycline, and daptomycin. Antibiotic duration should be 7–10 days.
In neonates, infants and children, if CA-MRSA is suspected or confirmed, or the patient has a penicillin allergy, trimethoprim/sulfamethoxazole or clindamycin can be used. Doxycycline may only be used if the child is >8 years old. Vancomycin can be used in more severe cases and in hospitalized patients where hospital-acquired MRSA is suspected. The antibiotic treatment course should also be 7 to 10 days. The decision to start oral or intravenous antimicrobials at the time of initial presentation depends on clinical judgement and the severity of illness.
The diagnosis and treatment will need to be re-assessed, with adjustment made if there is no response to antibiotics within 48 hours. Antibiotic therapy should be adjusted depending on the specific pathogen(s) isolated. If gram-negative bacilli are isolated, a quinolone (e.g., levofloxacin) can be used, if the patient is not breastfeeding. Alternatively, a third-generation cephalosporin (e.g., ceftriaxone or cefotaxime) can be used for infection with gram-negative bacilli.
Surgical intervention
Surgical intervention is required for mature fluctuant abscesses. Needle aspiration (18- to 21-gauge needle) using adequate local anesthesia, with or without ultrasound guidance can be used to drain an abscess (46–52) (Figure 3). Once the pus has been aspirated, the abscess cavity should be irrigated with approximately 50 mL of 1% lidocaine and adrenaline (or serum physiologic solution) (5). Aspiration gives excellent palliation and cosmesis. Multiple aspirations over time (daily aspiration for 5 to 7 days) may be necessary for complete drainage, which can be followed by ultrasound scan if available. Aspiration is continued until no further fluid is visible in the abscess cavity or the fluid aspirated does not contain pus. The majority of lactational breast abscesses can be managed in this manner. If the skin overlying the abscess is compromised and is thin and shiny or necrotic a mini-I&D (Figure 4) should be performed by infiltrating local anesthetic into the skin overlying the abscess and then a small stab incision with a number 15 blade should be made over the point of maximum fluctuation (ultrasound guidance may be of assistance) (5). Any necrotic tissue should be excised. The contents of the cavity should be drained and then the cavity irrigated with local anesthetic solution. This should be repeated every couple of days until there is no evidence of leakage, it is possible to get wound closure and no pus remains. On the majority of occasions, this is possible under local anesthetic in the outpatient clinic setting. Large I&D (which usually requires general anesthesia (6) is not normally necessary and the small incision gives excellent cosmesis. Large I&D should be reserved for patients in whom aspiration/small incision fails and/or for large abscesses (>5 cm in diameter) (52). The placement of percutaneous drains and/or insertion of packing rarely has a role in the modern day management of breast abscesses (5). However, in cases where a larger volume of pus is involved, the placement of an additional drainage catheter may be beneficial (53).
Figure 3.
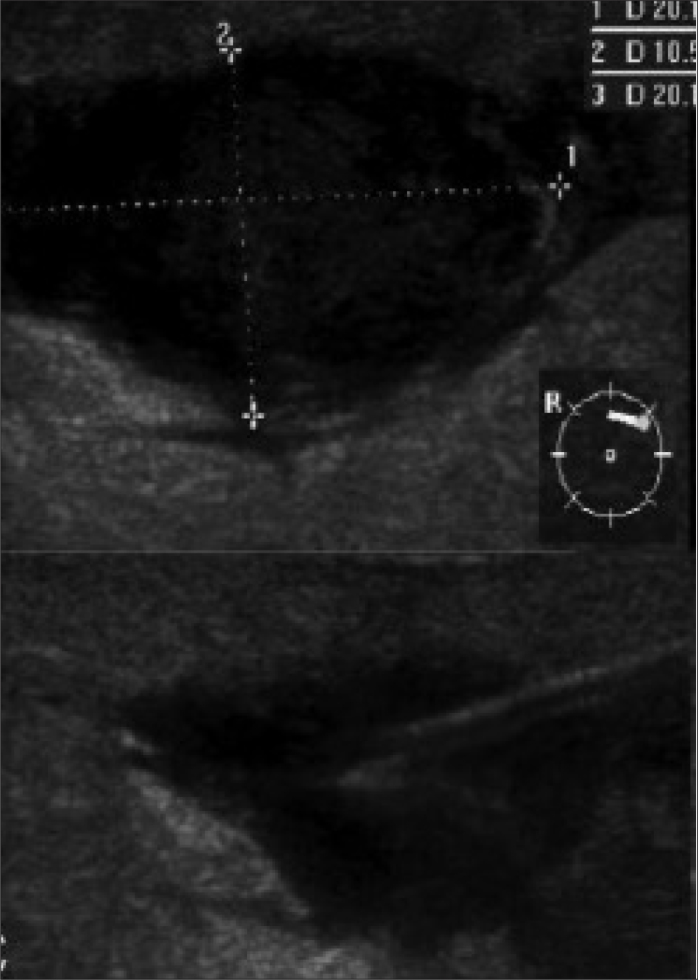
US-guided aspiration of a breast abscess
Figure 4. a, b.
