Abstract
Background
Orthotopic liver transplantation (OLT) is a potential curative treatment in patients with hepatocellular carcinoma (HCC); however, treatment options for recurrent HCC after OLT are limited. Immune checkpoint inhibitors, such as nivolumab, an inhibitor of programmed cell death protein 1, have been successfully used for metastatic HCC but data on safety of nivolumab following solid organ transplantation are limited.
Methods
We report a 53-year-old woman with HCC who was treated with OLT. After 2 years, HCC recurred. Initial treatment with sorafenib was discontinued due to side effects and disease progression. Progressive HCC in the lung and lymph nodes was subsequently treated with nivolumab. One week after the first nivolumab dose, rapid progressive liver dysfunction was noted. Liver biopsy revealed severe cellular graft rejection prompting treatment with intravenous steroids and tacrolimus. Liver function continued to decline, leading to severe coagulopathy. The patient succumbed to intracranial hemorrhage.
Results
A systematic PubMed search revealed 29 cases treated with a checkpoint inhibitor following solid organ transplantation. Loss of graft was described in 4 (36%) of 11 cases with OLT and in 7 (54%) of 13 cases after kidney transplantation. However, cases with favorable outcome were also described. Eighteen cases with adverse events were identified upon searching the World Health Organization database VigiBase, including 2 cases with fatal outcome in liver transplant recipients due to graft loss.
Conclusion
Experience with checkpoint inhibitors in solid organ transplant recipients is limited. Published cases so far suggest severe risks for graft loss as high as 36% to 54%.
Hepatocellular carcinoma (HCC) frequently occurs in patients with liver cirrhosis. In selected cases, orthotopic liver transplantation (OLT) is the best curative option. Hepatocellular carcinoma recurs in about 16%1,2 of patients after OLT. If OLT is not an option, therapeutic options for HCC recurrence include the tyrosine kinase inhibitor sorafenib.3 Sorafenib has also been used for patients after OLT, but the therapeutic benefit has not been clearly established.4 Usage of sorafenib is in addition limited due to severe side effects, including hand-foot syndrome, nausea, emesis and wasting in a large percentage of patients.3,5
Recently, checkpoint inhibitors have been introduced for immune activation in patients with metastatic cancer, resulting in tumor regression and even remission in a subgroup of patients.6 Nivolumab, an inhibitor of programmed cell death protein 1 (PD-1), was successfully established as first or second line treatment in various malignancies, such as melanoma, squamous cell skin carcinoma, non–small-cell lung carcinoma, kidney carcinoma, and classical Hodgkin lymphoma.7 Case series also demonstrate benefits of nivolumab in HCC on an off-label basis.8 However, solid organ transplant recipients were excluded from checkpoint inhibitor registration and there is limited experience with the application of nivolumab in this patient population.9,10 We here present a case of fulminant liver transplant failure with cellular rejection and fatal outcome in a patient treated with nivolumab for recurrent HCC.
MATERIALS AND METHODS
Patient's relatives provided written informed consent to publication.
We performed a systematic Pubmed literature search with the following complementary search strategies (February 22, 2018): (nivolumab OR ipilimumab OR pembrolizumab OR atezolizumab) AND (transplantation OR transplant OR rejection) yielding 210 publications; (pd-1 AND checkpoint inhibitor) AND (organ transplant recipient OR transplantation) yielding 53 publications; Immune checkpoint inhibitor AND liver transplant yielding 13 publications. All identified publications were screened and redundant reports were excluded. Publications describing patients after solid organ transplantation treated with 1 or a combination of the 4 checkpoint inhibitors, nivolumab, ipilimumab, pembrolizumab, or atezolizumab with sufficient information regarding the outcome for the transplanted organ were included. Altogether, our literature research identified 25 publications with 29 cases. The final list is provided in Table 1.
TABLE 1.
Summary of solid organ transplant recipients treated with checkpoint inhibitors

We searched for individual case safety reports (ICSRs) in the World Health Organization (WHO) global database VigiBase. In this database, spontaneously reported adverse drug reactions are collected as ICSR. We included all ICSRs with the substances, nivolumab, ipilimumab, pembrolizumab, or atezolizumab and the reactions “transplant rejection”, “graft rejection” as a preferred term, low-level term or high-level term reported in the database until August 6, 2017 (total number of ICSRs 15 160.275). Matching of patients from VigiBase to case reports in Table 1 was not possible and potentially redundant cases could not be excluded.
