Abstract
Background
Recovery from pain after surgery is faster after cesarean delivery than after other abdominal procedures. We hypothesized that recovery in rats after surgery could be reversed by antagonism of spinal oxytocin or vasopressin receptors, that there may be a sex difference, and that spinal oxytocin innervation could change after surgery.
Methods
Male and female rats underwent partial spinal nerve ligation surgery. Effects of nonselective and selective oxytocin and vasopressin 1A receptor antagonists on mechanical hypersensitivity during partial recovery were assessed (n=8-14/group). Oxytocin immunoreactivity in the dorsal horn of the spinal cord (n=7-8/group) and mRNA expression for oxytocin-binding receptors in dorsal root ganglia and spinal cord (n=8/group) were measured.
Results
Intrathecal injection of oxytocin and vasopressin receptor antagonists were similarly effective at reducing withdrawal threshold (in all experiments from 22 [19,26] median [1st,3rd quartile]) g to 8.3 [6.4,12] g after injection) in both sexes while having no or minimal effects in animals without surgery. Oxytocin fiber immunoreactivity was 3-5 fold greater in lumbar than other regions of the spinal cord and was increased over 2-fold in lumbar cord ipsilateral to surgery. Injury was also associated with a 6.5-fold increase in oxytocin receptor and a 2-fold increase in vasopressin 1A receptor mRNA expression in the L4 dorsal root ganglion ipsilateral to surgery.
Discussion
These findings suggest that the capacity for oxytocin signaling in the spinal cord increases after surgery and that spinal oxytocin signaling plays ongoing roles in both sexes in recovery from mechanical hypersensitivity after surgery with known nerve injury.
Introduction
Better understanding of the processes regulating recovery from painful injury, including surgery, is critical to developing strategies to speed recovery and prevent chronic pain. In humans, the speed of recovery from pain and disability after major surgery varies greatly between individuals1 and factors including female sex and degree of nerve injury are consistently associated, although weakly, with likelihood of chronic pain after surgery.2,3 Similarly in rats, recovery from mechanical hypersensitivity after surgery varies considerably between individuals,4 although there is not a sex difference in the speed of recovery.5 A key goal of research in this area is to understand the mechanisms underlying this variability and risk factors in recovery from surgery.
Surgery induces sensitization of peripheral and central nervous system structures and circuits involved in pain transduction and transmission, leading to mechanical hypersensitivity in animals and humans that may partially underlie pain after surgery. The extent and duration of mechanical hypersensitivity in the first weeks after surgery correlate with presence of pain months and years later,6,7 yet mechanisms which regulate resolution of this hypersensitivity are not well described. Some studies in animals suggest that recovery from mechanical hypersensitivity may reflect a new balance between enhancement of both inhibition and facilitation rather than simply resolution of sensitization processes. For example, weeks after resolution from hypersensitivity induced by surgery or inflammation, acute blockade of spinal opioid8 or noradrenergic4 signaling results in renewed mechanical hypersensitivity. In the case of noradrenergic systems this enhanced inhibition during recovery reflects injury-induced increases in noradrenergic innervation of the region of the spinal cord receiving input from the injury9 and is associated with changes in G protein coupling of noradrenergic receptors.10 Destruction of spinal noradrenergic innervation results in slowing of resolution of mechanical hypersensitivity after surgery.4
The current study examines the role of oxytocin in recovery from hypersensitivity after surgery, and follows from clinical observations in obstetrics. Women following complicated vaginal or cesarean delivery have a remarkably low incidence of pain ascribed to the delivery itself one year later compared to other abdominal, pelvic, or perineal surgical procedures.11 In animals the speed of recovery from mechanical hypersensitivity following surgery with nerve injury is faster if the injury occurs in the immediate postpartum period,5 a time of upregulation in oxytocin signaling. Since this quicker recovery is abolished if the pups are separated from the dams immediately after birth and transiently reversed following weaning of pups or by intrathecal injection of atosiban, a nonselective antagonist of oxytocin and vasopressin 1A receptors,12 spinal oxytocin likely participates in this quicker recovery. Based on these and other studies of oxytocin in spinal pain neurotransmission, we examined the role of spinal oxytocin signaling in recovery from mechanical hypersensitivity outside the postpartum period, focusing on potential plasticity of oxytocin innervation as well as oxytocin and vasopressin 1A receptor expression and behavioral pharmacology of oxytocin signaling in both male and female rats.
Materials and Methods
Animals and general aspects of study design
A total of 120 male and 56 female Sprague-Dawley rats were obtained from Harlan Industries (Indianapolis, IN) for these studies. Animals were pair-housed under a standard 12:12 hour light-dark (light from 6 a.m. to 6 p.m.) cycle and provided with food and water ad libitum. All experiments were approved and executed in accordance to guidelines by the Institutional Animal Care and Use Committee at Wake Forest University (Winston Salem, NC, USA).
In all studies the primary outcome measures, minimum biologically meaningful difference, power analysis (based on published or pilot experiments) to detect this difference, and statistical analysis plan including definition of outliers for data exclusion were defined prior to experimentation. Animals were randomly allocated by cage-pair groups and all experiments were performed with investigator blinded to treatment group. In some studies, several cohorts were required, in which case each cohort contained all randomized groups.
L5 partial spinal nerve ligation (pSNL) surgery
Partial ligation of the L5 spinal nerve was performed as previously described in 7 week aged rats.13 Animals were anesthetized with 1.75-3% isoflurane in oxygen. A 2.5 cm incision was made lateral to the lumbar spine and the right L6-transverse process was removed to expose the L5 spinal nerve. One third of the L5 spinal nerve was ligated with 8-0 nylon suture (Ethicon, Cincinnati, OH, USA) under a dissecting microscope. To avoid paralysis, the L4 spinal nerve was left untouched. The skin was closed with 5-0 suture and treated with a topical antibiotic (neomycin, polymyxin and bacitracin, Johnson & Johnson, New Brunswick, NJ, USA). After surgery and emergence from anesthesia, animals were returned to paired housing. Animals were weighed regularly for 1 week following surgery and a 30-40 g weight gain was considered evidence of normal surgical recovery.
Behavioral assessments and intrathecal drug administration
Paw withdrawal threshold
Animals were placed in plastic chambers atop a mesh surface and were acclimated to the behavioral test room for 30 minutes. Paw withdrawal threshold was determined by applying calibrated von Frey filaments to the plantar hindpaw. Filaments (Touch Test sensory probes, Stoelting, Wood Dale, IL, USA) of increasing bending force (0.6, 1.0, 2.0, 4.0, 6.0, 8.0, 15.0 and 26.0 g) were applied on the footpad until the filament bent. Paw withdrawal response was defined as a brisk withdrawal of the hindpaw within 5 seconds of the filament’s application at bending force. If animals did not respond to the final 26.0 g filament, they were assigned a paw withdrawal threshold of 26.0 g. Paw withdrawal threshold was the force that resulted in a 50% probability of withdrawal as calculated using a previously described up-down method.14
Intrathecal antagonist studies during recovery from pSNL
Two independent experiments were performed: one in which animals were randomized to receive vehicle or one of two doses of atosiban and another in which animals were randomized to receive vehicle or one of two doses of an oxytocin and a vasopressin 1A receptor antagonist. The first experiment with atosiban was to test whether there was any behavioral effect of blocking both spinal oxytocin and vasopressin 1A receptors. Since there was an effect, the second experiment was performed to distinguish which of these receptors was responsible for the behavioral effect. Within each experiment there were 2 equally sized cohorts of male and of female no-surgery animals (no-surgery animals were age-matched to pSNL animals) and 2 other, equally sized cohorts of male and of female animals after pSNL. Animals were randomly allocated by cage-pair groups upon arrival to the pSNL or no-surgery group prior to behavioral testing. Any animal that had paw withdrawal threshold ≤13 g in the two measures prior to the day of surgery was excluded from the experiment. Animals underwent paw withdrawal threshold testing weekly.