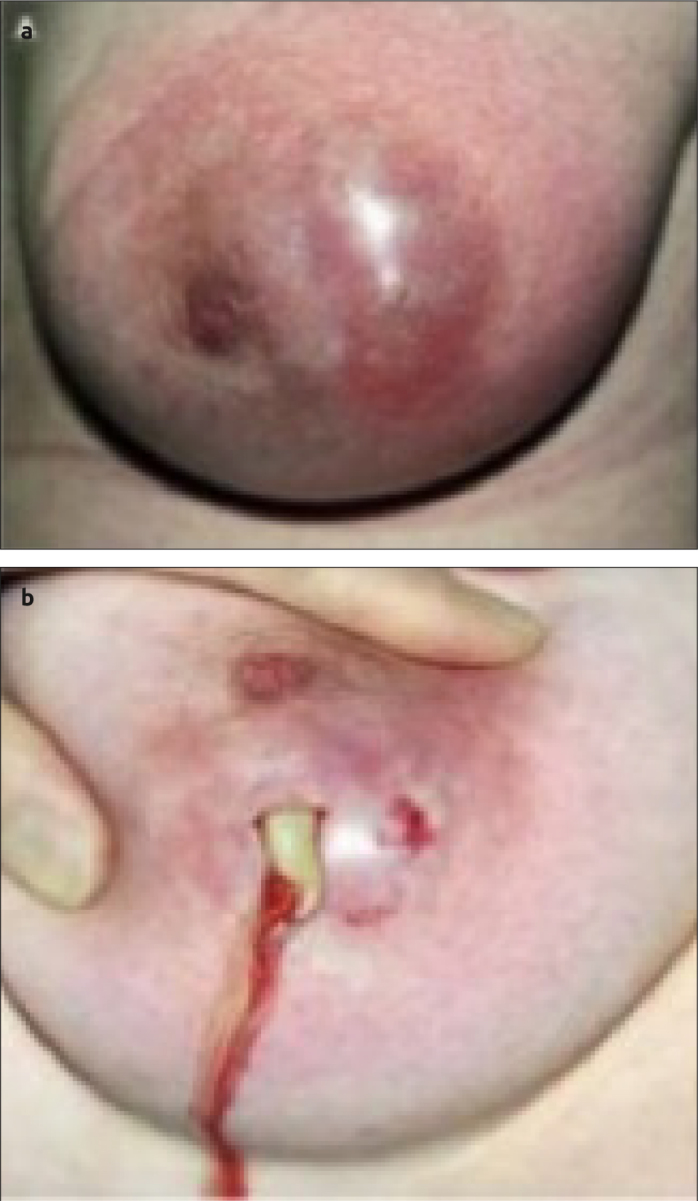
Surgical management of lactational breast abscesses (a) shows a lactational breast abscess with erythema, thin overlying skin and necrotic tissue (b) small I&D of a breast abscess
Granulomatous mastitis should be treated with corticosteroids and then surgical excision two weeks following the end of medical treatment (45).
Purulent material should be sent for microbiology studies and cytological examination. Antibiotics should be continued for up to 10 days after drainage. If the abscess is <5 cm in diameter and there is no associated cellulitis, antibiotics may not be required providing drainage is successful. If the incision does not interfere with breastfeeding, a lactating mother can continue to nurse. If the incision does interfere with nursing on an affected breast, milk can be regularly removed with a breast pump.