Case Presentation
A 53-year-old woman of central African origin received domino-liver transplantation 36 months ago for HCC that developed on the basis of liver cirrhosis due to chronic hepatitis C. Immunosuppression after OLT included prednisone for 3 months on a tapering scheme, mycophenolate mofetil (MMF) 1 to 2 g/d for 3 months and everolimus 1 mg/d. In 4 posttransplant biopsies within the first 5 months no rejection was shown. Hepatitis C virus was successfully treated with sofosbuvir 400 mg/ledipasvir 90 mg for 12 weeks. Two years after OLT, HCC recurred with pulmonary, pulmonary bihilar and retroperitoneal lymph node metastases. The patient received sorafenib for 2 months but stopped the treatment due to intolerable nausea, emesis, and tumor progression. No hepatic recurrences could be detected. After thorough discussion of benefits and potential adverse drug reactions—including risk of graft loss and fatal outcome—treatment with nivolumab was initiated. A change of immune suppression was not performed because a recent randomized controlled study failed to show survival benefits upon sirolimus treatment for patients with HCC treated after transplantation.36 After administration of a single dose of 200 mg nivolumab (3 mg/kg body weight), an increase in liver function tests was noted 1 week after administration (Figure 1). Liver biopsy was performed 2 weeks after administration of nivolumab and demonstrated acute cellular transplant Rejection Activity Index (RAI) 7 (Figure 2) under immunosuppression with everolimus (trough level, 3.3 μg/L) and MMF. Intravenous high-dose steroid therapy (methylprednisolone 500 mg daily) for 5 days was administered but liver function tests continued to increase. Steroids were changed to prednisone 40 mg per day orally and tacrolimus 6 mg per day (aiming for a trough level of 5 μg/L) was added. No antiplatelet agent and only prophylactic anticoagulation with subcutaneous low molecular weight heparin was used.
FIGURE 1.
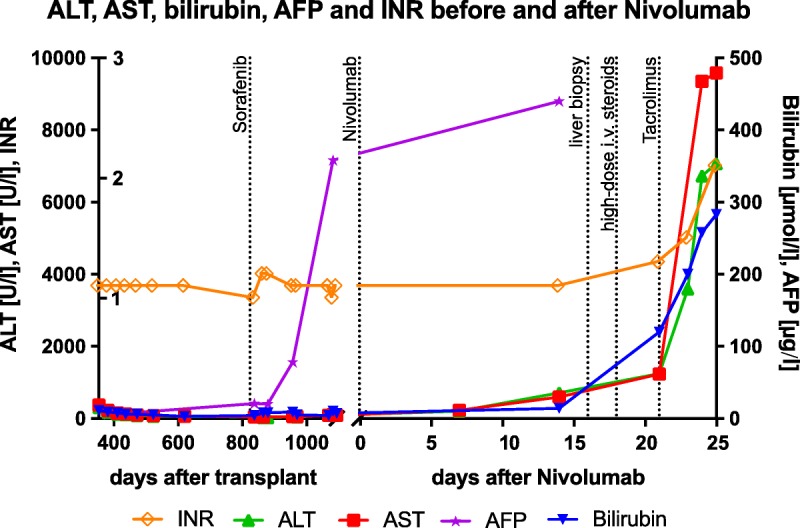
Time course for liver function tests and tumor markers before and after nivolumab treatment.
FIGURE 2.