To investigate the ongoing activity of spinal oxytocin and vasopressin 1A receptors during recovery from pSNL, intrathecal administration of receptor-selective antagonists was performed during a window of time of partial recovery, beginning 5 weeks after surgery and only when the paw withdrawal threshold ipsilateral to surgery had recovered to at least 13 g for 2 testing days in a row. Animals which did not demonstrate paw withdrawal threshold ≥13 g by 9 weeks were excluded. This time window and level of recovery were chosen based on prior experience with the pSNL model which indicated that about 80% of animals recover to this extent during this time window (historical data, not shown). We also excluded animals from further analysis that had not recovered to at least 13 g after the previous drug injection.
For antagonist studies, drugs were administered over a period of 2 weeks, with injections separated by 2-3 days. Intrathecal injections were performed percutaneously between the L6-7 vertebrae of the spine using a 30-gauge 1-inch needle under brief (5-10 minutes in total) 2% isoflurane anesthesia. Successful entry into the intrathecal space was evidenced by a tail flick response upon the insertion of the needle. All drugs were delivered in a volume of 12 μL sterile saline solution. Drug solutions were injected with a sterile needle and syringe. Paw withdrawal threshold was assessed prior to and 0.5, 1, 2, and 3 hours after injection.
Atosiban (Sigma-Aldrich, St. Louis, MO, USA)4 was dissolved in sterile saline before use and stored at 4°C. The low atosiban dose in this study was that previously determined to induce hypersensitivity after partial recovery from surgery in postpartum female rats.15 A 5-fold higher dose of atosiban was also studied after pilot studies showed no behavioral toxicity from this dose (data not shown). The selective oxytocin receptor antagonist (desGly-NH2,d(CH2)5[D-Tyr2, Thr4]OVT) and vasopressin 1A receptor antagonist (d(CH2)5[Tyr(Me)2, Dab5]AVP) were graciously provided by Dr. Maurice Manning (Professor of Biochemistry and Cancer Biology, University of Toledo College of Medicine, Toledo, OH).16,17 The low dose oxytocin receptor antagonist was 3.52 μg and the high dose was 11.74 μg. The low dose vasopressin 1A receptor antagonist was 4.1 μg and the high dose was 13.65 μg. Drugs were dissolved in sterile saline and stored at 4°C.
In the atosiban experiment, animals were assigned to receive three injections (saline, low dose atosiban and high dose atosiban) using a block randomization with 3 blocks. In the selective antagonist experiment, animals were assigned to receive five injections of saline, low and high dose oxytocin receptor antagonist and low and high dose vasopressin 1A receptor antagonist using a block randomization with 5 blocks.
Immunohistochemistry
Nerve injury and age-matched male rats were anesthetized with 5% isoflurane / 95% O2 and injected with intraperitoneal Beauthanasia-D (195 mg pentobarbital sodium, 25 mg phenytoin sodium in 0.5 mL solution; Schering-Plough Corp., Kenilworth, NJ, USA). Tissues were fixed for immunohistochemistry by intracardiac perfusion using 0.01M phosphate buffered saline (PBS) containing 1% sodium nitrite followed by fixation with 0.01M PBS containing 4% formaldehyde. The entire spinal cord was dissected. The tissue was cryoprotected for 36 hours in 0.01M PBS containing 30% sucrose and embedded in OCT cryoprotectant before sectioning the cervical (C2-5), thoracic (T3-8), and lumbar (L4-6) spinal cord into 20 μm slices. Tissue sections were washed without agitation with 0.1M PBS and blocked for 1 hour at room temperature with 3% Normal Donkey Serum with 0.3% Triton X-100 in 0.1M PBS to prevent non-specific binding. All antibodies were prepared in 0.1% Normal Donkey Serum with 0.1% Triton X-100 0.1M PBS. Tissue sections were incubated in 1:100 1° monoclonal mouse antibody to rat oxytocin-neurophysin (PS 38 ATCC CRL-1950, American Type Culture Collection, Manassas, VA, USA)18 overnight at 4°C. The specificity of this antibody for oxytocin over vasopressin has been previously demonstrated18 and this antibody was recently used to demonstrate to support largely non-overlapping populations of oxytocin- and vasopressin-containing neurons in the paraventricular nucleus in the mouse.19 We confirmed a similar pattern of largely non-overlapping populations of oxytocin- and vasopressin-expressing neurons in the paraventricular nucleus of Sprague-Dawley rats (data not shown). Sections were washed without agitation in 0.1M PBS and then incubated with 2° donkey antibody to mouse conjugated to CY2 1:500 for 2 hours at room temperature without mechanical agitation (Jackson Immunoresearch, West Grove, PA, USA). Images were captured with a Nikon Ni-U Eclipse microscope and assessed for oxytocin immunoreactivity using NIS Elements software (Nikon Instruments, Inc., Melville, NY, USA). The area containing laminae I and II was determined in each image by dark field microscopy and the outlines of this area superimposed on epifluorescent images of oxytocin immunoreactivity. Deep laminae were defined as the region between the defined superficial laminae border and laminae V. The proportion of areas above a fixed threshold was calculated to determine oxytocin fiber density. The values from 4 sections at each spinal level and side per animal were averaged to yield one value for the oxytocin immunoreactive area for each level for every animal at each laminar division.
mRNA measurement
Tissue Preparation and RNA Extraction
Tissue was collected from animals 2 or 10 weeks after pSNL or from age-matched no-surgery male animals. To collect tissue, animals were anesthetized with 5% isoflurane / 95% O2 and rapidly decapitated. The L4-6 region of the spinal cord was dissected and the superficial ipsilateral and contralateral sides were collected in ice-cold 0.1M PBS. L4 and L5 dorsal root ganglia were dissected from the ipsilateral side.
The total RNA was extracted using TRIZOL (Invitrogen, Carlsbad, CA, USA). Contaminating genomic DNA was removed by DNase I digestion using DNA-free RNA kit (ZYMO Research, Irvine, CA, USA). The quality and concentration of RNA samples were assessed using a NanoDrop 2000c spectrophotometer (ThermoFisher Scientific, Waltham, MA, USA).
Reverse transcription
Reverse transcription was performed using a high-capacity cDNA reverse transcription kit (Applied Biosystems, Foster City, CA, USA). Total RNA (2 μg) was converted to single stranded cDNA. Reverse transcription without reverse transcriptase was also performed to assess genomic DNA contamination.
Quantitative real-time PCR (qPCR)
Primers were designed using qPCR primer designing software from Integrated DNA Technology and listed in Table 1 (Integrated DNA Technology Inc., Coralville, Iowa, USA). qRT-PCR was used to validate oxytocin receptor and vasopressin 1A receptor primers using oxytocin and vasopressin 1A receptor neuroblastoma (N2A)-derived hyper-expression cell lines and a non-receptor expressing N2A cell line. Primers amplified mRNA only for their respective receptors and not the other or in the N2A cell lines. Primers were designed to minimize amplification from contaminating genomic DNA.
Table 1.
Primers used
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| Oxytocin receptor | F: 5′-GGATCTACATGCTCTTCACAGG -3′ | R: 5′- CAGGACAAAGGTGGATGAGTT-3′ |
| Vasopressin 1a receptor | F: 5′- GGTCGCCTTCTTCCAAGTATTA -3′ | R: 5′- TGTCATCACCACCAGCATATAG-3′ |
| Actin | F: 5′-ACAGGATGCAGAAGGAGATTAC -3′ | R: 5′-ACAGTGAGGCCAGGATAGA -3′ |
qPCR was performed using All-in-One qPCR SYBR Green Master Mix (GeneCopoeia Inc., Rockville, MD, USA) in a 96-well format on an ABI PRISM 7500 Fast real-time PCR System (Applied Biosystems, Forester City, CA, USA). PCR reactions contained 0.2 μM of primers and 20 ng of reverse transcribed total RNA in 20 μL. PCR was performed with an initial 3-minute denaturation at 95°C followed by 40 cycles of PCR (15 seconds at 95°C, 30 seconds at 60°C and 15 seconds at 72°C). Melt curve analysis performed at the end of qPCR reproducibly showed a single peak for each gene in each sample. The relative change in the target gene expression was analyzed using 2−ΔΔCT method as previously described.20 Samples containing no cDNA template and no reverse transcriptase were run as negative controls for contamination and amplification of genomic DNA, respectively. All samples were run in triplicate. For each gene, qPCR reactions for no-surgery and injury groups were run concurrently on the same 96-well plate. The mRNA levels of oxytocin and vasopressin 1A receptors were normalized to mRNA levels of actin in each sample.
