Abstract
Background
This study tested the hypothesis that ad lib running wheel exercise in the tibia fracture model of complex regional pain syndrome can reverse hindlimb nociceptive sensitization and inflammation in mice.
Methods
Three weeks after tibia fracture, the cast was removed and hindlimb von Frey thresholds and unweighting were tested, then the mice were randomized to either ad lib access to a running wheel for 4 weeks or no wheel access. After 4 weeks the behavioral testing was repeated and then skin, sciatic nerve, and spinal cord tissues collected for polymerase chain reaction and enzyme immunoassay measurements of neuropeptide and inflammatory mediator levels. A similar protocol was used in fracture mice treated with exercise for 4 weeks and then the running wheel was removed for 2 weeks. Memory and anxiety were measured in both groups using the open field, zero maze, and novel objects recognition assays.
Results
At 7 weeks post fracture the mice with no wheel access exhibited hindlimb allodynia and unweighting, anxiety and memory loss, up-regulated spinal neuropeptide signaling, and increased hindpaw and spinal inflammatory mediator expression, but the post fracture mice allowed to exercise for 4 weeks exhibited none of these changes (n=12/cohort). When exercise was stopped for 2 weeks after 4 weeks of running, hindlimb allodynia and unweighting were rekindled and this nociceptive sensitization was associated with increased sciatic nerve neuropeptide levels and hindpaw skin interleukin-6 and nerve growth factor expression (n=12/cohort).
Conclusions
Daily exercise reversed nociceptive sensitization, inflammation, anxiety, and memory loss after tibia fracture.
1. Introduction
Chronic pain after surgery and trauma is being increasingly scrutinized regarding its frequency, severity and costs, and will be given its own diagnostic category in the upcoming International Classification of Diseases, ICD-11.1 Estimates of chronic pain after surgery vary enormously, affecting from 5 to 85% of patients, with some of the highest rates observed amongst patients after amputation, herniorrhaphy, thoracotomy and breast surgery.2,3 One specific form of chronic limb pain observed after trauma and surgery is complex regional pain syndrome (CRPS). CRPS can develop after a variety of upper and lower extremity surgical procedures.4,5
The mechanisms mediating CRPS are unknown, but limb immobilization is probably a factor. The traumatized limb is usually immobilized in casts, splints, or fixators prior to the development of CRPS 6,7 and patients guard the affected limb to prevent movement-induced pain.8 Furthermore, aggressive mobilization of the limb has been reported to alleviate CRPS symptoms,8 but a recent review noted a lack of high quality clinical trial data supporting exercise therapy for CRPS.9 Contrariwise, 4 weeks of forearm cast immobilization in normal subjects caused skin warmth, hyperalgesia, and movement-evoked pain, symptoms partially mimicking CRPS.10 These data support the hypothesis that prolonged immobilization contributes to the development of CRPS and that exercise and early mobilization is beneficial.
Distal limb fracture is the most common cause of CRPS,11,12 and a rodent distal tibia fracture model (TFM) recapitulates many of the nociceptive, vascular, trophic and cognitive features of CRPS.13,14 Using the TFM, we previously demonstrated that immobilization contributed to the development of post fracture nociceptive and inflammatory changes and that early mobilization reversed these changes.15 Tibia fracture with 4 weeks cast immobilization in rats resulted in hindpaw allodynia, unweighting, warmth, edema, increased sciatic nerve SP and CGRP protein, increased skin SP NK1 receptors, and increased in inflammatory mediator protein expression in the hindpaw skin (TNF, IL-1, IL-6, NGF) and cord (IL-1, NGF).15 After 4 weeks of cast immobilization alone these same changes occurred, except spinal IL-1 levels were not elevated.15 Treating cast only rats with an SP NK1 receptor antagonist inhibited development of nociceptive and inflammatory changes, similar to the NK1 receptor antagonist effects observed in the fracture cast rats.15 CRPS-like symptoms such as warmth and mechanical allodynia resolved much earlier in the cast immobilized (no fracture) rats than in the fracture casted rats.16,17 When tibia fracture rats were treated with intramedullary pinning instead of casting, they began weight bearing within days and by 4 weeks post fracture nociceptive sensitization resolved and neuropeptide signaling and inflammatory mediator expression returned to normal.15 These data indicate that immobilization alone caused changes in nociception, neuropeptide signaling, and inflammatory mediator expression similar to, but less robust than the changes observed after fracture and casting, and early mobilization after fracture inhibited these changes.
The current study used the mouse TFM to determine whether daily running exercise for 4 weeks can reverse post fracture CRPS-like changes, including nociceptive sensitization, exaggerated SP and CGRP signaling, inflammatory changes in the hindlimb and lumbar cord, anxiety, and memory loss.
2. Materials and methods
2.1 Animals and drugs
These experiments were approved by the Veterans Affairs Palo Alto Health Care System Institutional Animal Care and Use Committee (Palo Alto, CA, USA) and followed the animal subjects guidelines laid out in the Guide for the Care and Use of Laboratory Animals of the National Academy of Sciences. Three-month-old male C57BL/6J mice (#000664, Jackson Laboratory, Bar Harbor, ME) were used in these experiments. The mice were housed individually under pathogen-free conditions with soft bedding and were given food and water ad libitum, with a 12:12 light:dark cycle. During the experimental period the animals were fed Teklad lab rodent diet 2018 (Harlan Laboratories, Indianapolis, IN), which contains 1.0% calcium, 0.7% phosphorus, and 1.5 IU/g vitamin D3, and were kept under standard conditions with a 12-h light-dark cycle. Data collection was conducted blind to group assignment.
To assess if IL-6 supported allodynia in 9 week fracture mice that had or had not been previously exercised between weeks 3 and 7 post fracture, both groups of fracture mice were treated with TB-2-081 (2mg/kg, s.c., provided by Dr Kenner Rice, NIDA, Bethesda). TB-2-081 is an orally active small molecule IL-6 receptor antagonist originally isolated from the skin of a toad. Hindpaw von Frey testing was performed prior to and 15 min after TB-2-081 injection. In another experiment, additional cohorts of 9 week fracture mice that had or had not been previously exercised were injected with anti-NGF (10mg/kg i.p., (muMab 911, provided by Dr David Shelton, Rinat/Pfizer, San Francisco) and 7 days later underwent von Frey testing. This anti-NGF antibody is a TrkA-immunoglobulin G (TrkA-IGG) fusion molecule that binds to the NGF molecule, thus blocking the binding of NGF to the TrkA and p75 NGF receptors and inhibiting TrkA autophosphorylation.
2.2. Surgery
The TFM was performed in 3 month-old male mice as previously described 13. Under isoflurane anesthesia a hemostat was used to make a closed fracture of the right tibia just distal to the middle of the tibia. The hindlimb was then wrapped in casting tape (Delta-Lite, BSN Medical, Hamburg, Germany) so the hip, knee and ankle were all fixed. The cast extended from the metatarsals of the hindpaw up to a spica formed around the abdomen. A window was left open over the dorsal paw and ankle to prevent constriction when post-fracture edema developed. After fracture and casting, the mice were given subcutaneously 2 days of buprenorphine (0.05 mg/kg) and baytril (5 mg/kg) as well as 1.0 ml of normal saline. At 3 weeks after surgery the mice were anesthetized with isoflurane and the cast removed. All mice had union at the fracture site by manual inspection.
2.3. Hindpaw nociceptive testing
To measure mechanical allodynia in the mice, an up-down von Frey testing paradigm was used as we have previously described.13 Briefly, mice were placed on wire mesh platforms in clear cylindrical plastic enclosures 10 cm in diameter and 40 cm in height, and after 15 minutes of acclimation von Frey fibers of sequentially increasing stiffness were applied against the hindpaw plantar skin at approximately midsole, taking care to avoid the tori pads, and pressed upward to cause a slight bend in the fiber and left in place for 5 sec. Withdrawal of or licking the hindpaw after fiber application was scored as a response. When no response was obtained the next stiffest fiber in the series was applied to the same paw; if a response was obtained a less stiff fiber was applied. Testing proceeded in this manner until 4 fibers had been applied. Estimation of the mechanical withdrawal threshold by data fitting algorithm permitted the use of parametric statistics for analysis.18 Hind paw mechanical nociceptive thresholds were analyzed as the difference between the fracture side and the contralateral untreated side.
An incapacitance device (IITC Inc. Life Science, Woodland Hills, CA) was used to measure hind paw unweighting. The mice were manually held in a vertical position over the apparatus with the hind paws resting on separate metal scale plates, and the entire weight of the rat was supported on the hind paws. The duration of each measurement was 6s, and 6 consecutive measurements were taken at 10 s intervals. All 6 readings were averaged to calculate the bilateral hind paw weight-bearing values.13 Right hindpaw weight-bearing data were analyzed as a ratio between the right hindpaw weight and the sum of right and left hindpaw values ((2R/(R + L)) × 100%).
2.4. Hindpaw temperature testing
The temperature of the hindpaw was measured using a fine wire thermocouple (Omega Engineering, Stamford, CT) applied to the paw skin, as previously described previously13 The investigator held the wire using an insulating Styrofoam block. Three sites were tested over the dorsum of the hindpaw: the space between the first and second metatarsals (medial), the second and third metatarsals (central), and the fourth and fifth metatarsals (lateral). After a site was tested in one hindpaw the same site was immediately tested in the contralateral hindpaw. The testing protocol was medial dorsum right then left, central dorsum right then left, lateral dorsum right then left, medial dorsum left then right, central dorsum left then right, and lateral dorsum left then right. The six measurements for each hindpaw were averaged for the mean temperature. Hindpaw temperature data were analyzed as the difference between the fractured side and the contralateral unfractured side.
2.5. Hind paw volume testing
A laser sensor technique was used to determine the dorsal-ventral thickness of the hind paw, as we have previously described.13 The measurement sensor device used in these experiments (Limab, Goteborg, Sweden) has a measurement range of 200 mm with a 0.01 mm resolution. Hind paw volume data were analyzed as absolute value or the difference between the fracture side and the contralateral untreated side.
2.6. Running wheel exercise protocols
Mice underwent behavioral testing for allodynia, unweighting, warmth, and edema at baseline, and then underwent distal tibia fracture and casting for 3 weeks. On the day following cast removal the mice underwent repeat behavioral testing and then were randomized into 2 cohorts, one group had running wheels placed in their cages and the other group had no wheels in their cages. The mice were individually housed and the exercise group had ad lib access to the running wheels 24 hours/day, 7 days a week. Behavioral testing was repeated at 4, 5, 6, and 7 weeks after fracture, then the wheels were removed and behavioral testing was repeated at 9 weeks post-fracture. A computerized activity wheel (AWM software, version 6.9.2057.18763; Lafayette Instrument, Lafayette, IN) allowed monitoring the daily distances the mice ran and in one experiment was used to restrict the running distance to 0.5 km/daily.