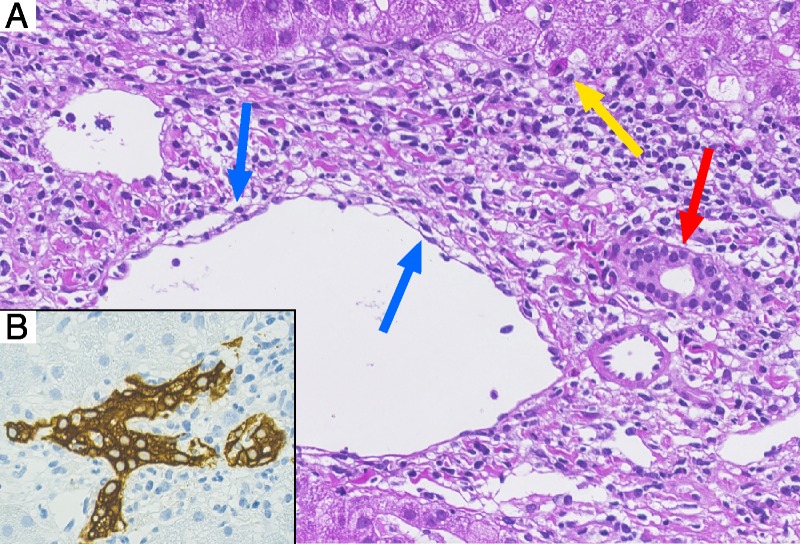
Liver biopsy 16 days after nivolumab. A, HE staining illustrating prominent portal mixed inflammation with interface activity and isolated hepatocyte necrosis (yellow arrow), cytoplasmatic vacuolization of the duct epithelium consistent with bile duct damage (red arrow) and subendothelial lymphocytic inflammation with lifting up of the endothelium compatible with endothelitis (blue arrow); 400×. B, Cytokeratin-7 immunohistochemical staining highlighting the influx of inflammatory cells in the ductal epithelium associated with dysmorphic changes of the bile duct (magnification, 400×).
Over the next 3 days, the patient's condition continued to deteriorate with tiredness, severe nausea and emesis, diffuse upper abdominal pain, night sweats, alcoholic stools, dark urine, scleral icterus but no signs of neurological dysfunction. The patient was admitted to the hospital. Laboratory tests revealed a rapid increasing of liver enzymes (aspartate aminotransferase, 9347 U/L; norm, <35 U/L; alanine aminotransferase, 6730 U/L; norm, <35 U/L), signs of coagulopathy (international normalized ratio, 2.1; norm, <1.2; factor V, 28%; norm, 50-150%), acute renal dysfunction (creatinine, 190 μmol/L; norm, 44-80 μmol/L), most likely due to dehydration, secondary to vomiting. Therapeutic levels of everolimus (3.3 μg/L) and tacrolimus (5.1 μg/L) were documented. Tests for viral infections, including CMV, hepatitis B, C, and E, were negative; diagnostic tests for HSV and autoimmune hepatitis were not performed due to low pretest probability. We noted a low-level Epstein-Barr virus viremia of 2323 IE/mL (detection limit, <122 IE/mL); however, similar levels have been noted before on several occasions after OLT. Ultrasound confirmed regular blood supply to the liver. Acetylcystein treatment and encephalopathy prophylaxis with rifaximine and lactulose were started.
On the second day of admission, the patient suddenly lost consciousness (Glasgow coma scale, 3), prompting transfer to the intensive care unit and intubation. Computed tomography scan demonstrated extensive liver necrosis and intracerebral bleeding with signs of increased cerebral pressure (Figure 3). After discussion with her family, therapy was discontinued and the patient passed away on the same day, 25 days after first administration of nivolumab.
FIGURE 3.
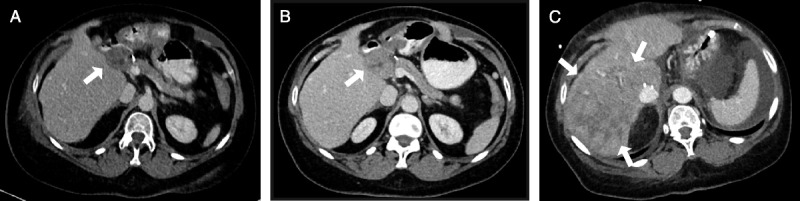
Abdominal imaging. A, Contrast-enhanced CT scan demonstrating an enlarged lymph node in the liver hilum (arrow) due to HCC recurrence 27 months after liver transplantation. B, CT scan 9 months later with stable lymph node before nivolumab treatment 35 months after liver transplantation. C, CT scan at the day of death 25 days after nivolumab treatment with inhomogeneous hypodense areas in the right hepatic lobe (arrows) suggestive for parenchymal necrosis. No signs of intrahepatic HCC recurrence. CT, computed tomography.
Systematic Literature Search
Our systematic literature research for the use of checkpoint inhibitors in solid organ recipients revealed 25 articles describing 29 patients, including our presented case (Table 1, for search strategy see methods). Fourteen of 29 cases were kidney transplant recipients, 11 cases were liver transplant recipients, 3 were heart transplant recipients, and 1 patient had received cornea transplantation.
