Supplemental digital content is available in the text.
Abstract
Background
Chronic lung allograft dysfunction (CLAD) is the major limiting factor for long-term survival in lung transplant recipients. Viral respiratory tract infection (VRTI) has been previously associated with CLAD development. The main purpose of this study was to evaluate the long-term effects of VRTI during the first year after lung transplantation in relation to CLAD development.
Method
Ninety-eight patients undergoing lung transplantation were prospectively enrolled between 2009 and 2012. They were monitored for infections with predefined intervals and on extra visits during the first year, the total follow-up period ranged between 5 and 8 years. Nasopharyngeal swab and bronchoalveolar lavage samples were analyzed using a multiplex polymerase chain reaction panel for respiratory pathogens. Data regarding clinical characteristics and infectious events were recorded.
Results
Viral respiratory tract infection during the first year was identified as a risk factor for long-term CLAD development (P = 0.041, hazard ratio 1.94 [1.03-3.66]) in a time-dependent multivariate Cox regression analysis. We also found that coronavirus in particular was associated with increased risk for CLAD development. Other identified risk factors were acute rejection and cyclosporine treatment.
Conclusions
This study suggests that VRTI during the first year after lung transplantation is associated with long-term CLAD development and that coronavirus infections in particular might be a risk factor.
Lung transplantation (LTx) is the only available therapeutic option for end-stage, nonmalignant lung disease. The yearly number of procedures has increased, but long-term survival has not improved markedly over the years.1 The main limiting factor for long-term survival is chronic lung allograft dysfunction (CLAD), commonly in the form of bronchiolitis obliterans syndrome (BOS).1 In recent years, restrictive allograft syndrome has also gained recognition.2
The underlying pathophysiology of CLAD development is multifactorial and infections are a major risk factor.3 Other risk factors include acute rejection (AR), antibody-mediated rejection, gastroesophageal reflux, and air pollution exposure.4 The importance of viral respiratory tract infections (VRTI) for CLAD development and graft loss has been highlighted.5-8 However, a meta-analysis of 34 articles was unable to establish an association between VRTI and CLAD or graft loss,9 possibly due to differences in diagnostic methods between the included studies. More recently, the development of multiplex real-time polymerase chain reaction (PCR) methods has promoted a more sensitive and a standardized detection of respiratory viral agents.10
We previously showed an association between detection of viral pathogens in bronchoalveolar lavage (BAL) samples taken during the first year after LTx and the development of BOS.11 Others have recently reported an increased risk of CLAD after VRTI,12,13 but the long-term effects of early VRTI as well as the importance of VRTI compared with other risk factors remain unclear.
The aim of the present study was to evaluate the impact of VRTI during the first year after LTx on long-term CLAD development and graft loss, in a prospective trial with a 5-year follow-up period.
MATERIAL AND METHODS
Patients and Study Design
All patients over the age of 18 years, who received a single or double lung transplant between January 2009 and April 2012, at the Sahlgrenska University Hospital Transplant Centre, were Swedish residents and survived the initial postoperative intensive care period, were asked to participate. The study was approved by the regional ethical review board in Gothenburg (Dnr791-08), and all subjects provided written and verbal informed consent.
Data were collected prospectively. A standardized follow-up protocol was used for all patient visits, as outlined in Figure 1. Induction therapy consisted of rabbit antithymocyte globulin which was given for 1 to 3 consecutive days together with methylprednisolone IV. Posttransplantation immunosuppression included prednisone, 0.3 mg/kg/day and mycophenolate mofetil, 2 g/d. The patients then received either oral cyclosporine (CSA) (1-2 mg/kg) adjusted to maintain a serum level of 300 to 350 ng/mL or tacrolimus (TAC), 0.075 mg/kg given orally divided in 2 doses daily adjusted to maintain a serum level of 14 to 16 ng/mL. The dosage of immunosuppression was gradually lowered during FU. Further changes in immunosuppressive therapy were based on clinical presentation. Antimicrobial prophylaxis is detailed in the supplementary material (S1 http://links.lww.com/TXD/A117). Extra follow-up visits and additional bronchoscopies were carried out upon suspicion of respiratory infections or AR based on clinical presentation.
FIGURE 1.