Breastfeeding advice
In lactating mothers milk stasis is often a risk factor for the development of mastitis and subsequent breast abscesses. It is essential that milk is removed frequently from the affected breast in order to manage it effectively (1, 54). The rate of abscess formation in lactating women with mastitis increases with the sudden cessation of breast feeding (1). Multiple studies have shown that the infant can continue to feed from the affected breast even when the causative organism is S. aureus (54).
Incomplete resolution or recurrence
After the acute phase of a breast abscess has subsided, chronically infected tissue and the major lactiferous duct associated with the abscess leading to the nipple may need to be excised (16). Recurrence may occur with therapy that is too short, delayed or inappropriate, and in Staphylococcus carriers. Recurrent mastitis or abscesses may be due to an underlying breast lesion and should be investigated appropriately. For lactational mastitis/breast abscess, the clinician should identify any predisposing factors such as nipple damage and ensure they have appropriate breast-feeding advice. Nasal carriage of S. aureus should be assessed by nasal swab of the woman and infant and decolonization should be administered if they are carriers. Granulomatous mastitis has a high recurrence rate. Smoking cessation should also be encouraged, to minimize the risk of recurrence.
Follow up
For women >40 years of age, breast imaging studies such as mammography or ultrasound should be performed after resolution of the acute process to exclude unsuspected underlying breast cancer.
Complications
Complications from mastitis and/or breast abscesses can be divided into acute and chronic complications. Acutely, breast infection may lead to the cessation of breastfeeding and support from healthcare workers and family are important (1). Breast infections may be associated with bacteremia leading to sepsis, immunocompromised people are particularly vulnerable. Mastitis may be the initiating factor for a breast abscess (less than 10% of patients with mastitis are likely to develop a breast abscess) or more seriously necrotizing fasciitis, especially in children. In addition, people with S. aureus mastitis are at increased risk for subsequent skin infections at extra-mammary sites. Mastitis and breast abscesses can occasionally be fatal if inadequately treated, especially in women who are immunocompromised (7).
Chronic complications include scarring; breast infection, including an abscess that is inadequately treated, may lead to significant breast scarring (Figure 5). Surgical intervention other than needle aspiration may cause a post-operative scar. Recurrent infections, TB, and granulomatous mastitis can cause significant breast deformity. In some patients the infection or treatment may result in a functional mastectomy (a breast that is unable to effectively lactate secondary to tissue destruction). In infants, damage to the breast bud from scarring and/or surgical intervention may cause subsequent breast asymmetry and/or hypoplasia. Recurrent mastitis may lead to chronic inflammation and disfigurement of the breast. Patients with S. aureus mastitis are at risk for subsequent skin infections at extra-mammary sites.