2.7. Enzyme immunoassay
After 4 weeks of wheel running exercise (7 weeks post fracture) or at 2 weeks after removing the wheels from the cages (9 weeks post fracture), the mice were euthanized by CO2 inhalation and cervical dislocation and the mouse hind paw dorsal skin and sciatic nerve were collected and frozen immediately on dry ice. All tissues were cut into fine pieces in ice-cold phosphate buffered saline, pH 7.4, containing a cocktail of protease inhibitors (Roche Applied Science, Indianapolis, IN) and then homogenized using a Bio-Gen PRO200 homogenizer (PRO Scientific, Oxford, CT). The homogenates were centrifuged for 15 minutes at 12,000g, 4°C. The supernatants were aliquoted and stored at −80°C until required for ELISA performance. Total protein contents in all tissue extracts were measured by using the DC Protein Assay kit (Bio-Rad, Hercules, CA) to normalize mediator levels. Mouse interleukin 1 beta (IL-1), interleukin 6 (IL-6), tumor necrosis factor-alpha (TNF), nerve growth factor (NGF), chemokine (C-C motif) ligand 2 (CCL2), substance P (SP), and calcitonin gene-related peptide (CGRP) protein levels were measured in duplicate by using mouse IL-1, IL-6, TNF, and CCL2 (R&D Systems, Minneapolis, MN), NGF (Millipore Merck, Darmstadt, Germany), SP (MyBioSource, San Diego, CA), and CGRP (Peninsula Laboratories, San Carlos, CA) ELISA kits following the manufacturer’s instructions. The results of all assays were confirmed by repeating the experiment twice.
2.8. Quantitative real-time polymerase chain reaction
After 4 weeks of wheel running exercise (7 weeks post fracture) or at 2 weeks after removing the running wheels from the cages (9 weeks post fracture), the mice were euthanized by CO2 inhalation and cervical dislocation and the fracture limb hind paw skin, DRG (L3-S1), and corresponding spinal cord (L4 and 5 lumber enlargement) were collected. Controls tissues were collected from control nonfracture mice and from fracture mice that did not have wheels placed in their cages, at 7 and 9 weeks post fracture. Total RNA was extracted by using the RNeasy Mini Kit (Qiagen, Hilden, Germany), and the purity and concentration were determined spectrophotometrically. Then cDNA was synthesized from 1 μg RNA using an iScript cDNA Synthesis Kit (Bio-Rad Laboratories, Hercules, CA). Real-time polymerase chain reactions (PCRs) were conducted using the SYBR Green PCR master mix (Applied Biosystems, Waltham, MA). Real-time PCR amplification of IL-1, IL-6, TNF, NGF, CCL2, TAC1, TACR1, RAMP1, CALCA, CALCB, CALCRL and 18S was performed on an ABI 7900HT sequencing detection system (Applied Biosystems, Waltham, MA). To validate the primer sets used (Table 1), we performed dissociation curves to document single product formation, and agarose gel analysis was conducted to confirm the size. The data from real-time PCR experiments were analyzed as described in the manufacturer’s manual for the ABI 7900HT sequencing detection systems. All results were confirmed by repeating the experiment 3 times.
Table 1.
Primers used for real-time PCR
| Gene | GenBank Accession # | Forward primer | Reverse primer | Product Size(bp) |
|---|---|---|---|---|
| IL-1β | NM_031512 | agtctgcacagttccccaac | agacctgacttggcagagga | 230 |
| IL-6 | NM_012589 | cacaagtccggagaggagac | acagtgcatcatcgctgttc | 168 |
| TNF-α | NM_012675 | ctcccagaaaagcaagcaac | cgagcaggaatgagaagagg | 210 |
| NGF | XM_227525 | acctcttcggacactctgga | gtccgtggctgtggtcttat | 168 |
| CCL2 | NM_031530 | tcccacttcctgctgcttctctta | agcaaaggctgctggtctcatagt | 86 |
| TAC1 | NM_012666 | tttgcagaggaaatcggtgccaac | ggcattgcctccttgatttggtca | 83 |
| TACR1 | NM_012667 | ctggaaagaggagccttgtg | ctgagacggaaaggaacagc | 205 |
| RAMP1 | NM_031645 | ggcaaacaagattggctgtt | aatggggagcacaatgaaag | 154 |
| CALCA | NM_017338 | agaagagatcctgcaacactgcca | ggcacaaagttgtccttcaccaca | 94 |
| CALCB | NM_138513 | cccagaagagatcctgcaac | agttcctcagacccgaaggt | 158 |
| CALCRL | NM_012717 | tcattgtggtggctgtgttt | aatgggaccatggatgatgt | 176 |
| 18S | NR_046237 | tcaactttcgatggtagtcgccgt | tccttggatgtggtagccgtttct | 108 |
2.9. Open field, zero maze, and novel object recognition assays
The open field arena measured 40 × 40 × 40 cm and was made of opaque plastic material. Luminosity inside the arena was measured to be 50 lux. The mice were placed into the arena and allowed to explore for 10 min. Total locomotor activity (distance traveled) and the time spent in the central portion (11% of total area) were determined for each mouse. All recordings were automatically analyzed in real time by TopScan software (Clever Sys Inc, Reston, VA). The time spent in the center area was used as an index of thigmotaxis, a measure often used for evaluating general anxiety levels in rodents.19
The zero maze was used to measure anxiety, following previously published methods.20 The maze had an outer diameter of 61 cm, inner diameter of 51 cm, it was situated 61 cm above the floor, and the closed quadrants had 15 cm tall walls. Luminosity inside the open quadrant was measured to be 50 lux, whereas that inside the closed quadrant was measured to be 20 lux. Mice were placed facing one of the closed quadrants of the maze at the beginning testing and the number of entries into the open quadrants over a 5-min test period was recorded using TopScan software.
Working memory was assessed using novel object recognition testing in the same arena as the one used for the open field test.21 Exploration behavior was used to assess object recognition. Mice were placed into the arena and presented with two identical objects for 10 min (habituation). Then, during a 5-min trial one of the objects was moved to a new location and exploratory behavior (investigation time) was recorded using TopScan software. Subsequently, the mice were returned to their home cages for a 5-min period and were then returned to the arena after one of the previous identical objects had been replaced with a novel one. The novel object had a distinct shape and size different from the original object pairs. Exploration behavior during the first 5-min was recorded. These experiments were performed under 50 lux luminosity.
2.10. Statistical Analysis
Statistical analysis was done using Prism 4.02 (GraphPad Software). Sample sizes were been based on a power analysis of preliminary and previously published data generated from using each of the proposed assays in fracture animals. Based on this analysis we calculated that the proposed experiments would require 8 animals per cohort to provide 80% power to detect a 25% difference between group means (using a two-tailed test with a 0.05 significant level). Animals were randomized to experimental groups using computer generated random numbers and all testing was performed in a blinded fashion when possible. In response to reviewer concerns, an additional exercise nonfracture control group was added to the cognitive studies (Figs 9, 10), thus the cognitive behavioral experiments performed in these mice were not performed in blinded fashion.
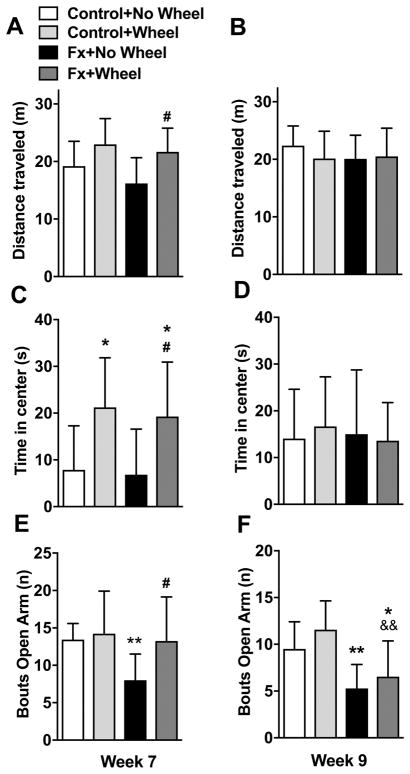
Figure 9. Exercise improved post fracture locomotor activity and reduced anxiety.
Fracture mice were treated with 4 weeks of wheel running (FX+Wheel, Week 7) or no wheel access (FX+No Wheel, Week 7) and nonfracture mice with and without access to running wheels were used as controls (Control+Wheel, Control+No Wheel, Week 7). In an additional set of experiments, fracture and control mice were given 4 weeks of wheel access and then the wheel was removed for 2 weeks (FX+Wheel, Control+Wheel, Week 9) or were not given access to a running wheel and were tested at 9 weeks post fracture (FX+No Wheel, Week 9). In the open field test, compared to nonfracture controls, there was no reduction in the distance traveled by the FX+No Wheel cohort at 7 (A) or 9 (B) weeks post fracture. Wheel exercise (FX+Wheel) did increase the distance traveled, compared to FX+No Wheel (A), but this effect was lost after stopping exercise for 2 weeks (B). The exercise fracture mice spent increased time in the center of the open field assay, compared to no exercise fracture mice or controls (C), but this effect was lost after stopping exercise for 2 weeks (D). After 4 weeks of wheel exercise control nonfracture mice also spent increased time in the center of the open field assay, compared to controls lacking wheel access, and this effect was also lost after stopping exercise for 2 weeks (D). Compared to no fracture controls, the FX+No Wheel mice were less likely to enter the open arms of the zero maze assay, signifying increased anxiety at 7 (E) and 9 (F) weeks post fracture. Four weeks of wheel exercise reversed anxiety behavior in the zero maze test in fracture mice (FX+Wheel), but had no effect on controls (E), and this anxiolytic effect was lost after stopping exercise for 2 weeks (F). Exercising the fracture mice cause an increase the time spent in the open arms of the zero maze (E), but this effect was lost after stopping exercise for 2 weeks (F). Values are means ± SD, n=11–15 per cohort. One-way analysis of variance with Bonferroni post hoc testing. * P< 0.05, ** P< 0.01, for FX+No Wheel or FX+Wheel vs Control, # P< 0.05, ## P< 0.01, for FX+Wheel vs FX+No Wheel, && P< 0.01 for FX+Wheel vs Control+Wheel.
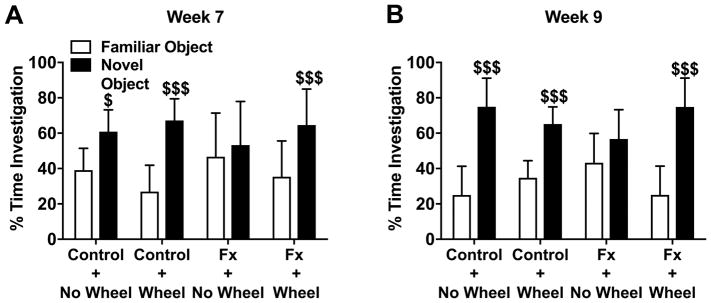
Figure 10. Exercise improved post fracture memory impairment.
Object recognition memory testing was performed with 5 min intervals between acquisition and retrieval trials. Fracture mice (FX+No Wheel) exhibited reduced object recognition memory compared to nonfracture Control+No Wheel mice (A), and 4 weeks of exercise reversed this memory impairment in fracture mice (FX+Wheel). After discontinuing exercise for 2 weeks the beneficial effects of prior exercise were still present (B). Exercise in nonfracture Control+Wheel mice had no effect on object recognition memory (A). All recordings were analyzed in real time by automated software. Values are means ± SD, n=11–15 per cohort. One-way analysis of variance with Bonferroni post hoc testing. $ P<0.05, $$$ P<0.001 for novel vs familiar object.
