Supplemental digital content is available in the text.
Abstract
Background
Efforts to maximize transplantation by matching organ quality to recipient longevity require reliable tools. The US kidney allocation system uses the Kidney Donor Risk Index (KDRI) for this purpose, and many centers additionally rely on donor biopsies. The Leuven score combines donor age with procurement histology (glomerulosclerosis and interstitial fibrosis/tubular atrophy) to predict allograft survival.
Methods
We compared KDRI with Leuven scores for associations with kidney discard, delayed graft function, and allograft function and survival. We used Cox, modified Poisson, and linear regression to calculate risks based on KDRI and (separately) Leuven scores, adjusting for important transplant and recipient variables.
Results
From 890 donors, 1729 kidneys were procured and biopsied. Five hundred eighty-five (34%) kidneys were discarded. Median donor age was 53 years (interquartile range [IQR], 44-61 years). Median KDRI and Leuven scores were 1.56 (IQR, 1.28-1.90) and 59 (IQR, 49-69). Relative risk for discard was 1.21 (95% confidence interval [CI], 1.17-1.24) per 0.2-unit increase in KDRI and 1.38 (1.31-1.46) per 10-unit increase in Leuven score. Adjusted relative risks for delayed graft function were 0.98 (95% CI, 0.94-1.02) and 0.94 (95% CI, 0.90-0.99), adjusted hazard ratios for graft failure were 1.10 (95% CI, 1.04-1.16) and 1.11 (95% CI, 1.02-1.21), and adjusted linear regression coefficients for 3-year estimated glomerular filtration rate were −3.88 (−4.63 to −3.13) and -5.18 (−6.19 to −4.18).
Conclusions
In kidneys clinically selected for procurement biopsy, the Leuven score was more strongly associated with discard but performed similarly to KDRI for predicting transplant outcomes, suggesting the need to reevaluate current procurement biopsy practices. Given modest associations for both tools; however, neither KDRI nor the Leuven score should be used in isolation for individual organ acceptance decisions.
Approximately 100 000 individuals currently await kidney transplantation in the United States. Roughly 30 000 adults are added to the waiting list each year, but the number of transplants has remained steady over the past decade at around 17 000 annually, leading to increasing mortality on the waiting list.1 To expand the number of kidneys for transplantation, organ procurement organizations (OPOs) have increasingly used less than ideal deceased donors. In addition, the Organ Procurement and Transplantation Network (OPTN) sought to improve matching between organ quality and expected recipient survival in hopes of reducing retransplantation rates by initiating a new kidney allocation system in December 2014. The OPTN now uses the Kidney Donor Risk Index (KDRI) as the measure of organ quality for the matching algorithm; however, many transplant centers in the United States also request procurement kidney biopsies to aid in kidney offer decision-making.
The primary concerns about performing procurement biopsies are that they delay decisions and prolong cold ischemia, which could further injure already “marginal” kidneys, encourage unnecessary discards, and increase healthcare costs. There is also a lack of standardization in how procurement biopsies are performed, processed, examined, and reported, as well as uncertainty about their predictive utility.2,3 Procurement kidney biopsies are rarely performed in Europe despite higher transplantation rates for “marginal” kidneys with similar outcomes compared with the United States.4 These and other concerns have led some to suggest abandoning procurement biopsies altogether.5 Prior studies have evaluated donor kidney biopsy features but have reported inconsistent results for associations with posttransplant outcomes.6
Despite their limitations, procurement biopsies are being performed more often in the United States and are associated with increasing rates of discard. For example, Stewart et al7 demonstrated that much of the rise in kidney discards during the last decade (from 13% in 1999 to 19% in 2009) can be attributed to more biopsies in addition to changing donor characteristics. Primary reasons to request a procurement biopsy are likely linked to underlying clinical concerns about donor quality. Because these underlying indications for biopsy may confound relationships with posttransplant outcomes, we performed a large cohort study of kidneys selected for procurement biopsy to compare the utility of a well-described clinicohistopathological score to that of KDRI itself primarily for predicting kidney discard. We secondarily compared its performance for the development of delayed graft function (DGF) and 3-year allograft function and survival.
MATERIALS AND METHODS
Cohort and Data Sources
This is an ancillary study to an ongoing multicenter deceased organ donor cohort (ClinicalTrials.gov identifier: NCT01848249). Details regarding donor enrollment and data collection are available in prior publications.8-13 Briefly, 5 participating OPOs enrolled donors between May 2010 and December 2013. Detailed donor and organ characteristics were extracted from OPO charts. We excluded donors younger than 5 years and those that were not biopsied or had missing data necessary for the analysis.