Figure 5. a, b.
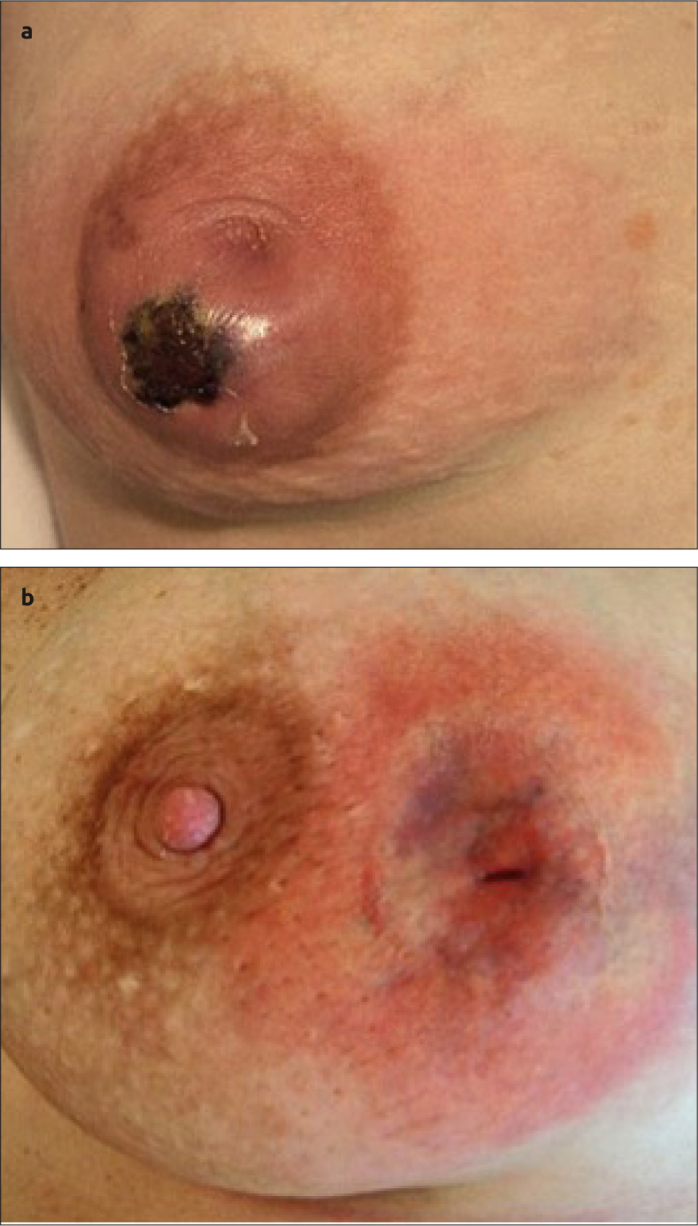
(a) Shows severe necrosis due to delayed management, with (b) post management, with significant asymmetry/scarring
If rupture of an abscess occurs, this can lead to a draining sinus with a resulting mammary fistula. Mammary fistula is a chronic condition that represents the final step in what has been termed “mammary duct associated inflammatory disease sequence.” The treatment is primarily surgical and may include healing by secondary intention or primary closure with or without antibiotics (55).
Conclusion
Breast infection is common and if managed appropriately will usually resolve with antibiotics alone. Breast abscesses require minimally invasive aspiration in combination with antibiotics to give the most favorable outcome. If managed appropriately invasive I&D is rarely required when managing an uncomplicated breast abscess. It is important that clinicians in primary and secondary care are aware of the current management pathways and make urgent referrals for any patient for which resolution does not rapidly occur with a single course of appropriate antibiotics. Delay in referral or appropriate management can have serious consequences on residual morbidity and cosmesis.
Footnotes
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - E.B., N.K.; Design - E.B., N.K.; Supervision - N.K.; Resources - E.B., N.K.; Materials - E.B., N.K.; Data Collection and/or Processing - E.B., N.K.; Analysis and/or Interpretation - E.B.; Literature Search - E.B; Writing Manuscript - E.B., N.K., A.W.; Critical Review - E.B., N.K., A.W., N.J.
Conflict of Interest: The authors have no conflicts of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Amir LH. ABM clinical protocol #4: Mastitis, revised March 2014. Breastfeed Med. 2014;9:239–243. doi: 10.1089/bfm.2014.9984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rizzo M, Gabram S, Staley C, Peng L, Frisch A, Jurado M, Umpierrez G. Management of breast abscesses in nonlactating women. Am Surg. 2010;76:292–295. [PubMed] [Google Scholar]
- 3.Efrat M, Mogilner JG, Iujtman M, Eldemberg D, Kunin J, Eldar S. Neonatal mastitis--diagnosis and treatment. Isr J Med Sci. 1995;31:558–560. [PubMed] [Google Scholar]
- 4.Bharat A, Gao F, Aft RL, Gillanders WE, Eberlein TJ, Margenthaler JA. Predictors of primary breast abscesses and recurrence. World J Surg. 2009;33:2582–2586. doi: 10.1007/s00268-009-0170-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dixon JM, Khan LR. Treatment of breast infection. Bmj. 2011;342:d396. doi: 10.1136/bmj.d396. [DOI] [PubMed] [Google Scholar]
- 6.Scott-Conner CE, Schorr SJ. The diagnosis and management of breast problems during pregnancy and lactation. Am J Surg. 1995;170:401–405. doi: 10.1016/S0002-9610(99)80313-4. [DOI] [PubMed] [Google Scholar]
- 7.Organisation WH. Causes and management. 2000. Mastitis. [Google Scholar]
- 8.Foxman B, D’Arcy H, Gillespie B, Bobo JK, Schwartz K. Lactation mastitis: occurrence and medical management among 946 breastfeeding women in the United States. Am J Epidemiol. 2002;155:103–114. doi: 10.1093/aje/155.2.103. [DOI] [PubMed] [Google Scholar]