No animals were excluded after enrollment into the experimental cohorts and no data was excluded from statistical analysis. Normal distribution of the data was confirmed using the D’Agostino-Pearson omnibus normality test and two-tailed test assumptions were used in all analyses with a 0.05 significance level. Figure 1 data was analyzed using a two-way repeated measures analysis of variance (ANOVA) followed by Bonferonni post hoc multiple comparison testing. All data other data was evaluated using a one way ANOVA followed by Bonferroni post hoc multiple comparison testing. Data are presented as the mean ± standard deviation (SD).
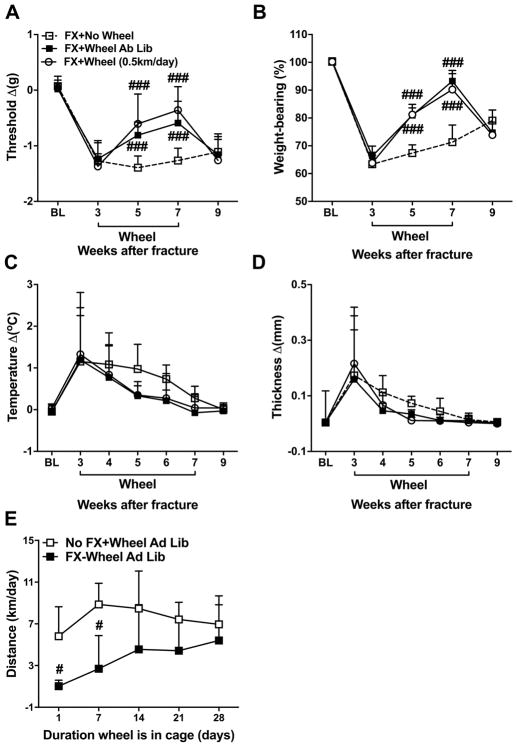
Figure 1. Effects of running exercise on post fracture nociception and vascular changes.
Mouse hindpaw von Frey fiber withdrawal thresholds (A), weight bearing (B), temperature (C), and paw thickness (D) were measured at baseline (BL), then the mice underwent right tibia fracture and hindlimb casting for 3 weeks, then the cast was removed and the mice retested the following day, then one cohort of mice had a running wheel placed in their cage for 4 weeks. There were 3 experimental groups, fracture with no wheel (FX+No Wheel, n = 12), fracture with ad lib access to wheel (FX+Wheel Ad Lib, n = 12), and fracture with only 0.5 km/day wheel access (FX+Wheel 0.5 km/day, n = 7). Allodynia (A), unweighting (B), warmth (C), and edema (D) were observed at 3 weeks post fracture. The mice with access to the running wheel had reduced hind paw allodynia and unweighting over the duration of the exercise interval (post fracture weeks 3–7). When the running wheel was removed from the cage for 2 weeks (post fracture weeks 7–9) the hindpaw allodynia and unweighting were exacerbated, returning to the same levels as seen in fracture mice not treated with running wheel access. Limited access (0.5 km/day) to the running wheel between post fracture weeks 3–7 resulted in the same beneficial effects as ad lib access to the wheel. Another experiment measured the distance that mice ran at 1, 7, 14, 21, and 28 days after placing the running wheel in their cages and allowing ad lib access to the wheel (E). The fracture mice ran 1.0 km/day the first day of ad lib wheel access (FX+Wheel Ad Lib, n=8), and this gradually increased to 5.4 km/day over 4 weeks. After 2 weeks of ad lib wheel access there was no significant difference between the distances the fracture mice were running daily and the distances that nonfracture control mice (No FX+Wheel Ad Lib, n = 8) were running. The hindpaw von Frey mechanical nociceptive threshold, temperature, and thickness were analyzed as the difference between the fracture side and the contralateral untreated side, thus a negative value for von Frey testing indicates allodynia, a positive value for temperature and thickness represents warmth and edema, respectively. Right hindpaw weight bearing data were analyzed as a ratio between twice the right hindpaw weight bearing and the sum of the right (R) and left (L) hindpaw weight bearing values ((2R/(R + L)) × 100%), thus a percentage less than 100% indicates unweighting. Values are means ± SD. Two-way analysis of variance with Bonferroni post hoc testing. #P< 0.05, ## P< 0.01 and ### P< 0.001 for FX+Wheel Ad Lib, or FX+Wheel (0.5 km/day), vs FX+No Wheel.
The hindpaw von Frey mechanical nociceptive threshold, temperature, and thickness were analyzed as the difference between the fracture side (right, R) and the contralateral untreated side (left, L). Right hindpaw weight bearing data were analyzed as a ratio between twice the right hindpaw weight bearing and the sum of the right (R) and left (L) hindpaw weight bearing values ((2R/(R + L)) × 100%).
3. Results
3.1. Exercise reversed pain behaviors, warmth, and edema in the fracture hindpaw
When the cast was removed at 3 weeks post fracture the mice exhibited ipsilateral hindpaw von Frey allodynia (−1.3 ± 0.4 vs 0.1 ± 0.2 Δg, P<0.001), unweighting (63 ± 4 vs 100 ± 1 %, P<0.001), warmth (1.2 ± 1.3 vs 0.1± 0.1 Δ°C, P=0.010), and edema (0.17 ± 0.21 vs 0.001 ± 0.01 Δmm, P=0.017), compared to baseline (Fig. 1A–D). When exercise wheels were placed in cages of the 3 week post fracture mice for 4 weeks (between post fracture weeks 3–7) the mice quickly began using the exercise wheels at night and after 4 weeks there were no significant differences in the average distances run by the fracture mice vs nonfracture control mice (5.4 ± 4.2 vs 6.9 ± 1.9 km/day, P=0.481, Fig. 1E). Hindpaw allodynia (week 5: −0.8 ± 0.2 vs −1.4 ± 0.2 Δg, P<0.001, week 7: −0.6±0.4 vs −1.3 ± 0.2 Δg, P<0.001) and unweighting (week 5: 81 ± 4 vs 67 ± 3 %, P<0.001, week 7: 93 ± 4 vs 71 ± 4 %, P<0.001) were progressively reversed with running exercise, compared to fracture mice not provided running wheel access. When the running wheel was removed from the cages of the fracture mice at 7 weeks post fracture the hindpaw allodynia (week 9: −0.8 ± 0.2 vs −1.1 ± 0.3 Δg, P=1.000) and unweighting (week 9: 81 ± 4 vs 67 ± 3 %, P=0.804) were rekindled within 2 weeks, compared to fracture mice not provided with running wheel access (Fig. 1A, B). When 3 week post fracture mice were given exercise wheels for 4 weeks that were computer controlled to lock after running 0.5 km/day, their hindpaw allodynia and unweighting were progressively reversed, similar to the effects seen with ad lib wheel running (Fig. 1A–D).
3.2 Exercise reversed post fracture increases in IL-1 and CCL2 mRNA and protein in the hindpaw skin
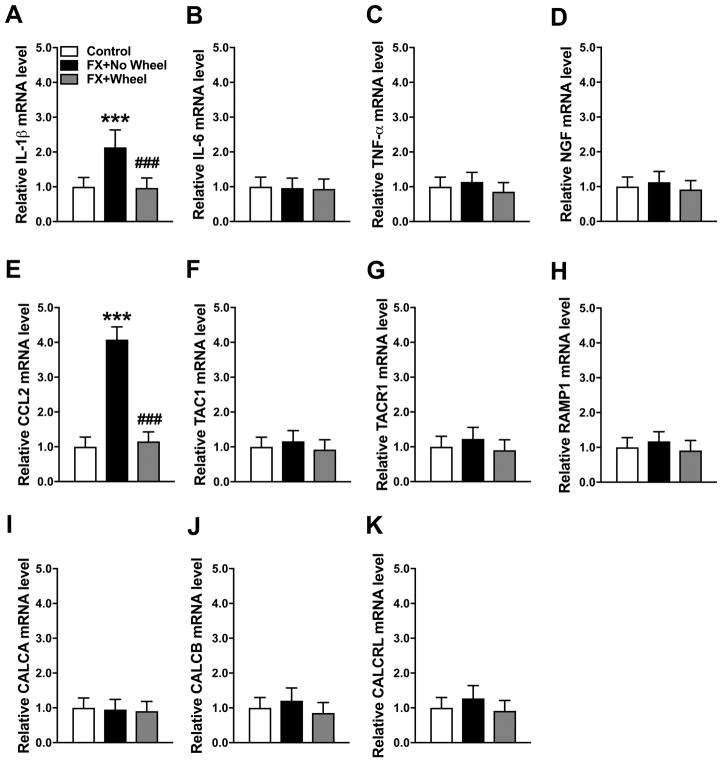
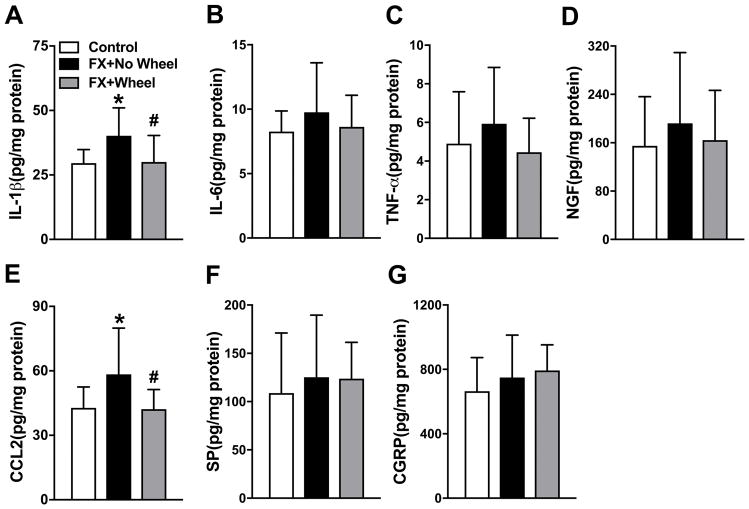
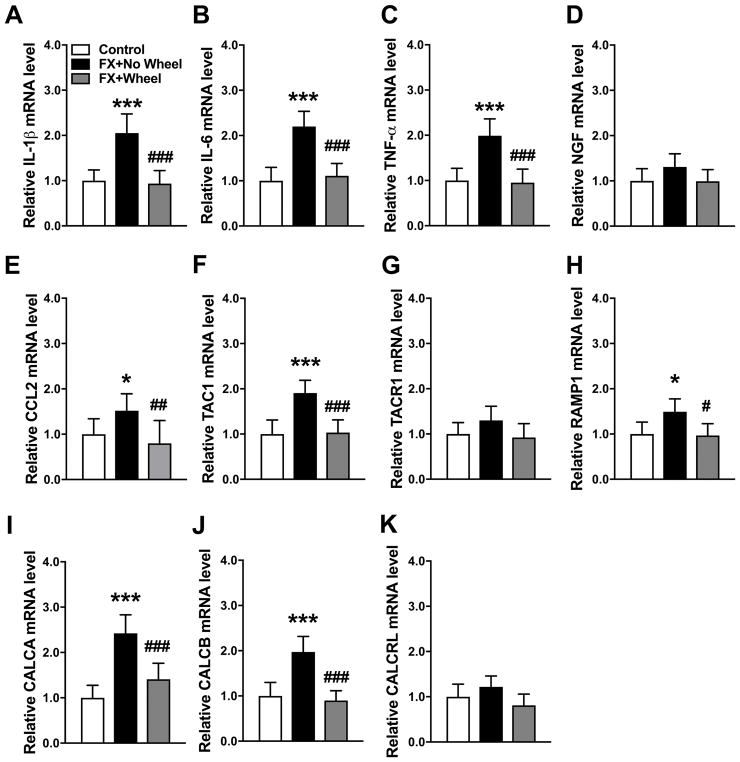
At 7 weeks post fracture neuropeptide and inflammatory mediator gene expression in the fracture limb hindpaw skin was measured by real time PCR (Fig. 2). Compared to nonfracture mouse mRNA expression, IL-1 and CCL2 mRNA levels were elevated at 7 weeks post fracture in nonexercised mice (110% and 310%, respectively), and 4 weeks of wheel running exercise reversed this increase (Fig. 2A, E). Hindpaw skin mRNA levels for IL-6, TNF, NGF, TAC1, TACR1, RAMP1, CALCA, CALCB, and CALCRL were unchanged at 7 weeks post fracture, versus control nonfracture mice, and wheel running had no effect on the expression of these mediators (Fig. 2B–D, F–K). Protein levels for inflammatory mediators in the hindpaw skin were also evaluated by EIA (Fig. 3). Similar to gene expression, IL-1 (Control: 29.6 ± 5.3 vs FX+No Wheel: 40.2 ± 10.8, P=0.040) and CCL2 (Control: 42.8 ± 9.8 vs FX+No Wheel: 58.4 ± 21.5, P=0.038) protein levels were increased at 7 weeks post fracture, when compared to nonfracture mouse protein levels, and wheel running reversed this increase (IL1: FX+No Wheel: 40.2 ± 10.8 vs FX+Wheel; 30.0 ± 10.3 pg/mg, P=0.021 and CCL2: FX+No Wheel: 58.4 ± 21.5 vs FX+Wheel; 42.2 ± 9.2 pg/mg, P=0.034). Hindpaw skin protein levels for IL-6, TNF, NGF, SP, and CGRP were unchanged at 7 weeks post fracture, versus control nonfracture mice, and wheel running had no effect on the expression of these mediators (Fig. 2B–D, F–K).