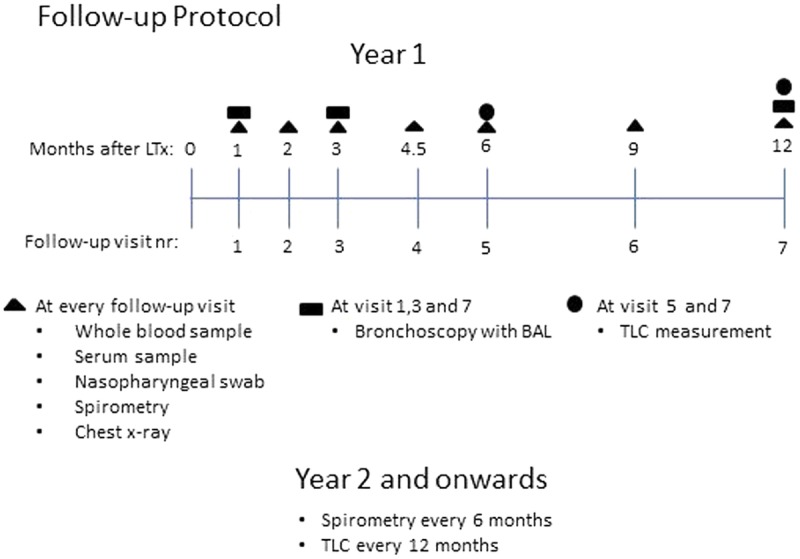
Visualization of follow-up protocol. Symbols representing the tests and sampling made at respective time point. TLC, total lung capacity.
At every visit, symptoms of VRTI were recorded (fever, cough, sputum, coryza, headache, wheezing, shortness of breath, muscular pain, rash, eye redness, and sore throat). If the patient presented 2 or more of these symptoms, a VRTI was considered symptomatic. Clinical data from follow-up visits and infectious events, including details regarding immunosuppression management, were recorded in an electronic case report form. All patients were in regular contact with transplant nurses and followed until the end of the study period, or graft loss.
Primary Endpoints
Chronic lung allograft dysfunction was defined as an irreversible loss of at least 20% from baseline of forced expiratory volume during the first second, if other reasons for the decline were excluded. When forced vital capacity was reduced with at least 20%, or the total lung capacity was reduced by at least 10% the function loss was considered to be predominantly restrictive.14 Lung function was screened weekly using a home spirometer and a conventional spirometry was performed if a loss of more than 10% in forced expiratory volume during the first second or forced vital capacity was identified. This was arranged by the transplant nurses as part of the clinical routine. Two experienced transplant pulmonologists reviewed each patient separately for CLAD development using all available spirometry data. The results were compared, and a consensus was reached on discrepancies. Graft loss was defined as either death or retransplantation.
VRTI Sampling and Cultures
A trained nurse obtained nasopharyngeal (NPH) samples using E-Swab (Copan, Brescia, Italy). The swab was put in a container with 1 mL of Amies medium and immediately transported to the laboratory or frozen at −80°C until analyzed. All NPH and BAL samples were tested using a multiplex real-time PCR panel for respiratory pathogens in addition samples were tested with real-time PCR for detection of Legionella pneumophila, Pneumocystis jirovecii and cytomegalovirus (CMV). Bronchoscopies for BAL were performed in a standardized manner (S2 http://links.lww.com/TXD/A117). All NPH were performed before bronchoscopies and all bronchoscopies were performed using either laryngeal or tracheal tubes to avoid cross-contamination. All BAL samples were tested with conventional bacterial and fungal cultures. If serious infection was suspected, blood and urine cultures were also performed.
Infectious Events and AR Episodes
Upper or lower VRTI was defined as detection of a viral pathogen by real-time PCR in either an NPH or BAL fluid sample. Subjects who had a positive PCR test for at least 1 viral pathogen in either BAL or NPH samples during the first year were considered “VRTI positive.” A bacterial respiratory tract infection was defined as a positive culture for bacteria in BAL or sputum and symptoms consistent with respiratory tract infection, as evaluated by an experienced transplant pulmonologist. A fungal infection was defined as significant presence of aspergillus or candida in BAL and/or sputum culture and symptoms and/or bronchoscopic findings consistent with fungal infection. Significant CMV viremia was defined as increasing levels of CMV-DNA in the blood. For seronegative transplant recipients, when CMV-DNA was detected for the first time, for CMV-seropositive recipients when levels increased above 3.0 log10 copies/mL. Repeated elevated Epstein-Barr virus (EBV)-DNA (REED) was defined as 2 separate EBV-DNA measurements above 3.1 log10 copies/mL at least 1 month apart. Acute rejection was defined as either a lung biopsy showing rejection of the International Society for Heart and Lung Transplantation grade A1 or higher,15 or, in the absence of a biopsy (n = 3), typical physical and radiological findings followed by a prompt response to high-dose corticosteroid therapy.
Real-time PCR for Quantification of CMV- and EBV-DNA
The levels of CMV- and EBV-DNA were determined in whole blood using a real-time PCR in a 7900 real-time PCR system (Applied Biosystems, Foster City, CA) as previously described.16 The primer and probe sequences are presented in the supplemental materials (S3 http://links.lww.com/TXD/A117).
Multiplex Real-time PCR for Detection of Respiratory Pathogens
NPH or BAL samples were obtained and the nucleic acids were isolated as described earlier.16 Samples were analyzed using a multiplex PCR system designed to detect adenovirus, bocavirus, Chlamydophila pneumoniae, human coronaviruses (CoV) NL63, HKU1, OC43 and 229E, human enterovirus, human metapneumovirus, human rhinovirus (hRV), influenza A and B, Mycoplasma pneumoniae, parainfluenza virus (1, 2, and 3) and respiratory syncytial virus (RSV). The multiplex PCR method has been described previously.17
Ex Vivo Lung Perfusion
During the study period, a novel management of donor organs was introduced in the form of ex vivo lung perfusion (EVLP) (S4, http://links.lww.com/TXD/A117),18 therefore a post hoc analysis of the impact of EVLP in our cohort was added.