- 9.Amir LH, Forster D, McLachlan H, Lumley J. Incidence of breast abscess in lactating women: report from an Australian cohort. BJOG. 2004;111:1378–1381. doi: 10.1111/j.1471-0528.2004.00272.x. [DOI] [PubMed] [Google Scholar]
- 10.Betzold CM. An update on the recognition and management of lactational breast inflammation. J Midwifery Womens Health. 2007;52:595–605. doi: 10.1016/j.jmwh.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 11.Marchant DJ. Inflammation of the breast. Obstet Gynecol Clin North Am. 2002;29:89–102. doi: 10.1016/S0889-8545(03)00054-8. [DOI] [PubMed] [Google Scholar]
- 12.Jahanfar S, Ng CJ, Teng CL. Antibiotics for mastitis in breastfeeding women. Cochrane Database Syst Rev. 2009;1:CD005458. doi: 10.1002/14651858.CD005458.pub2. [DOI] [PubMed] [Google Scholar]
- 13.AbdelHadi MSA, Bukharie HA. Breast infections in non-lactating women. J Family Community Med. 2005;12:133–137. [PMC free article] [PubMed] [Google Scholar]
- 14.Hamit HF, Ragsdale TH. Mammary tuberculosis. J R Soc Med. 1982;75:764–765. doi: 10.1177/014107688207501002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Al-Khaffaf B, Knox F, Bundred NJ. Idiopathic granulomatous mastitis: a 25-year experience. J Am Coll Surg. 2008;206:269–273. doi: 10.1016/j.jamcollsurg.2007.07.041. [DOI] [PubMed] [Google Scholar]
- 16.Meguid MM, Oler A, Numann PJ, Khan S. Pathogenesis-based treatment of recurring subareolar breast abscesses. Surgery. 1995;118:775–782. doi: 10.1016/S0039-6060(05)80049-2. [DOI] [PubMed] [Google Scholar]
- 17.Dixon JM. Breast infection. Bmj. 2013;347:f3291. doi: 10.1136/bmj.f3291. [DOI] [PubMed] [Google Scholar]
- 18.Kataria K, Srivastava A, Dhar A. Management of Lactational Mastitis and Breast Abscesses: Review of Current Knowledge and Practice. Indian J Surg. 2013;75:430–435. doi: 10.1007/s12262-012-0776-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Versluijs-Ossewaarde FN, Roumen RM, Goris RJ. Subareolar breast abscesses: characteristics and results of surgical treatment. Breast J. 2005;11:179–182. doi: 10.1111/j.1075-122X.2005.21524.x. [DOI] [PubMed] [Google Scholar]
- 20.Moazzez A, Kelso RL, Towfigh S, Sohn H, Berne TV, Mason RJ. Breast abscess bacteriologic features in the era of community-acquired methicillin-resistant Staphylococcus aureus epidemics. Arch Surg. 2007;142:881–884. doi: 10.1001/archsurg.142.9.881. [DOI] [PubMed] [Google Scholar]
- 21.Branch-Elliman W, Golen TH, Gold HS, Yassa DS, Baldini LM, Wright SB. Risk factors for Staphylococcus aureus postpartum breast abscess. Clin Infect Dis. 2012;54:71–77. doi: 10.1093/cid/cir751. [DOI] [PubMed] [Google Scholar]
- 22.Rudoy RC, Nelson JD. Breast abscess during the neonatal period. A review. Am J Dis Child. 1975;129:1031–1034. doi: 10.1001/archpedi.1975.02120460019005. [DOI] [PubMed] [Google Scholar]
- 23.Brook I. The aerobic and anaerobic microbiology of neonatal breast abscess. Pediatr Infect Dis J. 1991;10:785–786. doi: 10.1097/00006454-199110000-00014. [DOI] [PubMed] [Google Scholar]
