Abstract
Background:
Phototherapy has been a mainstay in the treatment of mycosis fungoides (MF). However, the recent findings of UV-induced p53 mutations in advanced MF suggest that phototherapy may contribute to disease progression.
Objective:
The objective of this study was to evaluate the effect of phototherapy on the time to tumor progression and overall survival in MF.
Materials and methods:
Retrospective analysis of patients seen at the University of Pittsburgh Cutaneous Lymphoma Clinic from 1979 to 2016.
Results:
345 patients with MF were identified. 258 (74.8%) were diagnosed at stage IA or IB. 43 out of 258 (16.6%) progressed to tumor stage. Before tumor development, 30 out of 43 (69.8%) patients received phototherapy, and 13 (30.2%) did not. Patients who received phototherapy had a longer median time to tumor progression than those who did not: 3.5 years (interquartile range = 1.9 – 5.7) versus 1.2 years (0.2 – 2.3) (p =0.001). Patients who received phototherapy also survived longer: 6.9 years (interquartile range = 4.3 – 9.5) versus 3.8 years (3.0 – 4.5) (p = 0.014).
Limitations:
Limited information on specific phototherapy start dates, durations, and treatment protocols.
Conclusions:
The therapeutic effects of phototherapy, with longer times to tumor progression and increased overall survival, appear to outweigh its potential adverse effects.
Keywords: mycosis fungoides, cutaneous T cell lymphoma, tumor stage, phototherapy, rate of progression, PUVA, NB UVB
INTRODUCTION
Mycosis fungoides (MF) is the most common type of cutaneous T-cell lymphoma (CTCL) in which the initial lesions tend to appear as patches and plaques on the non-photoexposed skin. Most patients’ disease rarely progress beyond early stages, but some go on to develop tumors and may have extracutaneous involvement of the lymph nodes, blood, and visceral organs.
Phototherapy, particularly psoralen plus ultraviolet A (PUVA) and narrowband ultraviolet B (NBUVB) [1], has been a longstanding cornerstone in the treatment of MF, particularly for patch-plaque disease occupying more than 10% body surface area (BSA) without lymph node involvement (stage IB). The use of PUVA in the treatment of MF was first reported in 1976 [2] and was found to be efficacious even for plaques. In a 2005 survey study of 1399 practitioners, PUVA was ranked the most effective and preferred treatment for stage IA and IB MF [3]. Similarly, UVB has been used to treat MF since 1982 [4]. When used as monotherapy for patch-plaque disease, NBUVB is reported as having clinical response rates ranging from 54– 90% [5].
However, phototherapy is not without its side effects, and questions have been raised about its potential carcinogenicity. While NBUVB has not been associated with a significantly increased risk of non-melanoma skin cancers (NMSCs), studies have shown that patients who receive both NBUVB and PUVA have a higher risk of developing basal cell carcinomas (BCCs) [6, 7]. On the other hand, PUVA has been associated with an increased risk of squamous cell carcinoma (SCC) [8, 9], and a possible increased risk of malignant melanoma (MM) [10, 11, 12, 13]. Though most studies on photocarcinogenicity have been done in patients with psoriasis, in a recent investigation of 104 patients with stage IA to IIA MF treated with chronic PUVA therapy, 26% developed NMSCs [14].
The goal of our study was to evaluate from a clinical perspective the effect of phototherapy on the rate of progression to tumor stage MF and on overall survival times.
MATERIALS AND METHODS
Study design
We queried the cutaneous lymphoma database on patients with early stage (stage IA and IB) diagnosed and treated at the University of Pittsburgh Medical Center in the Cutaneous Lymphoma Clinic at the University of Pittsburgh over the last 37 years from 1979 to 2016. Patients with reactive lymph nodes were excluded. Only patients who progressed to tumor stage were included in this study. The electronic medical record or paper charts were reviewed by one of us (J.W.H., T.K., and O.E.A.) to establish the history of phototherapy. Clinical features included age at diagnosis, sex, Fitzpatrick skin type, duration of symptoms before diagnosis, stage at diagnosis, the number of skin cancer, number and type of treatments prior to tumor development, the presence of large cell transformation in tumors, and progression beyond stage IIB. The study was approved by the Institutional Review Board at the University of Pittsburgh (PRO15110413) with a waiver of informed consent.