Statistics
Comparisons of the group levels for numerical variables were performed using the Mann-Whitney U test, and for categorical variables, the Fisher exact test (2-sided). Kaplan-Meier analysis and the log-rank test were used to calculate and compare the CLAD-free survival and time until graft loss for time-independent covariates. Acute rejection, bacterial infection, fungal infection, VRTI, CMV viremia, and REED were handled as time-dependent covariates for appropriate assignment of hazard. A univariate Cox proportional hazards model was used for examining the association between covariates and CLAD-free survival and graft loss respectively. Covariates with a P value less than 0.2 in the univariate analysis were included in the multivariate model. The SPSS software package version 22.0.0 (IBM, Armonk, NY) was used for all statistical analyses, and a P value less than 0.05 was considered significant.
RESULTS
Between February 23, 2009, and April 11, 2012, 98 patients were included in the study. The last day of follow-up was March 12, 2017. No patient was lost to follow-up. During the first 365 days after LTx, 629 outpatient visits were recorded, of which 574 were scheduled follow-up visits and 55 were extra visits. Each patient had a median of 7 visits with viral sampling (range 2-9) during the first year, and the median follow-up time was 80 months.
VRTI
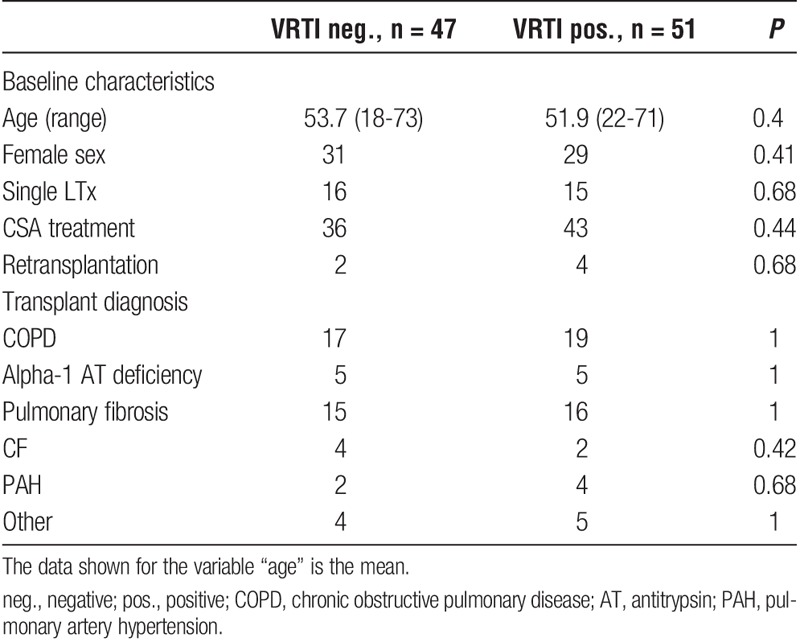
There were 51 VRTI-positive patients, and no statistically significant differences regarding baseline characteristics between VRTI-positive and -negative patients (Table 1).
TABLE 1.
Baseline characteristics VRTI positive and negative patients
During the first year of follow-up VRTI was detected in 111 of 629 visits, of which 38 of 111 were lower VRTIs. Of the 251 visits in which surveillance bronchoscopies were performed, the same virus was detected in both upper (NPH) and lower (BAL) airways in 24 cases of which 7 were symptomatic.
The median rate of viral infections was 1/patient; 30 patients suffered more than 1 viral infection, whereas 47 patients had no viral infections. Six patients were positive for more than 1 virus in at least 1 sample, in total, 14 such samples were detected. In 19 patients, the same virus was found in at least 2 consecutive samples at least 3 weeks apart. For the VRTI-positive patients, the mean time to first viral infection after LTx was 16.5 weeks. A majority of the detected viruses were hRV (57.6%), followed in frequency by CoV (19.2%) (Figure 2). Human rhinovirus were detected across all seasons and CoV mostly during winter and spring (Figure 3). In 10 samples, RSV was detected, and in another 6 samples, enterovirus was detected. Metapneumovirus, parainfluenza and adenovirus were all detected in 5 samples or fewer. Only 3 cases of influenza virus were detected. No patients were treated with antiviral drugs. All patients were vaccinated against influenza before transplantation.
FIGURE 2.