2.7. Statistics
Power analysis
Target group sizes (11 for behavioral studies and 8 for mRNA and immunohistochemistry studies) were determined prior to experimentation using power analyses on preliminary data to observe a 20% effect size in the primary outcome measures (α=0.05, [1-β] =0.8, IBM SPSS Sample Power) (IBM, Armonk, NY, USA). For the intrathecal antagonist studies, we accounted for up to a 25% exclusion rate for pSNL animals due to lack of recovery to 13 g within 5-9 weeks after surgery and received approval to prepare up to 14 animals per group to accommodate these potential exclusions.
Growth curve analysis of recovery from hypersensitivity after pSNL
Paw withdrawal threshold over time in animals allocated to the intrathecal antagonist studies but prior to their first intrathecal injection were modeled using a previously described growth curve approach using SAS version 9.2 (SAS Institute, Inc., Cary, NC, USA).5 Linear, log-linear, and quadratic functions were examined.
Intrathecal antagonist studies during recovery from pSNL
Data were not normally distributed and are presented as medians and quartiles in graphs. For both the atosiban and the selective antagonist experiments we developed models that best fit our data to analyze nested variables. The primary analyses for the experiments were clustered, where models were constructed for no-surgery females, pSNL females, no-surgery males, and pSNL males for each side in the atosiban experiment (ipsilateral and contralateral), resulting in 8 clusters and for the ipsilateral side in the selective antagonist experiment, resulting in 4 clusters. In each cluster, we developed Generalized Estimating Equations to test the factors of drug, time, and injection number using IBM SPSS version 22 (IBM, Armonk, NY, USA). Drug, time and injection number were nested variables for each animal. GEE models are most appropriate to accommodate the skewness of the data and allowed comparisons including nested measures from the same animals. The GEE analyses were best fit to the data when a log-link function was applied to the models. The residual series were examined to determine the fit of the models. Bonferroni correction was applied for comparisons within study drug and condition groups for paw withdrawal threshold at all times compared to the baseline for that group. In the atosiban experiment the corrected P was 0.0042 (0.05/12 test comparisons for significance), and in the selective antagonist experiment the corrected P was 0.0025 (0.05/20 test comparisons for significance). Secondary, exploratory analyses were the effects of all factors on the hindpaw contralateral to injury in the atosiban experiment.
Immunohistochemistry
To test the oxytocin fiber outcome between nerve injury and no-surgery groups, accommodate the skewness of the data, and compare multiple nested measures from the same animals (3 dermatomal levels and 2 sides of the spinal cord per each animal), a GEE analysis was used. The residual series were examined to determine the fit of the model. Ipsilateral and contralateral sides were examined independently with Bonferroni correction for multiple comparisons within side. All hypothesis testing was two-tailed with P values of 0.05/4= 0.0125 considered statistically significant. Pairwise comparisons were used to determine the differences between no-surgery and pSNL animals described in the results. Age was initially included as a variable, but later removed as it had no bearing on any outcomes in the experiment.
mRNA expression
Relative expression levels of mRNA for oxytocin and vasopressin 1A receptors were analyzed with 2-way ANOVAs to observe effects of surgical group and location and their interaction, and Bonferroni corrections were applied for multiple comparisons. Tukey’s post hoc analyses were performed to assess pairwise comparisons between groups at each location.
Results
Animal and data exclusion
Of the 64 animals in studies excepting the intrathecal antagonist behavioral experiments, data from one animal in the immunohistochemistry study (pSNL group 10 weeks after surgery) were excluded for data outlier (defined a priori as > 2 SD from the mean). Exclusions for the intrathecal antagonist studies are shown in Figure 1. “Exclusions by Drug” in this figure represents failure to regain a paw withdrawal threshold ≥ 13 g after the previous randomly-allocated drug and dose injection. There was no association between the nature of the previous injection (saline or drug, dose of drug) and hypersensitivity on the next testing day leading to exclusion, arguing against a residual effect only from the antagonists as a cause of these exclusions.
Figure 1. Animals included in atosiban and selective antagonist experiments.
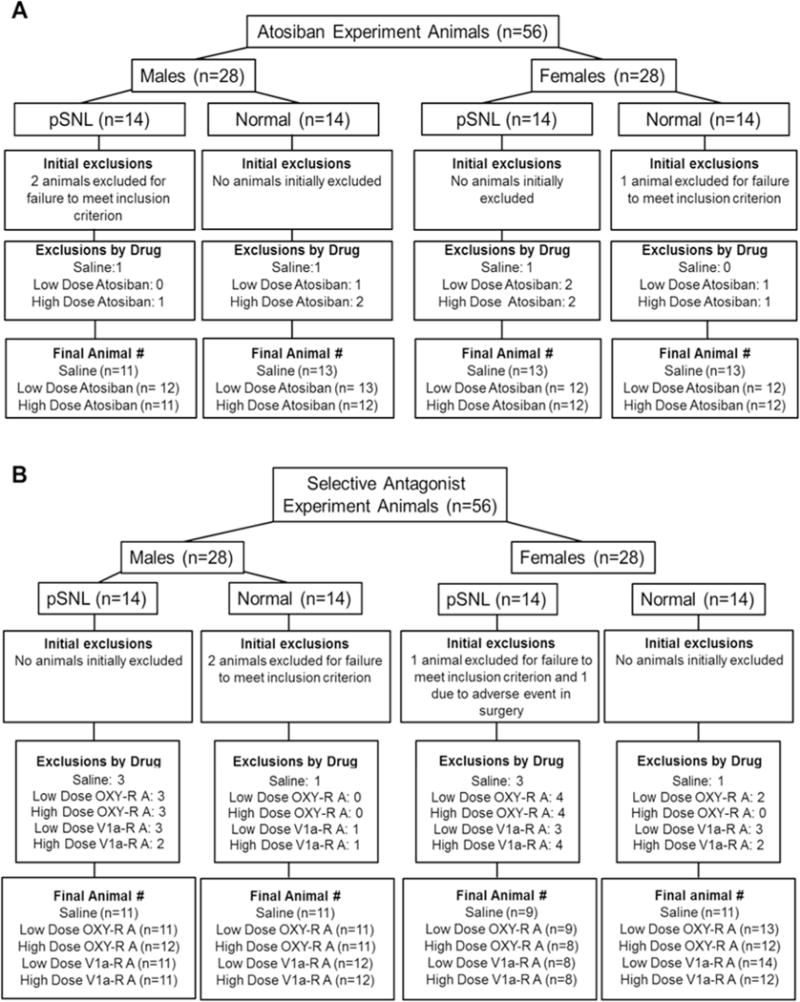
Flow diagram of animals included in A) atosiban and B) selective oxytocin receptor (OXY-R) and vasopressin 1A receptor (V1a-R) antagonist studies. pSNL: partial spinal nerve ligation.
Spinal oxytocin receptor and vasopressin 1A receptor antagonism during partial recovery from pSNL
The time course of paw withdrawal threshold after pSNL is shown in Figure 2. Paw withdrawal threshold over time was best fit by a quadratic function. There was no sex difference in the modeled change of paw withdrawal threshold over time. Only 6 of 112 animals failed to meet inclusion criteria for the first intrathecal injection.