Figure 2. The effects of exercise on post fracture gene expression of cutaneous inflammatory mediators.
Expression of inflammatory mediators in the fracture limb hindpaw skin were measured by real-time PCR. Interleukin-1β (IL-1β, A) and chemokine (C-C motif) ligand 2 (CCL2, E) gene expression were up-regulated at 7 weeks post fracture (FX+No Wheel), compared to nonfractured control mice (Control), and this increase was reversed in FX mice provided with 4 weeks of ad lib access to a running wheel (FX + Wheel), starting at 3 weeks post fracture. There were no changes in the hindpaw skin expression of interleukin-6 (IL-6, B), tumor necrosis factor-α (TNF-α, C), nerve growth factor (NGF, D), substance P (TAC1, F), the substance P NK 1 receptor (TACR1, G), the calcitonin gene-related peptide (CGRP) RAMP1 receptor (RAMP1, H), CGRP (CALCA, I and CALCB, J) and the CGRP receptor (CALCRL, K) at 7 weeks post fracture (FX+No Wheel), compared to control nonfracture mice. Exercise had no effects on the post-fracture expression of IL-6, TNF-α, NGF, TAC1, TACR1, RAMP1, CALCA, CALCB, or CALCRL. Values are means ± SD, n=8. One-way analysis of variance with Bonferroni post hoc testing. *** P< 0.001 for FX+No Wheel or FX + Wheel vs Control. ### P< 0.001 for FX + Wheel vs FX+No Wheel.
Figure 3. The effects of exercise on post fracture protein expression of cutaneous inflammatory mediators.
Protein levels of IL-1β, TNF-α, IL-6, NGF, CCL2, SP and CGRP in the fracture limb hind paw skin were determined by enzyme immunoassay (EIA). Similar to the changes observed in gene expression (Fig. 2), IL-1β (A) and CCL2 (E) protein expression were up-regulated at 7 weeks post fracture (FX+No Wheel), compared to nonfractured control mice (Control), but returned to normal levels after 4 weeks of wheel running, starting at 3 weeks post fracture (FX+Wheel). Hindpaw skin protein levels for IL-6, TNF-α, NGF, SP and CGRP (B–D, F, G) were not elevated at 7 weeks post fracture, compared to nonfractured control mice, and 4 weeks of wheel running in fracture mice had no effect. Values are means ± SD, n=8 per cohort. One-way analysis of variance with Bonferroni post hoc testing * P< 0.05 for FX vs Control. # P< 0.05 for FX + Wheel vs FX.
3.3 Exercise had no effect on sciatic nerve neuropeptide expression
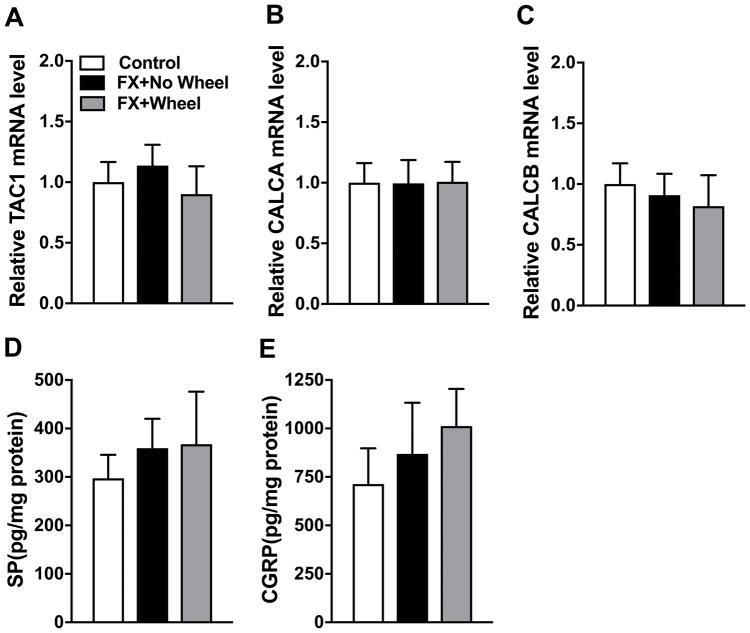
At 7 weeks post fracture SP and CGRP gene (TAC1, CALCA, and CALCB) expression in the fracture limb lumbar DRGs were measured by real time PCR and SP and CGRP protein levels in the sciatic nerve were measured by EIA (Fig. 4). There were no increases in DRG TAC1, CALCA, and CALCB mRNA levels and no increases in sciatic nerve SP and CGRP protein levels at 7 weeks post fracture. Four weeks of wheel running exercise had no effect on the expression of TAC1, CALCA, CALCB, SP, and CGRP.
Figure 4. Exercise had no effects on post fracture neuropeptide expression in the sciatic nerve.
Gene expression levels for SP (TAC1, A) and CGRP (CALCA, B and CALCB, C) were determined in the lumbar DRGs by real time PCR and SP (D) and CGRP (E) protein levels in the sciatic nerve were determined by EIA. No changes in DRG SP and CGRP gene expression or sciatic nerve protein expression were observed in the fracture limb at 7 weeks post fracture (FX+No Wheel), compared to nonfractured control mice (Control), and 4 weeks of wheel running in fracture mice had no effect (FX+Wheel). Values are means ± SD, n=8 per cohort. One-way analysis of variance with Bonferroni post hoc testing.
3.4 Exercise reversed post fracture increases in IL-1, IL-6, TNF, CCL2, TAC1, RAMP1, CALCA, and CALCB mRNA in the ipsilateral lumbar cord
At 7 weeks post fracture neuropeptide and inflammatory mediator gene expression in the lumbar cord innervating the fracture limb was measured by real time PCR (Fig. 5). Compared to nonfracture mice, IL-1 (110%), IL-6 (120%), TNF (100%), CCL2 (50%), TAC1 (90%), RAMP1 (50%), CALCA (140%), and CALCB (100%) mRNA levels were elevated at 7 weeks post fracture and 4 weeks of wheel running exercise reversed this increase (Fig. 5A–C, E, F, H–J). Spinal cord mRNA levels for NGF, TACR1, and CALCRL were unchanged at 7 weeks post fracture, versus control nonfracture mice, and wheel running had no effect on the expression of these mediators (Fig. 5D, G, K). Lumbar cord protein levels for these inflammatory mediators were also evaluated by EIA, but levels were below the sensitivity thresholds for the assay kits used in this study.
Figure 5. The effects of exercise on post fracture gene expression of spinal cord inflammatory mediators.
Inflammatory mediator expression in the lumbar cord innervating the fracture limb was measured by real-time PCR. IL-1β, IL-6, TNF-α, CCL2, TAC1, RAMP1, CALCA and CALCB (A–C, E–F, H–J) gene expression were up-regulated at 7 weeks post fracture (FX+No Wheel), compared to nonfractured control mice (Control). All these increases were reversed by voluntary wheel running for 4 weeks starting at day 21 after fracture (FX + Wheel). There were no changes in the hindpaw skin expression of NGF (D), TACR1 (G) and CALCRL (K) at 7 weeks post fracture, compared to nonfracture control mice, and exercise had no effects on the post fracture expression of NGF, TACR1, or CALCRL. Values are means ± SD, n=8 per cohort. One-way analysis of variance with Bonferroni post hoc testing. * P< 0.05, ** P< 0.01, *** P< 0.001 for FX+No Wheel or FX + Wheel vs Control, # P< 0.05, ## P< 0.01, ### P< 0.001 for FX + Wheel vs FX+No Wheel.