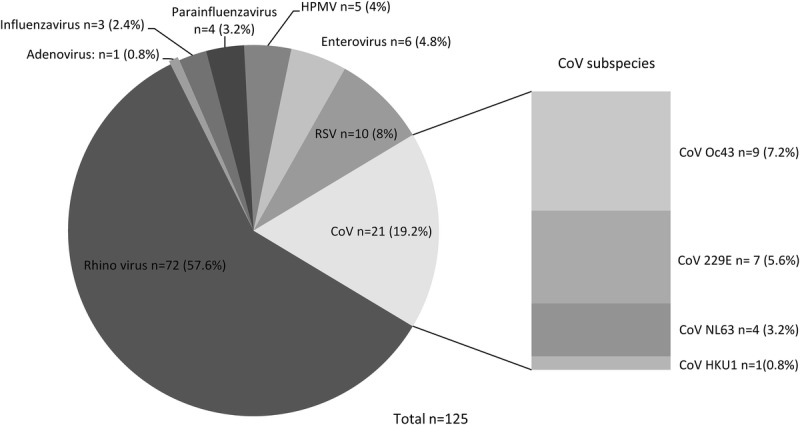
Total amount of detected viruses, divided into viral species. HMPV, human metapneumovirus.
FIGURE 3.
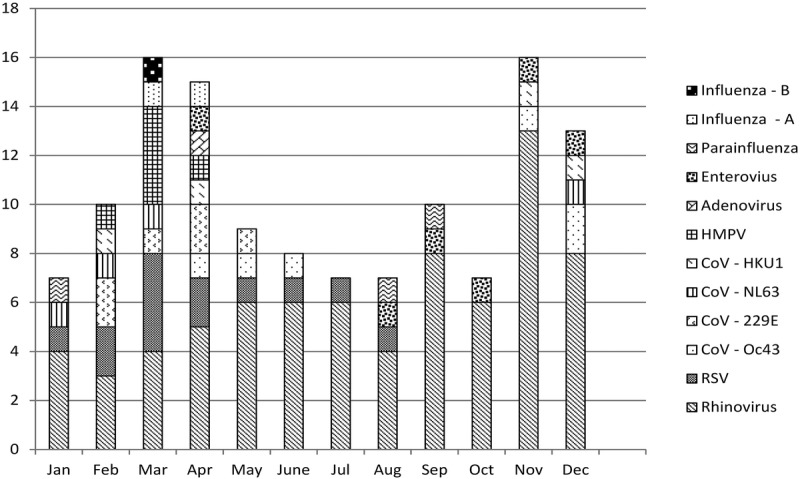
Total number of viral infections during follow-up, per calendar month, divided into viral species.
Infectious Events and AR
In total, 26 patients suffered an AR during the first year, 3 of these patients suffered more than 1 AR before developing CLAD. Bacterial infections affected 41 patients and consisted of Pseudomonas aeruginosa in 20 cases, and the remainder were other bacteria. Fungal infections affected 13 patients with 2 cases of Candida glabrata, 1 case of Candida krusei and the rest consisted of Aspergillus species. A total of 14 patients showed CMV antibody mismatch; 46 patients had at least 1 episode of CMV viremia during the first year, but only 4 patients developed CMV disease during the follow-up period. Repeated elevated EBV-DNA was common, with 29 patients suffering from more than 1 episode of Epstein-Barr viremia. Three patients developed posttransplant lymphoproliferative disease during follow-up, all responded favorably to treatment. Acute rejection and infectious events are outlined in Table 2. No case of positive PCR for Legionella pneumophila, Pneumocystis jirovecii, Chlamydophila pneumoniae, or Mycoplasma pneumoniae was detected.
TABLE 2.
Infectious events, acute rejection, and endpoint distribution for VRTI positive and negative patients
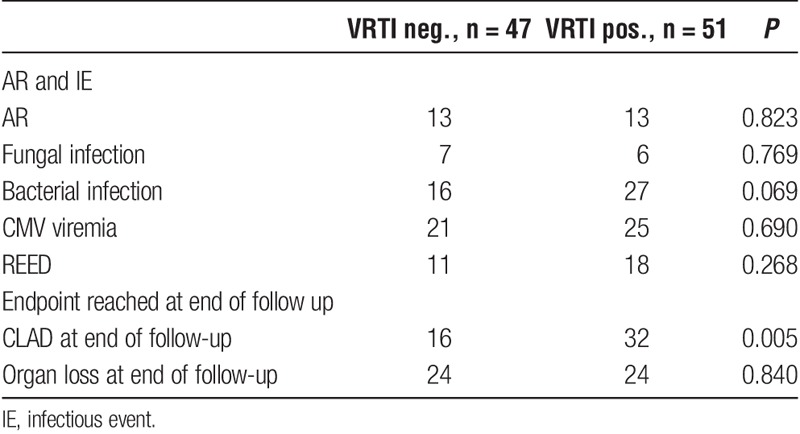
CLAD and Organ Loss
At the end of follow-up, 48 patients had been diagnosed with CLAD (Table 2). Of these, 11 were predominantly restrictive and 37 were predominantly obstructive. A total of 48 patients suffered organ loss, of which 42 patients died and 6 had a retransplantation. Twelve of the deaths occurred during the first year. The major cause for organ loss was CLAD (n = 25), and other significant causes were infections (n = 7) and malignancy (n = 6). Chronic lung allograft dysfunction development during the follow-up period was more common among the VRTI positive subjects (P = 0.005) (Table 2), and it was significantly more common that CLAD was cause of organ loss among those who had suffered from a VRTI (P = 0.008). We found no significant difference regarding graft survival at the end of follow-up between patients with and without VRTIs (P = 0.84) (Table 2). The VRTI-positive subjects had a significantly higher hazard ratio (HR) for CLAD development in multivariate analysis (P = 0.041) (Table 3), but we found no significant difference in time until graft loss (P = 0.86). However, among those who suffered from organ loss, time between CLAD development and organ loss were significantly longer for those who had 1 or more VRTI before developing CLAD, compared with those who had none (P = 0.021). Bacterial (P = 0.013) and fungal (P = 0.001) infections were associated with shorter time to graft loss.