Statistical analysis
Chi-squared tests or Fisher’s Exact tests were performed to determine whether there were significant differences between the phototherapy and the non-phototherapy groups with regards to categorical variables: gender, Fitzpatrick skin type, stage at diagnosis, the number of skin cancers, death from MF complications, large cell transformation, progression beyond tumor stage, and treatment specifics before tumor development. Mann-Whitney U tests were performed to determine whether there were significant differences between the phototherapy and non-phototherapy groups with regards to continuous variables: age at diagnosis, number of treatments used prior to tumor development, and duration of symptoms prior to diagnosis. Univariate and multivariable linear regression analyses adjusting for age at diagnosis were performed to test the log-transformed time to progression to tumor stage between the phototherapy and non-phototherapy groups. Survival analyses were performed using the log-rank (Mantel-Cox) test, and results are shown in Kaplan-Meier curves. Cox regression analyses were conducted to adjust for the effect of age at diagnosis with regards to overall survival. SPSS Version 24 (IBM Corp., Armonk, NY) was used to run the statistical analyses and p < 0.05 was considered statistically significant.
RESULTS
Patient characteristics
Of the 652 patients seen in the Cutaneous Lymphoma Clinic at the University of Pittsburgh from 1979 to 2016, we identified 345 patients with MF. Two hundred and fifty-eight patients (74.7%) were initially diagnosed with either stage IA or IB MF. The study group was comprised of the 43 patients (17.0%) who progressed from stage IA or IB to stage IIB MF during their follow-up. Eight out of 43 (18.6%) patients had stage IA at diagnosis, and 35 (81.4%) had stage IB. The mean age at diagnosis was 63.9 years old with a standard deviation of 13.0. Our study population was made up of 30 men (69.8%) and 13 women (30.2%). Thirty-seven patients (86.0%) had Fitzpatrick skin type I-III and 6 (14.0%) had type IV-VI.
The patients were divided into two groups, based on whether or not they were treated with phototherapy prior to tumor development. Thirty out of 43 patients (69.8%) received phototherapy—defined as NBUVB, PUVA, and/or intentional sunlight—prior to developing tumors, and 13 (30.2%) did not. Six out of 30 patients (20.0%) received NBUVB and 19 (63.3%) received PUVA. One received both NBUVB and PUVA (3.3%), and 4 (13.4%) treated their MF with natural sunlight. To our knowledge, the patients who were encouraged to use natural sunlight did not use tanning beds. Eleven out of the 30 patients who received phototherapy also received oral bexarotene prior to developing tumors.
There were no statistically significant differences between the phototherapy and the non-phototherapy groups with regards to gender, Fitzpatrick skin type, duration of symptoms prior to diagnosis, or stage at diagnosis (Table 1). The only statistically significant differences between the two groups were with regards to age at diagnosis and number of treatments used prior to tumor development (Table 1).