Graft loss was defined as severe transplant dysfunction resulting in death in patients after liver or heart transplantation and hemodialysis in patients after kidney transplantation, respectively. Because in all patients uncontrolled neoplasia was present, retransplantation has not been an option. Acute rejection was defined graft dysfunction reversed by adequate medical therapy (1 case by Dueland et al).
Rates of graft loss are reported according to the checkpoint inhibitor used; patients receiving combination therapies are considered for both drugs.
Nivolumab Treatment in Transplant Patients
Loss of transplant function after nivolumab administration alone or in combination with ipilimumab15-17,22-29,33,34 occurred in 9 (56%) of 16 cases: graft loss was observed in 4 of 8 cases of kidney transplant recipients, in 3 of 5 cases of liver transplant recipients and 1 of 2 cases after heart transplantation. In the case of cornea transplant nivolumab resulted in graft dysfunction.
Overall cancer response is known in only 11 of the 16 cases: in 5 cases, progressive disease was described; in 3 cases, regression was reported; and in 3 cases, stable disease was reported.
Ipilimumab Treatment in Transplant Patients
Loss of transplant function after ipilimumab administration alone or in combination with nivolumab or pembrolizumab11-14,20-24,32 occurred in 4 (36%) of 11 cases: ipilimumab administration after kidney transplant resulted in graft loss in 3 out of 6 cases and in liver transplant recipients in 1 of 4 cases. One heart transplanted patient showed no rejection after ipilimumab.
Cancer response was reported in all cases: in 7 of 11 cases progressive disease was described, 1 case showed stable disease and 3 cases showed tumor regression.
Pembrolizumab Treatment in Transplant Patients
Loss of transplant function after pembrolizumab administration alone or in combination with ipilimumab14,18,19,28,30,31 occurred in 2 (33%) of 6 cases: pembrolizumab resulted in graft loss in 2 of 3 cases of kidney transplant recipients but in no rejection in 3 cases after liver transplantation.
Cancer response was reported in all cases: in 2 of 6 cases, progressive disease was described, the remaining 4 cases showed cancer regression.
A search in VigiBase, the WHO database for pharmacovigilance, revealed worldwide 9 ICSRs of transplant rejection after using the checkpoint inhibitor nivolumab between 2015 and August 2017. Transplant rejections of the kidney (n = 6), cornea (n = 1), or without organ specification (n = 2) were reported. In all cases, nivolumab was labeled as the suspected drug. In 1 patient, the cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) inhibitor ipilimumab was coadministered. Fatal outcome was documented in 1 female patient with nivolumab treatment for liver carcinoma, who died after transplant rejection. Further details are not provided in the database.
DISCUSSION
We report a single case of fatal liver failure 3 weeks after administration of a single dose of nivolumab in a patient with recurrent HCC 2 years after liver transplantation. Our systematic literature search identified additional cases with graft loss after checkpoint inhibitor treatment. Overall, graft loss or acute rejection, as discussed above, was described in 13 (45%) of 29 cases across all checkpoint inhibitors. For nivolumab, ipilimumab, and pembrolizumab, rejection rates of 56%, 36%, and 33%, respectively, were apparent. Loss of graft function for liver allografts occurred in 3 (37%) of 11 cases, for kidney allografts in 7 (50%) of 14 cases, and heart allografts in 1 (33%) of 3 cases.
Therefore, our data suggest a severe risk of transplant rejection after use of checkpoint inhibitors in patients after solid organ transplantation. Even though the rates of rejection were highest with nivolumab, our data do not allow to compare relative risks of 1 checkpoint inhibitor versus the other. Furthermore, because our study relies on publication of case reports, the possibility of publication bias has to be considered which might result in overestimation or underestimation of rejection rates.
The safety of nivolumab or other checkpoint inhibitors in solid organ recipients has not been established because this patient population was excluded from relevant clinical trials.9,10 Formal assessment of causality of nivolumab exposure and fatal liver transplant rejection in our case suggests a “probable” relationship according to the WHO/Council for International Organizations of Medical Sciences criteria.37 Chronological order of drug exposure and adverse event, existence of additional cases with nivolumab treatment and graft rejection, pharmacological plausibility, and exclusion of other nonpharmacological explanations suggest a causal relationship between nivolumab treatment and graft rejection.