Figure 2. Time course of recovery from hypersensitivity after surgery.
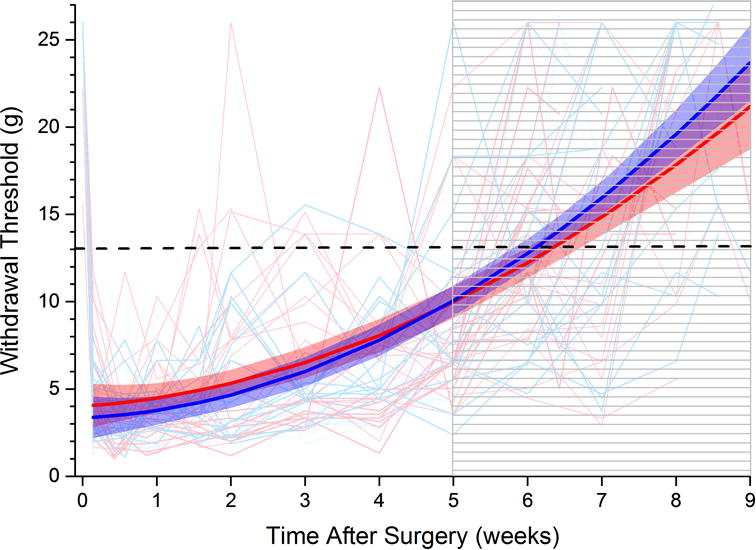
Paw withdrawal threshold before and after partial spinal nerve ligation surgery in individual female (fine pink lines) and male (fine blue lines) rats. (Time and withdrawal threshold for eligibility to enter into intrathecal antagonist studies shown by striped area and dashed line, respectively. Thick lines and shaded areas represent mean and 95% confidence limits for growth curve modeled trajectory in females (red) and males (blue). No sex difference.
Atosiban
A statistically significant interaction was found in GEE analyses for the main effect of Drug*Time*Injection in the contralateral and ipsilateral sides to injury in the overall analyses for both males and females after pSNL injury and in no-surgery females when compared to their baseline paw withdrawal threshold (Table 2). No main effect of Drug*Time*Injection was found in no-surgery male animals on the contralateral or ipsilateral side. These effects describe an overall analysis where all Drug*Time*Injections were compared within surgery, sex and side clusters. The main effects of the Drug*Time*Injection analyses were further analyzed to compare paw withdrawal thresholds over time after administration of atosiban or saline (saline, low dose atosiban, high dose atosiban) compared to the baseline paw withdrawal threshold for each drug.
Table 2.
Main effects from General Estimating Equation analysis in primary outcome comparison for intrathecal atosiban and antagonist experiments.
| Main Effect Source: Drug*Time*Injection | ||||||
|---|---|---|---|---|---|---|
| Experiment | Sex | Surgery | Side | Wald X2 | df | Significance |
| Atosiban | Female | Normal | Contralateral | 1.5 × 1011 | 11 | <1 × 10−15* |
| Female | Normal | Ipsilateral | 3.6 × 1013 | 11 | <1 × 10−15* | |
| Female | pSNL | Contralateral | 1.6 × 103 | 9 | <1 × 10−15* | |
| Female | pSNL | Ipsilateral | 2.2 × 1012 | 10 | <1 × 10−15* | |
| Male | Normal | Contralateral | 1.6 × 101 | 10 | 0.096 | |
| Male | Normal | Ipsilateral | 1.4 × 101 | 10 | 0.159 | |
| Male | pSNL | Contralateral | 2.8 × 1013 | 11 | <1 × 10−15* | |
| Male | pSNL | Ipsilateral | 3.4 × 1013 | 13 | <1 × 10−15* | |
|
| ||||||
| Selective | Female | Normal | Ipsilateral | 7.5 × 107 | 11 | <1 × 10−15* |
| Antagonist | Female | pSNL | Ipsilateral | 1.1 × 1010 | 11 | <1 × 10−15* |
| Male | Normal | Ipsilateral | 2.0 × 1013 | 10 | <1 × 10−15* | |
| Male | pSNL | Ipsilateral | 4.5 × 1012 | 13 | <1 × 10−15* | |
In females with partial recovery after pSNL, intrathecal atosiban reduced paw withdrawal threshold ipsilateral and, to a lesser extent, contralateral to injury (Figure 3A and 3B) whereas in males intrathecal atosiban reduced paw withdrawal threshold only ipsilateral to injury (Figure 3C and 3D). Intrathecal atosiban did not affect paw withdrawal threshold in no-surgery male or female rats (Figure 3). Baseline values differed between normal and pSNL groups, but did not differ among intrathecal treatments within each of these groups.
Figure 3. Effects of saline and high and low doses of intrathecal atosiban on paw withdrawal thresholds in male and female rats after intermediate recovery from partial spinal nerve ligation (pSNL) and in age-matched, no-surgery controls.
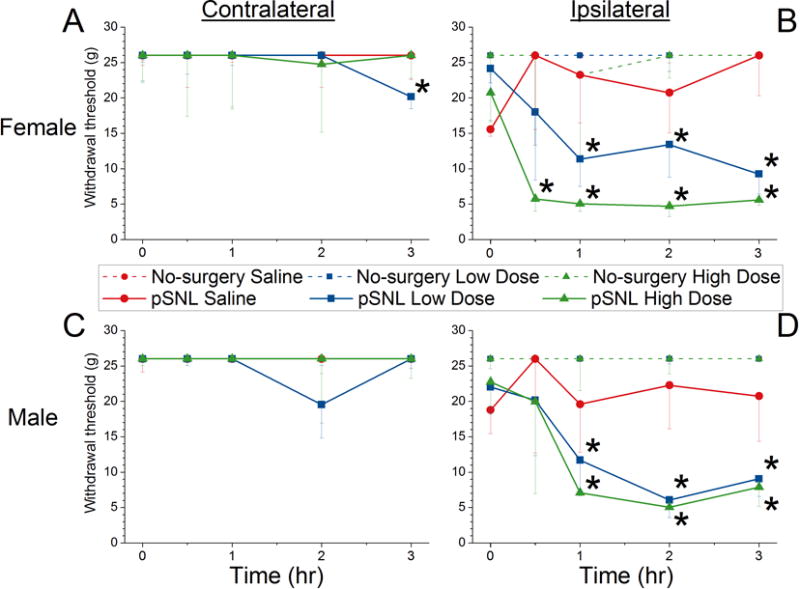
Female (A=contralateral, B=ipsilateral) and male (C=contralateral, D=ipsilateral) animals after administration at time 0 of saline (red), low dose atosiban (blue) or high dose atosiban (green). Dotted lines represent no-surgery animals and solid lines represent pSNL animals.
Data are presented as median and 25th percentile values.
* P<0.0042 vs baseline within drug group. See Figure 1A for group sizes.
Selective antagonists
A statistically significant interaction was found in GEE analyses for the main effect of Drug*Time*Injection in the ipsilateral to injury sides in the overall analyses for both males and females after pSNL injury and in no-surgery males and females compared to their baseline paw withdrawal threshold (Table 2). The main effects of the Drug*Time*Injection analyses were further analyzed to compare paw withdrawal thresholds over time after administration of selective antagonists or saline (saline, low dose oxytocin receptor antagonist, high dose oxytocin receptor antagonist, low dose vasopressin 1A receptor antagonist and high dose vasopressin 1A receptor antagonist) compared to the baseline paw withdrawal threshold for each drug.
Both antagonists reduced paw withdrawal threshold in animals of both sexes that had partially recovered from pSNL surgery. In all cases, both low- and high-dose intrathecal antagonists selective for either vasopressin 1A or oxytocin receptors reduced paw withdrawal threshold (Figure 4). Small effects of intrathecal saline were observed at isolated time points, with a decrease in paw withdrawal threshold in males 1 hr after injection increase in females 1 and 3 hr after injection (Figure 4).