This study also used data from the OPTN. The OPTN data system includes information on all donors, waitlisted candidates, and transplant recipients in the United States, submitted by the members of the OPTN and is more fully described elsewhere.14 The Health Resources and Services Administration, US Department of Health and Human Services provides oversight to the activities of the OPTN contractor. Recipient characteristics and outcomes were ascertained using data submitted by transplant centers to the United Network for Organ Sharing (UNOS), the current OPTN contractor, through routine transplantation and follow-up forms.
Based on individual OPO practices, wedge or needle biopsies were performed on ex vivo kidneys immediately after procurement. Tissue samples were not collected for this study. On-call donor hospitals evaluated frozen sections from procurement biopsies to generate reports that were uploaded to the secure, Web-based DonorNet maintained by UNOS for review by transplant centers during kidney allocation. Data from these clinically available pathology reports were abstracted to calculate the previously described “3-year prediction” Leuven Donor Risk Score (Leuven score).15
The Leuven score is calculated from donor age (years), percent glomerulosclerosis (GS) and interstitial fibrosis/tubular atrophy (IFTA) grade according to the Banff ‘07 classification16 using the following equation: age + (2×GS) + (10×IFTA), where GS <10% = 0, ≥10% = 1; and IFTA ≤5% = 0, 6-25% = 1, 26-50% = 2, >50% = 3. We specifically chose this clinicohistopathological score for the following reasons. First, the score was initially developed using multivariable modeling in a cohort of 181 procurement biopsies that were not clinically available for allocation or discard decisions. Second, the score was validated in a separate group of 367 procurement biopsies and found to provide “sufficient predictive performance to guide kidney allocation” with an area under the receiver-operating characteristic curve (AUC) for 3-year allograft survival of 0.70. Third, we could calculate the score from clinically available procurement kidney biopsy reports, which provided variable summary information about the degree of GS, IFTA, arteriosclerosis, and acute tubular necrosis. Thus, based on current practices in the United States, transplant centers can similarly calculate the Leuven score from procurement kidney biopsy reports without requesting additional morphologic measurements.
We chose kidney discard (ie, kidneys procured for the purpose of transplantation but not transplanted) and time to allograft failure (defined as initiation/return to chronic dialysis, retransplantation, or death of the recipient) as coprimary outcomes. Secondary outcomes of interests were DGF (defined as any dialysis in the first week of transplant), allograft survival at 3 years, and allograft function at 1 and 3 years. Allograft function was assessed via estimated glomerular filtration rate (eGFR) calculated from serum creatinine values reported to UNOS using the Chronic Kidney Disease Epidemiology Collaboration equation.17 We carried forward the last eGFR reported for recipients that died with functional allografts and imputed eGFR as 10 mL/min per 1.73 m2 after instances of death-censored graft failure.
We adhered to the ethical principles of the Declaration of Helsinki,18 and the institutional review boards and/or scientific committees for all participating organizations approved the study. Data for deceased donors were collected if donor surrogates agreed to research, and institutional review board waiver of consent was approved for recipient outcomes given our use of deidentified OPTN data. The clinical and research activities reported here are also consistent with the principles outlined in the Declaration of Istanbul on Organ Trafficking and Transplant Tourism.19
Statistical Analyses
We calculated descriptive statistics and reported values as mean (SD) or median [interquartile range] for continuous variables and as frequency (percentage) for categorical variables. We used Wilcoxon rank-sum tests to compare continuous variables between groups and χ2 or Fisher exact tests as appropriate for categorical comparisons. We calculated the KDRI as described by Rao et al20 with mapping to the Kidney Donor Profile Index (KDPI) relative to all US deceased donors in 2010.21
Using modified Poisson regression to estimate relative risk (RR) and control for possible correlation of outcomes between kidneys from the same donor, we fit separate models using KDRI and then the Leuven score for the dichotomous outcomes of kidney discard, DGF, and 3-year allograft survival. We fit Cox proportional hazards models for time to allograft failure and linear mixed regression models for 1- and 3-year eGFRs. Based on the cohort distribution of KDRIs and Leuven scores, we calculated risk for each outcome per 0.2-unit increase in KDRI and per 10-unit increase in Leuven score. Because the aim of this study was to compare these clinical summary measures of donor/organ quality, no additional characteristics were used to adjust the models for kidney discard. Models for all other (posttransplant) outcomes, however, were subsequently adjusted for the use of machine perfusion, cold ischemia time (hours) and the following recipient variables: age (years), black race, sex, previous kidney transplant, diabetes as the cause of end stage renal disease, need for pretransplant blood transfusion, number of HLA mismatches, percent panel-reactive antibody (PRA), body mass index (weight/height2, kg/m2), and preemptive transplant status. For kidney discard and allograft survival, we additionally controlled for KDRI to assess potential independent associations for the Leuven score.