3.5 Stopping exercise induced the up-regulation of SP and CGRP in the sciatic nerve and IL-1 and NGF in the hindpaw skin
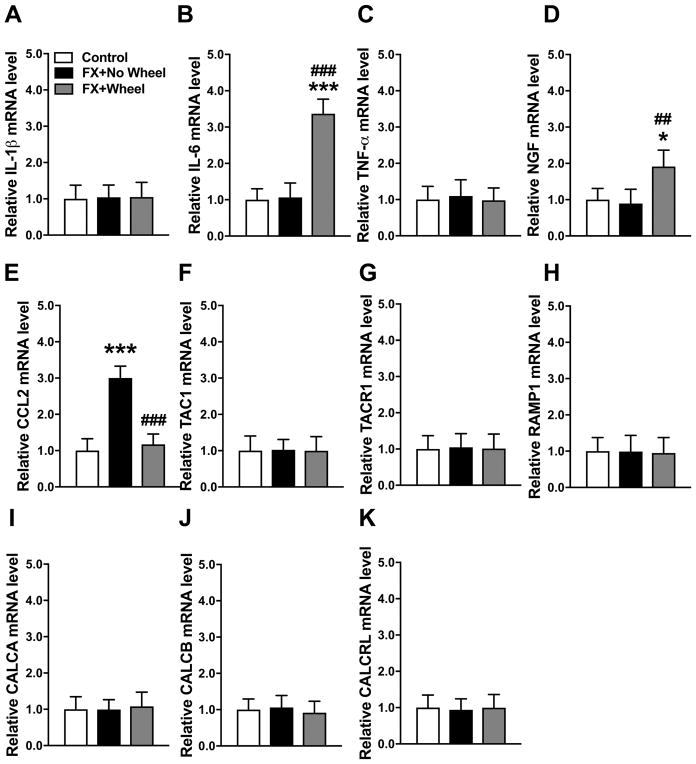
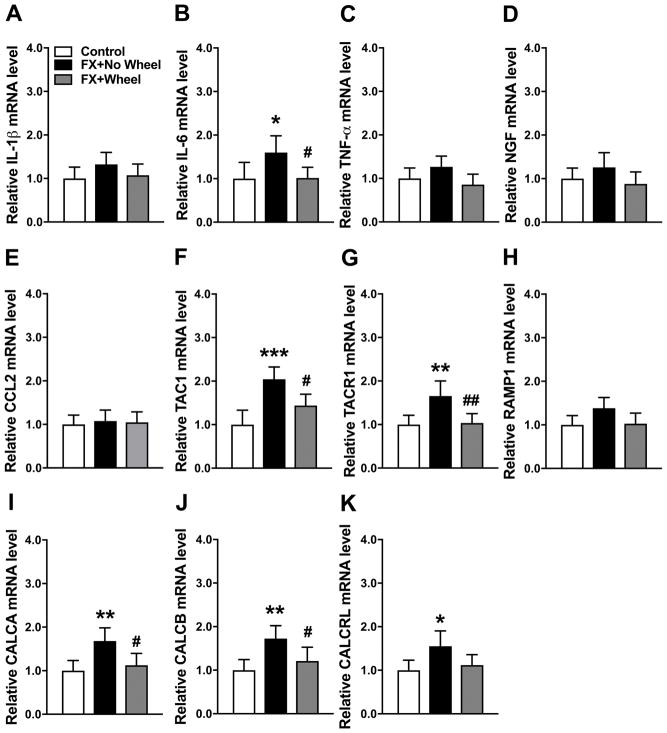
A cohort of fracture mice were treated with 4 weeks ad lib running wheel exercise (between post fracture weeks 3–7), then the running wheels were removed from the cages for 2 weeks and the mice were retested and then sacrificed (9 weeks after fracture). Control fracture mice had no access to running wheels and were also sacrificed at 9 weeks after fracture. At 9 weeks post fracture neuropeptide and inflammatory mediator gene expression in the fracture limb hindpaw skin was measured by real time PCR (Fig. 6). Compared to the nonfracture control mice, only CCL2 (200%) mRNA levels were elevated at 9 weeks post fracture in the nonexercised fracture mice and 4 weeks of wheel running exercise persistently reversed this increase even after exercise had been stopped for 2 weeks (Fig. 6E). Interestingly, hindpaw skin mRNA levels for IL-6, TNF, NGF, TAC1, TACR1, RAMP1, CALCA, CALCB, and CALCRL were unchanged at 9 weeks post fracture in control mice that had no running wheel treatment, versus control nonfracture mice, but mice that had 4 weeks running wheel treatment and then 2 weeks no running wheel access had increased IL-6 (240%) and NGF (90%) mRNA levels in the hindpaw skin, relative to nonfracture control mice (Fig. 6B, D). Wheel running for 4 weeks and then no exercise for 2 weeks did not affect the expression of TNF, TAC1, TACR1, RAMP1, CALCA, CALCB, and CALCRL mRNA in hindpaw skin (Fig. 6C, F–K).
Figure 6. The effects of stopping exercise on cutaneous gene expression of IL-6 and NGF.
Figure 1 illustrates the reoccurrence of allodynia and unweighting in exercised mice after stopping exercise for 2 weeks. In this experiment a cohort of fracture mice (FX+Wheel) were treated with 4 weeks ad lib access to a running wheel, starting at 3 weeks post fracture, then the wheel was removed for 2 weeks and then the animals sacrificed (at 9 weeks post fracture) and the skin collected. Control fracture mice not provided access to a running wheel (FX+No Wheel) were also sacrificed at 9 weeks post fracture and the skin collected. The expression of inflammatory mediators in the fracture limb hindpaw skin were measured by real-time PCR. Only CCL2 (E) gene expression was up-regulated at 9 weeks post fracture (FX+No Wheel), compared to nonfractured control mice (Control), and this increase was reversed in FX mice provided with 4 weeks of ad lib access to a running wheel (FX + Wheel), starting at 3 weeks post fracture and this effect persisted after stopping exercise for 2 weeks (week 9 post fracture). At 9 weeks post fracture (FX+No Wheel) hindpaw skin IL-1β (A), IL-6 (B), TNF-α (C), NGF (D), TAC1 (F), TACR1 (G), RAMP1 (H), CALCA (I), CALCB (J), and CALCRL (K) mRNA levels did not significantly differ from control nonfracture mice (Control). Stopping exercise for 2 weeks resulted in increased cutaneous IL-6 (B) and NGF (D) mRNA levels at 9 weeks post fracture, but did not affect the expression of IL-1β, IL-6, TNF-α, NGF, TAC1, TACR1, RAMP1, CALCA, CALCB, or CALCRL mRNA. Values are means ± SD, n=8. One-way analysis of variance with Bonferroni post hoc testing. * P< 0.05, ** P< 0.01, *** P< 0.001 for FX+No Wheel or FX + Wheel vs Control, # P< 0.05, ## P< 0.01, ### P< 0.001 for FX + Wheel vs FX+No Wheel.
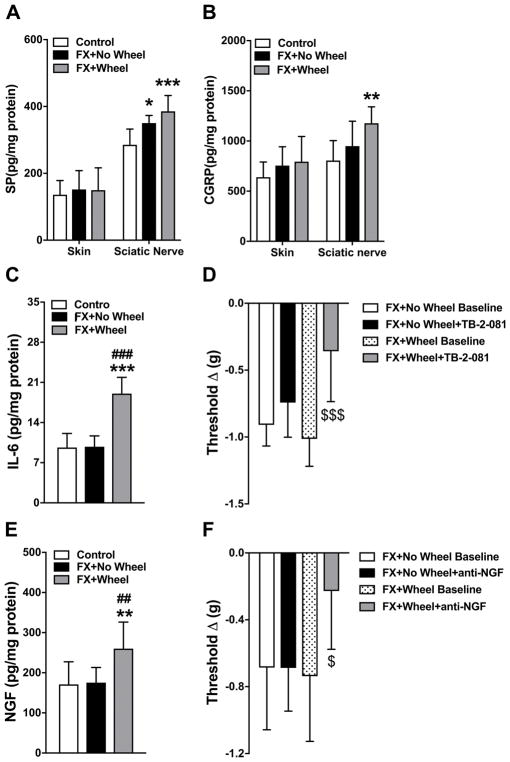
Protein levels for SP and CGRP in the sciatic nerve and for IL-6 and NGF in the hindpaw skin were also evaluated by EIA. At 9 weeks post fracture sciatic nerve SP levels were increased vs controls (Control: 285 ± 47 vs FX+No Wheel: 350 ± 22, P=0.013), but CGRP (Control: 805 ± 199 vs FX+No Wheel: 949 ± 248, P=0.529) levels were not increased at 9 weeks post fracture vs controls (Fig. 7A, B). When fracture mice were allowed to run for 4 weeks and then stopped exercising for 2 weeks there was an increase in sciatic nerve SP (Control: 285 ± 47 vs FX+Wheel; 386 ± 47 pg/mg, P<0.001) and CGRP protein levels (Control: 805 ± 199 vs FX+Wheel; 1177 ± 164 pg/mg, P=0.005) vs controls (Fig. 7A, B). Similar to gene expression, IL-6 and NGF protein levels were not increased at 9 weeks post fracture vs control levels (Fig. 7C, E), but in the fracture mice who ran for 4 weeks and then stopped exercising for 2 weeks there was an increase in IL-6 (Control: 9.6 ± 2.4 vs FX+Wheel; 19.0 ± 2.8 pg/mg, P=0.001, FX+No Wheel: 9.8 ± 1.9 vs FX+Wheel; 19.0 ± 2.8 pg/mg, P<0.001) and NGF (Control: 171 ± 56 vs FX+Wheel; 260 ± 66 pg/mg, P=0.001 and FX+No Wheel: 175 ± 3.8 vs FX+Wheel; 260 ± 66 pg/mg, P=0.002) protein levels in the skin. Postulating that IL-6 and NGF up-regulation in the fracture hindpaw contributed to the rekindling of nociceptive sensitization after stopping exercise, the effects of a single injection of an IL-6 receptor antagonist (TB-2-081) or an anti-NGF antibody were tested in the 9 week fracture mice. The 9 week post fracture mice not treated with wheel exercise had no change in hindpaw allodynia after TB-2-081 or anti-NGF antibody injections, but the 9 week post fracture wheel treated mice with rekindled nociceptive sensitivity after stopping exercise had reduced allodynia after TB-2-081 (FX+Wheel Baseline: −1.0 ± 0.2 vs FX+Wheel+TB-2-081: −0.4 ± 0.4 Δg, P<0.001) or anti-NGF antibody (FX+Wheel Baseline: −0.7 ± 0.4 vs FX+Wheel+anti-NGF: −0.2 ± 0.3 Δg, P=0.027) injections (Fig. 7D, F).
Figure 7. The effects of stopping exercise on sciatic nerve expression of SP and CGRP and the cutaneous pronociceptive mediators IL-6 and NGF.
Figure 1 illustrates the reoccurrence of allodynia and unweighting in exercised mice after stopping exercise for 2 weeks. In these experiments a cohort of fracture mice (FX+Wheel) were treated with 4 weeks ad lib access to a running wheel, starting at 3 weeks post fracture, then the wheel was removed for 2 weeks and then the animals sacrificed (at 9 weeks post fracture) and the sciatic nerve and skin collected. Control fracture mice not provided access to a running wheel (FX+No Wheel) were sacrificed (at 9 weeks post fracture) and the tissues collected. Sciatic nerve SP and CGRP proteins and hindpaw skin IL-6 and NGF proteins were measured by EIA. Neither SP (A), CGRP (B), IL-6 (C), or NGF (E) protein were up-regulated at 9 weeks post fracture in the untreated fracture mice (FX+No Wheel), compared to nonfractured control mice (Control). Stopping exercise for 2 weeks in the wheel treated fracture mice caused an increased expression of SP (A), CGRP (B), IL-6 (C), and NGF (E) proteins at 9 weeks post fracture. At 9 weeks post fracture there were no differences in baseline hindpaw mechanical allodynia (von Frey thresholds) between fracture mice that were treated with wheel exercise for 4 week and then the wheel was removed for 2 weeks and fracture mice that were never given wheel access (D,F). When either an IL-6 receptor antagonist (TB-2-081) or an anti-NGF antibody (anti-NGF) was injected into the 9 week fracture mice, there was no effect in the fracture mice that had not previously been given wheel access, but both drugs reversed mechanical allodynia in fracture mice that had been given 4 weeks running exercise and then the exercise was discontinued for 2 weeks. Values are means ± SD, n=8. One-way analysis of variance with Bonferroni post hoc testing. * P< 0.05, ** P< 0.01, *** P< 0.001 for FX+No Wheel or FX+Wheel vs Control, # P< 0.05, ## P< 0.01, ### P< 0.001 for FX+Wheel vs FX+No Wheel, $ P< 0.05, $$$ P< 0.001 for FX+Wheel Baseline vs FX+Wheel+TB-2-081.