Immune checkpoint inhibitors, including nivolumab and ipilimumab, revolutionized the treatment of various malignancies. Nivolumab (Opdivo) is a human monoclonal IgG4 antibody which blocks PD-1 receptor on activated T cells, B cells, natural killer T cells, monocytes, and dendritic cells.38 Programmed cell death protein 1 receptor has 2 ligands, PD-L1 and PD-L2, which are expressed on tumor cells and antigen-presenting cells in tumor microenvironment. Binding of the receptor with its ligand (programmed cell death 1 ligand 1 [PD-L1]) leads to negative regulation of T cells and lack of T cell response. Therefore, prevention of PD1 and PD-L1 interaction by nivolumab can restore T cell–mediated tumor suppression.26 The mechanism of ipilimumab (Yervoy) is different from nivolumab because ipilimumab inhibits CTLA-4 activity. Generally, CTLA-4 modulates T-cell activation during the initial phase of the immune response, whereas PD-1 acts during the effector phase.
Induction of immune tolerance of organ-transplanted patients is crucial after solid organ transplantation. Both PD-1 and CTLA-4 signaling pathways contribute to immune tolerance of transplanted organ. PD-L1 expression was proposed to be the key component of graft tolerance after liver transplantation, because higher expression provides a negative feedback, creating a protective shield from human T-cell responses.39 Additionally, PD-1 plays a crucial role in both induction and maintenance of peripheral transplant tolerance by its ability to alter the balance between pathogenic and regulatory T cells and it is also involved in T-cell exhaustion—another emerging mechanism in transplant tolerance.40-44
On the other hand, blocking CTLA-4 in early phases after transplantation led to transplant rejection in a murine model, whereas late blockade seems not to affect transplant survival.45,46
Therefore, inhibition of either CTLA-4 or PD-1 after solid organ transplantation might trigger immune-mediated organ failure. However, which checkpoint inhibitor would be more favorable in organ transplant recipients remains unclear. It also remains unclear whether the time passed after transplantation before checkpoint inhibitor treatment might decrease the risk for graft loss.
Patients with and without organ failure did not significantly differ regarding age, time after transplantation, sex, graft (liver/kidney/heart) and treatment with nivolumab, ipilimumab, or pembrolizumab. We noticed a minor trend for more organ failure in younger patients, which failed to reach statistical significance.
Our case and the 2 cases described by Friend et al are remarkable for an extreme severity of liver transplant rejection leading to rapid transplant failure, nonresponsive to 5 days high-dose steroids. Hepatic side effects of nivolumab are frequent also in patients without OLT, and immune-mediated hepatitis with a median time to onset of 3.3 months (range, 6 days to 9 months) is the main hepatic adverse effect of nivolumab. However, nivolumab-induced hepatitis typically resolves after discontinuation of treatment47 and usually begins much later than 16 days after administration as in our patient. It has different histologic features than described in the liver biopsy in our patient, as it is characterized by an acute lobular hepatitis with isolated or confluent necrosis and a predominantly lymphocytic infiltrate. In contrast, in our case, portal tract inflammation was dominated by a mixed infiltrate with interface activity, bile duct injury and endothelitis compatible with acute cellular rejection (RAI: 3/2/2 = 7). Taken together, nivolumab-induced hepatitis seems an unlikely explanation for liver failure in our patient, and histological changes favor an acute cellular rejection (Figure 2).
However, the clinical course in our patient also remains highly unusual for an acute cellular rejection because this complication only infrequently occurs more than 1 year after transplantation, especially in patients with good therapeutic adherence and no prior history of cellular rejection. Further, acute cellular rejection typically responds to steroid treatment and virtually never progresses to fulminant liver failure. It therefore seems probable that nivolumab exposure and PD-1 inhibition had induced a cellular rejection of unusual severity, leading to fulminant liver transplant failure. However, the underlying immunological mechanisms are unknown.
Our patient ultimately succumbed to fatal intracranial bleeding. Whether this complication is related to coagulopathy associated with liver failure remains unclear because permission to perform autopsy in this patient was not granted.
Management of checkpoint inhibitor-associated solid allograft failure is unclear. Acute graft rejection resolves upon high-dose steroid treatment in 70% to 80% of all cases.48 However, neither in our patient nor in those patients described by Friend et al or in other cases identified in our literature search did high-dose steroid treatment result in clinical improvement. Rapid clinical deterioration in our patient precluded alternative treatment options, such as infliximab,49 antithymoglobuline,50 or cyclosporine. Plasmapheresis was not used in the cases described involving organ failure after checkpoint inhibitor treatment. Plasmapheresis has been used for treatment of acute humoral rejection after OLT51 but it will likely not stop the T cell–mediated immune rejection after checkpoint inhibitor treatment. However, plasmapheresis would remove the checkpoint inhibitor from the circulation, which might potentially be beneficial. Taken together, no medical treatment for graft failure after checkpoint inhibitor treatment has been identified.