Recipient follow-up was calculated from the date of transplant to all-cause allograft failure and administratively censored at the end of the study period on July 6, 2016. For dichotomous outcomes, we used each regression model to calculate AUCs. Analyses were completed using SAS 9.4 statistical software for Windows (SAS Institute, Cary, NC) and R 3.1.2 (R Foundation for Statistical Computing, Vienna, Austria). We used a 2-tailed significance value less than 0.05 and controlled for possible correlation of outcomes between kidneys from the same donor for all statistical tests.
RESULTS
Figure 1 depicts the cohort generation. Table S1 (SDC, http://links.lww.com/TXD/A119) shows the characteristics for the 1729 kidneys from 890 donors included in the analysis (ie, procured for the purpose of transplantation and biopsied) compared with the 1469 kidneys from 774 donors that were excluded (ie, no procurement biopsy). Kidneys from donors enrolled in the parent cohort that underwent biopsy tended to come from older donors with more comorbidities. There was substantial variation in procurement biopsy rate between OPOs (range, 16-87%; Table S2, SDC, http://links.lww.com/TXD/A120). However, there were no clear correlations between OPO biopsy rates and severity of reported findings. One hundred twenty-two (8%) of the kidneys that were not biopsied (excluded from further analyses) were eventually discarded compared with 585 (34%) kidney discards for those that were biopsied and included in subsequent analyses (P < 0.001).
FIGURE 1.
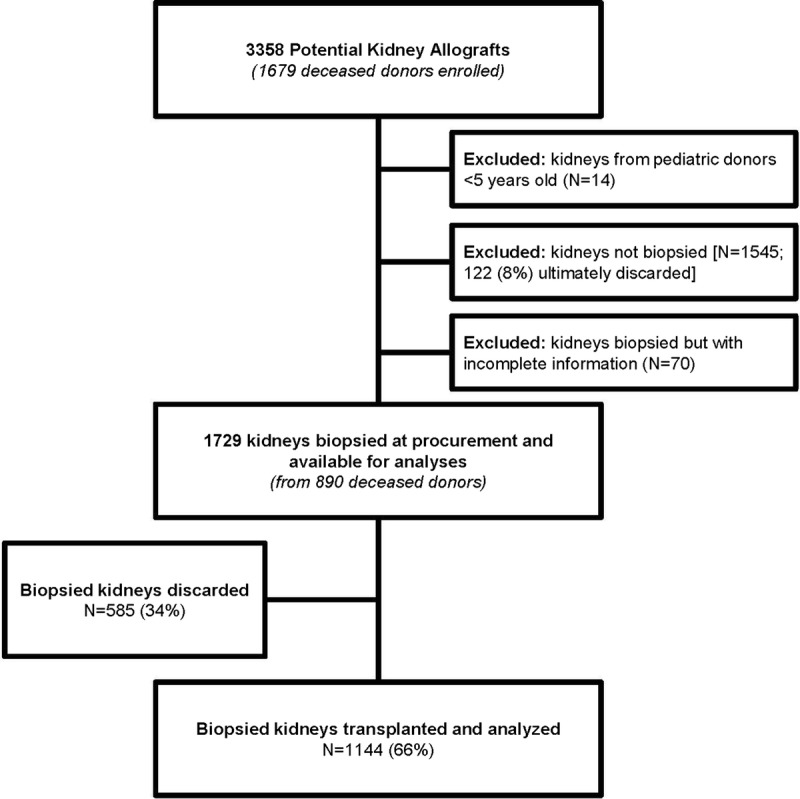
Cohort generation.
A total of 1144 (66%) biopsied kidneys were transplanted. Table 1 provides donor characteristics according to the number (0, 1, or 2) of resulting transplants. Median donor age was 53 years (44-61 years), 56% were male, 18% were black, 15% donated after cardiovascular determination of death, and 29% had KDPI greater than 85%. Median KDRI and Leuven score were 1.57 (1.29-1.91) and 59 (49-69), respectively. There was overlap between KDRIs and Leuven scores as shown in Figure 2.
TABLE 1.
Donor demographics by number of kidneys transplanted
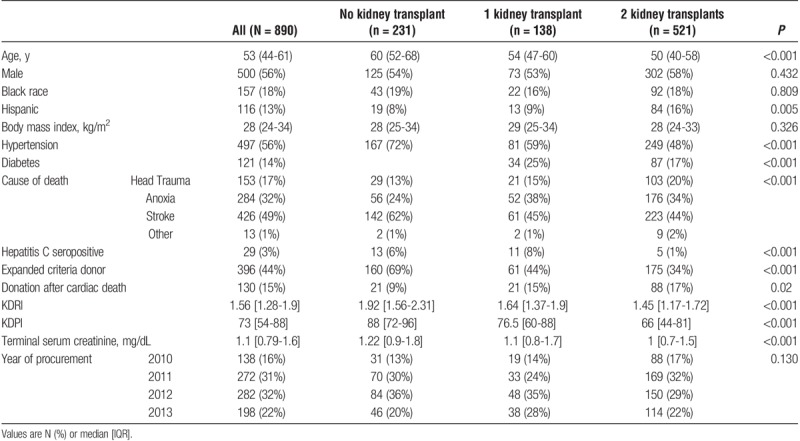
FIGURE 2.