TABLE 3.
Univariate and multivariate Cox Proportional Hazards model for time to CLAD with time- dependent covariates
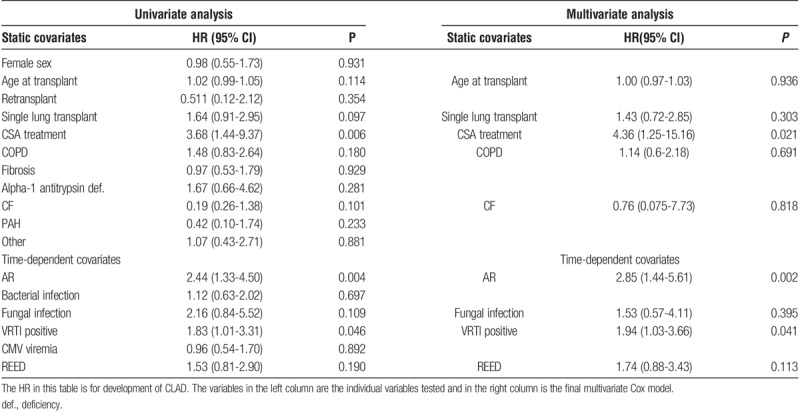
Association Between VRTI and CLAD
From the univariate analysis, the following variables were included in the multivariate model: immunosuppressive treatment with CSA (as opposed to TAC), age at transplant, single lung transplant, REED, COPD diagnosis, VRTI positive or at least 1 episode of AR and cystic fibrosis (CF) diagnosis were included in the multivariate analysis (Table 3). In the multivariate analysis, VRTI-positive (HR, 1.94), CSA treatment (HR, 4.36) and at least 1 episode of AR (HR = 2.85) remained independently predictive of CLAD. Figure 4 shows the effect of CSA treatment in a Kaplan-Meier plot.
FIGURE 4.
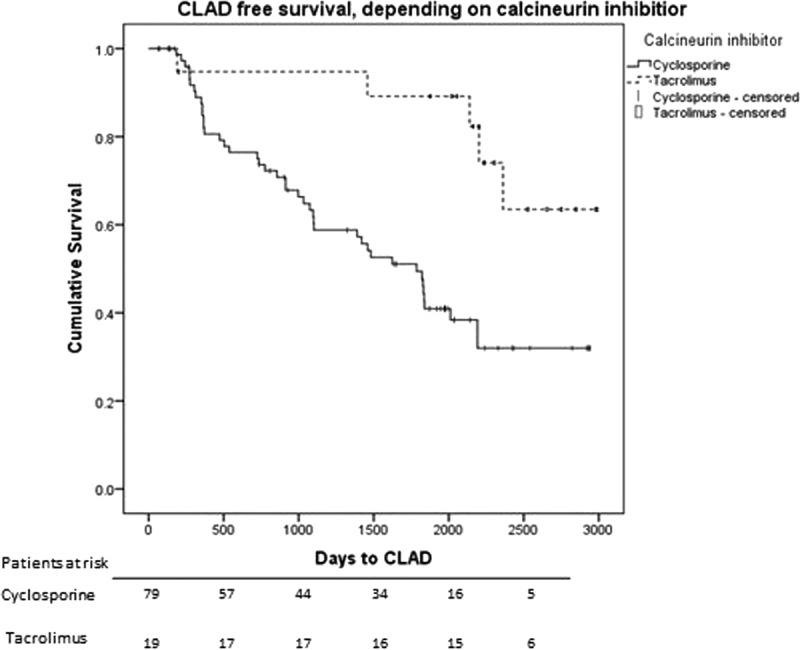
Kaplan-Meier plot of CLAD-free survival after LTx for patients treated with CSA versus TAC. The difference is significant with Log-rank test (P = 0.004). Below are patients at risk.