- 24.Gollapalli V, Liao J, Dudakovic A, Sugg SL, Scott-Conner CE, Weigel RJ. Risk factors for development and recurrence of primary breast abscesses. J Am Coll Surg. 2010;211:41–48. doi: 10.1016/j.jamcollsurg.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 25.Pantanowitz L, Connolly JL. Pathology of the breast associated with HIV/AIDS. Breast J. 2002;8:234–243. doi: 10.1046/j.1524-4741.2002.08409.x. [DOI] [PubMed] [Google Scholar]
- 26.Barrett GS, MacDermot J. Breast abscess: a rare presentation of typhoid. Br Med J. 1972;2:628–629. doi: 10.1136/bmj.2.5814.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singh G, Dasgupta M, Gautam V, Behera A, Ray P. Bilateral Breast Abscesses due to Salmonella Enterica Serotype Typhi. J Glob Infect Dis. 2011;3:402–404. doi: 10.4103/0974-777X.91069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnson PE, Hanson KD. Acute puerperal mastitis in the augmented breast. Plast Reconstr Surg. 1996;98:723–725. doi: 10.1097/00006534-199609001-00021. [DOI] [PubMed] [Google Scholar]
- 29.Jacobs VR, Golombeck K, Jonat W, Kiechle M. Mastitis nonpuerperalis after nipple piercing: time to act. Int J Fertil Womens Med. 2003;48:226–231. [PubMed] [Google Scholar]
- 30.Johnstone KJ, Robson J, Cherian SG, Wan Sai Cheong J, Kerr K, Bligh JF. Cystic neutrophilic granulomatous mastitis associated with Corynebacterium including Corynebacterium kroppenstedtii. Pathology. 2017;49:405–412. doi: 10.1016/j.pathol.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 31.Bundred NJ, Dixon JM, Lumsden AB, Radford D, Hood J, Miles RS, Chetty U, Forrest AP. Are the lesions of duct ectasia sterile? Br J Surg. 1985;72:844–845. doi: 10.1002/bjs.1800721023. [DOI] [PubMed] [Google Scholar]
- 32.Dixon JM. Periductal mastitis/duct ectasia. World J Surg. 1989;13:715–720. doi: 10.1007/BF01658420. [DOI] [PubMed] [Google Scholar]
- 33.Berens PD. Prenatal, intrapartum, and postpartum support of the lactating mother. Pediatr Clin North Am. 2001;48:365–375. doi: 10.1016/S0031-3955(08)70030-0. [DOI] [PubMed] [Google Scholar]
- 34.Zuska JJ, Crile G, Jr, Ayres WW. Fistulas of lactifierous ducts. Am J Surg. 1951;81:312–317. doi: 10.1016/0002-9610(51)90233-4. [DOI] [PubMed] [Google Scholar]
- 35.Martin JG. Breast abscess in lactation. J Midwifery Womens Health. 2009;54:150–151. doi: 10.1016/j.jmwh.2008.07.015. [DOI] [PubMed] [Google Scholar]
- 36.Li S, Grant CS, Degnim A, Donohue J. Surgical management of recurrent subareolar breast abscesses: Mayo Clinic experience. Am J Surg. 2006;192:528–529. doi: 10.1016/j.amjsurg.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 37.Benson EA. Management of breast abscesses. World J Surg. 1989;13:753–756. doi: 10.1007/BF01658428. [DOI] [PubMed] [Google Scholar]
- 38.Borders H, Mychaliska G, Gebarski KS. Sonographic features of neonatal mastitis and breast abscess. Pediatr Radiol. 2009;39:955–958. doi: 10.1007/s00247-009-1310-x. [DOI] [PubMed] [Google Scholar]
- 39.Thomsen AC, Espersen T, Maigaard S. Course and treatment of milk stasis, noninfectious inflammation of the breast, and infectious mastitis in nursing women. Am J Obstet Gynecol. 1984;149:492–495. doi: 10.1016/0002-9378(84)90022-X. [DOI] [PubMed] [Google Scholar]
- 40.van Overhagen H, Zonderland HM, Lameris JS. Radiodiagnostic aspects of non-puerperal mastitis. Rofo. 1988;149:294–297. doi: 10.1055/s-2008-1048345. [DOI] [PubMed] [Google Scholar]