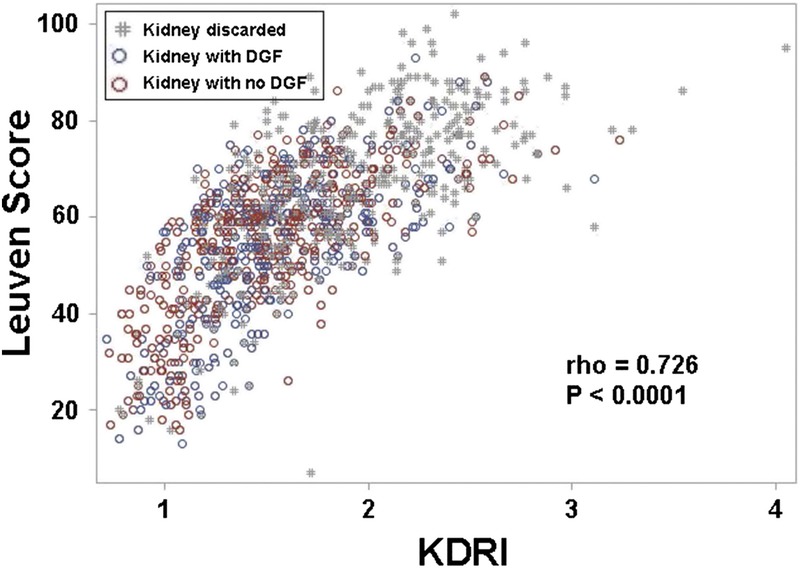
Scatterplot of Leuven scores and KDRI.
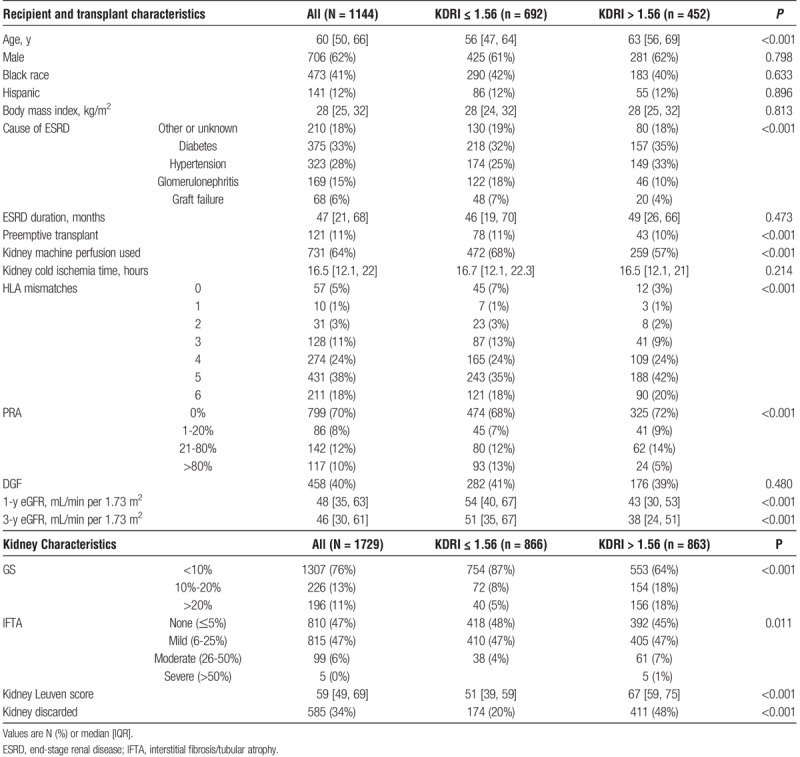
Median recipient age was 60 [50-66] years, 41% were male, 27% were black, 7% were preemptive transplants, 4% had prior transplants and 10% had PRA >80%. Table 2 provides recipient and transplant characteristics by median KDRI. Table S3 (SDC, http://links.lww.com/TXD/A121 provides the same information by median Leuven score. Of transplanted kidneys, 458 (40%) developed DGF and 251 (22%) failed by 3 years, including 25 (2%) with primary nonfunction, 130 (11%) with death-censored graft failure, 96 (8%) deaths with functioning grafts, and 37 (3%) deaths after graft failure. Median follow-up was 3.5 [3-4.5] years.
TABLE 2.
