By increasing use of mammography, significant number of small and node negative cancers are diagnosed earlier makes axillary lymph node dissection (ALND) unnecessary in approximately 75% of patients with operable breast cancer (1). In patient with breast cancer and clinically negative axilla, sentinel lymph node biopsy (SLNB) should be the first choice and in clinically positive axilla neoadjuvant systemic treatment should be considered regarding to decrease the need of ALND. ALND is performed for locally advanced breast cancer, inflammatory breast cancer, mastectomy with positive lymph node, positive lymph node patients who will have accelerated partial breast irradiation (APBI) treatment and patients who have positive lymph nodes after receiving neoadjuvant systemic treatment. However, while ALND is a routine procedure, it has a higher complication rate than SLNB. ALMANAC trial had 1031 patients who were divided into two groups as SLNB and ALND. The incidence of lymphedema and sensation loss were lower in the SLNB group than the ALND group. For 12-month postoperative period, patients with SLNB, the amount of drainage, length of hospital stay and time to return normal activity was statistically low (2). In NSABP B-32 trial, ALND was compared to SLNB among patients who were clinically node negative. Results were either positive or negative .26% of patients who had clinically negative lymph nodes were found to have positive SLN. In patients who underwent to ALND due to SLN positivity, more than 60% had no additional positive lymph nodes. The B32 trial showed us that there is no difference in disease free survival (DFS), overall survival (OS) and locoregional recurrence rate among SLN negative patients who had ALND or SLNB alone (3).
ACOSOG has done two important studies; Z0010 and Z0011. ACOSOG Z0010 study included 5539 patients with T1-2 tumors who underwent breast conserving surgery (BCS) and whole breast irradiation (WBI) (4). Z0011 study is a continuation of Z0010, done to define if there is a need for ALND in patients with positive SLNs; the exclusion criteria were patients who has neoadjuvant systemic treatment, mastectomy or lumpectomy without RT or lumpectomy with APBI. Patients with 1–2 positive lymph node(s) were divided into two groups; ALND and SLNB only. In a 10-year follow up, there was no difference among OS (83.6% and 86.3%, p=0.72) and DFS (78.2% and 80.2%, p=0.51). Survival without locoregional recurrence was 83.0% at SLNB and 81.2% at ALND. The cumulative incidence of nodal recurrences in the ALND group was 0.5% and it was 1.5% in the SLND group (P=0.28). Patients at Z0011 had partially good prognostic characteristics; mean age was 55, 70% had T1, 82% were ER+, 71% had only one positive lymph node and 44 % has micrometastases (1, 5). Before ACOSOG Z0011, ALND was routine treatment for lymph node positive patients. After the Z0011 was published showing that there is no difference in OS in patients with only 1–2 positive lymph node(s), NCCN guidelines suggest ALND can be omitted (6). Parallel to this, ASBS suggested that there is no need for ALND in patients who fit Z0011 criteria (7).
Another study questioning the need of ALND is the AMAROS trial. In this study T1-2 clinically node negative patients were divided into two groups; ALND and axillary RT (ART). Eighty-two percent of patient underwent BCS and 18% had mastectomy with one or two positive SLNs. At 5-year follow up, there was no difference in nodal recurrence (ALND: 0.43%; ART: 1.19%), disease free survival (ALND: 86.9%; ART: 82.7%) and OS (ALND: 93.3%; ART: 92.5%) (8). Similar with Z0011, the IBCSG 23-01 trial included only patients with micrometastases at SLNB; patients were divided into two groups as ALND and no surgery. Unlike Z0011, mastectomy patients were not excluded. As a result, there was no difference in OS or locoregional recurrence rate among the two groups (9).
In general, when a positive lymph node was detected by frozen section during surgery, the surgeon goes directly to ALND. In Z0011 study, instead of frozen evaluation H&E stain was used. This decreased the rate of false positives, allowing for more accurate results. In breast cancer centers where the Z0011 study is considered as their current standard for the treatment of early breast cancer, there is no need for frozen section. It should be kept in mind that most of the cases where frozen section was positive there were no additional positive nodes with the H&E, and patients who undergo ALND have up to a 40% life time risk of developing lymphedema.
Breast cancer is a complex disease and a multidisciplinary approach is fundamental to diagnose and treat it. Most of the time, having ALND pathology results does not change the treatment with who gets systemic and radiation treatment. The current axillary treatment strategy is to avoid unnecessary ALND. In 2018 most of the centers have adapted that ALND should not be the routine treatment for SLN positive patients. We suggest an algorithm than can be used in daily clinic practice for breast cancer (Figure 1).
Figure 1.