CONCLUSIONS
Solid allograft failure is a severe or catastrophic complication after checkpoint inhibitor treatment. No medical treatment options have been identified. Our case and several published cases suggest a 36% to 54% risk of graft loss on checkpoint inhibitor treatment.
Footnotes
Published online 20 July, 2018.
F.J. and B.Mi. contributed equally to this report.
B.Mi. has received traveling fees from Novartis, Vifor, MSD; served on an advisory board from Gilead, Novigenix; speaking fees from Given Imaging, MSD, Vifor. B.Mü. has received consulting honoraria from Abbvie, BMS, Gilead, Janssen, Merck and MSD. J.M. has received consulting honoraria from Abbvie, Bayer, BMS, Gilead, Intercept, Janssen, Merck, MSD, Shire and Vifor. B.V., C.R., D.G., F.J., M.N., and S.W. have nothing to disclose.
No funding was received for this article.
D.G., J.M., B.Mi., C.R., B.V., M.N., F.J., and B.Mü. prepared the clinical findings for the case presentation. F.J., S.W., D.G., M.N., J.M., B.Mi. performed the systematic literature research and the WHO database search. D.G., F.J., C.R., B.V., and B.Mi. performed data analysis and prepared tables and figures. D.G., B.Mi., F.J., and S.W. wrote the article. All authors approved the final version of the article.
Partly, the data for this work were obtained from the WHO Collaborating Centre for International Drug Monitoring, Uppsala, Sweden. Data from spontaneous reporting are inhomogeneous as a result of different reporting policies worldwide and are vulnerable to underreporting and reporting bias. The information contained in this work related to the Pharmacovigilance Database is therefore not homogeneous. The conclusions drawn based on these data do not necessarily represent the opinion of the World Health Organization or of a competent authority.
REFERENCES
- 1.de'Angelis N, Landi F, Carra MC, et al. Managements of recurrent hepatocellular carcinoma after liver transplantation: a systematic review. World J Gastroenterol. 2015;21:11185–11198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–699. [DOI] [PubMed] [Google Scholar]
- 3.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. [DOI] [PubMed] [Google Scholar]
- 4.Castelli G, Burra P, Giacomin A, et al. Sorafenib use in the transplant setting. Liver Transpl. 2014;20:1021–1028. [DOI] [PubMed] [Google Scholar]
- 5.Li Y, Gao ZH, Qu XJ. The adverse effects of sorafenib in patients with advanced cancers. Basic Clin Pharmacol Toxicol. 2015;116:216–221. [DOI] [PubMed] [Google Scholar]
- 6.Wang C, Thudium KB, Han M, et al. In vitro characterization of the anti-PD-1 antibody nivolumab, BMS-936558, and in vivo toxicology in non-human primates. Cancer Immunol Res. 2014;2:846–856. [DOI] [PubMed] [Google Scholar]
- 7.Kudo M. Immune checkpoint inhibition in hepatocellular carcinoma: basics and ongoing clinical trials. Oncology. 2017;92(Suppl 1):50–62. [DOI] [PubMed] [Google Scholar]
- 8.Mamdani H, Wu H, O'Neil BH, et al. Excellent response to anti-PD-1 therapy in a patient with hepatocellular carcinoma: case report and review of literature. Discov Med. 2017;23:331–336. [PubMed] [Google Scholar]
- 9.Gbolahan OB, Schacht MA, Beckley EW, et al. Locoregional and systemic therapy for hepatocellular carcinoma. J Gastrointest Oncol. 2017;8:215–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389:2492–2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morales RE, Shoushtari AN, Walsh MM, et al. Safety and efficacy of ipilimumab to treat advanced melanoma in the setting of liver transplantation. J Immunother Cancer. 2015;3:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ranganath HA, Panella TJ. Administration of ipilimumab to a liver transplant recipient with unresectable metastatic melanoma. J Immunother. 2015;38:211. [DOI] [PubMed] [Google Scholar]