Recipient, transplant, and kidney characteristics and outcomes by KDRI median
Kidney Discard
Compared with KDRI, there was a stronger association between increasing Leuven score and kidney discard risk. RR (95% confidence interval [CI]) for discard was 1.20 (95% CI, 1.17-1.24) and 1.38 (95% CI, 1.31-1.46) for each 0.2-unit increase in KDRI and each 10-unit increase in Leuven score. Controlling for KDRI, adjusted RR (aRR) for discard decreased to 1.22 (1.13-1.31) with each 10-unit increase in Leuven score. For predicting discard, AUCs for KDRI and Leuven score were no different at 0.72.
Allograft Failure
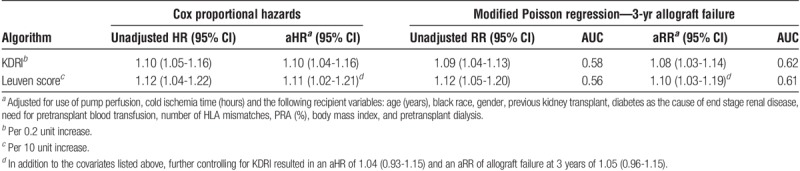
Increasing values for either KDRI or the Leuven score were independently associated with allograft failure with adjusted hazard ratios (aHR) of 1.10 (1.04-1.16) for each 0.2-unit increase in KDRI and 1.11 (1.02-1.21) for each 10-unit increase in Leuven score (Table 3). The aRRs for 3-year allograft failure were also similar for KDRI and Leuven score at 1.08 (1.03-1.14) and 1.10 (1.03-1.19) and with AUCs of 0.62 and 0.61, respectively. The baseline clinical model of transplant and recipient factors (ie, excluding donor factors) provided an AUC for 3-year allograft failure of 0.60. Statistical significance for the Leuven score was lost after controlling for KDRI in each clinical model (aHR, 1.04; 95% CI, 0.93-1.15; aRR for 3-year allograft failure, 1.05; 95% CI, 0.96-1.15).
TABLE 3.
Risk of allograft failure by KDRI or Leuven score
Secondary Outcomes
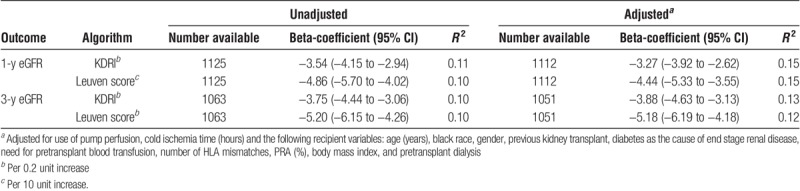
Neither KDRI nor the Leuven score predicted DGF in this cohort with unadjusted AUCs of 0.5 and 0.53, respectively (Table 4). The aRRs for DGF with KDRI and the Leuven score were 0.98 (0.94-1.02) and 0.94 (0.90-0.99), respectively. However, both scores were independently associated with worse allograft function at 1 and 3 years posttransplant (Table 5). Based on linear regression R2 values, either score accounted for 10% to 11% of the variation in 1- and 3-year eGFRs, whereas multivariable models using either score and adjusting for transplant and recipient factors accounted for 12% to 15% of eGFR variation.
TABLE 4.
Risk of DGF by KDRI or Leuven score
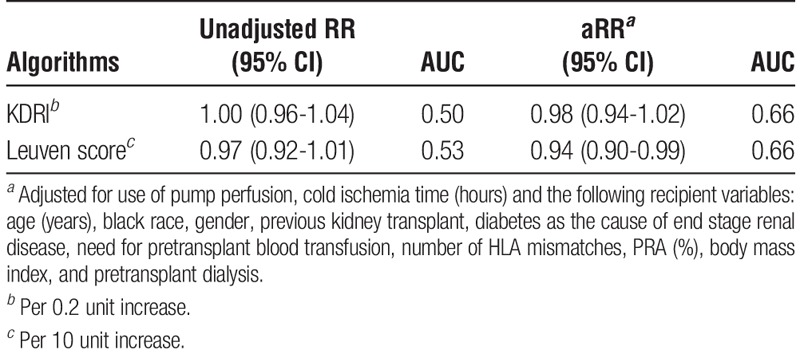
TABLE 5.
Linear regression coefficients on allograft function by KDRI or Leuven score
DISCUSSION
Current deceased-donor kidney transplant practices, on average, improve and prolong the lives of recipients, but the process to consider kidney offers involves individualized and often complex clinical decisions. In this large, multicenter study of deceased-donor kidneys selected to undergo biopsy, we found that the Leuven score (1) can be easily calculated from donor age and 2 histopathological findings available from procurement kidney biopsy reports and (2) performs no better than the 10-variable KDRI for predicting kidney discard as well as posttransplant allograft survival and function. Neither the Leuven score nor KDRI accurately predicted DGF in this cohort. We believe these findings support the notion that procurement biopsies, at least as they are currently performed and reported in the United States, lead to more kidney discards and provide no added value for predicting transplant outcomes.