3.6 Exercise induced inhibition of post fracture up-regulated IL-6, TAC1, TACR1, CALCA, CALCB, and CALCRL mRNA expression in the spinal cord persisted after stopping exercise
At 9 weeks post fracture neuropeptide and inflammatory mediator gene expression in the lumbar cord innervating the fracture limb was measured by real time PCR (Fig. 8). IL-6 (60%), TAC1 (100%), TACR1 (70%), CALCA (70%), CALCB (70%), and CALCRL (60%) mRNA levels were elevated at 9 weeks post fracture in mice that had no running wheel treatment, versus control nonfracture mice, but the fracture mice who ran for 4 weeks and then stopped exercise for 2 weeks had persistent reversal of post fracture up-regulated neuropeptide and inflammatory mediator expression (Fig. 8B, F, G, I–K).
Figure 8. The effects of stopping exercise on post fracture gene expression of spinal cord inflammatory mediators.
Figure 1 illustrates the reoccurrence of allodynia and unweighting in exercised mice after stopping exercise for 2 weeks. In this experiment a cohort of fracture mice (FX+Wheel) were treated with 4 weeks ad lib access to a running wheel, starting at 3 weeks post fracture, then the wheel was removed for 2 weeks and then the animals sacrificed (9 weeks post fracture) and the skin collected. Control fracture mice not provided access to a running wheel (FX+No Wheel) were sacrificed (9 weeks post fracture) and the skin collected. Inflammatory mediator expression in the lumbar cord innervating the fracture limb was measured by real-time PCR. IL-6 (B), TAC1 (F), TACR1(G), CALCA (I), CALCB (J), and CALCRL (K) mRNA levels were up-regulated at 9 weeks post fracture, compared to nonfractured control mice. All these increases in inflammatory mediator gene express were reversed by 4 weeks wheel running (Fig. 5) and this reversal persisted after stopping wheel running. There were no changes in the hindpaw skin expression of IL-1β (A), TNF-α (C), NGF (D), CCL2 (E), and RAMP1 (H) at 9 weeks post fracture (no wheel), compared to nonfracture control mice, and exercise had no effects on the 9 weeks post fracture expression of these genes. Values are means ± SD, n=8 per cohort. One-way analysis of variance with Bonferroni post hoc testing. * P< 0.05, ** P< 0.01, *** P< 0.001 for FX+No Wheel or FX + Wheel vs Control, # P< 0.05, ## P< 0.01, ### P< 0.001 for FX + Wheel vs FX+No Wheel.
3.7 Exercise reversed post fracture anxiety and working memory impairment
Consistent with our previous results in fracture mice,14 the distance traveled in the open field assay did not significantly differ between the no exercise fracture mice and the no fracture controls at 7 or 9 weeks post fracture (Fig. 9A,B). However, fracture mice having access to the running wheel for 4 weeks displayed significant increases in distance traveled compared to their no exercise fracture counterparts (week 7: 21.7 ± 4.1 vs 16.2 ± 4.5 meters, P=0.011). At 2 weeks after stopping exercise, locomotor activity in the exercised fracture mice returned to levels similar to those observed in the control or no exercise fracture groups (Fig. 9A,B). Similarly, there were no significant differences between the no exercise fracture mice and controls in the time spent in the center of the open field assay at 7 or 9 weeks (Fig. 9C,D). The exercised nonfracture controls (week 7: 21.2 ± 10.6 vs 7.8 ± 9.4 seconds, P=0.036 ) and the exercised fracture mice (week 7: 19.3.2 ± 11.7 vs 6.8 ± 11.7 seconds, P=0.017) displayed increased center time compared to the no exercise fracture mice or controls, but after stopping exercise for 2 weeks this effect resolved (Fig. 9C,D).
Previously we noted decreased risk taking behavior in fracture mice using the zero maze assay,14 and we observed similar results in the no exercise fracture mice. At 7 and 9 weeks after fracture the no exercise fracture mice were less likely to enter the open arms of the maze compared to nonfractured controls (week 7: 8.0 ± 3.5 vs 13.4 ± 2.1 bouts, P=0.004, and week 9: 5.3 ± 2.6 vs 9.5 ± 2.9 bouts, P=0.004), consistent with neophobic anxiety (Fig. 9E,F). Exercising the fracture mice for 4 weeks increased the number of entries into the open arms of the maze (week 7: 13.3 ± 5.9 vs 8.0 ± 3.5 bouts, P=0.048), but after stopping exercise for 2 weeks this effect resolved.
Similar to our previous findings in post fracture mice,14 the no exercise fracture mice had impaired object recognition working memory at both 7 weeks (FX+No Wheel: 39 ± 12% familiar vs 60 ± 12% novel, P=1.000) and 9 weeks (FX+No Wheel: 43 ± 17% familiar vs 57 ± 17% novel, P=0.109) after fracture (Fig. 10A,B), and this impairment was reversed after wheel exercise at both 7 weeks (FX+Wheel: 35 ± 20% familiar vs 65 ± 20% novel, P=0.001) and 9 weeks (FX+Wheel: 25 ± 16% familiar vs 75 ± 16% novel, P<0.001 after fracture. Unlike the transient effects of exercise on reducing anxiety in fracture mice, working memory improvements were still present 2 weeks after stopping exercise (Fig. 10A,B).
4. Discussion
The tibia fracture mice exhibited hindpaw allodynia, unweighting, warmth, and edema (Fig. 1), and these pain behaviors spontaneously resolve over a 4-month period and the inflammatory symptoms recover within 6 weeks.13 Four weeks of ad lib wheel running, starting at the time of cast removal, accelerated the resolution of allodynia, unweighting, warmth, and edema, but when exercise was stopped for 2 weeks the pain behaviors were rekindled (Fig. 1). Four weeks of ad lib wheel running (gradually increasing to 5.6 km/day) had the same effect as running 0.5 km/day for 4 weeks (Fig. 1). Rodent exercise studies almost uniformly show analgesic effects in a variety of pain models using various types and intensities of exercise.22–27 Some studies have reported reoccurrence and others observed no reoccurrence of neuropathic pain after stopping exercise.25,26 No prior exercise studies have utilized the TFM or examined exercise effects on skin, nerve, and spinal cord neuropeptide or cytokine expression.
Neurogenic inflammation is mediated by neuronal release of SP and CGRP, neurotransmitters that activate their vascular receptors to induce extravasation and vasodilatation. Electrically evoked extravasation and vasodilatation responses are enhanced in CRPS patients, and when SP is microdialyzed in CRPS skin there is an exaggerated extravasation response.28,29 Furthermore, serum levels of SP and CGRP are elevated in CRPS patients.30–32 Similarly, in the TFM there is increased SP and CGRP expression in the sciatic nerve, spinal cord, and serum, up-regulated SP NK1 receptor expression in endothelial cells and keratinocytes in hindpaw skin and in spinal cord homogenates, and enhanced SP evoked extravasation and edema responses in the fracture limb.33,34 Systemic treatment with an SP receptor antagonist reduced hindpaw allodynia, warmth, and edema in fracture rats, and intrathecal treatment with an SP or CGRP receptor antagonist reduced nociceptive sensitization.16,34,35 Additionally, at 3 weeks post fracture wildtype mice exhibited hindpaw allodynia, unweighting, warmth, and edema, but in SP deficient fracture mice allodynia and unweighting were attenuated and there was no warmth and edema.13 Fracture mice lacking the CGRP RAMP1 receptor had a similar presentation. These data support the hypothesis that neuropeptide signaling is amplified in the affected skin, vasculature, and spinal cord after fracture, as well as in CRPS patients, and that this facilitated signaling contributes to the development of CRPS-like changes.
Exercise effects on neuropeptide signaling in the fracture hindlimb and spinal cord were evaluated. There was no post fracture change in the expression of the SP gene (TAC1), the SP neurokinin 1 (NK1) receptor gene (TACR1), the alpha CGRP gene (CALCA) and beta CGRP gene (CALCB), the calcitonin receptor-like receptor (CRLR) gene (CALCLR), and the CGRP receptor activity-modifying protein (RAMP1) gene (RAMP1) in the ipsilateral hindpaw skin and DRGs of unexercised 7 week fracture mice (Figs. 2, 3, 4). The spontaneous resolution of facilitated neuropeptide signaling in the injured hindlimb at 7 weeks post fracture is consistent with the temporal resolution of up-regulated neuropeptide signaling in the injured limb observed between 4 and 16 weeks after fracture in rats.17,33 The spinal expression of TAC1, CALCA, CALCB, and RAMP1 was up-regulated at 7 weeks post fracture (Fig. 5), exercise inhibited this up-regulation (Fig. 5), and this inhibitory effect persisted even after stopping exercise for 2 weeks (Fig. 8).
When SP or CGRP is microdialyzed into human skin there is no immediate pain, supporting the premise that SP and CGRP act as intermediate mediators in development of inflammatory pain.36 When applied to keratinocytes in vitro, SP and CGRP stimulated expression of TNF, IL-1, IL-6, and NGF.37 Plantar injection of SP induced a sequential increase in keratinocyte expression of TNF, IL-1, IL-6, and NGF in rat skin in vivo that resolved after 48 hours, temporally correlating with the development and resolution of allodynia.38 Furthermore, at 3 weeks post fracture mice expressed elevated levels of TNF, IL-1, IL-6, CCL2, and NGF in the hindpaw skin and spinal cord, but in SP and CGRP RAMP1 receptor deficient mice the post fracture pain behaviors were attenuated and only IL-6 levels were increased in the hindpaw skin.13,34 These results demonstrate that neuropeptide signaling can evoke keratinocyte and spinal cord expression of inflammatory mediators in vitro and in vivo.
At 3–4 weeks after fracture in mice and rats there is increased expression of TNF, IL-1, IL-6, NGF, and CCL2 mRNA and protein in the ipsilateral skin and spinal cord.13,15,17,33,34,39,40 Keratinocytes are the primary cellular source of these inflammatory mediators in the fracture hindpaw skin41 and in CRPS affected skin.42 Spinal cord microglia and astrocytes have been identified as the primary source of spinal inflammatory mediators in a variety of chronic pain models43 and both microglia and astrocytes are chronically activated in the TFM35. Furthermore, intraplantar injection of each of these inflammatory mediators into normal paw skin rapidly induced prolonged sensitization.41,44 Systemic or intrathecal treatment with inhibitors/antagonists for TNF, IL-1, IL-6, NGF, or CCL2 all reduced hindpaw allodynia and unweighting in 4-week fracture rats, indicating a role for cytokine and growth factor signaling in post fracture sensitization.34,40,44,45 Collectively, these data suggest that inflammatory mediators can act at the cutaneous nociceptor and spinal cord levels to induce post fracture nociceptive sensitization.
Previously we observed that TNF, IL-1, and IL-6 and NGF protein levels in the hindpaw skin and spinal cord were elevated at 4 weeks, but by 16 weeks post fracture cutaneous inflammatory mediator levels returned to baseline and spinal levels of TNF, IL-1, and NGF were persistently elevated.17 In addition, intrathecal, but not systemic injection of an IL-1 receptor antagonist or anti-NGF antibody reduced nociceptive behaviors at 16 weeks. These results indicate that fracture caused increased peripheral and central inflammatory mediator production acutely, but that spinal inflammation may be more important for persistent nociceptive sensitization in the TFM.