Table 1:
Patient Characteristics
| No Phototherapy N = 13 | Phototherapy N = 30 | P value | |
|---|---|---|---|
| Male | 11 (84.6%) | 19(63.3%) | p = 0.279 |
| Female | 2(15.4%) | 11 (30.2%) | |
| Fitzpatrick I-III | 12 (92.3%) | 25 (83.3%) | p = 0.649 |
| Fitzpatrick IV-VI | 1 (7.7%) | 5 (16.7%) | |
| Median age at dx (Interquartile range) | 69.9 years (65.0 – 75.3 years) | 61.9 years (56.3 – 71.8 years) | p = 0.048 |
| Median duration of symptoms prior to dx (Interquartile range) | 3.0 years (2.0 – 6.0 years) | 2.0 years (0.8 to 5.5 years) | p = 0.387 |
| Stage IA at dx | 3 (23.1%) | 5 (16.7%) | p = 0.681 |
| Stage IB at dx | 10 (76.9%) | 25 (83.3%) | |
| Number of patients with skin cancer, any type | 1(7.7%) | 5 (16.7%) | p = 0.649 |
| Non-melanoma skin cancer | 1(7.7%) | 4(13.3%) | |
| Melanoma | 0 (0.0%) | 3 (10.0%) | |
| Multiple skin cancers | 1 (7.7%) | 3 (10.0%) | |
| Median number of treatments prior to tumor development (Interquartile range) | 2.4 treatments (1.6 – 3.2) | 3.7 treatments (3.1 −4.4) | p = 0.019 |
The median age at diagnosis for patients in the phototherapy group was significantly lower than the non-phototherapy group: 61.9 years old (interquartile range = 56.3 – 71.8) versus 69.9 years old (65.0 – 75.3) (p = 0.048). The age at diagnosis for patients in the phototherapy group also had a wider range (28.9 to 90.1 years) than the non-phototherapy group (57.6 to 78.9 years).
The patients in our cohort received a range of treatments prior to tumor development, including: phototherapy, topical steroids, topical nitrogen mustard, radiation (including local electron beam therapy, total skin electron beam, and x-ray), extracorporeal photopheresis (ECP), bexarotene, interferon alpha-2a, methotrexate, pralatrexate, romidepsin, vorinostat, and denileukin diftitox. As to be expected with the addition of phototherapy, patients who received phototherapy received more treatments than those who did not: a median of 3.7 treatments (interquartile range = 3.1 – 4.4) versus 2.4 treatments (1.6 – 3.2), respectively (p = 0.019). Importantly, there were no statistically significant differences between the phototherapy and the non-phototherapy groups with regards to percent of patients who received systemic medications (46.2% vs. 56.7%, p = 0.526), ECP (84.6% vs. 73.3%, p = 0.696), radiation (69.2% vs. 73.3%, p = 1.000), or topical nitrogen mustard (61.5% vs. 63.3%, p = 1.000).
Of the 43 patients in this study, 6 developed NMSCs. Two developed both NMSCs and melanoma in situ, and one patient had an invasive melanoma. The rate of skin cancer was higher in the phototherapy group—5 out of 30 patients (16.7%) versus 1 out of 13 (7.7%)—but this was not statistically significant (p = 0.681). There were no melanomas observed in patients who did not receive phototherapy.
Time to progression to tumors
Patients who received phototherapy took a median of 3.5 years (interquartile range = 1.9 – 5.7) to progress to stage IIB (calculated as the time from date of tissue diagnosis to date of first documented tumor). Patients who did not receive phototherapy took a median of 1.2 years (interquartile range = 0.2 – 2.3) to progress to stage IIB. This difference was statistically significant (p = 0.001), and is represented in a Kaplan-Meier curve (Figure 1).
Figure 1. Effect of phototherapy on time to progression from IA/IB to stage IIB measured in years.
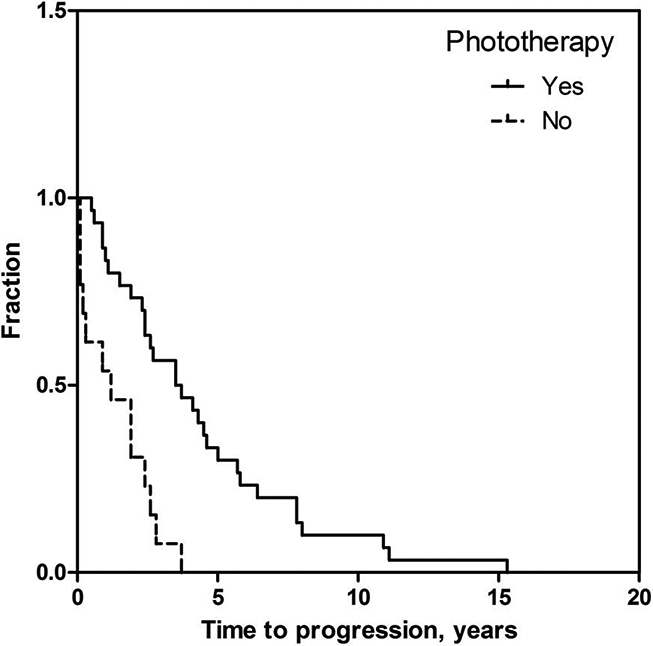
Kaplan-Meier curve of the percentage of the patient treated or not treated with phototherapy as a function of time after diagnosis to progression to tumors.