It is important to note that over half of the donors enrolled in the parent cohort were biopsied with wide variation between OPOs. Kidneys from donors that underwent biopsy tended to have more unfavorable clinical characteristics and were much more likely to result in discard compared with those that did not undergo procurement biopsy. Because we cannot know the clinical outcomes for discarded kidneys had they instead been transplanted, theoretical arguments against discarding kidneys because of biopsy findings can be quite tenuous. Consequently, some OPOs obtain procurement kidney biopsies from most donors, and many centers request biopsies if not already available as permitted by OPTN Policy 2.11.A(2).22 In fact, Stewart et al7 noted an increase in biopsies from about 23% in 1999 to 50% in 2009—a substantial increase despite concomitant decreases in median donor age and KDRI over the same period.
In some transplant centers, GS is the primary donor biopsy information consistently reviewed because it provides a convenient cutoff (eg, >10%) for automatic offer turndowns. However, Banff guidelines for procurement biopsies discourage the use of “rigidly defined histologic cutoffs” during organ allocation.2 This seems appropriate given the analyses by Massie et al,23 which showed selected candidates experience improved survival with early acceptance of lower-quality (ie, high-KDPI) offers. Notwithstanding, whether a center should accept a lower-quality offer for an individual patient can be a challenging question, and there are high-volume centers that use procurement biopsies as part of their decision making process for select offers. Centers have also used standardized procurement biopsies to calculate histopathology scores to determine whether to transplant lower-quality kidneys from 1 donor into 2 recipients versus 1 as a dual-kidney transplant, resulting in acceptable outcomes.24,25
With regard to organ utilization at the national level, however, medical environments for transplantation in the United States are complex. Thus, variables affecting organ discard are complex and likely include regulatory oversight and diverse geographic, reimbursement, ethnosocioeconomic, and even behavioral economic issues, such as loss and risk aversion.26 Nevertheless, procurement kidney biopsy is an important factor.7 We noted a 34% discard rate for kidneys that underwent procurement biopsy—substantially higher than the 8% discard rate for nonbiopsied (excluded) kidneys. Although these divergent rates were comparable to national averages (31.4% for biopsied kidneys vs 6.8% for nonbiopsied kidneys),1 the tendency to request procurement biopsies based on donor quality complicates analyses of organ utilization because of confounding by indication.
Confounding by indication in this setting was supported by our finding of reduced RR for discard with increasing Leuven score after adjusting for KDRI. Decreasing from an unadjusted RR of 1.38, the aRR of 1.22 remained statistically significant, however, indicating a persistent independent association between the Leuven score (ie, procurement biopsy) and kidney discard. Moreover, we were interested to find that the utility of the procurement biopsy Leuven score was essentially no different than that of KDRI for predicting allograft failure. In fact, both scores performed only modestly in this cohort with unadjusted AUCs that were less than the KDRI C-statistic reported as 0.62 for all deceased-donor kidney-only transplants in the OPTN database from 1995 to 2005.20 Lending further support for confounding by biopsy indication, statistical significance for the Leuven score for predicting allograft failure was abolished after controlling for KDRI.
There are limitations to this study. Though we controlled for factors known to associate with allograft survival, residual confounding is possible given our observational design and use of OPTN data. Although the Banff Working Group recommended using frozen sections from wedge biopsies, the guidelines also described the limitations to this approach, including limited interobserver concordance for GS and IFTA.2 Without biopsy slides, we could not assess other histologic scores from the literature, such as the chronic allograft damage index,27 the Remuzzi score,28 all chronic changes described in the Banff '97 classification,29 or the Maryland aggregate pathology index.30 Although prior studies have suggested that renal vascular changes based on these and other scoring systems have prognostic value,31-34 the procurement biopsy reports used for the current study contained inadequate information for meaningful analyses on vessel pathology. Although independent, standardized reviews of biopsy slides could not be performed, we believe this study provides an innovative analysis of the pathology reports that are currently used during organ allocation. By definition, only transplanted kidneys have posttransplant outcomes, which make selection bias possible and underscores our decision to analyze discard as a primary outcome.
In conclusion, our data highlight some of the “real-world” clinical shortcomings of procurement biopsy reports and reveal their limited predictive utility. Given sufficient equipoise and the fact that deceased-donor organs are a national resource, we believe a properly designed donor trial is needed to collect procurement biopsy data to determine how to appropriately obtain and use this information. An example could be a large cluster trial for organ utilization and allograft outcomes in which OPOs are randomized to current biopsy practice versus standardized biopsies from prespecified donors (eg, KDPI >50%) with rapid fixation,35,36 whole-slide imaging,37 and centralized pathology evaluation in real-time.