- 41.Crowe DJ, Helvie MA, Wilson TE. Breast infection. Mammographic and sonographic findings with clinical correlation. Invest Radiol. 1995;30:582–587. doi: 10.1097/00004424-199510000-00003. [DOI] [PubMed] [Google Scholar]
- 42.Reddin A, McCrea ES, Keramati B. Inflammatory breast disease: mammographic spectrum. South Med J. 1988;81:981–984. 988. doi: 10.1097/00007611-198808000-00010. [DOI] [PubMed] [Google Scholar]
- 43.Muttarak M. Abscess in the non-lactating breast: radiodiagnostic aspects. Australas Radiol. 1996;40:223–225. doi: 10.1111/j.1440-1673.1996.tb00390.x. [DOI] [PubMed] [Google Scholar]
- 44.Kamal RM, Hamed ST, Salem DS. Classification of inflammatory breast disorders and step by step diagnosis. Breast J. 2009;15:367–380. doi: 10.1111/j.1524-4741.2009.00740.x. [DOI] [PubMed] [Google Scholar]
- 45.Gurleyik G, Aktekin A, Aker F, Karagulle H, Saglamc A. Medical and surgical treatment of idiopathic granulomatous lobular mastitis: a benign inflammatory disease mimicking invasive carcinoma. J Breast Cancer. 2012;15:119–123. doi: 10.4048/jbc.2012.15.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Karstrup S, Solvig J, Nolsoe CP, Nilsson P, Khattar S, Loren I, Nilsson A, Court-Payen M. Acute puerperal breast abscesses: US-guided drainage. Radiology. 1993;188:807–809. doi: 10.1148/radiology.188.3.8351352. [DOI] [PubMed] [Google Scholar]
- 47.O’Hara RJ, Dexter SP, Fox JN. Conservative management of infective mastitis and breast abscesses after ultrasonographic assessment. Br J Surg. 1996;83:1413–1414. doi: 10.1002/bjs.1800831028. [DOI] [PubMed] [Google Scholar]
- 48.Tan SM, Low SC. Non-operative treatment of breast abscesses. Aust N Z J Surg. 1998;68:423–424. doi: 10.1111/j.1445-2197.1998.tb04791.x. [DOI] [PubMed] [Google Scholar]
- 49.Hook GW, Ikeda DM. Treatment of breast abscesses with US-guided percutaneous needle drainage without indwelling catheter placement. Radiology. 1999;213:579–582. doi: 10.1148/radiology.213.2.r99nv25579. [DOI] [PubMed] [Google Scholar]
- 50.Schwarz RJ, Shrestha R. Needle aspiration of breast abscesses. Am J Surg. 2001;182:117–119. doi: 10.1016/S0002-9610(01)00683-3. [DOI] [PubMed] [Google Scholar]
- 51.Ozseker B, Ozcan UA, Rasa K, Cizmeli OM. Treatment of breast abscesses with ultrasound-guided aspiration and irrigation in the emergency setting. Emerg Radiol. 2008;15:105–108. doi: 10.1007/s10140-007-0683-0. [DOI] [PubMed] [Google Scholar]
- 52.Eryilmaz R, Sahin M, Hakan Tekelioglu M, Daldal E. Management of lactational breast abscesses. Breast. 2005;14:375–379. doi: 10.1016/j.breast.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 53.Fahrni M, Schwarz EI, Stadlmann S, Singer G, Hauser N, Kubik-Huch RA. Breast Abscesses: Diagnosis, Treatment and Outcome. Breast Care (Basel) 2012;7:32–38. doi: 10.1159/000336547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Organisation WH. Causes and management. 2000. Mastitis. [Google Scholar]
- 55.Beechey-Newman N, Kothari A, Kulkarni D, Hamed H, Fentiman IS. Treatment of mammary duct fistula by fistulectomy and saucerization. World J Surg. 2006;30:63–68. doi: 10.1007/s00268-005-0116-8. [DOI] [PubMed] [Google Scholar]