In the phototherapy group, the 19 patients who received PUVA took a median of 2.6 years (95% CI = 1.5 – 3.8) to progress to stage IIB. The 6 patients who received NBUVB took a median of 4.5 years, and the 1 patient who received both PUVA and NBUVB took 3.6 years. These differences were not statistically significant (p = 0.098).
In the univariate regression analysis, patients who received phototherapy took 4.2 times longer to develop tumors (95% CI = 2.1 – 8.6, p < 0.001) than patients who did not receive phototherapy. As previously mentioned, patients in the non-phototherapy group were almost a decade older at the time of diagnosis than patients in the phototherapy group. Since the risk of disease progression in patients with the T2 disease over ten years was previously shown in the literature to be approximately 72% [15], a multivariable linear regression was performed on log-in order to adjust for age at diagnosis. In the multivariable analysis, where age at diagnosis was no longer significant (p = 0.129), patients who received phototherapy took 3.6 times longer to develop tumors (95% CI = 1.7 – 7.5, p = 0.001).
Overall survival
We also looked at the effect of phototherapy on patient survival (Table 2). Patients who received phototherapy had a median overall survival of 6.9 years (95% CI = 4.3 – 9.5) after the initial diagnosis of MF. Patients who did not receive phototherapy had a median survival of 3.8 years (95% CI = 3.0 – 4.5) after diagnosis. This difference was statistically significant (p = 0.014) and is represented in a Kaplan-Meier curve (Figure 2). We adjusted for the difference in age at diagnosis with a Cox regression. In the analysis, patients who received phototherapy still survived longer, but this was not statistically significant (HR = 0.50, 95% CI = 0.23 – 1.06, p = 0.072). Older age at diagnosis was correlated with decreased survival time (HR = 1.03, 95% CI = 1.00– 1.06, p = 0.035).
Table 2:
Survival Data
| No Phototherapy N =13 | Phototherapy N =30 | P value | |
|---|---|---|---|
| Median time to progression to tumors (Interquartile range) | 1.2 years (0.2 – 2.3 years) | 3.5 years (1.9 – 5.7 years) | p< 0.001 |
| Median overall survival time (95% CI) | 3.8 years (3.0 – 4.5 years) | 6.9 years (4.3 – 9.5 years) | p = 0.014 |
| Median survival time after tumor development (95% CI) | 1.5 years (0–3.9 years) | 2.6 years (1.5 – 3.6 years) | p = 0 173 |
| Death from MF complications (Total deaths 34) | 6/11 (54.5%) | 8/24 (34.8%) | p = 0.458 |
| Large cell transformation | 4 (30.8%) | 11 (36.7%) | p= 1.000 |
| Progression beyond IIB | 8(61.5%) | 14 (46.7%) | p = 0.370 |
Figure 2. Effect of phototherapy on overall survival.