Supplementary Material
ACKNOWLEDGMENTS
The authors are grateful for the study participation of partners at 5 OPOs: Gift of Life Donor Program in Philadelphia, Gift of Life Michigan, LiveOnNY, NJ Sharing Network, and New England Donor Services. The data reported here have been supplied by UNOS as the contractor for the OPTN. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy of or interpretation by the OPTN or the US Government. These organizations were not involved in study design, analysis, interpretation, or article creation.
Footnotes
Published online 13 July, 2018.
This work was supported by the National Institutes of Health grant R01DK-93770, grant K24DK090203, a Roche Organ Transplantation Research Foundation Award, and the George M. O'Brien Kidney Center at Yale grant P30DK079310 to Dr. Parikh; a Fellow-To-Faculty award from the American Heart Association to Dr. Hall; and the Health Resources and Services Administration contract 234-2005-37011C.
The authors declare no conflicts of interest.
I.E.H. participated in the design and analyses for this study, interpreted the results and drafted the article. C.R.P. conceived of the parent study, participated in the design of this study, interpreted the results and helped write the article. B.S. participated by contributing study subjects for the parent study, interpreting results and helping with article revisions. F.L.W. participated by contributing study subjects for the parent study, interpreting results and helping with article revisions. Y.J. participated by performing the statistical analyses and helping with article revisions. H.T.-P. participated by providing important statistical feedback and helping with article revisions. P.P.R. participated by contributing study subjects for the parent study, interpreting results and helping with article revisions. M.D.D. participated by contributing study subjects for the parent study, helping design this study, interpreting results and helping with article revisions.
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantationdirect.com).
REFERENCES
- 1.Hart A, Smith JM, Skeans MA, et al. OPTN/SRTR 2015 annual data report: kidney. Am J Transplant. 2017;17(Suppl 1):21–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liapis H, Gaut JP, Klein C, et al. Banff histopathological consensus criteria for preimplantation kidney biopsies. Am J Transplant. 2017;17:140–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Naesens M. Zero-time renal transplant biopsies: a comprehensive review. Transplantation. 2016;100:1425–1439. [DOI] [PubMed] [Google Scholar]
- 4.Hopfer H, Kemeny E. Assessment of donor biopsies. Curr Opin Organ Transplant. 2013;18:306–312. [DOI] [PubMed] [Google Scholar]
- 5.Kasiske BL, Stewart DE, Bista BR, et al. The role of procurement biopsies in acceptance decisions for kidneys retrieved for transplant. Clin J Am Soc Nephrol. 2014;9:562–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang CJ, Wetmore JB, Crary GS, et al. The donor kidney biopsy and its implications in predicting graft outcomes: a systematic review. Am J Transplant. 2015;15:1903–1914. [DOI] [PubMed] [Google Scholar]
- 7.Stewart DE, Garcia VC, Rosendale JD, et al. Diagnosing the decades-long rise in the deceased donor kidney discard rate in the United States. Transplantation. 2017;101:575–587. [DOI] [PubMed] [Google Scholar]
- 8.Hall IE, Reese PP, Doshi MD, et al. Delayed graft function phenotypes and 12-month kidney transplant outcomes. Transplantation. 2017;101:1913–1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hall IE, Reese PP, Weng FL, et al. Preimplant histologic acute tubular necrosis and allograft outcomes. Clin J Am Soc Nephrol. 2014;9:573–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hall IE, Schroppel B, Doshi MD, et al. Associations of deceased donor kidney injury with kidney discard and function after transplantation. Am J Transplant. 2015;15:1623–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parikh CR, Hall IE, Bhangoo RS, et al. Associations of perfusate biomarkers and pump parameters with delayed graft function and deceased donor kidney allograft function. Am J Transplant. 2016;16:1526–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Puthumana J, Hall IE, Reese PP, et al. YKL-40 associates with renal recovery in deceased donor kidney transplantation. J Am Soc Nephrol. 2017;28:661–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reese PP, Hall IE, Weng FL, et al. Associations between deceased-donor urine injury biomarkers and kidney transplant Outcomes. J Am Soc Nephrol. 2016;27:1534–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dickinson DM, Bryant PC, Williams MC, et al. Transplant data: sources, collection, and caveats. Am J Transplant. 2004;4(Suppl 9):13–26. [DOI] [PubMed] [Google Scholar]
- 15.De Vusser K, Lerut E, Kuypers D, et al. The predictive value of kidney allograft baseline biopsies for long-term graft survival. J Am Soc Nephrol. 2013;24:1913–1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Solez K, Colvin RB, Racusen LC, et al. Banff 07 classification of renal allograft pathology: updates and future directions. Am J Transplant. 2008;8:753–760. [DOI] [PubMed] [Google Scholar]
- 17.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Medical Association. WMA Declaration of Helsinki - Ethical Principles for Medical Research Involving Human Subjects. Available at http://www.wma.net/en/30publications/10policies/b3/. Accessed September 10, 2013. [PubMed]
- 19.The Declaration of Istanbul on Organ Trafficking and Transplant Tourism. Istanbul Summit April 30–May 2, 2008. Nephrol Dial Transplant. 2008;23:3375–3380. [DOI] [PubMed] [Google Scholar]
- 20.Rao PS, Schaubel DE, Guidinger MK, et al. A comprehensive risk quantification score for deceased donor kidneys: the Kidney Donor Risk Index. Transplantation. 2009;88:231–236. [DOI] [PubMed] [Google Scholar]
- 21.Organ Procurement and Transplantation Network. A Guide to Calculating and Interpreting KDPI. 2016. Available at https://optn.transplant.hrsa.gov/media/1512/guide_to_calculating_interpreting_kdpi.pdf. Accessed August 30, 2016.