Figure 4. Effects of saline and high and low doses of oxytocin receptor(OXY-R) and vasopressin 1a receptor (V1a-R) antagonist on paw withdrawal thresholds in male and female rats in male and female rats after intermediate recovery from partial spinal nerve ligation (pSNL) and in age-matched, no-surgery controls.
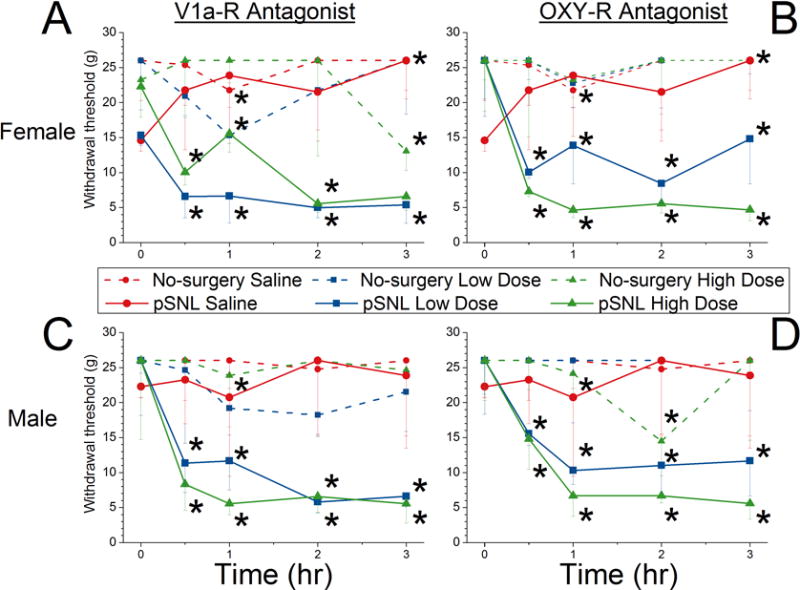
Female (A=OXY-R antagonist, B=V1A-R antagonist) and male (C=OXY-R antagonist, D=V1A-R antagonist) animals after administration at time 0 of saline (red), low dose (blue) or high dose (green) antagonist. Dotted lines represent no-surgery animals and solid lines represent pSNL animals.
Data are presented as median and 25th percentile values.
* P<0.0025 vs baseline within drug group. See Figure 1B for group sizes.
In no-surgery animals, intrathecal antagonists affected paw withdrawal threshold only at a few isolated time points, including a decreased paw withdrawal threshold in females 3 hours after administration of the high dose of vasopressin 1A receptor antagonist (Figure 4A) and 0.5 hours after administration of the high dose of oxytocin receptor antagonist (Figure 4B). In males the high dose of oxytocin receptor antagonist decreased paw withdrawal threshold 2 hours after administration of (Figure 4D).
Effect of surgical injury on oxytocin innervation of the spinal cord
Paw withdrawal threshold decreased in animals randomized to pSNL from 24 ± 1.0 gm before surgery to 14 ± 2.4 gm (P<0.05) at the time of tissue harvest after surgery. Representative images of oxytocin immunoreactivity in the cervical, thoracic, and lumbar spinal cord at 10 weeks after pSNL (ipsilateral to injury) and age-matched no-surgery animals (right side) show fiber staining concentrated in the superficial dorsal horn that is greater in lumbar than cervical or thoracic regions and is increased in the injured animals (Figure 5A). The GEE analyses indicated a significant main effect of Surgical Group in the deep laminae of the ipsilateral and contralateral side, a main effect of Spinal Cord Level in the superficial laminae of the contralateral side and in the superficial and deep laminae of the ipsilateral side. A statistically significant interaction was found in GEE analyses for Surgical Group*Spinal Level in the superficial laminae of both contralateral and ipsilateral sides. Quantitative analysis confirmed an approximate 2-fold greater oxytocin immunoreactive area in the superficial dorsal horn of the lumbar cord compared to other areas, and an increase in oxytocin immunoreactivity ipsilateral, but not contralateral to injury in the lumbar cord (Figure 5B). There was also a decrease in oxytocin immunoreactivity ipsilateral to injury in the cervical spinal cord (Figure 5B).
Figure 5. Anatomy of spinal cord oxytocin innervation and effect of nerve injury.
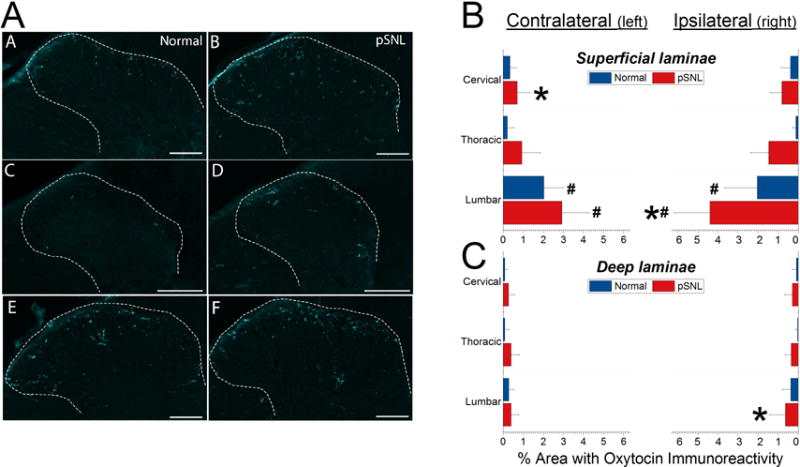
A) Representative images of cervical, thoracic, and lumbar oxytocin immunoreactivity on the right side of the spinal cord in no-surgery animals in left panels and animals 10 weeks after partial spinal nerve ligation (pSNL) injury in right panels. Scale bar represents 100 μm. B and C provide quantitative results of proportion of area occupied by oxytocin immunoreactivity in superficial and deep laminae of the spinal cord dorsal horn, respectively, in pSNL animals (n=15, ages combined) compared to age matched no-surgery animals (n=16, ages combined).
Data are presented as mean + SD.
# P<0.001 compared to cervical or thoracic regions
* P=0.012 (superficial laminae) or P=0.007 (deep laminae) compared to normal animals
Although there was a smaller proportion of deeper laminae occupied by oxytocin immunoreactivity than in the superficial dorsal horn, there was a similar cephalocaudal distribution, with greater oxytocin immunoreactivity in lumbar compared to thoracic and cervical regions (Figure 5C).
Effect of surgical injury on oxytocin and vasopressin 1A receptor mRNA in spinal cord and dorsal root ganglia
Paw withdrawal threshold decreased in animals randomized to pSNL surgery from 22 ± 1.4 gm before surgery to 12 ± 2.6 gm (P<0.05) at the time of tissue harvest after surgery whereas it did not change in no-surgery animals (26 ± 0 to 24 ± 1.4 gm). mRNA expression did not differ in tissues from animals 2 or 10 weeks after surgery, so these results were combined for analysis. Two-way ANOVAs revealed main effects and their interactions between surgical group and location for both oxytocin (P<0.001) and vasopressin 1A (P=0.002) receptor mRNA expression levels. Injury was associated with a 6.5-fold increase of oxytocin receptor mRNA level in the L4 dorsal root ganglion ipsilateral to injury and 1.7-fold increase in the lumbar spinal cord contralateral to injury compared to no-surgery animals (Figure 6; P=0.002 and P=0.046, respectively). Injury was also associated with a 2.3-fold increase in vasopressin 1A receptor mRNA level in the L4 dorsal root ganglion ipsilateral to surgery compared to no-surgery animals (Figure 6; P=0.013).
Figure 6. Oxytocin receptor (OXY-R) and vasopressin 1A receptor (V1A-R) mRNA expression.