Of the individual viral agents, CoV was identified as a time-dependent risk factor (P = 0.026; HR, 2.30 [1.10-4.80]) for CLAD development. This association persisted in a multivariate analysis using the same cofactors as the previous analysis, (P = 0.007; HR, 2.95 [1.34-6.49]). There were too few observations to separately evaluate the different CoV subtypes. Having more than 1 type of virus simultaneously detected in a single test was associated with CLAD in multivariate Cox performed with the same covariates as previous analysis (P = 0.026, HR, 3.35 [1.16-9.70]) (S5 http://links.lww.com/TXD/A117). Symptomatic VRTI (P = 0.16) or VRTI in BAL fluid (P = 0.10) were not associated with CLAD. The post hoc analysis of transplant recipients who received lungs treated with EVLP (n = 8) showed no significant difference in risk for CLAD development (P = 0.36) or graft loss (P = 0.55) compared with non–EVLP-treated lungs.
DISCUSSION
In the present study, VRTI during the first year of follow-up after LTx was a significant risk factor for CLAD development, but we found no association with graft survival. AR during the first year was an independent risk factor for CLAD development, as expected. Interestingly, CSA treatment was also significantly associated with CLAD.
In 2003, Khalifah et al6 presented data suggesting that respiratory viral infections are a distinct risk for BOS. More recently, Allyn et al19 reported that CLAD development was hastened by viral pneumonia defined as symptomatic viral infection and a radiographic infiltrate without a clear alternative explanation. Their study included BAL samples, expectorated sputum and tracheal suction, as well as NPH wash samples, primarily analyzed with cultures from 2000 to 2008, and with PCR from 2009 onward. It is indicated that a majority of the samples were BAL samples, and the main detection method was culture-based, which might explain the lower proportion of VRTI-positive subjects (25%) compared with our study (52%) and other prospective studies (51-61%).7,20-22 Fisher et al12 published a retrospective study of 250 patients with at least 1-year follow-up, in which tests for VRTI were performed upon clinical suspicion but no surveillance testing was carried out. The samples were tested using different methods including PCR, fluorescence microscopy and cultures. They found a time-dependent relationship with CLAD development at 3, 6, and 12 months after VRTI, with an HR declining with time after the event. They also showed that AR was a significant risk factor for CLAD development.
Our data suggest that in particular coronaviruses might contribute to CLAD developing sooner after transplantation which has not been previously reported. Although hRV was the most commonly detected viral agent, it seemed to contribute less to CLAD development (S6 http://links.lww.com/TXD/A117). The 4 coronavirus subtypes (229E, OC43, NL63, and HKU1) all usually replicate in NPH epithelial cells and generally cause mild symptoms in otherwise healthy patients,23 but it is not clear if this is the case among immunocompromised hosts.24 In our study, we found all the coronavirus subtypes in the lower airways. These findings fit with previous studies reporting CoV-HKU1 to have a preference for type 2 pneumocytes25 and 229E for alveolar macrophages,26 both known to trigger the innate immune system and a T cell mediated adaptive immune response.25,26 However, the number of each CoV subtype, as well as the number of viruses of other viral families were too few for subgroup analysis.
Our finding that AR is a risk factor for CLAD development is uncontroversial, and the effect of the choice of calcineurin inhibition on CLAD development has been previously published.27 At our center in general there is a selection bias toward Tacrolimus instead of CSA, for younger patients and CF patients. Also if a patient is retransplanted, TAC is selected more often (S7 http://links.lww.com/TXD/A117). The high HR for CLAD development associated with CSA treatment is somewhat unexpected, but our study was not designed for comparing the impact of different types of immunosuppression and the result must be interpreted witch caution.
We did not find any association between VRTI and time until graft loss; however, the majority of the CLAD population with previous VRTI exposure suffered from a relatively slowly progressing BOS compared with those in the CLAD population with no previous VRTI exposure. This could suggest that the CLAD phenotype triggered by VRTI is less aggressive than CLAD triggered by other causes.
We found no association between lower VRTI and CLAD in contrast to previous findings. However, the survey bronchoscopies that detected lower VRTI with a simultaneous finding of the same virus in upper airways were performed on asymptomatic patients in 70% of the cases. It is likely that some of the patients with asymptomatic upper VRTI, detected at surveillance sampling without bronchoscopy, also had a lower VRTI thus the true impact of lower VRTI may have been underestimated. A hypothetic mechanism for the graft damage is a disturbed repair mechanism due to decreased regulatory T (Treg) cell activity caused by the immunosuppressive treatment. A function of Treg cells is to suppress proliferation of fibroblasts triggered by cytotoxic T cells (CD8+ T cells) attacking infected respiratory epithelial cells. An absence of Treg cells in immunosuppressed patients might lead to poor control of inflammatory responses and dysregulated epithelial repair mechanisms.28 Thus, immune response induced by VRTI early after lung transplant might not stop with clearance of the virus and cause epithelial injury and fibroproliferation leading to reduced lung function and CLAD.29
This study has several limitations. First, the study was designed before the release of the standardization of definitions of infections in cardiothoracic transplant recipients, released by International Society for Heart and Lung Transplantation in 2011,30 which would have been preferable for future reference. Only episodes that resulted in contact with a physician were recorded in the case report form, and no systematically recorded clinical data, except spirometry data, were available from these visits. The study did not evaluate other known risk factors, such as gastroesophageal reflux or environmental pollution exposure after LTx. Furthermore, with respect to the high number of asymptomatic VRTIs, it would have been preferable to keep sampling intervals constant, as increased time between sampling at end of the first year might introduce a bias toward the effect of early infections. More frequent surveillance bronchoscopies to analyze the frequency of asymptomatic lower VRTI after LTx would also have been preferable. No viral sequencing was performed which makes it difficult to be certain whether the 19 patients with the same virus in sequential samples suffered from persistent infections or experienced multiple reinfections with different subtypes of virus.