- 22.Organ Procurement and Transplantation Network. OPTN Policies. 2018. Available at https://optn.transplant.hrsa.gov/governance/policies/. Accessed May 14, 2018.
- 23.Massie AB, Luo X, Chow EK, et al. Survival benefit of primary deceased donor transplantation with high-KDPI kidneys. Am J Transplant. 2014;14:2310–2316. [DOI] [PubMed] [Google Scholar]
- 24.Fernandez-Lorente L, Riera L, Bestard O, et al. Long-term results of biopsy-guided selection and allocation of kidneys from older donors in older recipients. Am J Transplant. 2012;12:2781–2788. [DOI] [PubMed] [Google Scholar]
- 25.Mallon DH, Riddiough GE, Summers DM, et al. Successful transplantation of kidneys from elderly circulatory death donors by using microscopic and macroscopic characteristics to guide single or dual implantation. Am J Transplant. 2015;15:2931–2939. [DOI] [PubMed] [Google Scholar]
- 26.Heilman RL, Green EP, Reddy KS, et al. Potential impact of risk and loss aversion on the process of accepting kidneys for transplantation. Transplantation. 2017;101:1514–1517. [DOI] [PubMed] [Google Scholar]
- 27.Isoniemi HM, Krogerus L, von Willebrand E, et al. Histopathological findings in well-functioning, long-term renal allografts. Kidney Int. 1992;41:155–160. [DOI] [PubMed] [Google Scholar]
- 28.Remuzzi G, Grinyo J, Ruggenenti P, et al. Early experience with dual kidney transplantation in adults using expanded donor criteria. Double Kidney Transplant Group (DKG). J Am Soc Nephrol. 1999;10:2591–2598. [DOI] [PubMed] [Google Scholar]
- 29.Racusen LC, Solez K, Colvin RB, et al. The Banff 97 working classification of renal allograft pathology. Kidney Int. 1999;55:713–723. [DOI] [PubMed] [Google Scholar]
- 30.Munivenkatappa RB, Schweitzer EJ, Papadimitriou JC, et al. The Maryland aggregate pathology index: a deceased donor kidney biopsy scoring system for predicting graft failure. Am J Transplant. 2008;8:2316–2324. [DOI] [PubMed] [Google Scholar]
- 31.Lehtonen SR, Taskinen EI, Isoniemi HM. Histopathological findings in renal allografts at time of transplantation and correlation with onset of graft function. APMIS. 1999;107:945–950. [DOI] [PubMed] [Google Scholar]
- 32.Remuzzi G, Cravedi P, Perna A, et al. Long-term outcome of renal transplantation from older donors. N Engl J Med. 2006;354:343–352. [DOI] [PubMed] [Google Scholar]
- 33.Sis B, Dadras F, Khoshjou F, et al. Reproducibility studies on arteriolar hyaline thickening scoring in calcineurin inhibitor-treated renal allograft recipients. Am J Transplant. 2006;6:1444–1450. [DOI] [PubMed] [Google Scholar]
- 34.Philosophe B, Malat GE, Soundararajan S, et al. Validation of the Maryland Aggregate Pathology Index (MAPI), a pre-implantation scoring system that predicts graft outcome. Clin Transplant. 2014;28:897–905. [DOI] [PubMed] [Google Scholar]
- 35.Chafin D, Theiss A, Roberts E, et al. Rapid two-temperature formalin fixation. PLoS One. 2013;8:e54138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Katoh K. Microwave-assisted tissue preparation for rapid fixation, decalcification, antigen retrieval, cryosectioning, and immunostaining. Int J Cell Biol. 2016;2016:7076910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pantanowitz L, Sinard JH, Henricks WH, et al. Validating whole slide imaging for diagnostic purposes in pathology: guideline from the College of American Pathologists Pathology and Laboratory Quality Center. Arch Pathol Lab Med. 2013;137:1710–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