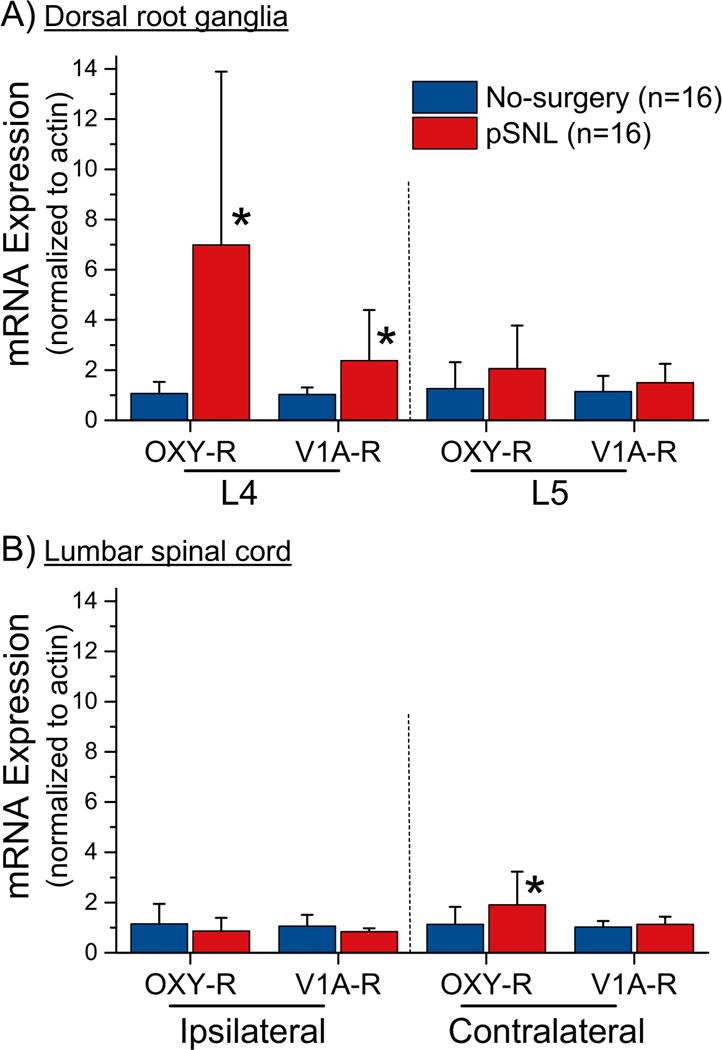
mRNA in A) dorsal root ganglia and B) lumbar spinal cord in rats after partial spinal nerve ligation (pSNL) and age matched no-surgery controls.
Data are presented as mean +SD.
*P<0.05 (Bonferroni corrected) compared to normal
Discussion
Preventing chronic pain after surgery is a major focus of preclinical and clinical research, yet there are few clinical signals to guide mechanistic work in animals, and much of previous preclinical research has focused on minor surgical procedures with rapid recovery (incision) or major nerve injury with exceedingly slow recovery (spinal nerve ligation). Clinical trials of drugs active to transiently treat pain behaviors after major nerve injury and in patients with chronic neuropathic pain such as gabapentinoids or monoamine reuptake inhibitors have shown mixed efficacy to speed recovery or reduce chronic pain after surgery.21,22 The key contribution of the current study is to extend the clinical observation of protection from chronic pain after surgery in women in the postpartum period11 to a role for spinal oxytocin plasticity and signaling in recovery from surgical injury-induced hypersensitivity in both sexes outside this period.
Acute reversal of recovery from mechanical hypersensitivity
Oxytocin and vasopressin 1A receptor agonists reduce hypersensitivity after inflammatory, chemical, or surgical injuries by actions in the periphery, spinal cord, and supraspinal areas.23 The novel contribution of the current study is the demonstration of ongoing activation of these receptors in the spinal cord and/or sensory afferents during the recovery phase after injury. These data are consistent with accelerated recovery from surgery-induced hypersensitivity in postpartum rats which is transiently reversed when oxytocin release is reduced during weaning of pups or when spinal oxytocin and vasopressin 1A receptors are blocked by intrathecal injection of a non-selective antagonist.12 Whether the site(s) of oxytocin signaling in this setting reside on interneurons in the spinal cord24 or on primary afferents25 cannot be determined by intrathecal injection, which affects both.
Clear sex differences exist for some supraspinal actions of oxytocin, such as pair-bonding, in which the sex difference reflects differences in oxytocin and vasopressin 1A receptor localization rather than oxytocin neuronal innervation patterns.26 Both oxytocin and vasopressin 1A receptors are present in the spinal cord of male and female rodents,27 although methodologic limitations in these studies preclude the definitive exclusion of a subtle sex difference if it exists. Similarly, there is not a sex difference in the anti-mechanical hypersensitivity effect of intrathecal oxytocin itself in rats, although its duration of action is shorter in females.28 Our anatomic and behavioral data do not support a major sex difference in tonic spinal oxytocin signaling during recovery from surgery and confirm our previous findings5 of a lack of sex difference in speed of recovery after surgery. Ongoing clinical trials of intrathecal oxytocin29 will determine whether there is a sex difference in humans.
Evidence in various species, injuries, drugs, and routes of administration support a primary mechanism of oxytocin on pain neurotransmission to reflect actions on oxytocin receptors,30 vasopressin 1A receptors,25 or both.23 The current study comparing dose responses after intrathecal injection of two highly selective peptide antagonists16,17 suggests that both receptors are activated to reduce hypersensitivity during recovery from surgery. We recognize that potential differences in drug disposition and dispersion after intrathecal injection limit this interpretation and are currently developing and validating selective conditional knockdown of these receptors to more definitively address this question. In addition, whether simultaneous activation of both receptors by oxytocin results in synergistic activity after surgery is not addressed in the current study.
Surgical injury-induced alterations of spinal oxytocin expression
Peripheral nerve injury results in a plethora of anatomic and biochemical changes in the spinal cord in response to factors released from injured and nearby uninjured sensory afferents and from inflammatory products generated by glia.31 Among these is a brain derived growth factor-dependent increase in norepinephrine content and noradrenergic fiber immunoreactivity in the superficial dorsal horn restricted to dermatomes surrounding those receiving input from injury and which begins within days of injury and lasts for weeks.32 The current results suggest that a similar response occurs with oxytocin innervation in the spinal cord, since oxytocin fiber immunoreactivity was increased in a similar, dermatomally restricted pattern at 2 and 10 weeks after surgery. Although we interpret this increase in fiber immunoreactivity to reflect an anatomic change, other interpretations are possible, including injury-induced increased neurotransmitter synthesis and storage or increased antigen availability or presentation to the antibody. The factors driving this change in immunoreactivity are currently unknown.
We focused in these studies on oxytocin rather than vasopressin immunoreactivity for two reasons. First, recovery from surgical injury in rodents and humans occurs more rapidly when injury occurs at or near the time of delivery,11,12 a time of increased oxytocin, but not vasopressin activity. Second, anatomic, physiologic, and stimulation studies indicate that the primary source of dorsal horn spinal oxytocin comes from fibers descending from the paraventricular nucleus of the hypothalamus.33–35 In contrast, descending vasopressin fibers primarily innervate the intermediolateral cell column, with scant presence in the dorsal horn.36
Surgical injury-induced alterations in oxytocin and vasopressin 1A receptor mRNA expression
Both oxytocin and vasopressin 1A receptors are expressed on primary sensory afferent cell bodies in dorsal root ganglia in rodents and humans,37 and both receptors have been implicated in antinociceptive or anti-hypersensitive actions on sensory afferents.38,39 We observed an upregulation of mRNA for oxytocin and, to a lesser extent, vasopressin 1A receptors in lumbar dorsal root ganglia ipsilateral to injury, but not in the spinal cord. In contrast to our findings, infraorbital nerve ligation in rats increases the proportion of trigeminal ganglion neurons which are immunoreactive for vasopressin 1A, but not oxytocin receptors.25 Whether this discrepancy reflects a difference between mRNA expression and immunoreactivity for protein, between injury procedures, or between trigeminal and dorsal root ganglion sites is not clear. Nonetheless, our observations of increased expression of both receptors upon with oxytocin acts in the dorsal root ganglion is consistent with behavioral results showing tonic activation of both during process of the recovery from surgery.