The current study examined the effects of 4 weeks ad lib wheel running on inflammatory mediator expression in the ipsilateral hindpaw skin and lumbar spinal cord of fracture mice. The 7-week post fracture mice that were not exercised had increased IL-1 and CCL2 mRNA and protein levels in the hindpaw skin (Figs. 2,3) and increased IL1, IL-6, TNF, and CCL2 mRNA levels in the spinal cord (Fig. 5). Four weeks of daily wheel running completely reversed the post fracture increases in skin and cord inflammatory mediators (Figs. 2,3,4) and resolved hindpaw allodynia and unweighting (Fig. 1), indicating that daily exercise can effectively alleviate inflammation and pain behaviors in this CRPS model. Other investigators have observed exercise inhibition of microglia and astrocyte proliferation in mouse neuropathic pain models23,26,46 and we postulate that exercise induced glial inhibitory effects in the TFM may mediate the reversal of post fracture spinal inflammatory mediator expression.
Stopping exercise for 2 weeks induced increased sciatic nerve SP and CGRP protein levels, up-regulated expression of IL-6 and NGF mRNA and protein in the hindpaw skin, and triggered the reoccurrence of allodynia and unweighting in the fracture limb (Figs. 1,6,7), without any change in spinal cord inflammatory mediator expression (Fig. 8). When either an IL-6 receptor antagonist (TB-2-081) or an anti-NGF antibody (anti-NGF) was injected into exercised fracture mice that had stopped exercising their hindpaw allodynia was reversed (Fig. 7). These results suggest that after stopping exercise hindpaw sciatic nerve neuropeptide signaling was up-regulated and cutaneous inflammation was rekindled, resulting in nociceptive sensitization. The fracture mice that were not exercised also had nociceptive sensitization at 9 weeks post fracture, but there was no up-regulation of cutaneous or spinal inflammatory mediators and NGF and IL-6 inhibitors/antagonists had no effect on this sensitization (Figs. 1, 7). We postulate that this reflects the spontaneous resolution of innate immune pronociceptive mechanisms by 9 weeks post fracture in nonexercised mice and that from 9–20 weeks post fracture the only pronociceptive mechanisms mediating sensitization in nonexercised mice are the adaptive autoimmune mechanisms recently identified in this model.47
Exercise also reduced anxiety on the open field and zero maze assays, but these effects were lost after stopping exercise for 2 weeks (Fig. 9). Post fracture object recognition memory impairment was also improved with exercise, and this effect persisted after stopping exercise (Fig. 10). Previously we observed similar anxiety-related behaviors and impairment in novel object recognition in 7–9 week post fracture mice, as well as structural changes and synaptic plasticity in the brain.14 Correspondingly, decreased memory, global cognitive impairments, and increased anxiety frequently occur in CRPS patients.48,49 Collectively, these results suggest that daily exercise could potentially ameliorate the CRPS pain experience by modifying its associated cognitive and emotional comorbidities.
In conclusion, daily exercise reversed the up-regulation of neuropeptide and inflammatory mediator expression in skin and spinal cord, as well as the pain behaviors, anxiety, and memory impairments observed in the tibia fracture mouse model of CRPS. The current study has several limitations, including those inherent to using animal models of pain and the translational value of the data acquired. Clinical investigations are required to determine whether exercise can inhibit up-regulated neuropeptide signaling and inflammatory mediator expression in skin and spinal cord and reverse nociceptive sensitization, anxiety and memory impairment in CRPS patients.
Table 2.
Exercise effects on post fracture up-regulation of neuropeptide signaling proteins and inflammatory mediators in skin, nerve, and spinal cord
| Neuropeptide Signaling | Cytokines and NGF | |||
|---|---|---|---|---|
|
| ||||
| 7 Weeks after fracture | 9 weeks after fracture | 7 Weeks after fracture | 9 weeks after fracture | |
|
| ||||
| Skin |
Skin mRNA/protein levels after fracture: No change in TAC1/SP, TACR1, RAMP1, CALCA/CGRP, CALCB/CGRP, CALCRL levels. |
Skin mRNA levels after fracture: No change in TAC1, TACR1, RAMP1, CALCA, CALCB, CALCRL levels. |
Skin mRNA/protein levels after fracture: ↑IL-1/IL-1, ↑CCL2/CCL2, and no change in IL-6/IL-6, TNF/TNF, NGF/NGF levels. |
Skin mRNA/protein levels after fracture: ↑CCL2 and no change in IL-1, IL-6/IL-6, TNF, NGF/NGF levels. |
| Exercise (weeks 3–7) had no effect on TAC1/SP, TACR1, RAMP1, CALCA/CGRP, CALCB/CGRP, CALCRL levels. | Exercise (weeks 3–7) had no effect on TAC1, TACR1, RAMP1, CALCA, CALCB, CALCRL levels. | Exercise (weeks 3–7) reversed the increase in IL-1/IL-1 and CCL2/CCL2 levels. | Exercise (weeks 3–7) caused an increase in IL-1/IL-1 and NGF/NGF levels. | |
|
| ||||
| Sciatic nerve |
Sciatic nerve protein levels after fracture: No change in SP or CGRP levels. |
Sciatic nerve protein levels after fracture: No change in SP or CGRP levels. |
||
| Exercise (weeks 3–7) had no effect on SP and CGRP levels. | Exercise (weeks 3–7) caused increased SP and CGRP levels. | |||
|
| ||||
| DRG |
DRG mRNA levels after fracture: No change in TAC1, CALCA, or CALCB levels. |
|||
| Exercise had no effect on TAC1, CALCA, or CALCB levels | ||||
|
| ||||
| Spinal Cord |
Cord mRNA levels after fracture: ↑TAC1, ↑RAMP1, ↑CALCA, ↑CALCB, and no change in TACR1 or CALCRL levels. |
Cord mRNA levels after fracture: ↑TAC1, ↑TACR1, ↑CALCA, ↑CALCB, ↑CALCRL, and no change in RAMP1 levels. |
Cord mRNA levels after fracture: ↑IL-1, ↑IL-6, ↑TNF, and ↑CCL2, no change in NGF levels. |
Cord mRNA levels after fracture: ↑IL-6, no change in IL-1, TNF, NGF, CCL2 levels. |
| Exercise (weeks 3–7) reversed the increase in TAC1, RAMP1, CALCA, and CALCB levels. | Exercise (weeks 3–7) reversed the increase in TAC1, TACR1, CALCA, CALCB, and CALCRL levels. | Exercise (weeks 3–7) reversed the increase in IL-1, IL-6, TNF, and CCL2 levels. | Exercise (weeks 3–7) reversed the increase in IL-6 levels. | |
CALCA: the CGRP calcitonin related polypeptide α gene, CALCB: the CGRP calcitonin related polypeptide β gene, CALCRL: the CRLR calcitonin gene-related peptide type 1 receptor precussor gene, CCL2: C-C motif chemokine 2 (MCP 1), CGRP: calcitonin gene-related peptide, CRLR: the CGRP calcitonin receptor-like receptor, IL-1: interleukin 1β, IL-6: interleukin 6, NGF: nerve growth factor, RAMP1: the CGRP receptor activity modifying protein 1 co-receptor, SP: substance P, TAC1: the SP tachykinin precursor 1 gene, TACR1: the tachykinin 1 receptor gene, TNF: tumor necrosis factor α
Acknowledgments
Funding Statement: This study was supported by the National Institutes of Health grants NS072143 and NS094438, the Department of Veterans Affairs, Rehabilitation Research and Development Merit grant I01RX001475, and the Intramural Research Programs of the National Institute on Drug Abuse and the National Institute on Alcoholism and Alcohol Abuse.
Footnotes
Conflicts of Interest: The authors do not have financial or other relationships that might lead to conflict of interest.