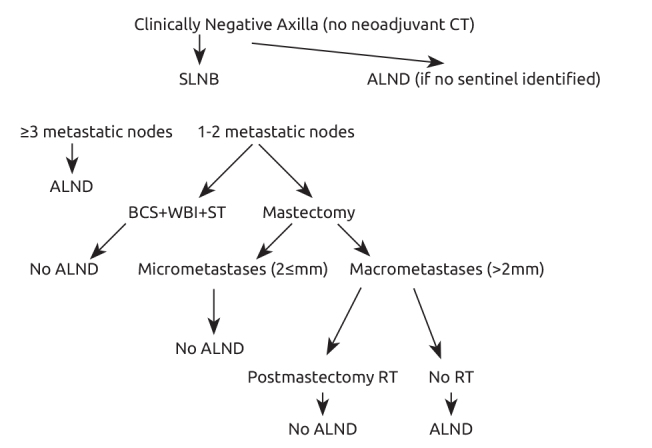
Clinically negative axilla approach
ALND: Axillary lymph node dissection; BCS: breast conserving surgery; CT: chemotherapy; RT: radiotherapy; SLNB: sentinel lymph node biopsy; ST: systemic therapy; WBI: whole breast irradiation
Footnotes
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - A.I., A.G., A.S.; Design - A.I., A.G., A.S.; Supervision - A.I., A.G., A.S.; Resources - A.I., A.G., A.S.; Materials - A.I., A.G., A.S.; Data Collection and/or Processing - A.I., A.G., A.S.; Analysis and/or Interpretation - A.I., A.G., A.S.; Literature Search - A.I., A.G., A.S.; Writing Manuscript - A.I., A.G., A.S.; Critical Review - A.I., A.G., A.S.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Giuliano AE, Ballman KV, McCall L, Beitsch PD, Brennan MB, Kelemen PR, Ollila DW, Hansen NM, Whitworth PW, Blumencranz PW, Leitch AM, Saha S, Hunt KK, Morrow M. Effect of AxillaryDissection vs No AxillaryDissection on 10-Year Overall Survival Among Women With Invasive Breast Cancer and Sentinel Node Metastasis: The ACOSOG Z0011 (Alliance) Randomized Clinical Trial. JAMA. 2017;318:918–926. doi: 10.1001/jama.2017.11470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mansel RE, Fallowfield L, Kissin M, Goyal A, Newcombe RG, Dixon JM, Yiangou C, Horgan K, Bundred N, Monypenny I, England D, Sibbering M, Abdullah TI, Barr L, Chetty U, Sinnett DH, Fleissig A, Clarke D, Ell PJ. Randomized multicenter trial of sentinel node biopsy versus standard axillary treatment in operable breast cancer: the ALMANAC Trial. J Natl Cancer Inst. 2006;98:599–609. doi: 10.1093/jnci/djj158. [DOI] [PubMed] [Google Scholar]
- 3.Krag DN, Anderson SJ, Julian TB, Brown AM, Harlow SP, Costantino JP, Ashikaga T, Weaver DL, Mamounas EP, Jalovec LM, Frazier TG, Noyes RD, Robidoux A, Scarth HM, Wolmark N. Sentinel-lymph-node resection compared with conventional axillary-lymph-node dissection in clinically node-negative patients with breast cancer: overall survival findings from the NSABPB-32 randomised phase 3 trial. Lancet Oncol. 2010;11:927–933. doi: 10.1016/S1470-2045(10)70207-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hunt KK, Ballman KV, McCall LM, Boughey JC, Mittendorf EA, Cox CE, Whitworth PW, Beitsch PD, Leitch AM, Buchholz TA, Morrow MA, Giuliano AE. Factors associated with local-regional recurrence after a negative sentinel node dissection: results of the ACOSOG Z0010 trial. Ann Surg. 2012;256:428–436. doi: 10.1097/SLA.0b013e3182654494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giuliano AE, Ballman K, McCall L, Beitsch P, Whitworth PW, Blumencranz P, Leitch AM, Saha S, Morrow M, Hunt KK. Locoregional Recurrence After Sentinel Lymph Node Dissection With or Without AxillaryDissection in Patients With Sentinel Lymph Node Metastases: Long-term Follow-up From the American College of Surgeons Oncology Group (Alliance) ACOSOG Z0011 Randomized Trial. Ann Surg. 2016;264:413–420. doi: 10.1097/SLA.0000000000001863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.The National Comprehensive Cancer Network. Available from: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf.
- 7.American Society of Breast Surgeons. Available from: https://www.breastsurgeons.org/new_layout/index.php.
- 8.Donker M, van Tienhoven G, Straver ME, Meijnen P, van de Velde CJ, Mansel RE, Cataliotti L, Westenberg AH, Klinkenbijl JH, Orzalesi L, Bouma WH, van der Mijle HC, Nieuwenhuijzen GA, Veltkamp SC, Slaets L, Duez NJ, de Graaf PW, van Dalen T, Marinelli A, Rijna H, Snoj M, Bundred NJ, Merkus JW, Belkacemi Y, Petignat P, Schinagl DA, Coens C, Messina CG, Bogaerts J, Rutgers EJ. Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer (EORTC 10981-22023 AMAROS): a randomised, multicentre, open-label, phase 3 non-inferiority trial. Lancet Oncol. 2014;15:1303–1310. doi: 10.1016/S1470-2045(14)70460-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galimberti V, Cole BF, Zurrida S, Viale G, Luini A, Veronesi P, Baratella P, Chifu C, Sargenti M, Intra M, Gentilini O, Mastropasqua MG, Mazzarol G, Massarut S, Garbay JR, Zgajnar J, Galatius H, Recalcati A, Littlejohn D, Bamert M, Colleoni M, Price KN, Regan MM, Goldhirsch A, Coates AS, Gelber RD, Veronesi U International Breast Cancer Study Group Trial 23-01 investigators. Axillarydissection versus no axillarydissection in patients with sentinel-node micrometastases (IBCSG 23-01): a phase 3 randomised controlled trial. Lancet Oncol. 2013;14:297–305. doi: 10.1016/S1470-2045(13)70035-4. [DOI] [PMC free article] [PubMed] [Google Scholar]