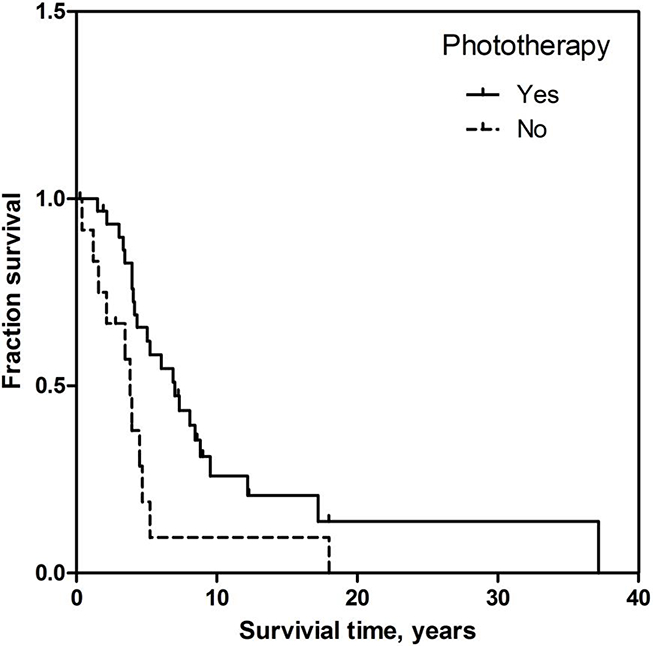
Kaplan-Meier curve of the percentage of the patient treated or not treated with phototherapy as a function of time after diagnosis to death. Overall survival measured in years. The patients who survived were censored.
We also evaluated the effect of phototherapy on survival after tumor development. While not statistically significant, patients who received phototherapy had longer median survival times after tumor development, fewer MF-related deaths (defined as metastases, sepsis from ulcerated tumors, and fatal drug reactions due to chemotherapy), and less progression beyond stage IIB (Table 2). There was no difference in the rate of large cell transformation between the two groups. Notably, of the 34 patients in this study who died, none died from NMSC or MM.
DISCUSSION
Both the etiology of MF and the pathogenesis of its progression from patch-plaque disease to advanced stages are still largely unknown and under investigation. MF is thought to be a multifactorial process, with genetic, immunologic, and environmental factors contributing to its development and clinical course. Various genetic abnormalities—the constitutive activation of oncogenes like STAT3, the inactivation of tumor suppressor genes like p53, p16, or PTEN [16, 17, 18, 19], and mutations in the tumor necrosis factor signaling pathway [20] — have been identified.
In our cohort of patients who progressed from stage IA or IB to stage IIB MF, those who received phototherapy had a statistically significantly longer time to tumor development than those who did not receive phototherapy. Before adjusting for age at diagnosis, patients who received phototherapy took 4.2 times longer (95% CI = 2.1 – 8.6, p < 0.001) to progress to tumors. After adjusting for age at diagnosis, patients who received phototherapy took 3.6 times longer to progress to tumors, suggesting that while age at diagnosis has an effect, phototherapy is still independently correlated with time to tumor progression. Within the phototherapy group, there were no significant differences in time to tumor progression or overall survival time between the PUVA and NBUVB groups, despite the fact that UVA is generally thought to be more carcinogenic than UVB. Our data suggests that the therapeutic effects of phototherapy on patch-plaque stage MF offset its potential oncogenic effects on the progression to tumor stage MF. This study may even underestimate the potential benefits of phototherapy because it does not include the numerous patients who received phototherapy and either achieved clinical remission or remained in patch-plaque stage.
UV light exerts its therapeutic effect by causing cell cycle arrest or apoptosis of keratinocytes, lymphocytes, and Langerhans cells, and by selectively decreasing the production of proinflammatory cytokines by T cells [21, 22, 23, 24]. Additionally, the psoralen component of PUVA cross-links with DNA, allowing not only for photosensitization but also for the independent inhibition of DNA replication and induction of cell cycle arrest [23]. Thus, while there is the risk of photocarcinogenesis, our findings suggest that the benefit of phototherapy on patch-plaque disease outweighs any of its potential effects on MF tumor development.
We believe the previous discovery of UV-induced mutations in MF is likely only a piece in the complicated picture of MF tumorigenesis. Moreover, the presence of UV-induced mutations does not necessarily indicate oncogenesis. Though UV-induced p53 DNPs have been implicated in the increased development of NMSCs [25, 26], both p53 mutations and p53 overexpression have also been found in the keratinocytes of chronically sun-exposed but otherwise phenotypically normal skin [27, 28]. A similar case can be made for the UV-induced DNPs found in MF tumors—that it is unclear whether the mutations played a role in MF tumorigenesis or are an incidental finding of life-long light exposure.