- 13.Dueland S, Guren TK, Boberg KM, et al. Acute liver graft rejection after ipilimumab therapy. Ann Oncol. 2017;28:2619–2620. [DOI] [PubMed] [Google Scholar]
- 14.Kuo JC, Lilly LB, Hogg D. Immune checkpoint inhibitor therapy in a liver transplant recipient with a rare subtype of melanoma: a case report and literature review. Melanoma Res. 2018;28:61–64. [DOI] [PubMed] [Google Scholar]
- 15.Friend BD, Venick RS, McDiarmid SV, et al. Fatal orthotopic liver transplant organ rejection induced by a checkpoint inhibitor in two patients with refractory, metastatic hepatocellular carcinoma. Pediatr Blood Cancer. 2017;64. [DOI] [PubMed] [Google Scholar]
- 16.De Toni EN, Gerbes AL. Tapering of immunosuppression and sustained treatment with nivolumab in a liver transplant recipient. Gastroenterology. 2017;152:1631–1633. [DOI] [PubMed] [Google Scholar]
- 17.Biondani P, De Martin E, Samuel D. Safety of an anti-PD-1 immune checkpoint inhibitor in a liver transplant recipient. Ann Oncol. 2018;29:286–287. [DOI] [PubMed] [Google Scholar]
- 18.Rammohan A, Reddy MS, Farouk M, et al. Pembrolizumab for metastatic hepatocellular carcinoma following live donor liver transplantation: the silver bullet? Hepatology. 2018;67:1166–1168. [DOI] [PubMed] [Google Scholar]
- 19.Schvartsman G, Perez K, Sood G, et al. Immune checkpoint inhibitor therapy in a liver transplant recipient with melanoma. Ann Intern Med. 2017;167:361–362. [DOI] [PubMed] [Google Scholar]
- 20.Lipson EJ, Bodell MA, Kraus ES, et al. Successful administration of ipilimumab to two kidney transplantation patients with metastatic melanoma. J Clin Oncol. 2014;32:e69–e71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jose A, Yiannoullou P, Bhutani S, et al. Renal allograft failure after ipilimumab therapy for metastatic melanoma: a case report and review of the literature. Transplant Proc. 2016;48:3137–3141. [DOI] [PubMed] [Google Scholar]
- 22.Herz S, Hofer T, Papapanagiotou M, et al. Checkpoint inhibitors in chronic kidney failure and an organ transplant recipient. Eur J Cancer. 2016;67:66–72. [DOI] [PubMed] [Google Scholar]
- 23.Miller DM, Faulkner-Jones BE, Stone JR, et al. Complete pathologic response of metastatic cutaneous squamous cell carcinoma and allograft rejection after treatment with combination immune checkpoint blockade. JAAD Case Rep. 2017;3:412–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spain L, Higgins R, Gopalakrishnan K, et al. Acute renal allograft rejection after immune checkpoint inhibitor therapy for metastatic melanoma. Ann Oncol. 2016;27:1135–1137. [DOI] [PubMed] [Google Scholar]
- 25.Barnett R, Barta VS, Jhaveri KD. Preserved renal-allograft function and the PD-1 pathway inhibitor nivolumab. N Engl J Med. 2017;376:191–192. [DOI] [PubMed] [Google Scholar]
- 26.Boils CL, Aljadir DN, Cantafio AW. Use of the PD-1 pathway inhibitor nivolumab in a renal transplant patient with malignancy. Am J Transplant. 2016;16:2496–2497. [DOI] [PubMed] [Google Scholar]
- 27.Kittai AS, Oldham H, Cetnar J, et al. Immune checkpoint inhibitors in organ transplant patients. J Immunother. 2017;40:277–281. [DOI] [PubMed] [Google Scholar]
- 28.Winkler JK, Gutzmer R, Bender C, et al. Safe administration of an anti-PD-1 antibody to kidney-transplant patients: 2 clinical cases and review of the literature. J Immunother. 2017;40:341–344. [DOI] [PubMed] [Google Scholar]
- 29.Ong M, Ibrahim AM, Bourassa-Blanchette S, et al. Antitumor activity of nivolumab on hemodialysis after renal allograft rejection. J Immunother Cancer. 2016;4:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lipson EJ, Bagnasco SM, Moore J, Jr, et al. Tumor regression and allograft rejection after administration of anti-PD-1. N Engl J Med. 2016;374:896–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kwatra V, Karanth NV, Priyadarshana K, et al. Pembrolizumab formetastatic melanoma in a renal allograft recipient with subsequent graft rejection and treatment response failure: a case report. J Med Case Reports. 2017;11:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gastman BR, Ernstoff MS. Tolerability of immune checkpoint inhibition cancer therapy in a cardiac transplant patient. Ann Oncol. 2016;27:2304–2305. [DOI] [PubMed] [Google Scholar]