References
- 1.Treede RD, Rief W, Barke A, Aziz Q, Bennett MI, Benoliel R, Cohen M, Evers S, Finnerup NB, First MB, Giamberardino MA, Kaasa S, Kosek E, Lavand’homme P, Nicholas M, Perrot S, Scholz J, Schug S, Smith BH, Svensson P, Vlaeyen JW, Wang SJ. A classification of chronic pain for ICD-11. Pain. 2015;156:1003–7. doi: 10.1097/j.pain.0000000000000160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Correll D. Chronic postoperative pain: recent findings in understanding and management. F1000Res. 2017;6:1054. doi: 10.12688/f1000research.11101.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Macrae WA. Chronic post-surgical pain: 10 years on. Br J Anaesth. 2008;101:77–86. doi: 10.1093/bja/aen099. [DOI] [PubMed] [Google Scholar]
- 4.Corradini C, Bosizio C, Moretti A. Algodystrophy (CRPS) in minor orthopedic surgery. Clin Cases Miner Bone Metab. 2015;12:21–5. doi: 10.11138/ccmbm/2015.12.3s.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pons T, Shipton EA, Williman J, Mulder RT. Potential risk factors for the onset of complex regional pain syndrome type 1: a systematic literature review. Anesthesiol Res Pract. 2015;2015:956539. doi: 10.1155/2015/956539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allen G, Galer BS, Schwartz L. Epidemiology of complex regional pain syndrome: a retrospective chart review of 134 patients. Pain. 1999;80:539–44. doi: 10.1016/S0304-3959(98)00246-2. [DOI] [PubMed] [Google Scholar]
- 7.Schwartzman RJ, Kerrigan J. The movement disorder of reflex sympathetic dystrophy. Neurology. 1990;40:57–61. doi: 10.1212/wnl.40.1.57. [DOI] [PubMed] [Google Scholar]
- 8.Oerlemans HM, Oostendorp RA, de Boo T, van der Laan L, Severens JL, Goris JA. Adjuvant physical therapy versus occupational therapy in patients with reflex sympathetic dystrophy/complex regional pain syndrome type I. Arch Phys Med Rehabil. 2000;81:49–56. [PubMed] [Google Scholar]
- 9.Smart KM, Wand BM, O’Connell NE. Physiotherapy for pain and disability in adults with complex regional pain syndrome (CRPS) types I and II. Cochrane Database Syst Rev. 2016;2:CD010853. doi: 10.1002/14651858.CD010853.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Terkelsen AJ, Bach FW, Jensen TS. Experimental forearm immobilization in humans induces cold and mechanical hyperalgesia. Anesthesiology. 2008;109:297–307. doi: 10.1097/ALN.0b013e31817f4c9d. [DOI] [PubMed] [Google Scholar]
- 11.de Mos M, de Bruijn AG, Huygen FJ, Dieleman JP, Stricker BH, Sturkenboom MC. The incidence of complex regional pain syndrome: a population-based study. Pain. 2007;129:12–20. doi: 10.1016/j.pain.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 12.Sandroni P, Benrud-Larson LM, McClelland RL, Low PA. Complex regional pain syndrome type I: incidence and prevalence in Olmsted county, a population-based study. Pain. 2003;103:199–207. doi: 10.1016/s0304-3959(03)00065-4. [DOI] [PubMed] [Google Scholar]
- 13.Guo TZ, Wei T, Shi X, Li WW, Hou S, Wang L, Tsujikawa K, Rice KC, Cheng K, Clark DJ, Kingery WS. Neuropeptide deficient mice have attenuated nociceptive, vascular, and inflammatory changes in a tibia fracture model of complex regional pain syndrome. Mol Pain. 2012;8:85. doi: 10.1186/1744-8069-8-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tajerian M, Leu D, Zou Y, Sahbaie P, Li W, Khan H, Hsu V, Kingery W, Huang TT, Becerra L, Clark JD. Brain neuroplastic changes accompany anxiety and memory deficits in a model of complex regional pain syndrome. Anesthesiology. 2014;121:852–65. doi: 10.1097/ALN.0000000000000403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guo TZ, Wei T, Li WW, Li XQ, Clark JD, Kingery WS. Immobilization contributes to exaggerated neuropeptide signaling, inflammatory changes, and nociceptive sensitization after fracture in rats. J Pain. 2014;15:1033–45. doi: 10.1016/j.jpain.2014.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo TZ, Offley SC, Boyd EA, Jacobs CR, Kingery WS. Substance P signaling contributes to the vascular and nociceptive abnormalities observed in a tibial fracture rat model of complex regional pain syndrome type I. Pain. 2004;108:95–107. doi: 10.1016/j.pain.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 17.Wei T, Guo TZ, Li WW, Kingery WS, Clark JD. Acute versus chronic phase mechanisms in a rat model of CRPS. J Neuroinflammation. 2016;13:14. doi: 10.1186/s12974-015-0472-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poree LR, Guo TZ, Kingery WS, Maze M. The analgesic potency of dexmedetomidine is enhanced after nerve injury: a possible role for peripheral alpha2-adrenoceptors. Anesth Analg. 1998;87:941–948. doi: 10.1097/00000539-199810000-00037. [DOI] [PubMed] [Google Scholar]
- 19.Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur J Pharmacol. 2003;463:3–33. doi: 10.1016/s0014-2999(03)01272-x. [DOI] [PubMed] [Google Scholar]
- 20.Shepherd JK, Grewal SS, Fletcher A, Bill DJ, Dourish CT. Behavioural and pharmacological characterisation of the elevated “zero-maze” as an animal model of anxiety. Psychopharmacology (Berl) 1994;116:56–64. doi: 10.1007/BF02244871. [DOI] [PubMed] [Google Scholar]
- 21.Mumby DG, Gaskin S, Glenn MJ, Schramek TE, Lehmann H. Hippocampal damage and exploratory preferences in rats: memory for objects, places, and contexts. Learn Mem. 2002;9:49–57. doi: 10.1101/lm.41302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chuganji S, Nakano J, Sekino Y, Hamaue Y, Sakamoto J, Okita M. Hyperalgesia in an immobilized rat hindlimb: effect of treadmill exercise using non-immobilized limbs. Neurosci Lett. 2015;584:66–70. doi: 10.1016/j.neulet.2014.09.054. [DOI] [PubMed] [Google Scholar]
- 23.Lopez-Alvarez VM, Modol L, Navarro X, Cobianchi S. Early increasing-intensity treadmill exercise reduces neuropathic pain by preventing nociceptor collateral sprouting and disruption of chloride cotransporters homeostasis after peripheral nerve injury. Pain. 2015;156:1812–25. doi: 10.1097/j.pain.0000000000000268. [DOI] [PubMed] [Google Scholar]
- 24.Luan S, Wan Q, Luo H, Li X, Ke S, Lin C, Wu Y, Wu S, Ma C. Running exercise alleviates pain and promotes cell proliferation in a rat model of intervertebral disc degeneration. Int J Mol Sci. 2015;16:2130–44. doi: 10.3390/ijms16012130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stagg NJ, Mata HP, Ibrahim MM, Henriksen EJ, Porreca F, Vanderah TW, Philip Malan T., Jr Regular exercise reverses sensory hypersensitivity in a rat neuropathic pain model: role of endogenous opioids. Anesthesiology. 2011;114:940–8. doi: 10.1097/ALN.0b013e318210f880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Almeida C, DeMaman A, Kusuda R, Cadetti F, Ravanelli MI, Queiroz AL, Sousa TA, Zanon S, Silveira LR, Lucas G. Exercise therapy normalizes BDNF upregulation and glial hyperactivity in a mouse model of neuropathic pain. Pain. 2015;156:504–13. doi: 10.1097/01.j.pain.0000460339.23976.12. [DOI] [PubMed] [Google Scholar]
- 27.Pitcher MH, Tarum F, Rauf IZ, Low LA, Bushnell C. Modest Amounts of Voluntary Exercise Reduce Pain- and Stress-Related Outcomes in a Rat Model of Persistent Hind Limb Inflammation. J Pain. 2017;18:687–701. doi: 10.1016/j.jpain.2017.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weber M, Birklein F, Neundorfer B, Schmelz M. Facilitated neurogenic inflammation in complex regional pain syndrome. Pain. 2001;91:251–7. doi: 10.1016/S0304-3959(00)00445-0. [DOI] [PubMed] [Google Scholar]
- 29.Leis S, Weber M, Isselmann A, Schmelz M, Birklein F. Substance-P-induced protein extravasation is bilaterally increased in complex regional pain syndrome. Exp Neurol. 2003;183:197–204. doi: 10.1016/s0014-4886(03)00163-8. [DOI] [PubMed] [Google Scholar]
- 30.Schinkel C, Gaertner A, Zaspel J, Zedler S, Faist E, Schuermann M. Inflammatory mediators are altered in the acute phase of posttraumatic complex regional pain syndrome. Clin J Pain. 2006;22:235–9. doi: 10.1097/01.ajp.0000169669.70523.f0. [DOI] [PubMed] [Google Scholar]
- 31.Birklein F, Schmelz M, Schifter S, Weber M. The important role of neuropeptides in complex regional pain syndrome. Neurology. 2001;57:2179–84. doi: 10.1212/wnl.57.12.2179. [DOI] [PubMed] [Google Scholar]
- 32.Blair SJ, Chinthagada M, Hoppenstehdt D, Kijowski R, Fareed J. Role of neuropeptides in pathogenesis of reflex sympathetic dystrophy. Acta Orthop Belg. 1998;64:448–51. [PubMed] [Google Scholar]
- 33.Wei T, Li WW, Guo TZ, Zhao R, Wang L, Clark DJ, Oaklander AL, Schmelz M, Kingery WS. Post-junctional facilitation of Substance P signaling in a tibia fracture rat model of complex regional pain syndrome type I. Pain. 2009;144:278–86. doi: 10.1016/j.pain.2009.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shi X, Guo TZ, Wei T, Li WW, Clark DJ, Kingery WS. Facilitated spinal neuropeptide signaling and upregulated inflammatory mediator expression contribute to postfracture nociceptive sensitization. Pain. 2015;156:1852–63. doi: 10.1097/j.pain.0000000000000204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li WW, Guo TZ, Shi X, Sun Y, Wei T, Clark DJ, Kingery WS. Substance P spinal signaling induces glial activation and nociceptive sensitization after fracture. Neuroscience. 2015;310:73–90. doi: 10.1016/j.neuroscience.2015.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weidner C, Klede M, Rukwied R, Lischetzki G, Neisius U, Skov PS. Acute effects of substance P and calcitonin gene-related peptide in human skin-a microdialysis study. J Invest Dermatol. 2000;115:1015–1020. doi: 10.1046/j.1523-1747.2000.00142.x. [DOI] [PubMed] [Google Scholar]
- 37.Shi X, Wang L, Clark JD, Kingery WS. Keratinocytes express cytokines and nerve growth factor in response to neuropeptide activation of the ERK1/2 and JNK MAPK transcription pathways. Regul Pept. 2013;186:92–103. doi: 10.1016/j.regpep.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wei T, Guo TZ, Li WW, Hou S, Kingery WS, Clark JD. Keratinocyte expression of inflammatory mediators plays a crucial role in substance P-induced acute and chronic pain. J Neuroinflammation. 2012;9:181. doi: 10.1186/1742-2094-9-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang L, Guo TZ, Wei T, Li WW, Shi X, Clark JD, Kingery WS. Bisphosphonates Inhibit Pain, Bone Loss, and Inflammation in a Rat Tibia Fracture Model of Complex Regional Pain Syndrome. Anesth Analg. 2016;123:1033–45. doi: 10.1213/ANE.0000000000001518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gallagher JJ, Tajerian M, Guo T, Shi X, Li W, Zheng M, Peltz G, Kingery WS, Clark JD. Acute and chronic phases of complex regional pain syndrome in mice are accompanied by distinct transcriptional changes in the spinal cord. Mol Pain. 2013;9:40. doi: 10.1186/1744-8069-9-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li WW, Guo TZ, Li XQ, Kingery WS, Clark JD. Fracture induces keratinocyte activation, proliferation, and expression of pro-nociceptive inflammatory mediators. Pain. 2010;151:843–52. doi: 10.1016/j.pain.2010.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Birklein F, Drummond PD, Li W, Schlereth T, Albrecht N, Finch PM, Dawson LF, Clark JD, Kingery WS. Activation of cutaneous immune responses in complex regional pain syndrome. J Pain. 2014;15:485–95. doi: 10.1016/j.jpain.2014.01.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Watkins LR, Milligan ED, Maier SF. Glial proinflammatory cytokines mediate exaggerated pain states: implications for clinical pain. Adv Exp Med Biol. 2003;521:1–21. [PubMed] [Google Scholar]
- 44.Li WW, Sabsovich I, Guo TZ, Zhao R, Kingery WS, Clark JD. The role of enhanced cutaneous IL-1beta signaling in a rat tibia fracture model of complex regional pain syndrome. Pain. 2009;144:303–13. doi: 10.1016/j.pain.2009.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li W, Shi X, Wang L, Guo T, Wei T, Cheng K, Rice KC, Kingery WS, Clark JD. Epidermal adrenergic signaling contributes to inflammation and pain sensitization in a rat model of complex regional pain syndrome. Pain. 2013;154:1224–36. doi: 10.1016/j.pain.2013.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cobianchi S, Marinelli S, Florenzano F, Pavone F, Luvisetto S. Short- but not long-lasting treadmill running reduces allodynia and improves functional recovery after peripheral nerve injury. Neuroscience. 2010;168:273–87. doi: 10.1016/j.neuroscience.2010.03.035. [DOI] [PubMed] [Google Scholar]
- 47.Guo TZ, Shi X, Li WW, Wei T, Clark JD, Kingery WS. Passive transfer autoimmunity in a mouse model of complex regional pain syndrome. Pain. 2017;158:2410–2421. doi: 10.1097/j.pain.0000000000001046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Libon DJ, Schwartzman RJ, Eppig J, Wambach D, Brahin E, Peterlin BL, Alexander G, Kalanuria A. Neuropsychological deficits associated with Complex Regional Pain Syndrome. J Int Neuropsychol Soc. 2010;16:566–73. doi: 10.1017/S1355617710000214. [DOI] [PubMed] [Google Scholar]
- 49.Speck V, Schlereth T, Birklein F, Maihofner C. Increased prevalence of posttraumatic stress disorder in CRPS. Eur J Pain. 2017;21:466–473. doi: 10.1002/ejp.940. [DOI] [PubMed] [Google Scholar]