Due to limitations in clinical documentation, it is hard to establish the exact duration of phototherapy in our patients as well as the specific phototherapy protocols used. In our analyses, patients were considered to be in the phototherapy group regardless of when they started or for how long they were on phototherapy prior to the development of tumors. Additionally, while we were able to adjust for the different age at diagnosis between the phototherapy and non-phototherapy group, there may be other confounding factors that we have not considered and were, therefore, unable to adjust for in our analysis.
In conclusion, our study shows that phototherapy does not lead to a more rapid progression to tumor stage MF, but may slow the disease progression and prolong overall survival. Our clinical findings support the continued use of phototherapy in patients with patch-plaque stage MF.
ACKNOWLEDGMENT
The authors would like to thank Sue McCann for clinical coordination and Vladimir Lamm for his help with data acquisition.
Funding source: This work was supported by National Cancer Institute grant 5P50CA121973– 08. The statistical analyses were performed by the Clinical Translational Science Institute at the University of Pittsburgh and were supported by the National Institutes of Health Grant Number UL-TR 000005 and UL1-TR-001857.
Footnotes
Conflict of Interest Disclosures: None reported.
REFERENCES
- 1.Carter J, Zug KA. Phototherapy for cutaneous T-cell lymphoma: online survey and literature review [Review]. Journal of the American Academy of Dermatology. 2009. January;60(1):39–50. doi: 10.1016/j.jaad.2008.08.043. PubMed PMID: ; eng. [DOI] [PubMed] [Google Scholar]
- 2.Gilchrest BA, Parrish JA, Tanenbaum L, et al. Oral methoxsalen photochemotherapy of mycosis fungoides [Case Reports]. Cancer. 1976. August;38(2):683–9. PubMed PMID: ; eng. [DOI] [PubMed] [Google Scholar]
- 3.Gettler SL, Fung MA. Efficacy of treatments for mycosis fungoides and Sezary syndrome: nationwide survey responses [Research Support, Non-U.S. Gov’t]. Dermatology online journal. 2005;11(3):6 PubMed PMID: ; eng. [PubMed] [Google Scholar]
- 4.Ramsay DL, Lish KM, Yalowitz CB, et al. Ultraviolet-B phototherapy for early-stage cutaneous T-cell lymphoma. Archives of dermatology. 1992. July;128(7):931–3. PubMed PMID: ; eng. [PubMed] [Google Scholar]
- 5.Olsen EA, Hodak E, Anderson T, et al. Guidelines for phototherapy of mycosis fungoides and Sezary syndrome: A consensus statement of the United States Cutaneous Lymphoma Consortium [Review]. Journal of the American Academy of Dermatology. 2016. January;74(1):27–58. doi: 10.1016/j.jaad.2015.09.033. PubMed PMID: ; eng. [DOI] [PubMed] [Google Scholar]
- 6.Hearn RM, Kerr AC, Rahim KF, et al. Incidence of skin cancers in 3867 patients treated with narrow-band ultraviolet B phototherapy [Research Support, Non-U.S. Gov’t]. The British journal of dermatology. 2008. September;159(4):931–5. doi: 10.1111/j.1365-2133.2008.08776.x. PubMed PMID: ; Eng. [DOI] [PubMed] [Google Scholar]
- 7.Lee E, Koo J, Berger T. UVB phototherapy and skin cancer risk: a review of the literature [Review]. International journal of dermatology. 2005. May;44(5):355–60. doi: 10.1111/j.1365-4632.2004.02186.x. PubMed PMID: ; Eng. [DOI] [PubMed] [Google Scholar]