The long-term effects of early VRTI and the dominance of asymptomatic VRTI indicated by our findings suggest that improved VRTI prophylaxis might promote a more favorable outcome after LTx. However, at present, few effective drugs exist and new antiviral compounds that could be evaluated for treating LTx recipients are needed.
In conclusion, this study suggests that VRTIs during the first postoperative year, in particular those caused by coronavirus, could be an important risk factor for long-term CLAD development. Using a systematic prospective recording of symptoms, we also found that a majority of these VRTIs were asymptomatic. Further mechanistic studies of viral interaction in transplanted lungs, and larger multicenter trials using standardized sampling and diagnostic methods, are warranted to better understand the effect of viral infections on long-term outcome after LTx.
Supplementary Material
ACKNOWLEDGMENTS
The authors wish to acknowledge the nursing staff at the Sahlgrenska lung transplant outpatient ward, Katarina Lindström, the staff at the viral detection laboratory and Biostatistician Kjell Pettersson for expert technical assistance.
Footnotes
Published online 3 July, 2018.
This study has been funded by the Swedish Heart-Lung foundation, the Region Västra Götaland Research Funds, (VGFOUREG-228341, VGFOUREG-82811, Regional ALF funds (ALFGBG-217671, ALFGBG-439391, ALFGBG-672131), the Gothenburg Medical Society Research Fund and Kungsbacka Hospital.
The authors declare no conflicts of interest.
J.M. participated in research design, sample collection, data collection, data analysis and writing of the article. J.W. participated in research design, data analysis and writing of the article. L-M.A. participated in research design, data analysis and writing of the article. M.L. participated in supervision and quality control of laboratory work, research performance and writing of the article. R.B.-L. participated in research design and writing of the article. R.N. participated in research performance, data analysis and writing of the article. G.C.R. was the main supervisor and participated in research design, sample collection, research performance, data analysis and writing of the article.
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantationdirect.com).
REFERENCES
- 1.Yusen RD, Edwards LB, Dipchand AI, et al. The Registry of the International Society for Heart and Lung Transplantation: thirty-third adult lung and heart-lung transplant report—2016; focus theme: primary diagnostic indications for transplant. J Heart Lung Transplant. 2016;35:1170–1184. [DOI] [PubMed] [Google Scholar]
- 2.Sato M, Waddell TK, Wagnetz U, et al. Restrictive allograft syndrome (RAS): a novel form of chronic lung allograft dysfunction. J Heart Lung Transplant. 2011;30:735–742. [DOI] [PubMed] [Google Scholar]
- 3.Verleden GM, Vos R, Vanaudenaerde B, et al. Current views on chronic rejection after lung transplantation. Transpl Int. 2015;28:1131–1139. [DOI] [PubMed] [Google Scholar]
- 4.Meyer KC, Raghu G, Verleden GM, et al. An international ISHLT/ATS/ERS clinical practice guideline: diagnosis and management of bronchiolitis obliterans syndrome. Eur Respir J. 2014;44:1479–1503. [DOI] [PubMed] [Google Scholar]
- 5.Billings JL, Hertz MI, Wendt CH. Community respiratory virus infections following lung transplantation. Transpl Infect Dis. 2001;3:138–148. [DOI] [PubMed] [Google Scholar]
- 6.Khalifah AP, Hachem RR, Chakinala MM, et al. Respiratory viral infections are a distinct risk for bronchiolitis obliterans syndrome and death. Am J Respir Crit Care Med. 2004;170:181–187. [DOI] [PubMed] [Google Scholar]
- 7.Milstone AP, Brumble LM, Barnes J, et al. A single-season prospective study of respiratory viral infections in lung transplant recipients. Eur Respir J. 2006;28:131–137. [DOI] [PubMed] [Google Scholar]
- 8.Gottlieb J, Schulz TF, Welte T, et al. Community-acquired respiratory viral infections in lung transplant recipients: a single season cohort study. Transplantation. 2009;87:1530–1537. [DOI] [PubMed] [Google Scholar]
- 9.Vu DL, Bridevaux PO, Aubert JD, et al. Respiratory viruses in lung transplant recipients: a critical review and pooled analysis of clinical studies. Am J Transplant. 2011;11:1071–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leland DS, Ginocchio CC. Role of cell culture for virus detection in the age of technology. Clin Microbiol Rev. 2007;20:49–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Magnusson J, Westin J, Andersson LM, et al. The impact of viral respiratory tract infections on long-term morbidity and mortality following lung transplantation: a retrospective cohort study using a multiplex PCR panel. Transplantation. 2013;95:383–388. [DOI] [PubMed] [Google Scholar]