- 33.Owonikoko TK, Kumar M, Yang S, et al. Cardiac allograft rejection as a complication of PD-1 checkpoint blockade for cancer immunotherapy: a case report. Cancer Immunol Immunother. 2017;66:45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Le Fournis S, Gohier P, Urban T, et al. Corneal graft rejection in a patient treated with nivolumab for primary lung cancer. Lung Cancer. 2016;102:28–29. [DOI] [PubMed] [Google Scholar]
- 35.Banff schema for grading liver allograft rejection: an international consensus document. Hepatology. 1997;25:658–663. [DOI] [PubMed] [Google Scholar]
- 36.Geissler EK, Schnitzbauer AA, Zulke C, et al. Sirolimus use in liver transplant recipients with hepatocellular carcinoma: a randomized, multicenter, open-label phase 3 trial. Transplantation. 2016;100:116–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meyboom R, Royer R. Causality classification at pharmacovigilance centres in the European Community. Pharmacoepidemiol Drug Saf. 1992;1:87–97. [Google Scholar]
- 38.Wanchoo R, Karam S, Uppal NN, et al. Adverse renal effects of immune checkpoint inhibitors: a narrative review. Am J Nephrol. 2017;45:160–169. [DOI] [PubMed] [Google Scholar]
- 39.Morita M, Fujino M, Jiang G, et al. PD-1/B7-H1 interaction contribute to the spontaneous acceptance of mouse liver allograft. Am J Transplant. 2010;10:40–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Riella LV, Paterson AM, Sharpe AH, et al. Role of the PD-1 pathway in the immune response. Am J Transplant. 2012;12:2575–2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dong Y, Sun Q, Zhang X. PD-1 and its ligands are important immune checkpoints in cancer. Oncotarget. 2017;8:2171–2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ito T, Ueno T, Clarkson MR, et al. Analysis of the role of negative T cell costimulatory pathways in CD4 and CD8 T cell-mediated alloimmune responses in vivo. J Immunol. 2005;174:6648–6656. [DOI] [PubMed] [Google Scholar]
- 43.Tanaka K, Albin MJ, Yuan X, et al. PDL1 is required for peripheral transplantation tolerance and protection from chronic allograft rejection. J Immunol. 2007;179:5204–5210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12:492–499. [DOI] [PubMed] [Google Scholar]
- 45.Li W, Zheng XX, Kuhr CS, et al. CTLA4 engagement is required for induction of murine liver transplant spontaneous tolerance. Am J Transplant. 2005;5:978–986. [DOI] [PubMed] [Google Scholar]
- 46.Judge TA, Wu Z, Zheng XG, et al. The role of CD80, CD86, and CTLA4 in alloimmune responses and the induction of long-term allograft survival. J Immunol. 1999;162:1947–1951. [PubMed] [Google Scholar]
- 47.Postow MA. Managing immune checkpoint-blocking antibody side effects. Am Soc Clin Oncol Educ Book. 2015;76–83. [DOI] [PubMed] [Google Scholar]
- 48.Adams DH, Neuberger JM. Treatment of acute rejection. Semin Liver Dis. 1992;12:80–88. [DOI] [PubMed] [Google Scholar]
- 49.Spain L, Diem S, Larkin J. Management of toxicities of immune checkpoint inhibitors. Cancer Treat Rev. 2016;44:51–60. [DOI] [PubMed] [Google Scholar]
- 50.Schmitt TM, Phillips M, Sawyer RG, et al. Anti-thymocyte globulin for the treatment of acute cellular rejection following liver transplantation. Dig Dis Sci. 2010;55:3224–3234. [DOI] [PubMed] [Google Scholar]
- 51.Kamar N, Lavayssiere L, Muscari F, et al. Early plasmapheresis and rituximab for acute humoral rejection after ABO-compatible liver transplantation. World J Gastroenterol. 2009;15:3426–3430. [DOI] [PMC free article] [PubMed] [Google Scholar]