- 8.Stern RS, Liebman EJ, Vakeva L. Oral psoralen and ultraviolet-A light (PUVA) treatment of psoriasis and persistent risk of nonmelanoma skin cancer. PUVA Follow-up Study [Clinical Trial Multicenter Study Research Support, U.S. Gov’t, P.H.S.]. Journal of the National Cancer Institute. 1998. September 2;90(17):1278–84. PubMed PMID: ; Eng. [DOI] [PubMed] [Google Scholar]
- 9.Stern RS, Laird N, Melski J, et al. Cutaneous squamous-cell carcinoma in patients treated with PUVA [Research Support, U.S. Gov’t, P.H.S.]. The New England journal of medicine. 1984. May 3;310(18):1156–61. doi: 10.1056/NEJM198405033101805. PubMed PMID: ; Eng. [DOI] [PubMed] [Google Scholar]
- 10.Morison WL, Baughman RD, Day RM, et al. Consensus workshop on the toxic effects of long-term PUVA therapy [Consensus Development Conference Research Support, Non-U.S. Gov’t Review]. Archives of dermatology. 1998. May;134(5):595–8. PubMed PMID: ; Eng. [DOI] [PubMed] [Google Scholar]
- 11.Chuang TY, Heinrich LA, Schultz MD, et al. PUVA and skin cancer. A historical cohort study on 492 patients [Research Support, U.S. Gov’t, Non-P.H.S. Research Support, U.S. Gov’t, P.H.S.]. Journal of the American Academy of Dermatology. 1992. February;26(2 Pt 1):173–7. PubMed PMID: ; Eng. [DOI] [PubMed] [Google Scholar]
- 12.Stern RS, Nichols KT, Vakeva LH. Malignant melanoma in patients treated for psoriasis with methoxsalen (psoralen) and ultraviolet A radiation (PUVA). The PUVA Follow-Up Study [Multicenter Study Research Support, U.S. Gov’t, P.H.S.]. The New England journal of medicine. 1997. April 10;336(15):1041–5. doi: 10.1056/NEJM199704103361501. PubMed PMID: ; Eng. [DOI] [PubMed] [Google Scholar]
- 13.Stern RS. The risk of melanoma in association with long-term exposure to PUVA [Multicenter Study Research Support, U.S. Gov’t, P.H.S.]. Journal of the American Academy of Dermatology. 2001. May;44(5):755–61. doi: 10.1067/mjd.2001.114576. PubMed PMID: ; Eng. [DOI] [PubMed] [Google Scholar]
- 14.Querfeld C, Rosen ST, Kuzel TM, et al. Long-term follow-up of patients with early-stage cutaneous T-cell lymphoma who achieved complete remission with psoralen plus UV-A monotherapy [Comparative Study]. Archives of dermatology. 2005. March;141(3):305–11. doi: 10.1001/archderm.141.3.305. PubMed PMID: ; Eng. [DOI] [PubMed] [Google Scholar]
- 15.Kim YH, Liu HL, Mraz-Gernhard S, et al. Long-term outcome of 525 patients with mycosis fungoides and Sezary syndrome: clinical prognostic factors and risk for disease progression. Archives of dermatology. 2003. July;139(7):857–66. PubMed PMID: ; eng. [DOI] [PubMed] [Google Scholar]
- 16.McGregor JM, Crook T, Fraser-Andrews EA, et al. Spectrum of p53 gene mutations suggests a possible role for ultraviolet radiation in the pathogenesis of advanced cutaneous lymphomas [Research Support, Non-U.S. Gov’t]. The Journal of investigative dermatology. 1999. March;112(3):317–21. doi: 10.1046/j.1523-1747.1999.00507.x. PubMed PMID: ; eng. [DOI] [PubMed] [Google Scholar]
- 17.Scarisbrick JJ, Woolford AJ, Russell-Jones R, et al. Loss of heterozygosity on 10q and microsatellite instability in advanced stages of primary cutaneous T-cell lymphoma and possible association with homozygous deletion of PTEN. Blood. 2000. May 1;95(9):2937–42. PubMed PMID: ; eng. [PubMed] [Google Scholar]