- 12.Fisher CE, Preiksaitis CM, Lease ED, et al. Symptomatic respiratory virus infection and chronic lung allograft dysfunction. Clin Infect Dis. 2016;62:313–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fisher CE, Mohanakumar T, Limaye AP. Respiratory virus infections and chronic lung allograft dysfunction: assessment of virology determinants. J Heart Lung Transplant. 2016;35:946–947. [DOI] [PubMed] [Google Scholar]
- 14.Verleden GM, Raghu G, Meyer KC, et al. A new classification system for chronic lung allograft dysfunction. J Heart Lung Transplant. 2014;33:127–133. [DOI] [PubMed] [Google Scholar]
- 15.Stewart S, Fishbein MC, Snell GI, et al. Revision of the 1996 working formulation for the standardization of nomenclature in the diagnosis of lung rejection. J Heart Lung Transplant. 2007;26:1229–1242. [DOI] [PubMed] [Google Scholar]
- 16.Kullberg-Lindh C, Olofsson S, Brune M, et al. Comparison of serum and whole blood levels of cytomegalovirus and Epstein-Barr virus DNA. Transpl Infect Dis. 2008;10:308–315. [DOI] [PubMed] [Google Scholar]
- 17.Andersson ME, Olofsson S, Lindh M. Comparison of the FilmArray assay and in-house real-time PCR for detection of respiratory infection. Scand J Infect Dis. 2014;46:897–901. [DOI] [PubMed] [Google Scholar]
- 18.Wallinder A, Ricksten SE, Hansson C, et al. Transplantation of initially rejected donor lungs after ex vivo lung perfusion. J Thorac Cardiovasc Surg. 2012;144:1222–1228. [DOI] [PubMed] [Google Scholar]
- 19.Allyn PR, Duffy EL, Humphries RM, et al. Graft loss and CLAD-onset is hastened by viral pneumonia after lung transplantation. Transplantation. 2016;100:2424–2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bridevaux PO, Aubert JD, Soccal PM, et al. Incidence and outcomes of respiratory viral infections in lung transplant recipients: a prospective study. Thorax. 2014;69:32–38. [DOI] [PubMed] [Google Scholar]
- 21.Kumar D, Husain S, Chen MH, et al. A prospective molecular surveillance study evaluating the clinical impact of community-acquired respiratory viruses in lung transplant recipients. Transplantation. 2010;89:1028–1033. [DOI] [PubMed] [Google Scholar]
- 22.Peghin M, Hirsch HH, Len O, et al. Epidemiology and immediate indirect effects of respiratory viruses in lung transplant recipients: a 5-year prospective study. Am J Transplant. 2017;17:1304–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mandell G, Bennett J, Dolin R. Principles and Practice of infectious diseases 7th edition. Churchill Livingston Elsevier; 2010. [Google Scholar]
- 24.Ogimi C, Waghmare AA, Kuypers JM, et al. Clinical significance of human coronavirus in bronchoalveolar lavage samples from hematopoietic cell transplant recipients and patients with hematologic malignancies. Clin Infect Dis. 2017;64:1532–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dominguez SR, Travanty EA, Qian Z, et al. Human coronavirus HKU1 infection of primary human type II alveolar epithelial cells: cytopathic effects and innate immune response. PLoS One. 2013;8:e70129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Funk CJ, Wang J, Ito Y, et al. Infection of human alveolar macrophages by human coronavirus strain 229E. J Gen Virol. 2012;93(Pt 3):494–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Treede H, Glanville AR, Klepetko W, et al. Tacrolimus and cyclosporine have differential effects on the risk of development of bronchiolitis obliterans syndrome: results of a prospective, randomized international trial in lung transplantation. J Heart Lung Transplant. 2012;31:797–804. [DOI] [PubMed] [Google Scholar]
- 28.Braciale TJ, Sun J, Kim TS. Regulating the adaptive immune response to respiratory virus infection. Nat Rev Immunol. 2012;12:295–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Palmer SM, Flake GP, Kelly FL, et al. Severe airway epithelial injury, aberrant repair and bronchiolitis obliterans develops after diacetyl instillation in rats. PLoS One. 2011;6:e17644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Husain S, Mooney ML, Danziger-Isakov L, et al. A 2010 working formulation for the standardization of definitions of infections in cardiothoracic transplant recipients. J Heart Lung Transplant. 2011;30:361–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