Limitations
There are limitations to these studies in addition to those already discussed. Hypersensitivity, although often present after surgery and correlated with risk of persistent pain 2,3, is not a measure of pain per se. We are in the process of validating novel motivated and operant behavioral methods that can be repeatedly examined over prolonged periods of time to more completely assess pain behavior during recovery from surgery. Also, sustained spinal delivery of antagonists would more directly address the role of spinal oxytocin signaling on the speed of recovery than transient effects after single bolus administration. The current study provides dose response data essential to the design of such studies, although whether prolonged intrathecal catheterization would in itself alter speed of recovery is unknown. The behavioral studies were not powered to observe subtle sex differences, immunoreactivity is only semi-quantitative and can be influenced by alterations in oxytocin synthesis or storage or by antigen presentation caused by injury rather than changes in fiber anatomy, and tissue measurement of mRNA precludes cellular localization. Finally, we chose a surgical procedure involving known nerve injury because of its time course of recovery and clinical observations that likelihood of chronic pain after surgery increases with extent of presumed nerve injury.40 We recognize that chronic pain can occur after surgery without known injury to large peripheral nerves and that chronic pain after surgery does not frequently present with neuropathic features. We chose age-matched normal animal control groups rather than sham surgery because our primary interest was in the differential effects in biochemical, anatomic, and behavioral measures in animals recovering from surgery compared to animals without other manipulations. As such, we cannot comment on the specific contribution of the nerve injury element of the surgical procedure.
Conclusions
In summary, acute antagonism in either female or male rats of oxytocin or vasopressin 1A receptors in the lumbar intrathecal compartment during the period of active recovery from hypersensitivity after peripheral nerve injury surgery transiently reverses this recovery. At the same time there is an increase in oxytocin fiber immunoreactivity in the lumbar spinal cord and in oxytocin and vasopressin 1A receptor mRNA in lumbar dorsal root ganglion ipsilateral to surgery. These data extend observations in females in the postpartum period to suggest that spinal oxytocin signaling is a factor which is involved in regulating the speed and extent of recovery from surgery in both sexes and which could be manipulated to speed such recovery.
Summary statement.
Spinal oxytocin innervation and receptor expression increase after surgery involving nerve injury in rats and spinal injection of antagonists of oxytocin signaling reverse partial recovery from mechanical hypersensitivity in both sexes.
Acknowledgments
Supported in part by grant R37 GM48085 to JCE from the National Institutes of Health, Bethesda, MD, USA.
Footnotes
Conflict of interest: In the past 36 months JCE has consulted to Adynxx (San Francisco, CA, USA) and TEVA Pharmaceutical Industries (North Wales, PA, USA) regarding preclinical and clinical analgesic development of non-oxytocin targets. The remaining authors declare no conflicts of interest.
References
- 1.Gaudilliere B, Fragiadakis GK, Bruggner RV, Nicolau M, Finck R, Tingle M, Silva J, Ganio EA, Yeh CG, Maloney WJ, Huddleston JI, Goodman SB, Davis MM, Bendall SC, Fantl WJ, Angst MS, Nolan GP. Clinical recovery from surgery correlates with single-cell immune signatures. Sci Transl Med. 2014;6:255ra131. doi: 10.1126/scitranslmed.3009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perkins FM, Kehlet H. Chronic pain as an outcome of surgery - A review of predictive factors. Anesthesiology. 2000;93:1123–1133. doi: 10.1097/00000542-200010000-00038. [DOI] [PubMed] [Google Scholar]
- 3.Yarnitsky D, Crispel Y, Eisenberg E, Granovsky Y, Ben-Nun A, Sprecher E, Best LA, Granot M. Prediction of chronic post-operative pain: pre-operative DNIC testing identifies patients at risk. Pain. 2008;138:22–28. doi: 10.1016/j.pain.2007.10.033. [DOI] [PubMed] [Google Scholar]
- 4.Peters CM, Hayashida KI, Suto T, Houle TT, Aschenbrenner CA, Martin TJ, Eisenach JC. Individual Differences in Acute Pain-induced Endogenous Analgesia Predict Time to Resolution of Postoperative Pain in the Rat. Anesthesiology. 2015;122:895–907. doi: 10.1097/ALN.0000000000000593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aschenbrenner CA, Houle TT, Gutierrez S, Eisenach JC. Modeling individual recovery after peripheral nerve injury in rats and the effects of parturition. Anesthesiology. 2014;121:1056–1067. doi: 10.1097/ALN.0000000000000360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eisenach JC. Preventing chronic pain after surgery: who, how, and when? Reg Anesth Pain Med. 2006;31:1–3. doi: 10.1016/j.rapm.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 7.Kaasa T, Romundstad L, Roald H, Skolleborg K, Stubhaug A. Hyperesthesia one year after breast augmentation surgery increases the odds for persistent pain at four years. A prospective four-year follow-up study. Scandinavian Journal of Pain. 2010;1:75–81. doi: 10.1016/j.sjpain.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 8.Corder G, Doolen S, Donahue RR, Winter MK, Jutras BL, He Y, Hu X, Wieskopf JS, Mogil JS, Storm DR, Wang ZJ, McCarson KE, Taylor BK. Constitutive mu-opioid receptor activity leads to long-term endogenous analgesia and dependence. Science. 2013;341:1394–1399. doi: 10.1126/science.1239403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma WY, Eisenach JC. Chronic constriction injury of sciatic nerve induces the up-regulation of descending inhibitory noradrenergic innervation to the lumbar dorsal horn of mice. Brain Research. 2003;970:110–118. doi: 10.1016/s0006-8993(03)02293-5. [DOI] [PubMed] [Google Scholar]
- 10.Bantel C, Eisenach JC, Duflo F, Tobin JR, Childers SR. Spinal nerve ligation increases a2-adrenergic receptor G-protein coupling in the spinal cord. Brain Research. 2005;1038:76–82. doi: 10.1016/j.brainres.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 11.Eisenach JC, Pan P, Smiley RM, Lavand’homme P, Landau R, Houle TT. Resolution of Pain after Childbirth. Anesthesiology. 2013;118:143–151. doi: 10.1097/ALN.0b013e318278ccfd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gutierrez S, Liu B, Hayashida K, Houle TT, Eisenach JC. Reversal of Peripheral Nerve Injury-induced Hypersensitivity in the Postpartum Period: Role of Spinal Oxytocin. Anesthesiology. 2013;118:152–159. doi: 10.1097/ALN.0b013e318278cd21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guan Y, Yuan F, Carteret AF, Raja SN. A partial L5 spinal nerve ligation induces a limited prolongation of mechanical allodynia in rats: an efficient model for studying mechanisms of neuropathic pain. Neurosci Lett. 2010;471:43–47. doi: 10.1016/j.neulet.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. Journal of Neuroscience Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 15.Gutierrez S, Hayashida K, Eisenach JC. The puerperium alters spinal cord plasticity following peripheral nerve injury. Neuroscience. 2013;228:301–308. doi: 10.1016/j.neuroscience.2012.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chan WY, Wo NC, Cheng LL, Manning M. Isosteric substitution of Asn5 in antagonists of oxytocin and vasopressin leads to highly selective and potent oxytocin and V1a receptor antagonists: new approaches for the design of potential tocolytics for preterm labor. J Pharmacol Exp Ther. 1996;277:999–1003. [PubMed] [Google Scholar]