- 18.Navas IC, Ortiz-Romero PL, Villuendas R, et al. p16(INK4a) gene alterations are frequent in lesions of mycosis fungoides. The American journal of pathology. 2000. May;156(5):1565–72. PubMed PMID: ; eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sommer VH, Clemmensen OJ, Nielsen O, et al. In vivo activation of STAT3 in cutaneous T-cell lymphoma. Evidence for an antiapoptotic function of STAT3 [Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S.]. Leukemia. 2004. July;18(7):1288–95. doi: 10.1038/sj.leu.2403385. PubMed PMID: ; eng. [DOI] [PubMed] [Google Scholar]
- 20.Tracey L, Villuendas R, Dotor AM, et al. Mycosis fungoides shows concurrent deregulation of multiple genes involved in the TNF signaling pathway: an expression profile study. Blood. 2003. August 1;102(3):1042–50. PubMed PMID: ; eng. [DOI] [PubMed] [Google Scholar]
- 21.El-Domyati M, Moftah NH, Nasif GA, et al. Evaluation of apoptosis regulatory proteins in response to PUVA therapy for psoriasis [Clinical Trial]. Photodermatology, photoimmunology & photomedicine. 2013. February;29(1):18–26. doi: 10.1111/phpp.12012. PubMed PMID: ; eng. [DOI] [PubMed] [Google Scholar]
- 22.Kohyama S, Morimoto Y, Nakai K, et al. Effectiveness of narrow-band ultraviolet-B phototherapy for prevention of intimal hyperplasia in a rat carotid balloon injury model [Research Support, Non-U.S. Gov’t]. Lasers in surgery and medicine. 2007. September;39(8):659–66. doi: 10.1002/lsm.20543. PubMed PMID: ; eng. [DOI] [PubMed] [Google Scholar]
- 23.Weichenthal M, Schwarz T. Phototherapy: how does UV work? [Research Support, Non-U.S. Gov’t Review]. Photodermatology, photoimmunology & photomedicine. 2005. Oct;21(5):260–6. doi: 10.1111/j.1600-0781.2005.00173.x. PubMed PMID: ; eng. [DOI] [PubMed] [Google Scholar]
- 24.Ozawa M, Ferenczi K, Kikuchi T, et al. 312-nanometer ultraviolet B light (narrow-band UVB) induces apoptosis of T cells within psoriatic lesions [Comparative Study Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S.]. The Journal of experimental medicine. 1999. February 15;189(4):711–8. PubMed PMID: ; PubMed Central PMCID: PMC2192929. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benjamin CL, Ananthaswamy HN. p53 and the pathogenesis of skin cancer [Research Support, N.I.H., Extramural Review]. Toxicol Appl Pharmacol. 2007. November 1;224(3):241–8. doi: 10.1016/j.taap.2006.12.006. PubMed PMID: ; PubMed Central PMCID: PMC2080850. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dumaz N, van Kranen HJ, de Vries A, et al. The role of UV-B light in skin carcinogenesis through the analysis of p53 mutations in squamous cell carcinomas of hairless mice [Comparative Study Research Support, Non-U.S. Gov’t]. Carcinogenesis. 1997. May;18(5):897–904. PubMed PMID: ; eng. [DOI] [PubMed] [Google Scholar]
- 27.Jonason AS, Kunala S, Price GJ, et al. Frequent clones of p53-mutated keratinocytes in normal human skin [Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S.]. Proceedings of the National Academy of Sciences of the United States of America. 1996. November 26;93(24):14025–9. PubMed PMID: ; PubMed Central PMCID: PMC19488. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stahl PL, Stranneheim H, Asplund A, et al. Sun-induced nonsynonymous p53 mutations are extensively accumulated and tolerated in normal appearing human skin [Comparative Study Research Support, Non-U.S. Gov’t]. The Journal of investigative dermatology. 2011. February;131(2):504–8. doi: 10.1038/jid.2010.302. PubMed PMID: ; eng. [DOI] [PubMed] [Google Scholar]