- 17.Manning M, Miteva K, Pancheva S, Stoev S, Wo NC, Chan WY. Design and synthesis of highly selective in vitro and in vivo uterine receptor antagonists of oxytocin: comparisons with Atosiban. Int J Pept Protein Res. 1995;46:244–52. doi: 10.1111/j.1399-3011.1995.tb00596.x. [DOI] [PubMed] [Google Scholar]
- 18.Ben-Barak Y, Russell JT, Whitnall MH, Ozato K, Gainer H. Neurophysin in the hypothalamo-neurohypophysial system. I Production and characterization of monoclonal antibodies. J Neurosci. 1985;5:81–97. doi: 10.1523/JNEUROSCI.05-01-00081.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Otero-Garcia M, Agustin-Pavon C, Lanuza E, Martinez-Garcia F. Distribution of oxytocin and co-localization with arginine vasopressin in the brain of mice. Brain Struct Funct. 2016;221:3445–73. doi: 10.1007/s00429-015-1111-y. [DOI] [PubMed] [Google Scholar]
- 20.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 21.Schmidt PC, Ruchelli G, Mackey SC, Carroll IR. Perioperative gabapentinoids: choice of agent, dose, timing, and effects on chronic postsurgical pain. Anesthesiology. 2013;119:1215–1221. doi: 10.1097/ALN.0b013e3182a9a896. [DOI] [PubMed] [Google Scholar]
- 22.YaDeau JT, Brummett CM, Mayman DJ, Lin Y, Goytizolo EA, Padgett DE, Alexiades MM, Kahn RL, Jules-Elysee KM, Fields KG, Goon AK, Gadulov Y, Westrich G. Duloxetine and Subacute Pain after Knee Arthroplasty when Added to a Multimodal Analgesic Regimen: A Randomized, Placebo-controlled, Triple-blinded Trial. Anesthesiology. 2016;125:561–72. doi: 10.1097/ALN.0000000000001228. [DOI] [PubMed] [Google Scholar]
- 23.Gonzalez-Hernandez A, Rojas-Piloni G, Condes-Lara M. Oxytocin and analgesia: future trends. Trends Pharmacol Sci. 2014;35:549–51. doi: 10.1016/j.tips.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 24.Breton JD, Veinante P, Uhl-Bronner S, Vergnano AM, Freund-Mercier MJ, Schlichter R, Poisbeau P. Oxytocin-induced antinociception in the spinal cord is mediated by a subpopulation of glutamatergic neurons in laminas I-II which amplify GABAergic inhibition. Mol Pain. 2008;4:19. doi: 10.1186/1744-8069-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kubo A, Shinoda M, Katagiri A, Takeda M, Suzuki T, Asaka J, Yeomans DC, Iwata K. Oxytocin alleviates orofacial mechanical hypersensitivity associated with infraorbital nerve injury through vasopressin-1A receptors of the rat trigeminal ganglia. Pain. 2016;158:649–659. doi: 10.1097/j.pain.0000000000000808. [DOI] [PubMed] [Google Scholar]
- 26.Young LJ. The neuroendocrinology of the social brain. Front Neuroendocrinol. 2009;30:425–428. doi: 10.1016/j.yfrne.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 27.Tribollet E, Barberis C, Arsenijevic Y. Distribution of vasopressin and oxytocin receptors in the rat spinal cord: sex-related differences and effect of castration in pudendal motor nuclei. Neuroscience. 1997;78:499–509. doi: 10.1016/s0306-4522(96)00591-x. [DOI] [PubMed] [Google Scholar]
- 28.Chow LH, Chen YH, Wu WC, Chang EP, Huang EY. Sex Difference in Oxytocin-Induced Anti-Hyperalgesia at the Spinal Level in Rats with Intraplantar Carrageenan-Induced Inflammation. PLoS One. 2016;11:e0162218. doi: 10.1371/journal.pone.0162218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eisenach JC, Tong C, Curry R. Phase 1 safety assessment of intrathecal oxytocin. Anesthesiology. 2015;122:407–13. doi: 10.1097/ALN.0000000000000539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Juif PE, Poisbeau P. Neurohormonal effects of oxytocin and vasopressin receptor agonists on spinal pain processing in male rats. Pain. 2013;154:1449–1456. doi: 10.1016/j.pain.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 31.Kuner R, Flor H. Structural plasticity and reorganisation in chronic pain. Nat Rev Neurosci. 2017;18:20–30. doi: 10.1038/nrn.2017.5. [DOI] [PubMed] [Google Scholar]
- 32.Hayashida K, Clayton BA, Johnson JE, Eisenach JC. Brain derived nerve growth factor induces spinal noradrenergic fiber sprouting and enhances clonidine analgesia following nerve injury in rats. Pain. 2008;136:348–355. doi: 10.1016/j.pain.2007.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martinez-Lorenzana G, Espinosa-Lopez L, Carranza M, Aramburo C, Paz-Tres C, Rojas-Piloni G, Condes-Lara M. PVN electrical stimulation prolongs withdrawal latencies and releases oxytocin in cerebrospinal fluid, plasma, and spinal cord tissue in intact and neuropathic rats. Pain. 2008;140:265–273. doi: 10.1016/j.pain.2008.08.015. [DOI] [PubMed] [Google Scholar]
- 34.Condes-Lara M, Martinez-Lorenzana G, Rojas-Piloni G, Rodriguez-Jimenez J. Branched oxytocinergic innervations from the paraventricular hypothalamic nuclei to superficial layers in the spinal cord. Brain Res. 2007;1160:20–29. doi: 10.1016/j.brainres.2007.05.031. [DOI] [PubMed] [Google Scholar]
- 35.Miranda-Cardenas Y, Rojas-Piloni G, Martínez-Lorenzana G, Rodríguez-Jiménez J, López-Hidalgo M, Freund-Mercier MJ, Condés-Lara M. Oxytocin and electrical stimulation of the paraventricular hypothalamic nucleus produce antinociceptive effects that are reversed by an oxytocin antagonist. Pain. 2006;122:182–189. doi: 10.1016/j.pain.2006.01.029. [DOI] [PubMed] [Google Scholar]
- 36.Rood BD, De Vries GJ. Vasopressin innervation of the mouse (Mus musculus) brain and spinal cord. J Comp Neurol. 2011;519:2434–74. doi: 10.1002/cne.22635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shepherd AJ, Mickle AD, Copits BA, Karlsson P, Kadunganattil S, Golden JP, Tadinada SM, Mack MR, Haroutounian S, de Kloet AD, Samineni VK, Valtcheva MV, McIlvried LA, Sheahan TD, Jain S, Ray PR, Usachev YM, Dussor G, Kim BS, Krause EG, Price TJ, Gereau RW, Mohapatra DP. Macrophage-to-sensory neuron crosstalk mediated by Angiotensin II type-2 receptor elicits neuropathic pain. bioRxiv. 2017 [Google Scholar]
- 38.Gong L, Gao F, Li J, Li J, Yu X, Ma X, Zheng W, Cui S, Liu K, Zhang M, Kunze W, Liu CY. Oxytocin-induced membrane hyperpolarization in pain-sensitive dorsal root ganglia neurons mediated by Ca(2+)/nNOS/NO/KATP pathway. Neuroscience. 2015;289:417–28. doi: 10.1016/j.neuroscience.2014.12.058. [DOI] [PubMed] [Google Scholar]
- 39.Qiu F, Qiu CY, Cai H, Liu TT, Qu ZW, Yang Z, Li JD, Zhou QY, Hu WP. Oxytocin inhibits the activity of acid-sensing ion channels through the vasopressin, V1A receptor in primary sensory neurons. Br J Pharmacol. 2014;171:3065–76. doi: 10.1111/bph.12635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gartner R, Jensen MB, Nielsen J, Ewertz M, Kroman N, Kehlet H. Prevalence of and factors associated with persistent pain following breast cancer surgery. JAMA. 2009;302:1985–1992. doi: 10.1001/jama.2009.1568. [DOI] [PubMed] [Google Scholar]


